Abstract
Myopericytoma is a rare perivascular tumor commonly arising in the superficial soft tissue and subcutaneous tissue of the distal extremities. We report the first case of myopericytoma occurring in the breast, focusing on the imaging and histopathological characteristics of the tumor. From an imaging perspective, myopericytoma presents a well-circumscribed, marked hypervascularity, and intense enhancement after injection of contrast material. Imaging examinations, such as ultrasonography and magnetic resonance imaging, can contribute to the detection of tumor invasion to adjacent structures or distant metastases, and provide evidence for a treatment plan.
Keywords: Breast, Myopericytoma, Imaging feature, Immunohistochemistry, Pathology
Introduction
Myopericytoma is a rare soft tissue tumor and generally benign. The spindle-shaped myopericytic cells proliferate around vessels with a characteristic concentric distribution. There are no characteristic clinical manifestations of the disease and the diagnosis mainly depends on histopathological examination. Most previous cases of myopericytoma were described with pathological findings, and few imaging findings have been established. Herein, we report a case of breast myopericytoma along with its imaging and pathological features. Based on the past clinical literature, this is the first case report of this rare tumor arising in the breast. We also present a review of the previous literature on myopericytoma to summarize the imaging features of the tumor.
Case report
A 63-year-old woman presented with a mass in the left breast for 3 months that had gradually enlarged during the last month. Physical examination revealed an approximately 5 × 4-cm immovable mass in the lower inner quadrant of her left breast, with no nipple discharge. No axillary lymphadenopathy was noted. She had undergone fibroadenoma resection in the right breast 10 years previously with no family history of breast cancer.
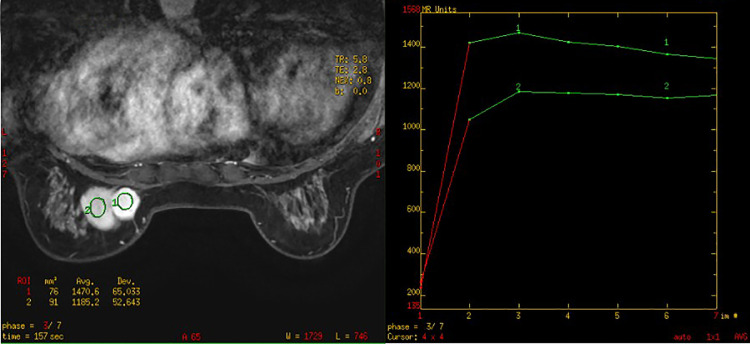
Sonographic examination demonstrated 2 well-demarcated heterogeneous hypoechoic adjacent solid masses of 4.2 × 1.5 cm and 2.0 × 1.2 cm in the left breast. Prominent color signals were noted within the 2 masses on color Doppler flow imaging. A syringe was used to inject 4.8 ml of SonVue suspension into the antecubital vein. On the resulting contrast-enhanced ultrasound images, the lesions exhibited enhancement from the periphery to the center (Fig. 1). On magnetic resonance imaging (MRI) examination, the 2 lesions appeared hypointense on T1-weighted sequences and hyperintense on T2-weighted sequences. The time-signal intensity curve obtained from breast dynamic contrast-enhanced MRI showed a plateau (type II) (Fig. 2). After gadolinium administration, the large mass showed peripheral enhancement and central irregular low contrast enhancement, while the small mass showed overall intense homogeneous contrast enhancement (Fig. 3).
Fig. 1.
Contrast-enhanced ultrasound images. (A) Central irregular non-enhancement of the lesion (arrow). (B) The lesion demonstrated marked abundant internal vascularity (arrowhead).
Fig. 2.
The time-signal intensity curve of the lesions showed a plateau (type II).
Fig. 3.
MRI findings. (A–F) Short TI inversion recovery images before (A, D) and after (B, C, E, F) contrast administration. (A) The large lesion. (B) The large lesion demonstrated avid, but heterogeneous, predominantly peripheral enhancement. (C) The large lesion showed central irregular non-enhancement. (D) The adjacent small lesion. (E, F) The small lesion showed overall intense homogeneous contrast enhancement.
An ultrasound-guided fine-needle aspiration biopsy revealed that the masses comprised oval-to-spindle-shaped cells with stroma and exhibited focal myxoid degeneration in their central regions. There were no atypia and mitotic cells.
The patient underwent surgical excision of the masses. The 2 masses were stuck together with well-circumscribed margins, but were not adherent to adjacent tissue. Histological examination revealed many thin-walled branching vessels surrounded by proliferative oval-to-spindle-shaped myoid cells in the peripheral area (Fig. 4). On immunochemical staining, the cells were positive for smooth muscle actin and vimentin. Immunostaining for other proteins, including desmin, CK5/6, and S-100 protein, was negative. The proliferation index Ki-67 was about 10%. Anti-CD34 antibodies only labeled vascular endothelial cells. Specialized staining including Verhoeff-Van Gieson, Hematoxylin-Eosin, and Masson indicated small amounts of fibers and blood vessels. Overall, the final diagnosis was myopericytoma. The patient remains alive without tumor recurrence or metastasis during follow-up at 29 months after surgery.
Fig. 4.
(A) Pathological examination revealed numerous thin-walled vessels surrounded concentrically by proliferative oval-to-spindle-shaped myoid cells (HE staining; original magnification, × 40). (B) The tumor cells were diffusely positive for smooth muscle actin.
Discussion
The term myopericytoma was first proposed in 1996 by Requena et al. [1] and later adopted in 1998 by Granter et al. [2] to describe a group of perivascular tumors consisting of myoid cells that they referred to as myopericytoma, glomangiopericytoma, and myofibromatosis in adults. The World Health Organization officially named this tumor type as myopericytoma in 2002, and classified it into the group of peripheral blood cell/vascular cell tumors [3]. Myopericytomas commonly occur in middle-aged male patients, with about 2-fold higher incidence compared with female patients [4]. The frequently affected areas are the superficial soft tissue and subcutaneous tissue of the distal extremities, with occasional occurrence in the head and neck region, spine, urinary tract, and female genital tract [4], [5], [6], [7]. Myopericytomas are usually benign, and their clinical manifestations are well-circumscribed, slow-growing, painless nodules. Although malignant myopericytomas are rare, they show typical malignant features such as nuclear anaplasia, increased mitosis, infiltrative growth, and poor prognosis [4,[8], [9], [10].
Most previous case reports on myopericytomas have focused on the clinical and pathological features, and only a few have described imaging findings. We summarize the imaging features for myopericytoma in the previous case reports [11], [12], [13], [14], [15], [16], [17], [18]. Ultrasonography is considered a widely available, noninvasive, and relatively inexpensive diagnostic tool. On ultrasonography examination, the masses were well-circumscribed with hypoechoic echogenicity and demonstrated prominent color signals on Doppler imaging [11], [12]. CT has obvious advantages for evaluation of tumors invading ambient structures [11,14,17]. Chu et al. [17] reported a case of myopericytoma that occurred in the parotid gland and CT examination demonstrated multiple smooth-margined and well-defined nodules in the periparotid areas and parapharyngeal spaces. On conventional MRI, the lesions usually appeared hypointense on T1-weighted sequences and hyperintense on T2-weighted sequences [12], [13], [14], [15]. FDG-PET/CT is conducive for detection of any evidence of distant metastatic disease. Shaikh et al. [18] reported a case of a typical myopericytic lesion in the ear. The lesion was moderately positive for GLUT-1 and identified as being hypermetabolic with intensely increased FDG uptake.
Ultrasound and MRI can provide morphological details and dynamic enhancement features to discriminate myopericytoma from hypervascular lesions originating from the breast. The differential diagnoses include breast phyllodes tumors (BPTs), fibroadenomas, and breast papillary carcinomas. BPTs usually appear as well-shaped lobulated masses with a radiolucent halo on ultrasound, and sometimes with coarse microcalcifications. Sonographically, round or cleft cavities with posterior acoustic enhancement are common and suggestive for a diagnosis of BPT [19]. BPTs frequently show a cystic component and heterogeneity on delayed-phase contrast-enhanced T1-weighted imaging [20]. The time-signal intensity curves for lesions frequently present the platform type [21]. Fibroadenomas are often small in size, and hypoechoic with well-demarcated margins on sonograms. On enhanced MRI, internal septations were a typical feature of fibroadenomas in up to 40% of cases [22]. However, other studies demonstrated that septations were also present in BPTs [22,23]. Papillary carcinomas usually present as round or microlobulated hypoechoic solid masses with well-defined margins on ultrasound. Large feeding vessels with branching vessels may be seen on Doppler imaging. On MRI, papillary carcinomas may show irregular heterogeneous enhancement because of cystic components or hemorrhage. Clinically, papillary carcinomas would be indistinguishable from a fibroadenoma in the early stage [24].
Surgical excision is the preferred treatment method for myopericytoma and results in good clinical outcomes, based on previous reports [4,5,10,16]. The local recurrence rate is low, and chemotherapy can be considered when metastases are present [4,16]. Histopathologic examination is the gold standard for diagnosis. The typical morphological and pathological characteristics of myopericytomas are ovoid or spindle-shaped tumor cells with eosinophilic cytoplasm, arranged circumferentially around vascular lumina in an “onion skin” pattern [2,4,25]. Immunohistochemical analysis of the tumor reveals myoid differentiation, with expression of vimentin and smooth muscle actin. Desmin is weakly positive in some cases [26], but S-100, EMA, CD31, and CD34 are generally negative [4,7,13,16].
Conclusion
In summary, we have described the clinical presentation, imaging features, and histopathologic features of the first reported case with myopericytomas in the breast. It is necessary for surgeons to understand the imaging characteristics of myopericytoma to review this uncommon entity and establish its clinical behavior.
Author contributions
Yang PeiPei: Conceptualization; Investigation; Formal analysis; Writing - Original Draft; Writing - Review & Editing.
Shi XianQuan: Investigation; Formal analysis.
Li JianMing: Investigation; Formal analysis.
Qian LinXue: Writing - Review & Editing; Supervision; Funding acquisition.
Statement of Ethics
This article does not contain any studies with human participants or animals performed by any of the authors. Informed consent was obtained from the patient.
Declaration of Competing Interest
The authors have stated that they have no conflicts of interest.
Acknowledgments
The authors thank Alison Sherwin, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac) for editing the English text of a draft of this manuscript.
Footnotes
This work was funded by the Beijing Municipal Administration of the Hospitals’ Ascent Plan (Code: DFL 20180102). The funding was used to support the article for publication.
References
- 1.Furio LRHK. Cutaneous adult myofibroma a vascular neoplasm. J Cutan Pathol. 1996;23(5):445–457. doi: 10.1111/j.1600-0560. [DOI] [PubMed] [Google Scholar]
- 2.Granter SR, Badizadegan K, Fletcher CD. Myofibromatosis in adults, glomangiopericytoma, and myopericytoma: a spectrum of tumors showing perivascular myoid differentiation. Am. J. Surg. Pathol. 1998;5(22):513–525. doi: 10.1097/00000478-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 3.CDM F, KK U, F M . IARC; Lyon: 2002. World Health Organization Classification of Tumors. Tumors of Soft Tissue and Bone; pp. 138–139. [Google Scholar]
- 4.Mentzel T, Tos APD, Sapi Z, Kutzner AH. Myopericytoma of skin and soft tissues clinicopathologic and immunohistochemical study of 54 cases. Am J Surg Pathol. 2006;30(1):104–113. doi: 10.1097/01.pas.0000178091.54147.b1. [DOI] [PubMed] [Google Scholar]
- 5.Ju W, Zhao T, Liu Y, Dong M, Wang L, Li J. Clinical and pathologic analysis of myopericytoma in the oral and maxillofacial region. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128(4):393–399. doi: 10.1016/j.oooo.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Borella F, Lucchino F, Bertero L, Ribotta M, Castellano I, Carosso A. Clinico-pathological features of gynecological myopericytoma: a challenging diagnosis in an exceptional location. Virchows Arch. 2019;475(6):763–770. doi: 10.1007/s00428-019-02645-2. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal N, Nag K. Myopericytoma of the thoracic spine: a case report and review of literature. Spine J. 2013;13(11):e23–e27. doi: 10.1016/j.spinee.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 8.Binesh F, Nafisi Moghadam R, Shabani M, Mortazavizadeh MR, Zare S. Malignant myopericytoma of shoulder: a rare lesion. APSP J Case Rep. 2016;7(3) doi: 10.21699/ajcr.v7i3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMenamin ME, Fletcher CDM. Malignant myopericytoma: expanding the spectrum of tumours with myopericytic differentiation. Histopathology. 2002;41(5):450–460. doi: 10.1046/j.1365-2559.2002.01537.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Han L, Pang H, Duan L, Zhao Z. Primary malignant myopericytoma with cancer cachexia. Medicine (Baltimore). 2017;96(49):e9064. doi: 10.1097/MD.0000000000009064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SK, Kwon SY. Gray-scale and power doppler sonography and CT findings of myopericytoma of the posterior cervical space. Am J Neuroradiol. 2009;30(8):1604–1606. doi: 10.3174/ajnr.A1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harish S, O Donnell P, Briggs TWR, Saifuddin A, Flanagan AM. Myopericytoma in Kager's fat pad. Skeletal Radiol. 2006;36(2):165–169. doi: 10.1007/s00256-006-0108-2. [DOI] [PubMed] [Google Scholar]
- 13.Rousseau A, Kujas M, van Effenterre R, Boch AL, Carpentier A, Leroy JP. Primary intracranial myopericytoma: report of three cases and review of the literature. Neuropath Appl Neuro. 2005;31(6):641–648. doi: 10.1111/j.1365-2990.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z, Liang W. Myopericytoma occurrence in the liver and stomach space: imaging performance. BMC Cancer. 2017;17(1) doi: 10.1186/s12885-017-3146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaskes MB, Bishop JW, Bobinski M, Farwell DG. Myopericytoma of the Neck Originating in the Middle Scalene Muscle: A Case Report. ENT Journal. 2017;96(10-11):E5–E7. doi: 10.1177/0145561317096010-1102. [DOI] [PubMed] [Google Scholar]
- 16.Holling M, Wildforster U, Verheggen R, Muller K, Stummer W, Jeibmann A. Myopericytoma: a series of 5 cases affecting the nervous system. World Neurosurg. 2015;84(5):1493–1495. doi: 10.1016/j.wneu.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 17.Chu Z, Yu J, Yang Z, Zhu Z, Yuan H. Myopericytoma involving the parotid gland as depicted on multidetector CT. Korean J Radiol. 2009;10(4):398–401. doi: 10.3348/kjr.2009.10.4.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shenfeng Qiu GL. Neurodevelopmental underpinnings of Angelman Syndrome. OMICS Journal of Radiology. 2014;06(06) doi: 10.4172/2167-7964.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rayzah M. Phyllodes tumors of the breast: a literature review. Cureus. 2020;12(9):e10288. doi: 10.7759/cureus.10288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yabuuchi TKYM, Masato NFMN, Kubo YYYE, Honda HYH. Differentiation between benign phyllodes tumors and fibroadenomas of the breast on MR imaging. Eur J Radiol. 2014;83(8):1344–1349. doi: 10.1016/j.ejrad.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Duman L, Gezer NS, Balcı P, Altay C, Başara I, Durak MG. Differentiation between phyllodes tumors and fibroadenomas based on mammographic sonographic and MRI features. Breast Care. 2016;11(2):123–127. doi: 10.1159/000444377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hochman Mary G., Orel Susan G., Powell Colleen M., Schnall Mitchell D., Reynolds Carol A., White Linda N. Fibroadenomas: MR Imaging Appearances with Radiologic-Histopathologic Correlation. Radiology. 1997;(204):123–129. doi: 10.1148/radiology.204.1.9205233. [DOI] [PubMed] [Google Scholar]
- 23.Wurdinger S, Herzog AB, Fischer DR, Marx C, Raabe G, Schneider A. Differentiation of phyllodes breast tumors from fibroadenomas on MRI. AJR Am J Roentgenol. 2005;185(5):1317. doi: 10.2214/AJR.04.1620. [DOI] [PubMed] [Google Scholar]
- 24.Eiada R, Chong J, Kulkarni S, Goldberg F, Muradali D. Papillary lesions of the breast: MRI, ultrasound, and mammographic appearances. AJR Am J Roentgenol. 2012;198(2):264. doi: 10.2214/AJR.11.7922. [DOI] [PubMed] [Google Scholar]
- 25.Mikami Y, Shiomi T, Manabe T. Perivascular myoma: Case report with immunohistochemical and ultrastructural studies. Pathol Int. 2002;52:69–74. doi: 10.1046/j.1440-1827.2002.01318.x. [DOI] [PubMed] [Google Scholar]
- 26.Choi GW, Yang JH, Seo HS, Kim WT, Lee MJ, Yoon JR. Myopericytoma around the knee: mimicking a neurogenic tumour. Knee Surg. Sports Traumatol. Arthrosc. 2016;24(9):2748–2751. doi: 10.1007/s00167-014-3390-x. [DOI] [PubMed] [Google Scholar]