Highlights
-
•
STUMP is a rare disease histologically, and giant STUMP is extremely rare.
-
•
To our knowledge, present case represents the largest STUMP reported.
-
•
After successful surgery, the patient is alive without evidence of recurrence.
Keywords: Smooth muscle tumor, Laparotomy, Myoma, Hysterectomy, Menorrhagia
Abstract
Background
Uterine smooth muscle tumor of uncertain malignant potential (STUMP) is a rare tumor belonging to a group of smooth muscle tumors that possess both benign and malignant features, complicating the diagnosis.
Case report.
We present the case of a 41-year-old primiparous woman who complained of heavy menstrual bleeding and severe pressure symptoms in the lower abdomen for 3 months. Magnetic resonance imaging revealed a large intramural myoma measuring 35 × 25 cm in the lower uterine corpus. A laparotomy including total hysterectomy was performed. Grossly, the uterine mass measured 38.5 × 35.4 × 20.4 cm in the largest diameter and weighed 18.3 kg. Pathological analysis revealed a uterine mass diagnosed as a smooth muscle tumor of uncertain malignant potential. The patient was normally discharged 7 days after surgery and decided to follow up without further treatment. At the time of this report, the patient had been followed up as an outpatient for 18 months without recurrence.
Conclusion
Giant uterine STUMP is extremely rare and difficult to diagnose on physical examination and imaging findings alone. It is important to consider the possibility of an underlying malignancy when performing a preoperative examination and to perform frozen biopsy if malignancy is suspected. During follow-up, patients should undergo consultation with a gynecologic oncologist and should be surveilled closely because of the possibility of recurrence or metastasis.
1. Introduction
According to the World Health Organization (2003), uterine smooth muscle tumor of uncertain malignant potential (STUMP) is a borderline tumor between benign leiomyoma and malignant leiomyosarcoma (Tavassoli et al., 2003). It is difficult for an even skilled pathologist to diagnose STUMP by identifying detailed markers such as cytologic atypia, mitotic activity, or tumor cell necrosis that distinguish leiomyoma from leiomyosarcoma (Berretta et al., 2008). The clinical features, prognostic factors, and optimal management of STUMP are poorly understood because of limited data associated with its rarity.
Patients with STUMP may have symptoms such as abnormal uterine bleeding, pelvic pain, and lower abdominal pressure consistent with benign uterine myoma, although there may be differences depending on the size of uterine mass (Ip et al., 2010). Because STUMP and benign uterine myoma are not significantly different in terms of preoperative radiologic imaging and laboratory tests, it is difficult to distinguish between these tumors prior to pathological confirmation at surgery. Conventional surgical management of STUMP includes myomectomy or hysterectomy. Myomectomy in limited cases may be considered in women who wish to preserve fertility (Vilos et al., 2012). Here, we report an unusual case of STUMP presenting as a giant uterine mass that was suspected to be a benign uterine myoma preoperatively.
2. Case presentation
A 41-year-old primiparous woman was admitted to our hospital with fatigue, mild dizziness, and lower abdominal pressure for 3 months. She had a history of heavy menstrual bleeding of more than 10 days over the previous few years. She had no systemic disease and no other history of surgical procedure except for one previous cesarean section. Physical examination revealed a solid mass of approximately 30 cm or more from the lower abdomen to the epigastric area; the uterus size was full-term. The mass was hard and generally round with limited mobility. Transabdominal ultrasound confirmed a 30 × 25 cm heterogenous mass in the uterus that was suspected to represent a uterine myoma. Magnetic resonance imaging (MRI) of the abdomen and pelvis confirmed a huge intramural myoma adjacent to the lower uterine corpus. Tumoral hemorrhage, necrosis, and secondary degeneration were not determined on MRI. Both ovaries were normal and there was no evidence of malignancy such as lymphadenopathy or ascites. The appearance was suggestive of a benign leiomyoma on MRI. Her body mass index was 39.6 kg/m2. Her baseline blood tests were normal except for anemia (hemoglobin 8.2 g/dL, reference range 12–16 g/dL). The level of CA-125 was 32.9 U/ml and that of CA 19–9 was 5.9 U/ml.
After discussing the various treatment options, the patient consented to total hysterectomy by laparotomy. This was performed through a 20-cm midline incision from symphysis pubis to upper umbilicus. We encountered a large intramural myoma arising from the low anterior wall of the uterus. We performed myomectomy first to secure the space, checking other organs (Fig. 1). We then performed total hysterectomy. There were no fluid collections and no other significant findings suggestive of malignancy throughout the abdomen. The surgery was difficult enough to require a red blood cell transfusion (2 units) because of bleeding at the time of myomectomy. Both internal iliac arteries were ligated using metal clips to reduce massive bleeding, and the estimated blood loss was 850 mL. The operation was completed safely, and the patient was discharged on day 7 without any complication.
Fig. 1.
Legend: Gross imaging after myomectomy in the operating room.
On gross examination, the lesion was 38.9 × 35.4 × 20.4 cm gray-whitish color mass that was firm and rubbery (Fig. 2). The cut surface revealed a trabecular pattern with several hemorrhagic areas. The weight of the mass was 18.3 kg, and the total weight of the uterus and other tissue was 19.1 kg. Histology revealed STUMP. The tumor showed no coagulative necrosis and low mitotic index with only one mitotic figure (MF) per 10 high-power fields (HPF). However, moderate cytological atypia and cellularity were reported (Fig. 3). After discussion with our gynecologic oncology team at the tumor board for the final pathologic report, the patient decided to follow up every 6 months with abdominal and chest CT. At 18 months follow-up, the patient was alive and doing well, without evidence of recurrent or metastatic disease.
Fig. 2.
Legend: 38.9 × 35.4 × 20.4 cm and 18.3 kg of uterine mass.
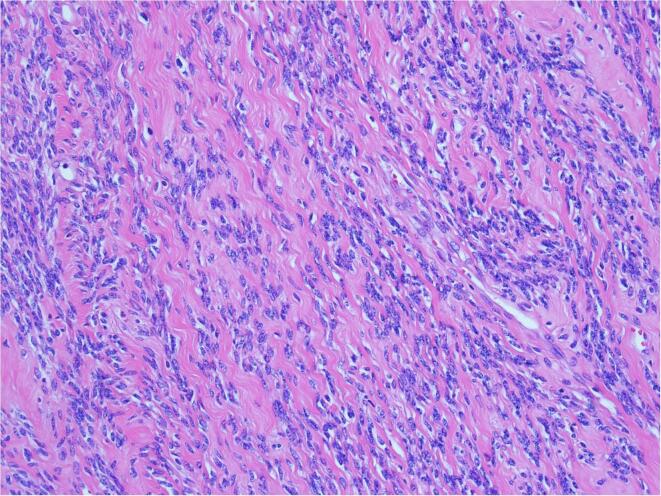
Fig. 3.
Legend: STUMP showed spindle-cell proliferation, moderate atypia, and absence of CTCN (H&E x200).
3. Discussion
The diagnostic classification of STUMP is poorly defined as a uterine smooth muscle tumor that is not characterized by either benign or malignant features (Guntupalli et al., 2009). Diagnosis of STUMP is difficult for several reasons. First, STUMP is extremely rare: appearing in 0.01% of patients who undergo myomectomy or hysterectomy for a presumptive benign leiomyoma (Picerno et al., 2016). Second, clinical features of STUMP include dysmenorrhea, heavy menstrual bleeding, and pelvic pain, all of which are similar to those of benign leiomyoma. Finally, it is difficult to distinguish STUMP from benign leiomyoma on imaging (Guntupalli et al., 2009, Ng et al., 2010). MRI can help to differentiate between leiomyoma and leiomyosarcoma utilizing differences in signal intensity, but it has limitations in imaging methods. Moreover, there is a lack of evidence to distinguish STUMP from leiomyoma using MRI (Schwartz et al., 1998). As a result, STUMP is often incidentally found after a hysterectomy or myomectomy. In the present case, benign leiomyoma was suspected because of the patient's age, size of the uterine mass, clinical features, and imaging results.
STUMP is characterized histologically as a slowly growing and late recurrence borderline tumor (Ip et al., 2010). The recurrence rate is low; however, depending on the subtype, recurrence rates range from 6.9% to 27%. (Guntupalli et al., 2009, Ip et al., 2010, Vilos et al., 2012). Three important histopathologic findings portends to increased risk of recurrence are presence of coagulative tumor cell necrosis (CTCN), degree of cytologic atypia, and mitotic index. STUMP can have a combination of these findings without meeting the diagnostic criteria for leiomyosarcoma (Ip et al., 2010, Stewart et al., 2017). A study proposed that extensive CTCN, expression of p16 and p53, and incomplete surgical margins are risk factors for STUMP recurrence (PETERS et al., 1994). Two studies have reported that extensive immunohistochemical staining of P16 and p53 was associated with recurrence; however, further reliable studies to confirm these markers are required (Atkins et al., 2008, Ip et al., 2009). Bell et al. reported three STUMP subdivisions according to histologically distinct groups with different clinical features: 1) “atypical leiomyoma with low risk of recurrence,” characterized by diffuse moderate to severe atypia, <10 MF per 10 HPF, and no CTCN; 2) “atypical leiomyoma but limited experience,” characterized by severe atypia, <20 MF per 10 HPF, and no CTCN; and 3) “smooth muscle tumors of low malignant potential,” characterized by mild to absent cytologic atypia, <10 MF per 10 HPF, and presence of CTCN (Bell et al., 1994). In our pathologic report, there was no CTCN, and a low mitotic index with only 1 MF per 10 HPF was observed, despite the fact that moderate cytological atypia and cellularity were reported. Our patient falls in the category of “atypical leiomyoma with low risk of recurrence.” In addition, our patient underwent total hysterectomy, and there was no gross residual lesion. In the present case, risk of recurrence was considered low because of the absence of risk factors. One worrying point is that hysterectonmy was performed after myomectomy, not performing hysterectomy including huge mass at once in consideration of underlying malignancies. However, it was an inevitable surgical decision because the size and weight of mass was too large and heavy that the surgical field of view was not secured, and the movement of uterus was restricted. As displayed in Table 1, STUMP can transform into low-grade or high-grade smooth muscle tumor and metastasize to other organ after several years, even in the absence of such recurrence risk factors as presence of CTCN or diffuse cytologic atypia (Bell et al., 1994, Robboy et al., 1990). In cases of recurrence with leiomyosarcoma, distant metastasis is often observed beyond the pelvis, even in extremities. Many patients with recurrence die of the disease. Most treatments are aggressive, involving debulking surgery followed by adjuvant chemotherapy. The standard postoperative follow-up period is every 6 months for 5 years with gynecological examinations and imaging modality by CT or MRI for evaluation of recurrence or metastasis. Our patient is adhering to this schedule of every 6 months; there was no evidence of recurrence or metastasis at 18 months postoperatively.
Table 1.
A summary of reports on clinical and histologic features and oncologic outcomes of STUMP patients relapsed with leiomyosarcoma.
| Reference | Histologic feature | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Mass size (cm) | Surgery | Cellularity | Atypia | MI | Necrosis | Subtype | Recurrence | Treatment | Outcome | |
| H. Sahin et al (Şahin et al.,) | 52 | 20 | Myomectomy | high | mild | 6 | present | SMT-LMP | LMS | Debulking + CTx | DOD |
| H.I. Ha et al (Ha et al., 2018) | 49 | 6 | Hysterectomy | high | mild | 0–3 | absent | N/A | LMS | Debulking + CTx | N/A |
| Ip PP et al. (Ip et al., 2009) | 50 | 5 | Hysterectomy | moderate | severe | 4 | infarct | AL-LE | LMS | Debulking + CTx | AAR |
| Ip PP et al. (Ip et al., 2009) | 39 | 5.8 | Hysterectomy | high | severe | 5 | infarct | AL-LE | LMS | Debulking + CTx | AAR |
| Basaran et al (Basaran et al., 2018) | 52 | N/A | Hysterectomy | mild | mild | 2–5 | present | SMT-LMP | LMS | Debulking + CTx | DOD |
| Basaran et al (Basaran et al., 2018) | 51 | 9 | Hysterectomy | mild | mild | < 2 | present | SMT-LMP | LMS | Debulking + CTx | AAR |
| Basaran et al (Basaran et al., 2018) | 38 | N/A | Myomectomy | moderate | none | 2–5 | present | SMT-LMP | LMS | Hysterectomy | AAR |
| A.Shapiro et al (Shapiro et al., 2004) | 46 | 4 | Hysterectomy | N/A | severe | 15 | absent | AL-LE | LMS | Mass excision | DOD |
| G.Sanchez et al (García-Sánchez et al., 2019) | 71 | 16 | Hysterectomy | high | moderate | 0–1 | present | N/A | LMS | Mass excision | AAR |
Abbreviations: SMT-LMP, smooth muscle tumors of low malignant potential; LMS, leiomyosarcoma; CTx, chemotherapy; DOD, dying of disease; AAR, alive after recurrence; AL-LE, atypical leiomyoma but limited experience; N/A, non-available.
Another noteworthy feature of the present case is the substantial size of the STUMP, 40 × 30 cm with a weight of about 20 kg. Although there are no specific criteria for the definition of “giant” STUMP, we reviewed the papers reporting substantial sizes of smooth muscle tumor. Among uterine smooth muscle tumors weighing more than 20 kg, the heaviest reported was 28.1 kg, followed by a tumor weighing 20 kg (Moris and Vernadakis, 2014, Rajender Prasad et al., 2015). While these two tumors were both benign leiomyomas, to our knowledge, we believe the present case represents the largest STUMP reported in the literature. It is unusual case for STUMP to weigh 20 kg with dimensions of 40 × 30 cm that exists only as single uterine mass without metastasis or another lesion.
In summary, STUMP is a rare disease, and giant STUMP is extremely rare. Despite the fact that consensus regarding management has not been clearly established because of low incidence and atypical clinical pattern, the best treatment strategy is surgery. Most patients undergo myomectomy or hysterectomy, and final diagnosis is confirmed on postoperative pathology. Hysterectomy is generally recommended; however, myomectomy may be a reasonable option when considering patient age and desire to maintain fertility. Because the recurrence rates after myomectomy and hysterectomy are similar and most STUMP have a benign clinical feature, STUMP is managed successfully using these two methods (Guntupalli et al., 2009). Nevertheless, in rare cases, STUMP transforms into leiomyosarcoma if the recurrence occurs during follow-up, and may metastasize, with fatal consequences. Therefore, patients with STUMP should undergo close surveillance and consultation with a gynecologic oncologist regarding several risk–benefit considerations, including age, desire to maintain fertility, and histopathologic results such as mitotic figures, degree of cellular atypia, and presence of CTCN.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
References
- Atkins K.A., Arronte N., Darus C.J., Rice L.W. The Use of p16 in Enhancing the Histologic Classification of Uterine Smooth Muscle Tumors. Am. J. Surgical Pathology. 2008;32(1):98–102. doi: 10.1097/PAS.0b013e3181574d1e. [DOI] [PubMed] [Google Scholar]
- Basaran D., Usubutun A., Salman M.C., Narin M.A., Boyraz G., Turkmen O., Comert Kimyon G., Karalok A., Bulbul D., Turan T., Ozgul N., Yuce K. The clinicopathological study of 21 cases with uterine smooth muscle tumors of uncertain malignant potential: centralized review can purify the diagnosis. Int. J. Gynecological Cancer. 2018;28(2):233–240. doi: 10.1097/IGC.0000000000001178. [DOI] [PubMed] [Google Scholar]
- Bell S.W., Kempson R.L., Hendrickson M.R. Problematic Uterine Smooth Muscle Neoplasms: A Clinicopathologic Study of 213 Cases. Am. J. Surgical Pathology. 1994;18(6):535–558. [PubMed] [Google Scholar]
- Berretta R., Rolla M., Merisio C., Giordano G., Nardelli G.B. Uterine smooth muscle tumor of uncertain malignant potential: a three-case report. Int. J. Gynecol. Cancer. 2008;18(5):1121–1126. doi: 10.1111/j.1525-1438.2007.01125.x. [DOI] [PubMed] [Google Scholar]
- García-Sánchez JM, Bauza M, Pérez-García A, et al. (2019) Secondary Leiomyosarcoma of the Lower Limb Following Uterine Smooth Muscle Tumor of Uncertain Malignant Potential. Clin Med Insights: Case Reports 12:1179547619857680. [DOI] [PMC free article] [PubMed]
- Guntupalli S.R., Ramirez P.T., Anderson M.L., Milam M.R., Bodurka D.C., Malpica A. Uterine smooth muscle tumor of uncertain malignant potential: A retrospective analysis. Gynecologic Oncology. 2009;113(3):324–326. doi: 10.1016/j.ygyno.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha H.I., Choi M.C., Heo J.H., Kim K.A., Jung S.G., Park H., Joo W.D., Song S.H., Kim T.H., Lee C. A clinicopathologic review and obstetric outcome of uterine smooth muscle tumor of uncertain malignant potential (STUMP) in a single institution. European J. Obstetrics Gynecol. Reproductive Biology. 2018;228:1–5. doi: 10.1016/j.ejogrb.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Ip P.P.C., Cheung A.N.Y., Clement P.B. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): a clinicopathologic analysis of 16 cases. Am. J. Surgical Pathology. 2009;33(7):992–1005. doi: 10.1097/PAS.0b013e3181a02d1c. [DOI] [PubMed] [Google Scholar]
- Ip P.P.C., Tse K.Y., Tam K.F. Uterine smooth muscle tumors other than the ordinary leiomyomas and leiomyosarcomas: a review of selected variants with emphasis on recent advances and unusual morphology that may cause concern for malignancy. Adv. Anatomic Pathol. 2010;17(2):91–112. doi: 10.1097/PAP.0b013e3181cfb901. [DOI] [PubMed] [Google Scholar]
- Moris D., Vernadakis S. Giant uterine leiomyoma mimicking pregnancy. Mayo Clin. Proc. 2014;89(6):e53–e54. doi: 10.1016/j.mayocp.2013.06.029. [DOI] [PubMed] [Google Scholar]
- Ng J.S., Han A., Chew S.H., Low J. A clinicopathologic study of uterine smooth muscle tumours of uncertain malignant potential (STUMP) Ann. Acad. Med. Singapore. 2010;39(8):625–628. [PubMed] [Google Scholar]
- Peters William.A., III, Howard Donald.R., Andersen Willie.A., Figge David.C. Uterine smooth-muscle tumors of uncertain malignant potential. Obstet. Gynecol. 1994;83(6):1015–1020. doi: 10.1097/00006250-199406000-00023. [DOI] [PubMed] [Google Scholar]
- Picerno T.M., Wasson M.N., Gonzalez Rios A.R., Zuber M.J., Taylor N.P., Hoffman M.K., Borowsky M.E. Morcellation and the incidence of occult uterine malignancy: a dual-institution review. Int. J. Gynecological Cancer. 2016;26(1):149–155. doi: 10.1097/IGC.0000000000000558. [DOI] [PubMed] [Google Scholar]
- Rajender Prasad S., Nikhil S., Kumud S. Giant uterine leiomyoma and review of literature. Int. J. Med. Sci. Clin. Invent. 2015;2:640–644. [Google Scholar]
- Robboy S.J., Kumudini M., Norris H.J. Malignant potential and pathology of leiomyomatous tumors of the uterus. Clin Consult Obstet Gynecol. 1990;1:2–9. [Google Scholar]
- Şahin H, Karatas F, Coban G, et al. (2019) Uterine smooth muscle tumor of uncertain malignant potential: fertility and clinical outcomes. J Gynecol Oncol 30(4). [DOI] [PMC free article] [PubMed]
- Schwartz L.B., Zawin M., Carcangiu M.L., Lange R., McCarthy S. Does pelvic magnetic resonance imaging differentiate among the histologic subtypes of uterine leiomyomata? Fertil. Steril. 1998;70(3):580–587. doi: 10.1016/s0015-0282(98)00193-9. [DOI] [PubMed] [Google Scholar]
- Shapiro A., Ferenczy A., Turcotte R., Bruchim I., Gotlieb W.H. Uterine smooth-muscle tumor of uncertain malignant potential metastasizing to the humerus as a high-grade leiomyosarcoma. Gynecol. Oncol. 2004;94(3):818–820. doi: 10.1016/j.ygyno.2004.05.049. [DOI] [PubMed] [Google Scholar]
- Stewart EA, Quade BJ, Laughlin-Tommaso SK (2017) Variants of uterine leimyomas (fibroids). Available from: http://www.uptodate.com. Accessed August 13, 2017.
- Tavassoli F.A., Devilee P. Iarc; 2003. World health organization classification of tumours: tumours of the breast and female genital organs; pp. 236–239. [Google Scholar]
- Vilos G.A., Marks J., Ettler H.C. Uterine smooth muscle tumors of uncertain malignant potential: diagnostic challenges ad therapeutic dilemmas. Report of 2 cases and review of the literature. J Minim Invasive Gynecol. 2012;19(3):288–295. doi: 10.1016/j.jmig.2011.12.025. [DOI] [PubMed] [Google Scholar]