Abstract
The end of 2019 saw the beginning of the coronavirus disease 2019 (COVID-19) pandemic that soared in 2020, affecting 215 countries worldwide, with no signs of abating. In an effort to contain the spread of the disease and treat the infected, researchers are racing against several odds to find an effective solution. The unavailability of timely and affordable or definitive treatment has caused significant morbidity and mortality. Acute respiratory distress syndrome (ARDS) caused by an unregulated host inflammatory response toward the viral infection, followed by multi-organ dysfunction or failure, is one of the primary causes of death in severe cases of COVID-19 infection. Currently, empirical management of respiratory and hematological manifestations along with anti-viral agents is being used to treat the infection. The quest is on for both a vaccine and a more definitive management protocol to curtail the spread. Researchers and clinicians are also exploring the possibility of using cell therapy for severe cases of COVID-19 with ARDS. Mesenchymal stromal cells are known to have immunomodulatory properties and have previously been used to treat viral infections. This review explores the potential of mesenchymal stromal cells as cell therapy for ARDS.
Introduction
The latter half of 2019 saw a sudden rise in pneumonia or severe respiratory infection in Wuhan, Hubei Province, China, secondary to a novel coronavirus—severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. The infectivity of SARS-COV-2 surpassed the pace of finding an effective treatment or preventive option, and as of October 27, 2020, there are 43,341,451 confirmed positive cases, with a mortality rate of 2.6% and a recovery rate of 73% (www.WHO.int).
A pathogen's basic reproduction number (R0) denotes the average number of people who can be infected by an infected individual. Though the R0 of coronavirus disease 2019 (COVID-19) differs between countries, it is higher than 1, suggesting an exponential infectivity potential of the virus, which has led to this pandemic [2,3]. The R0 of COVID-19 (2–3) and that of Spanish influenza is similar but higher than that of H1N1 influenza (1.46–1.52) and Middle East respiratory syndrome (0.3–0.8) [4], [5], [6].
Although a majority of patients with COVID-19 infection are asymptomatic, symptoms can range from mild to severe [7], [8], [9]. Pneumonia, respiratory distress, multi-organ dysfunction, sepsis, septic shock, loss of speech and movement are signs of severity [10]. The elderly and immune-compromised and those with comorbidities have a higher risk of developing severe symptoms with a fatal outcome [11,12]. The virus-induced cytokine storm results in COVID-specific acute respiratory distress syndrome (ARDS), multi-organ dysfunction syndrome and eventual death [13].
Currently, severely affected patients are being treated with anti-viral and anti-inflammatory drugs, besides supportive measures such as invasive and non-invasive mechanical ventilation [14]. Acute progressive renal injury, an early marker of multi-organ dysfunction syndrome, requires renal replacement therapy in advanced disease [15]. Horby et al. [16] found that dexamethasone reduced mortality in patients receiving invasive ventilation but not in those without any respiratory support. Although treatment with several anti-virals did not lead to any improvement [17,18], patients receiving remdesivir, an RNA polymerase inhibitor, demonstrated significant clinical improvement [19]. Over 50 clinical trials have been registered at ClinicalTrials.gov for investigating the safety and efficacy of the anti-viral favipiravir for COVID-19 treatment.
The usage of chloroquine and hydroxychloroquine for COVID-19 treatment remains inconclusive [20,21]. Anakinra, an IL-1 receptor antagonist, has shown beneficial effects in moderate to severe COVID-19 infections [22,23]. Tocilizumab and sarilumab, both IL-6 receptor antagonists, used in small cohorts, have alleviated clinical symptoms without oxygen supplementation [24]. Ongoing trials will clear ambiguity on tocilizumab dosage and mortality post-treatment. Janus kinase signal inducer pathway inhibitors ruxolitinib and baricitinib are also being investigated [25,26]. Convalescent plasma therapy also has potential, but safety and efficacy have to be established with larger studies [27], [28], [29].
With an increasing number of infections worldwide, there is a pressing need to find a method of prevention and treatment for COVID-19. Vaccines are being developed, with one from Oxford University in collaboration with AstraZeneca in a phase 3 trial [30,31]. Although 300 clinical trials for investigating anti-viral drugs and 163 for anti-inflammatory drugs are ongoing, it is imperative to look for newer and alternate modalities to treat COVID-19 patients. Researchers have explored the role of stem cells in suppressing ARDS during the cytokine storm since mesenchymal stromal cells are known to play an immunomodulatory role [1,32].
SARS-CoV-2 belongs to the Coronaviridae family, has a 5% genetic association with the SARS virus [33] and was given the nomenclature of COVID-19 by the Director General of the World Health Organization on January 30, 2020 [34]. The spike protein on the virus recognizes the spike protein present on angiotensin-converting enzyme 2 (ACE-2), making it the port of entry into the host cells [35]. The ACE-2 receptor is present ubiquitously and predominantly in the alveolar cells, making the lungs the most vulnerable to infection [36]. ACE-2 receptors have not been detected in bone marrow, lymph nodes, thymus, spleen, lymphocytes or macrophages [37]. Transmembrane protease serine 2 also plays a decisive role in viral entry into the host cells [38].
The overdrive that occurs in the host immune system in response to the virus also adversely affects the host cells [39]. Pro-inflammatory cytokines such as IL-7, IL-6, Il-2, tumor necrosis factor (TNF), MIP1A, interferon gamma-induced protein 10 and granulocyte colony-stimulating factor and chemokines such as CCL2, CCL3, CCL5, CXCL8, CXCL9 and CXCL10 are released during the infection [40,41]. The inflammatory response of the host can cause dysfunctional air exchange, pulmonary edema, cardiac injury and ARDS, eventually leading to death. Such an effect is called a cytokine storm and is reported in graft-versus-host disease during graft failure as well as in advanced stages of COVID-19 infection [42]. It has been reported to occur with a short median time of 8 days from the appearance of the first symptom to ARDS [43]. Hence, trials of multiple treatment modalities and strategies are being used, including anti-viral therapy, hydroxychloroquine, neutralizing antibodies, convalescent plasma therapy, repurposed anti-viral medications and blockers of ACE-2 receptor with antibodies [44,45].
Mesenchymal stromal cells as a potential therapeutic strategy
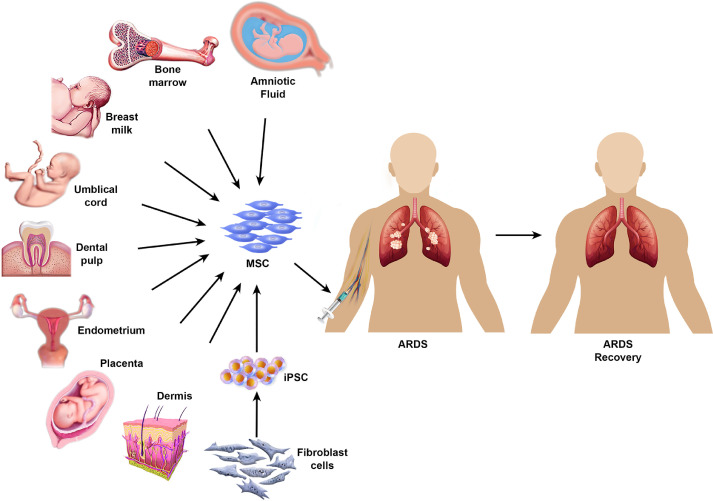
Mesenchymal stromal cells (MSCs) are multi-potent adult stem cells with immunomodulatory properties [46]. They are found in bone marrow, adipose tissue, dental pulp, umbilical cord, placenta, Wharton's jelly, amniotic fluid, skin, foreskin, salivary gland and cord blood (Figure 1 ) [47]. The versatility of the differentiation potential of MSCs is based on the tissue-specific source of the cells [46]. According to the International Society for Cell & Gene Therapy, MSCs are characterized by their ability to adhere to plastic surfaces as well as their expression of CD105, CD73 and CD90 and lack of CD45, CD34, CD14, CD11b, CD79, CD 19 and HLA-DR [48,49]. The multi-potency of MSCs is validated by their ability to differentiate into adipocytes, chondroblasts and osteoblasts [49]. They have been widely used to aid in the regeneration of damaged neurons or muscle fibers and to suppress immune reactions via anti-inflammatory macrophages and regulatory T cells [50]. MSCs express low levels of major histocompatibility complex class I but lack major histocompatibility complex class II on their surface [44]. They exert an anti-microbial role by dynamically balancing pro- and anti-inflammatory responses, secreting anti-microbial peptides and molecules such as indoleamine 2,3-dioxygenase and IL-17, in addition to their autocrine and paracrine functions [51,52].
Fig. 1.
Sources of MSCs and mode of MSC infusion in clinical trials. The diverse sources of MSCs and their application in ARDS recovery are shown. iPSCs, induced pluripotent stem cells. (Color version of figure is available online).
Bone marrow-derived MSCs (BM-MSCs) have been widely used, followed by umbilical cord-derived MSCs (UC-MSCs) and adipose-derived MSCs (AD-MSCs), for cytokine storm rescue. Apart from stromal vascular fraction cells and adipose-derived stromal cells (ADSCs), the stromal vascular fraction obtained after lipoaspirate contains endothelial cells, macrophages and pericytes, fulfilling the MSC definition described by the International Society for Cell & Gene Therapy [53,54]. ADSCs have high immunomodulatory, anti-inflammatory, proliferation, differentiation and regenerative potential compared with BM-MSCs [55]. They express fatty acid translocase marker CD36 but lack cell adhesion marker CD106 compared with BM-MSCs [56]. The paracrine effects of hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) and fibroblast growth factor 2 (FGF2) released by ADSCs aid in resolving the lung injury caused by COVID-19 infection by promoting type 2 alveolar cell regeneration and angiogenesis [57]. The immunomodulatory effects of ADSCs are driven primarily by anti-inflammatory cytokine IL-10 and conversion of inflammatory macrophage M1 to the anti-inflammatory and wound healing M2 type [55]. The extracellular matrix is maintained by ADSCs by regulating the levels of matrix metalloproteinase and tissue inhibitor matrix metalloproteinase. Ease of harvest, along with higher yield, longer life span and shorter doubling time, makes ADSCs a more preferred source of MSCs compared with BM-MSCs and UC-MSCs.
MSCs in viral infections
MSCs have been widely used in the management of both infectious and non-infectious etiologies owing to their immunomodulatory and regenerative potential.
Human immunodeficiency virus
Despite highly active anti-retroviral therapy and reduction in viral load, some HIV patients are vulnerable to opportunistic infections. These patients are categorized as non-immune responders (NIRs) [58]. In a pilot open-label clinical trial, Zhang et al. [59] administered three doses of UC-MSCs to seven NIR patients, and six NIRs served as controls. The results revealed an increase in circulating naive and central memory CD4 T-cell counts along with HIV-1-specific interferon γ and IL-2 generation. However, MSCs have been shown to reactivate latent HIV in macrophages and T-helper lymphocytes through PI3 kinase and nuclear factor kappa light chain enhancer of activated B-cell pathways using in vitro models [60]. Clinical trials with AD-MSCs (NCT02290041) and UC-MSCs (NCT01213186) are ongoing to evaluate the safety, efficacy and optimal dosage for reconstituting CD4 T cells. It has been shown that MSCs obtained from HIV patients harbor defective differentiation potential, thereby limiting the usage of autologous MSC transplantation [61]. Further studies are needed to determine the role of MSCs in immune restoration in NIR as well as HIV patients and whether MSCs can be administered as monotherapy or in combination with anti-viral therapy.
Hepatitis B virus
Liver disease is a major complication of chronic hepatitis B virus (HBV) infection, and orthotopic liver transplantation remains the only therapeutic strategy in end-stage disease, with artificial liver support systems serving as a temporary measure [62]. Although Xie et al. [63] found BM-MSCs to be resistant to HBV infection, Ma et al. [64] showed that BM-MSCs of HBV patients can be a virus reservoir. A single dose of autologous BM-MSCs in 53 patients with liver failure caused by HBV has been shown to be well tolerated, but the improvement is short-lived [65]. Zhong et al. [66] found that BM-MSCs of HBV patients had deranged proliferative capacity. A comparative study to investigate the differentiation ability and resistance to HBV was conducted by Wang et al. [67] in BM-MSCs and AD-MSCs. Both differentiated well into hepatocytes; however, only AD-MSCs were resistant to HBV. Phase 2 clinical trials by Ling et al.(NCT01223664) and Bingliang et al.(NCT01221454) are comparing the safety and efficacy of allogeneic BM-MSC transplantation in liver failure induced by chronic hepatitis in comparison to conventional treatment. In addition, Bingliang et al.are studying the effect of three different dosages of BM-MSCs (2 × 105 cells/kg, 1 × 106 cells/kg and 5 × 106 cells/kg) (NCT01322906). Gao et al.are evaluating the role of combination treatment with UC-MSCs and plasma exchange therapy for acute-on-chronic liver failure (NCT01724398) and investigating the short- and long-term outcomes of autologous BM-MSC transplantation in liver failure patients (NCT00956891). Jasirwan et al.(NCT04357600) and Fan et al.(NCT03826433) are evaluating the safety and efficacy of UC-MSCs in patients with liver failure due to chronic HBV infection. Multi-centric clinical trials with MSCs from different sources and long-term follow-up will help to obtain clarity on the safety and efficacy of MSCs in liver failure secondary to chronic HBV infection.
Clinical trials for cell therapy in COVID-19 management
The initial reports of stem cells as a therapeutic strategy for COVID-19 came from China, with the injection of human UC-MSCs into a 65-year-old woman on ventilation. After the second injection of 50 million MSCs, the patient showed improvement and received three infusions of MSCs 3 days apart. Serum bilirubin, liver function enzymes and C-reactive protein levels decreased, and CD3+ T cells, CD4+ T cells and CD8+ T cells increased to normal levels. The patient was weaned from the ventilator and 2 days after the third infusion tested negative for COVID-19 [68]. Seven more patients (one critically ill, four severely ill, two mildly ill) were injected intravenously with clinical-grade MSCs, and three severely ill COVID-19 patients were treated with placebo in a hospital in Beijing, China [69]. They were given a single injection of 1 × 106 stem cells/kg of weight and followed up for 14 days. Lung function improved on the second day, and C-reactive protein, CXCR3+CD4+T cells, CXCR3+CD8+T cells and CXCR3 plus natural killer cells decreased over a week post-injection. Regulatory dendritic cells CD14+CD11c+CD11b and IL-10 increased in the MSC-injected group. The study also revealed that MSCs did not express ACE-2 or transmembrane protease serine 2. The plausible mechanism by which the MSCs might have worked was by reducing the molecules that induce inflammation and triggering those that dampen inflammation.
The US Food and Drug Administration has allowed the use of MSCs as an investigational drug [70], [71], [72]. Over 50 clinical trials using MSCs or their products for COVID-19 are registered at ClinicalTrials.gov. The highest number of ongoing clinical trials are in the USA (18), followed by China (nine). Intravenous injection of MSCs ranging from 0.5 × 106 cells/kg to 750 × 106 cells/kg is being used in these clinical trials. Three trials have employed MSC-derived exosome vesicles, of which two used aerosols. Most trials are using MSCs derived from allogeneic umbilical cord (twenty one), followed by bone marrow (ten), adipose (ten), Wharton's jelly (six), dental pulp (two), olfactory mucosa (one) and unknown (six). Mount Sinai Hospital injected MSCs obtained from Mesoblast Ltd, an Australian biotech company, in 12 ventilator-dependent ARDS patients, with encouraging results. This prompted a randomized, double-blind, placebo-controlled trial with 300 patients [73,74] using an intravenous infusion of BM-MSCs 2 × 106 cells/kg (NCT4371393). Mesoblast is extending the use of MSCs to children from 2 months to 15 years of age [75]. The ongoing clinical trials are listed in Table 1 .
Table 1.
List of clinical trials on MSCs in COVID-19 from ClinicalTrials.gov.
| Sl. No. | Clinical trial no. | Number of patients | Study |
Source of biological material | |||||
|---|---|---|---|---|---|---|---|---|---|
| Arms | Type | Phase | Design | Purpose | Country | ||||
| 1 | NCT04276987 | 30 | Conventional plus aerosol inhalation of MSC-derived exosomes | Interventional | I | Single group treatment | Treatment | Spain | Allogeneic AD-MSC exosomes |
| 2 | NCT04400032 | 9 | Experimental with escalating dose (25 × 106 cells/kg, 50 × 106 cells/kg, 90 × 106 cells/kg) and three infusions | Interventional | I | Single group treatment | Dose-escalating safety | Canada | BM-MSCs |
| 3 | NCT4341610 | 40 | Experimental: 100 × 106 cells/kg Control: normal saline |
Interventional | I–II | Double-blind, randomized, placebo-controlled | Treatment | Denmark | Allogeneic AD-MSCs |
| 4 | NCT04445220 | 24 | Experimental: 1, low dose, 250 × 106 cells/kg 2, high dose, 750 × 106 cells/kg Control: sham In patients with acute kidney injury, MSC infusion by integration with continuous renal replacement therapy |
Interventional | I–II | Randomized, multi-center, double-blind, sham-controlled | Safety, treatment and tolerability | USA | Allogeneic MSC |
| 5 | NCT04466098 | 30 | Experimental: 300 × 106 cells/kg (three times) Control: placebo |
Interventional | II | Randomized, multi-center, placebo-controlled | Treatment | USA | Allogeneic MSC |
| 6 | NCT04299152 | 20 | Experimental: stem cell educator therapy Control: conventional therapy Patient blood separated by apheresis, and patient immune cells co-cultured with cord blood stem cells, followed by putting the educator immune cells back in patients |
Interventional | II | Partially masked and single center | Safety, feasibility and efficacy | USA | Human multipotent UC-MSCs |
| 7 | NCT04333368 | 40 | Experimental: 1 × 106 cells/kg (three times) Control: normal saline |
Interventional | I–II | Randomized parallel assignment | Treatment | France | UC Wharton's jelly |
| 8 | NCT04491240 | 90 | Experimental: 1, exosome inhalation (first type) 2, exosome inhalation (second type) Control: placebo inhalation |
Interventional | I–II | Randomized parallel assignment | Safety and treatment | Russia | AD-MSCs |
| 9 | NCT04447833 | 9 | Experimental: 1, MSC infusion 1 × 106 cells/kg 2, MSC infusion 2 × 106 cells/kg |
Interventional | I | Open-label dose escalation study of advanced therapy investigational medicinal product | Safety | Sweden | Allogeneic BM-MSCs |
| 10 | NCT04437823 | 20 | Experimental: MSC infusion 5 × 105 cells/kg (three times) Control: standard of care |
Interventional | II | Randomized open-label | Treatment | Pakistan | UC |
| 11 | NCT04269525 | 16 | Experimental: 3.3 × 107 cells/kg | Interventional | II | Single group assessment | Prevention and treatment | China | UC |
| 12 | NCT04389450 | 140 | Experimental: high dose (once and twice) and low dose (once) MSC infusion Control: placebo infusion (once and twice) |
Interventional | II | Randomized, multi-center, double-blind | Treatment | Israel | Placental mesenchymal-like adherent stromal cells |
| 13 | NCT03042143 | 18 (phase 1) and 60 (phase 2) | Experimental: 1, CD362-enriched MSCs, 100 × 106 cells/kg, 200 × 106 cells/kg, 400 × 106 cells/kg 2, highest dose of experimental arm 1. Control: placebo |
Interventional | I–II | Open-label dose escalation pilot study. Phase 1 double blind, randomized, placebo controlled. Phase 2 clinical trial | Treatment | UK | UC |
| 14 | NCT04361942 | 24 | Experimental: 1 × 106 cells/kg Control: placebo |
Interventional | II | Double-blind, randomized, placebo-controlled | Treatment | Spain | Allogeneic MSC |
| 15 | NCT04398303 | 70 | Experimental: 1, MSC infusion 1 × 106 cells/kg plus conventional treatment 2, conditioned medium plus conventional treatment Control: conventional treatment plus placebo |
Interventional | I–II | Randomized, placebo-controlled | Safety and treatment | USA | Allogeneic human UC-MSCs |
| 16 | NCT04467047 | 10 | Experimental: MSC infusion 1 × 106 cells/kg | Interventional | I | Open-label, single group assignment | Safety and feasibility | Brazil | BM-MSCs |
| 17 | NCT04392778 | 30 | Experimental: MSC infusion 3 × 106 cells/kg (three times) with ventilator Sham comparator Saline infusion (three times) with ventilator Untreated without ventilator |
Interventional | I–II | Randomized, double-blind, parallel assignment | Treatment | Turkey | Allogeneic UC |
| 18 | NCT04390139 | 30 | Experimental: MSC infusion 1 × 106 cells/kg Placebo comparator |
Interventional | I–II | Randomized, double-blind, parallel assignment | Safety and treatment | Spain | Wharton's jelly MSC |
| 19 | NCT04492501 | 600 | Experimental: 1, therapeutic plasma exchange 2, therapeutic plasma exchange plus MSC infusion 2 × 106 cells/kg or remdesivir 3, MSC infusion 2 × 106 cells/kg and/or remdesivir and/or tocilizumab (all alone or in combination) |
Interventional | I–II | Non-randomized, open-label, factorial assignment, case–control study | Treatment | Pakistan | BM-MSCs |
| 20 | NCT04345601 | 30 | Experimental: MSC infusion 1 × 108 cells/kg (twice) Control: standard of care |
Interventional | I | Randomized, open-label, parallel assignment | Treatment | USA | BM-MSCs |
| 21 | NCT4377334 | 40 | Experimental: MSC infusion Control: no intervention |
Interventional | II | Randomized, open-label, parallel assignment | Treatment | Germany | BM-MSCs |
| 22 | NCT04397796 | 45 | Experimental: MSC infusion Control: placebo |
Interventional | I | Randomized, double-blind, placebo-controlled | Safety and treatment | USA | BM-MSCs |
| 23 | NCT04494386 | 60 | Experimental: 1, MSC infusion 100 × 106 cells/kg (phase 1, open-label, one or two infusions) 2, MSC infusion (randomized, one or two infusions) 3, placebo infusion (one or two infusions) |
Interventional | I–II | Open-label, non-controlled trial Randomized, placebo-controlled trial |
Safety and treatment | USA | UC-MSCs |
| 24 | NCT04371393 | 300 | Experimental: MSC infusion 2 × 106 cells/kg (remestemcel-L) plus standard of treatment Control: placebo plus standard of treatment |
Interventional | III | Randomized, double-blind, parallel assignment, placebo-controlled | Safety and treatment | USA | BM-MSCs |
| 25 | NCT04452097 | 9 | Experimental: MSC infusion 0.5 × 106 cells/kg, 1 × 106 cells/kg, 1.5 × 106 cells/kg, BX-U001 | Interventional | I | Non-randomized, open-label, single arm, dose-escalating | Safety | USA | UC-MSCs |
| 26 | NCT04390152 | 40 | Experimental: MSC infusion 50 × 106 cells/kg (twice) plus hydroxychloroquine plus lopinavir/ritonavir or azithromycin Control: placebo plus hydroxychloroquine plus lopinavir/ritonavir or azithromycin |
Interventional | I–II | Randomized, double-blind, parallel assignment | Safety and treatment | USA | Wharton's jelly-derived MSCs |
| 27 | NCT04362189 | 100 | Experimental: MSC infusion 100 × 106 cells/kg (four infusions) Control: saline (four infusions) |
Interventional | II | Randomized, double-blind, placebo-controlled | Safety and treatment | USA | AD-MSCs |
| 28 | NCT04348461 | 100 | Experimental: MSC infusion 1.5 × 106 cells/kg (twice) Control: standard of treatment |
Interventional | II | Randomized, two-treatment, multi-center, controlled | Safety and treatment | Spain | AD-MSCs |
| 29 | NCT04371601 | 60 | Experimental: MSC infusion 1 × 106 cells/kg (four infusions) plus standard of care Control: standard of care |
Interventional | I | Randomized, parallel assignment, open-label | Safety and treatment | China | UC |
| 30 | NCT04461925 | 30 | Experimental: MSC infusion 1 × 106 cells/kg (twice) plus standard of care Control: standard of care |
Interventional | I–II | Non-randomized, open-label, parallel assignment | Safety and treatment | Ukraine | Placenta and UC |
| 31 | NCT04355728 | 24 | Experimental: MSC infusion 100 × 106 cells/kg (twice) plus standard of care plus heparin Control: standard of care plus vehicle plus heparin |
Interventional | I–II | Randomized, double-blind, parallel assignment | Safety and treatment | USA | UC |
| 32 | NCT04490486 | 21 | Experimental: MSC infusion 100 × 106 cells/kg (twice) Control: placebo |
Interventional | I | Randomized, double-blind, placebo-controlled | Safety and treatment | USA | UC |
| 33 | NCT04302519 | 24 | Experimental: MSC infusion 1 × 106 cells/kg (dose scaling) | Interventional | I | Open-label, single center, single arm | Safety and treatment | China | Dental pulp |
| 34 | NCT04352803 | 20 | Experimental: MSC infusion 5 × 105 cells/kg plus standard of care Control: standard of care |
Interventional | I | Non-randomized, open-label, sequential assignment, unmatched control | Safety and treatment | USA | Adipose tissue |
| 35 | NCT04457609 | 40 | Experimental: MSC infusion 1 × 106 cells/kg plus standard of treatment Control: standard of treatment |
Interventional | I | Randomized, double-blind, parallel assignment, controlled trial | Safety and treatment | Indonesia | UC |
| 36 | NCT04349631 | 56 | Experimental: MSC infusion (five times) | Interventional | II | Open-label, single center clinical trial | Safety and treatment | USA | Adipose tissue |
| 37 | NCT04428801 | 200 | Experimental: MSC infusion 200 × 106 cells/kg (three times) Control: placebo |
Interventional | II | Randomized, double-blind, multi-center, placebo-controlled | Treatment | USA | Adipose tissue |
| 38 | NCT04339660 | 30 | Experimental: MSC infusion 1 × 106 cells/kg plus standard of treatment Control: placebo plus standard of treatment |
Interventional | II–III | Randomized, double-blind, parallel assignment | Treatment | China | UC |
| 39 | NCT04366063 | 60 | Experimental: 1, MSC infusion 100 × 106 cells/kg (twice) 2, MSC infusion 100 × 106 cells/kg (twice) plus exosome vesicles (two infusions) Control: standard of treatment |
Interventional | II | Randomized, parallel assignment | Safety and treatment | Iran | NA |
| 40 | NCT04348435 | 100 | Experimental: 1, MSC infusion 200 × 106 cells/kg (five times) 2, MSC infusion 100 × 106 cells/kg (five times) 3, MSC infusion 50 × 106 cells/kg (five times) Control: placebo (five infusions) |
Interventional | I–II | Randomized, double-blind, placebo-controlled | Safety and treatment | USA | Adipose tissue |
| 41 | NCT04382547 | 40 | Experimental: MSC infusion plus standard of treatment Control: standard of treatment |
Interventional | II | Non-randomized, parallel assignment, open-label | Belarus | Olfactory mucosa | |
| 42 | NCT04273646 | 48 | Experimental: MSC infusion 0.5 × 106 cells/kg (four times) plus standard of treatment Control: placebo plus standard of treatment |
Interventional | I–II | Randomized, parallel assignment, open-label | Safety and treatment | China | UC |
| 43 | NCT04288102 | 100 | Experimental: MSC infusion 4 × 107 cells/kg (three times) plus standard of treatment Control: placebo plus standard of treatment |
Interventional | II | Randomized, multi-center, double-blind, placebo-control | Safety and treatment | China | UC |
| 44 | NCT04346368 | 20 | Experimental: MSC infusion 1 × 106 cells/kg plus standard of treatment Control: placebo plus standard of treatment |
Interventional | I–II | Randomized, parallel assignment, open-label | Safety and treatment | China | BM |
| 45 | NCT04336254 | 20 | Experimental: MSC infusion 3 × 107 cells/kg (three times) plus standard of treatment Control: saline plus standard of treatment |
Interventional | I–II | Randomized, parallel assignment, open-label | Safety and treatment | China | Dental pulp |
| 46 | NCT04313322 | 5 | Experimental: MSC infusion | Interventional | I | Open-label, direct study | Safety and treatment | Jordan | Wharton's jelly |
| 47 | NCT04252118 | 20 | Experimental: MSC infusion 3 × 107 cells/kg plus standard of treatment Control: standard of treatment |
Interventional | I | Non-randomized, parallel assignment, open-label | Safety and treatment | China | NA |
| 48 | NCT04366271 | 106 | Experimental: MSC infusion Control: standard of treatment |
Interventional | II | Randomized, multi-center, parallel assignment, open-label | Treatment | Spain | UC |
| 49 | NCT04366323 | 26 | Experimental: MSC infusion 80 × 106 cells/kg (twice) Control: no intervention |
Interventional | I–II | Randomized, multi-center, parallel assignment, open-label | Safety and treatment | Spain | Adipose tissue |
| 50 | NCT04456361 | 9 | Experimental: MSC infusion 1 × 108 cells/kg | Interventional | I | Open-label, pilot study, non-randomized, single group | Safety and treatment | Mexico | Wharton's jelly |
| 51 | NCT04315987 | 90 | Experimental: MSC infusion 2 × 107 cells/kg (four times) Control: placebo (four infusions) |
Interventional | II | Randomized, parallel assignment, double-blind | Treatment | Brazil | NA |
| 52 | NCT04429763 | 30 | Experimental: MSC infusion 1 × 106 cells/kg plus standard of treatment Control: placebo plus standard of treatment |
Interventional | II | Double-blind, controlled clinical trial, randomized, parallel assignment | Safety and treatment | USA | UC |
| 53 | NCT04416139 | 10 | Experimental: MSC infusion 1 × 106 cells/kg Control: standard of treatment |
Interventional | II | Non-randomized, parallel assignment, open-label | Treatment | Mexico | UC |
| 54 | NCT04444271 | 20 | Experimental: MSC infusion 2 × 106 cells/kg (once or twice) plus standard of treatment Control: placebo plus saline plus standard of treatment |
Interventional | II | Randomized, phase 2, parallel assignment, open-label | Treatment | Pakistan | BM |
Details of clinical trials as listed on ClinicalTrials.gov as of July 25, 2020.
NA, not applicable.
Intramuscular injection of placenta-derived mesenchymal-like cells cured six severely ill COVID-19 patients in a trial conducted by Pluristem Therapeutics Inc, an Israel-based biotech firm [76]. A randomized, double-blind, placebo-controlled, multi-center (USA and Israel), parallel assignment phase 2 trial with 140 patients is being conducted by Pluristem Theapeutics Inc, comparing high and multiple doses of intramuscular injections of MSCs (300 × 106 cells) with placebo treatment (NCT04389450). Novellus, Inc, and Citius Pharmaceuticals, Inc, propose to use MSCs derived from reprogrammed messenger RNA induced pluripotent stem cells generated from fibroblasts of a single individual (NoveCite MSCs). A randomized, placebo-controlled, dose-inducing study followed by a dose level expansion to assess the safety and efficacy of NoveCite MSCs in subjects with ARDS due to COVID-19 is in the pipeline [77]. An induced pluripotent stem cell bank would help overcome the scarcity or unavailability of MSCs.
Athersys, Inc, completed a phase 1/2 clinical trial of intravenous injection of their innovative product MultiStem in COVID-19 patients [78]. Phase 1, with a small initial dose, confirmed the safety, and phase 2, with a larger dosage, was a double-blind, placebo-controlled, randomized trial. A total of 36 patients were included in the study wherein six patients were treated with a small dose of MultiStem cells, 20 were intravenously injected with 900 × 106 MultiStem cells and 10 were treated with a placebo. The treatment group had lower mortality and lesser intensive care unit days, without any adverse reactions [79]. The group is now conducting a phase 2/3 clinical trial to investigate the safety and efficacy of MultiStem in COVID-19 patients with ARDS (NCT04367077) by recruiting 400 patients. The study will have two arms: experimental and placebo.
Cynata Therapeutics has initiated an open-label, randomized controlled clinical trial to evaluate the safety and efficacy of their Cymerus MSCs. These MSCs are derived from mesenchymal angioblasts. Using their proprietary technology, induced pluripotent stem cells are generated using transgene-, viral- and feeder-free techniques by de-differentiation of donated blood. These stem cells are further differentiated to mesenchymal angioblasts for the derivation of MSCs used in the infusion (NCT02923375). Of the 24 intensive care unit patients recruited, 12 random patients will be infused with Cymerus MSCs in addition to standard of care, and the other 12 receiving standard of care would serve as controls. The endpoint would be improvement in hypoxia at day 7 and safety/tolerability in 28 days [80].
Sanchez-Guijo et al. [81] treated 13 COVID-19 patients with AD-MSCs post anti-viral and anti-inflammatory treatment. Two patients received a single dose, 10 received double the dose and another received a single dose of 0.98 cells/kg body weight. The clinical analysis revealed improvement in the beneficial immune cell profile, with no adverse effects of the infusion (NCT04348461). Hope Biosciences is conducting three clinical trials using AD-MSCs in an attempt to address the dose-scaling effect of MSC infusion, starting with 50 × 106 cells/kg and going up to 200 × 106 cells/kg over 4–5 intravenous infusions, and to evaluate safety and efficacy in a phase 2 trial (NCT04362189, NCT04349631, NCT04348435). The START study (STem cells for ARDS Treatment) recently published a phase 2 safety administration trial with a single dose of intravenous MSCs [82]. Bari et al. [83] and Sanap et al. [84] advocate the use of MSC secretome as a cell-free treatment modality for COVID-19 patients with ARDS.
Protective mechanisms of MSCs in ARDS
Migration of MSCs is stimulated by the pro-inflammatory marker TNFα [85] and by the binding of ligands CD106 and CD62E with integrin α4/β1 (CD49δ/CD29) and CD44 receptors, respectively [86,87]. Trophic factors such as epithelial growth factor, transforming growth factors α and β, basic FGF2, HGF, insulin-like growth factor 1, VEGF, stem cell factor and stem cell-derived factor 1 and immunomodulatory factors such as prostaglandin E2, inducible nitric oxide synthase, indoleamine 2,3-dioxygenase, CCL2, IL-10 and IL-6 are some of the molecules released by MSCs [88,89]. The cytokine secretion profile of dendritic cells and macrophages is modulated by MSCs [90]. The anti-proliferative properties of MSCs play a role in limiting the proliferation of T lymphocytes, B cells, natural killer cells and microglial cells [91]. MSCs have been successfully transplanted in graft-versus-host disease and in multiple system atrophy [92,93].
MSCs have immunomodulatory functions, direct cell-to-cell interactions and secrete growth factors and extracellular vesicles. During inflammation, impaired barrier properties of epithelial cells are associated with an increase in the permeability of endothelial cells in the lungs [94]. It has been shown that intratracheal MSC administration in lipopolysaccharide-induced inflammatory conditions in mouse models leads to a reduction in inflammation [95]. This study also demonstrated that through the paracrine process MSCs can induce IL-10 via prostaglandin E2 and other secretory factors, such as granulocyte-macrophage colony-stimulating fact and granulocyte colony-stimulating factor, which help to recover the barrier properties of the lungs. Additionally, MSCs secrete anti-inflammatory factors IL-10 and IL-4 and suppress the activation of lymphocytes and inflammatory cytokines IL-1-α, IL-1-β, IL-6, IL-17, TNFα, TNFγ and interferon γ [96]. It has also been described that MSCs reduce the excessive secretion of neutrophil extracellular traps at the site of infection, thereby preventing further damage to lung tissues [97]. MSCs have the ability to reduce the excessive production of neutrophils that causes tissue damage and increase neutrophil-mediated phagocytosis in bacterial infections [98]. MSCs play a role in differentiating macrophages into M1 and M2 phenotypes. M1 activates phagocytosis, which has a pro-inflammatory function and aids in bacterial clearance, and M2 supports tissue repair by resolving inflammation at the infection site [99,100]. MSCs also suppress the proliferation of effector T cells and promote regulatory T cells, thereby reducing the immune response and resolving lung damage in ARDS [101].
In a sepsis mouse model, MSCs were shown to have transcriptional responses via the downregulation of Toll-like receptor-mediated nuclear factor kappa light chain enhancer of activated B cells and along with a simultaneous upregulation of the nuclear factor of activated T cells, calcium and calcineurin gene families regulating the transcription of cytokine genes [102]. In a lipopolysaccharide-induced acute lung injury mouse model, BM-MSCs established cell-to-cell contact with connexin 43 gap junction channels. The attached MSCs released mitochondria-containing microvesicles into alveolar epithelial cells. The mitochondrial transfer increased adenosine triphosphate concentrations in epithelial cells, thereby repairing alveolar epithelial and endothelial barriers in acute lung injury [103]. In addition, an Escherichia coli pneumonia model demonstrated that mitochondrial transfer from MSCs to macrophages partially occurs through tunneling nanotube-like structures [104]. The mitochondrial transfer enhances phagocytic activity, which establishes a mechanism for anti-microbial effect through cell-to-cell contact. MSCs also play a paracrine role by secreting soluble molecules.
In a rat ventilator-induced lung injury model, the MSC secretome (MSC-conditioned medium) reversed the lung injury via keratinocyte growth factor (KGF). KGF repairs epithelial cells by enhancing Na-K-ATPase, anti-inflammatory cytokine (IL-1α, matrix metallopeptidase 9) and macrophage activity via granulocyte-macrophage colony-stimulating factor [105,106]. Overexpression of certain MSC factors, such as PDGFβ, VEGF, basic FGF, angiogenin 1 and PDGF, induces cell proliferation and brings about lung repair [107]. In various studies, the overexpression of angiogenin 1, KGF, ACE-2, CXCR4 and HGF has reduced endotoxin-induced lung injury, edema formation, collagen deposition and fibrosis, in addition to enhancing the chemotactic and anti-inflammatory properties of MSCs [105,[108], [109], [110], [111]]. The plausible mechanism by which MSCs resolve ARDS is depicted schematically in Figure 2 .
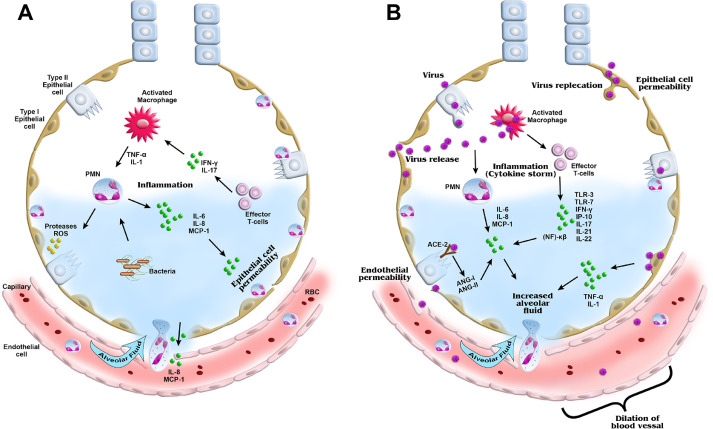
Fig. 2.
Schematic representation of ARDS in non-viral and viral conditions. (A) In non-viral-induced ARDS, macrophages and effector T cells are activated and cytokines induced, which in turn activates neutrophils and causes secretion of further inflammatory cytokines and chemokines. (B) In viral-induced ARDS, additional cytokines are produced, leading to a cytokine storm. The released proteases and inflammatory cytokines damage the epithelial and endothelial layers of the alveoli, causing increased epithelial/endothelial permeability, fibrosis, edema formation and vasodilation. ANG, angiogenin; IP, interferon gamma-induced protein; MCP, monocyte chemoattractant protein; NF-κB, nuclear factor kappa light chain enhancer of activated B cells; PMNs, polymorphonuclear cells; RBC, red blood cell; ROS, reactive oxygen species; TLR, Toll-like receptor. (Color version of figure is available online).
Experimental and pre-clinical evidence of the benefits of MSCs in immunomodulation of respiratory virus-induced lung injury is available, which may be helpful in the treatment of COVID-19. UC-MSCs are able to restore alveolar epithelial cell functions, as seen by increased alveolar fluid clearance and decreased protein permeability, in avian influenza virus (H5N1) lung injuries in mouse models [112]. MSCs are resistant to viral infections, and recently it has been established that intrinsically expressed interferon-stimulated genes (ISGs) protect stem cells against viral infection [113]. This study demonstrated that induction of intrinsic ISGs in human MSCs constitutively increased the expression of anti-virals (IFI6, ISG15, CCL2, SAT1, PMAIP1 and interferon-induced transmembrane protein 1 [IFITM1]). With regard to anti-viral mechanism, the IFITM family plays a major role in preventing the virus from crossing the lipid bilayer of cells. It has been demonstrated that IFITM prevents the entry of various viruses, including Ebola virus, dengue virus, influenza A virus, Rift Valley fever virus, reovirus and SARS-CoV, as well as replication in HIV-1 and hepatitis C virus [114,115]. Interestingly, it has been shown that SARS-CoV virus internalization is prevented by the host cell receptor ACE-2 in IFITM-expressing cells [114]. In the lungs, the ACE-2 receptor is expressed in alveolar type II cells and endothelial cells, and these cells play a role in preventing virus entry and reducing fibrosis and have anti-inflammatory and endothelial protective effects [116], [117], [118].
The COVID-19 mortality rate is higher in patients who have pre-existing systemic diseases, such as diabetes, renal disease and hypertension. In these conditions, the ACE-2 receptor plays an important role, as it is a major enzyme in the renin–angiotensin system, which has been localized in the apical surface area and glomeruli of the kidneys and in the acini and islets of the pancreas. In an in vivomouse model, it was demonstrated that an ACE-2 deficiency can cause decreased insulin secretion, leading to diabetes [119]. Plasma ACE-2 levels are lower in chronic kidney disease patients undergoing dialysis [120]. Adult hypertensive rats show decreased expression of ACE-2 in the kidneys [121].
The potential benefits of overexpression of ACE-2 receptors by MSCs in relation to COVID-19 need further exploration. The details regarding the underlying mechanisms involved in resolving COVID-19 in patients with infusion of MSCs are still unknown. The authors have schematically represented the plausible mechanism by which MSCs reduce the adverse effects of ARDS caused by COVID-19 based on the existing knowledge (Figure 3 ).
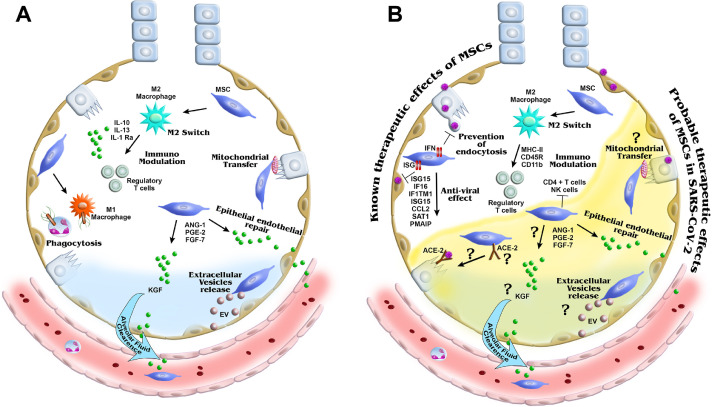
Fig. 3.
Schematic representation of the mechanism of action of MSCs in ARDS. Potential therapeutic mechanisms of MSCs in non-viral and viral ARDS recovery are shown. MSCs promote differentiate of macrophages from type M1 to M2 to induce anti-inflammatory cytokines and M1 macrophages with phagocytic activity. MSCs reduce the infiltration of neutrophils by secreting anti-inflammatory cytokines and regulating effector T cells. These anti-inflammatory cytokines and other secreted factors reduce the epithelial/endothelial permeability and influx of alveolar fluid. (A) MSCs are known to directly transfer mitochondria through tunneling nanotubules and microvesicles to transfer RNA and proteins for tissue repair. In viral ARDS, few mechanisms are understood, suggesting that intrinsically expressed genes and proteins may have anti-viral effects. (B) For SARS-CoV-2 virus infection, cell-based therapy with MSCs is being explored. ANG, angiogenin; EV, extracellular vesicle; MHC, major histocompatibility complex; PGE2, prostaglandin E2; PMAIP1, phorbol-12-myristate-13-acetate-induced protein 1; PMNs, polymorphonuclear cells; RBC, red blood cell; ROS, reactive oxygen species; SAT, spermidine/spermine N1-acetyltransferase. (Color version of figure is available online).
Discussion
The emergence of the COVID-19 pandemic and its sequelae have prompted clinicians and researchers to explore all possible preventive and treatment modalities since existing strategies target symptoms, rather than the underlying pathology. Anti-virals, pulmonary and renal support systems and immunomodulators are being used to treat the cytokine storm, which causes respiratory depression and multi-organ dysfunction. Until effective vaccines and specific treatment options are available, the high infectivity rate of COVID-19 makes limiting disease progression a challenge. MSCs serve as a potential therapeutic candidate for combating the cytokine storm owing to their primordial cell lineage and multi-potent functions, such as immunomodulation and anti-inflammatory activity, and their ability to secrete various growth factors and soluble vesicles. Encouraging results from ongoing trials would expand the clinical applicability of MSCs and provide hope for patients suffering with ARDS due to COVID-19 infection.
Clarity is lacking regarding the best source of MSCs, the method of application or mode of infusion of the cells to the patient, the stage of disease at which MSCs would work with the highest efficiency, the timeline of results expected post injection, the age group of patients, etc. A combination treatment approach with MSCs and supportive drugs might work synergistically to restrict the infectivity of the virus, in addition to preventing the progression of the infection to a severe form. Another approach may be administration of anti-viral drugs carrying nanoparticles loaded on stem cells with an affinity for ACE-2 receptor-harboring alveolar cells. The means to generate high-clinical-grade MSCs is the need of the hour. Apart from cell-based therapy, exosome vesicles as well as the culture secretome of MSC might be explored as an alternative.
Future research toward a better understanding of MSCs resident in lung tissue could pave the way for developing the means to activate host-specific resident stem cells to resolve site-specific ARDS. This would eliminate the need for infusion of allogeneic cell therapy. The results of ongoing clinical trials would help provide guidelines for cell monotherapy or combination therapy with non-cell-based treatment, enabling clinicians worldwide to better manage severely infected COVID-19 patients. With the fear of a second wave of infection looming large, it is a race against time for researchers worldwide to fight the challenge posed by COVID-19.
Acknowledgments
Funding
No funding was received.
Declaration of Competing Interest
The authors have no commercial, proprietary or financial interest in the products or companies described in this article.
Author Contributions
Conception and design of the study: RS and DD. Acquisition of data: MP, KC, CJ and DD. Analysis and interpretation of data: MP, KC, CJ and DD. Drafting or revising the manuscript: RS, DD, KC, HM, CJ and AG. All authors have approved the final article.
Acknowledgments
The authors thank Dr K Bhujang Shetty and Dr P Narendra for providing the necessary logistics for this review. The authors also thank the Narayana Nethralaya Foundation for its support. Finally, the authors thank the multimedia team, Narayana Nethralaya Eye Institute, for their help in making the schematic diagrams.
References
- 1.Metcalfe S.M. Mesenchymal stem cells and management of COVID-19 pneumonia. Medicine in drug discovery. 2020;5 doi: 10.1016/j.medidd.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majumder M., Mandl K. Early Transmissibility Assessment of a Novel Coronavirus in Wuhan, China. SSRN Electronic Journal. 2020 [Google Scholar]
- 3.Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. High Contagiousness and Rapid Spread of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis. 2020;26(7):1470–1477. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauch C.T., Lloyd-Smith J.O., Coffee M.P., Galvani A.P. Dynamically modeling SARS and other newly emerging respiratory illnesses: past, present, and future. Epidemiology. 2005;16(6):791–801. doi: 10.1097/01.ede.0000181633.80269.4c. [DOI] [PubMed] [Google Scholar]
- 5.Killerby M.E., Biggs H.M., Midgley C.M., Gerber S.I., Watson J.T. Middle East Respiratory Syndrome Coronavirus Transmission. Emerg Infect Dis. 2020;26(2):191–198. doi: 10.3201/eid2602.190697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikbakht R., Baneshi M.R., Bahrampour A., Hosseinnataj A. Comparison of methods to Estimate Basic Reproduction Number (R 0) of influenza, Using Canada 2009 and 2017-18 A (H1N1) Data. J Res Med Sci. 2019;24:67. doi: 10.4103/jrms.JRMS_888_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovato A., de Filippis C. Clinical Presentation of COVID-19: A Systematic Review Focusing on Upper Airway Symptoms. Ear, nose, & throat journal. 2020 doi: 10.1177/0145561320920762. 145561320920762. [DOI] [PubMed] [Google Scholar]
- 8.Shetty R., Ghosh A., Honavar S.G., Khamar P., Sethu S. Therapeutic opportunities to manage COVID-19/SARS-CoV-2 infection: Present and future. Indian journal of ophthalmology. 2020;68(5):693–702. doi: 10.4103/ijo.IJO_639_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clinical immunology. 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and Multiorgan Response. Current problems in cardiology. 2020;45(8) doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain A., Bhowmik B., do Vale Moreira N.C. COVID-19 and diabetes: Knowledge in progress. Diabetes research and clinical practice. 2020;162 doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahid Z., Kalayanamitra R., McClafferty B., Kepko D., Ramgobin D., Patel R., Aggarwal C.S., Vunnam R., Sahu N., Bhatt D., Jones K., Golamari R., Jain R. COVID-19 and Older Adults: What We Know. Journal of the American Geriatrics Society. 2020;68(5):926–929. doi: 10.1111/jgs.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 Does Not Lead to a “Typical”Acute Respiratory Distress Syndrome. American journal of respiratory and critical care medicine. 2020;201(10):1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEnery T., Gough C., Costello R.W. COVID-19: Respiratory support outside the intensive care unit. The Lancet. Respiratory medicine. 2020;8(6):538–539. doi: 10.1016/S2213-2600(20)30176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ronco C., Reis T., Husain-Syed F. Management of acute kidney injury in patients with COVID-19. The Lancet. Respiratory medicine. 2020;8(7):738–742. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. The New England journal of medicine. 2020 [Google Scholar]
- 17.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe COVID-19. The New England journal of medicine. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang D., Yu H., Wang T., Yang H., Yao R., Liang Z. Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borba M., Val F., Sampaio V., Alexandre M., Melo G., Brito M. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study) medRxiv. 2020 [Google Scholar]
- 21.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 22.Aouba A., Baldolli A., Geffray L., Verdon R., Bergot E., Martin-Silva N. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. 2020;79(10):1381–1382. doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
- 23.Huet T., Beaussier H., Voisin O., Jouveshomme S., Dauriat G., Lazareth I., Sacco E., Naccache J.-M., Bézie Y., Laplanche S., Le Berre A., Le Pavec J., Salmeron S., Emmerich J., Mourad J.-J., Chatellier G., Hayem G. Anakinra for severe forms of COVID-19: a cohort study. The Lancet Rheumatology. 2020;2(7):e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaspari V., Zengarini C., Greco S., Vangeli V., Mastroianni A. Side effects of ruxolitinib in patients with SARS-CoV-2 infection: Two case reports. Int J Antimicrob Agents. 2020;56(2) doi: 10.1016/j.ijantimicag.2020.106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizk J.G., Kalantar-Zadeh K., Mehra M.R., Lavie C.J., Rizk Y., Forthal D.N. Pharmaco-Immunomodulatory Therapy in COVID-19. Drugs. 2020;80(13):1267–1292. doi: 10.1007/s40265-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy M., Estcourt L., Grant-Casey J., Dzik S. International Survey of Trials of Convalescent Plasma to Treat COVID-19 Infection. Transfus Med Rev. 2020;34(3):151–157. doi: 10.1016/j.tmrv.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yigenoglu T.N., Hacibekiroglu T., Berber I., Dal M.S., Basturk A., Namdaroglu S. Convalescent plasma therapy in patients with COVID-19. J Clin Apher. 2020;35(4):367–373. doi: 10.1002/jca.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham S.P., McLean R.K., Spencer A.J., Belij-Rammerstorfer S., Wright D., Ulaszewska M., Edwards J.C., Hayes J.W.P., Martini V., Thakur N., Conceicao C., Dietrich I., Shelton H., Waters R., Ludi A., Wilsden G., Browning C., Bialy D., Bhat S., Stevenson-Leggett P., Hollinghurst P., Gilbride C., Pulido D., Moffat K., Sharpe H., Allen E., Mioulet V., Chiu C., Newman J., Asfor A.S., Burman A., Crossley S., Huo J., Owens R.J., Carroll M., Hammond J.A., Tchilian E., Bailey D., Charleston B., Gilbert S.C., Tuthill T.J., Lambe T. Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. NPJ vaccines. 2020;5:69. doi: 10.1038/s41541-020-00221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golchin A., Seyedjafari E., Ardeshirylajimi A. Mesenchymal Stem Cell Therapy for COVID-19: Present or Future. Stem cell reviews and reports. 2020;16(3):427–433. doi: 10.1007/s12015-020-09973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cucinotta D., Vanelli M. WHO Declares COVID-19 a Pandemic. Acta bio-medica: Atenei Parmensis. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) The Journal of pathology. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magrone T., Magrone M., Jirillo E. Focus on Receptors for Coronaviruses with Special Reference to Angiotensin-converting Enzyme 2 as a Potential Drug Target—A Perspective. Endocrine, metabolic & immune disorders drug targets. 2020;20(6):807–811. doi: 10.2174/1871530320666200427112902. [DOI] [PubMed] [Google Scholar]
- 37.Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infectious diseases of poverty. 2020;9(1):45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.052. 271-280 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. The Journal of infection. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitra P., Misra S., Sharma P. COVID-19 Pandemic in India: What Lies Ahead. Indian journal of clinical biochemistry: IJCB. 2020;35(3):380–381. doi: 10.1007/s12291-020-00886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. Journal of pharmaceutical analysis. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine & growth factor reviews. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singhal T. A Review of Coronavirus Disease-2019 (COVID-19) Indian journal of pediatrics. 2020;87(4):281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atluri S., Manchikanti L., Hirsch J.A. Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use. Pain physician. 2020;23(2):E71–E83. [PubMed] [Google Scholar]
- 45.Baden L.R., Rubin E.J. Covid-19—The Search for Effective Therapy. The New England journal of medicine. 2020;382(19):1851–1852. doi: 10.1056/NEJMe2005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ullah I., Subbarao R.B., Rho G.J. Human mesenchymal stem cells—current trends and future prospective. Bioscience reports. 2015;35(2):e00191. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hass R., Kasper C., Bohm S., Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell communication and signaling: CCS. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baghaei K., Hashemi S.M., Tokhanbigli S., Asadi Rad A., Assadzadeh-Aghdaei H., Sharifian A., Zali M.R. Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. Gastroenterology and hepatology from bed to bench. 2017;10(3):208–213. [PMC free article] [PubMed] [Google Scholar]
- 49.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 50.Newman R.E., Yoo D., LeRoux M.A., Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflammation & allergy drug targets. 2009;8(2):110–123. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- 51.Alcayaga-Miranda F., Cuenca J., Khoury M. Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies. Frontiers in immunology. 2017;8:339. doi: 10.3389/fimmu.2017.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gentile P., Sterodimas A. Adipose-derived stromal stem cells (ASCs) as a new regenerative immediate therapy combating coronavirus (COVID-19)-induced pneumonia. Expert opinion on biological therapy. 2020;20(7):711–716. doi: 10.1080/14712598.2020.1761322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gentile P., Sterodimas A., Pizzicannella J., Dionisi L., De Fazio D., Calabrese C., Garcovich S. Systematic Review: Allogenic Use of Stromal Vascular Fraction (SVF) and Decellularized Extracellular Matrices (ECM) as Advanced Therapy Medicinal Products (ATMP) in Tissue Regeneration. Int J Mol Sci. 2020;21(14):4982. doi: 10.3390/ijms21144982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gentile P., Sterodimas A., Pizzicannella J., Calabrese C., Garcovich S. Research progress on Mesenchymal Stem Cells (MSCs), Adipose-Derived Mesenchymal Stem Cells (AD-MSCs), Drugs, and Vaccines in Inhibiting COVID-19 Disease. Aging and disease. 2020;11(5):1191–1201. doi: 10.14336/AD.2020.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Copcu H.E. Potential Using of Fat-derived Stromal Cells in the Treatment of Active Disease, and also, in Both Pre- and Post-Periods in COVID-19. Aging and disease. 2020;11(4):730–736. doi: 10.14336/AD.2020.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gentile P., Sterodimas A. Adipose Stem Cells (ASCs) and Stromal Vascular Fraction (SVF) as a Potential Therapy in Combating (COVID-19)-Disease. Aging and disease. 2020;11(3):465–469. doi: 10.14336/AD.2020.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shete A., Dhayarkar S., Sangale S., Medhe U., Panchal N., Rahane G., Yelgate R., Dhamanage A., Gangakhedkar R. Incomplete functional T-cell reconstitution in immunological non-responders at one year after initiation of antiretroviral therapy possibly predisposes them to infectious diseases. Int J Infect Dis. 2019;81:114–122. doi: 10.1016/j.ijid.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Z., Fu J., Xu X., Wang S., Xu R., Zhao M., Nie W., Wang X., Zhang J., Li T., Su L., Wang F.S. Safety and immunological responses to human mesenchymal stem cell therapy in difficult-to-treat HIV-1-infected patients. AIDS. 2013;27(8):1283–1293. doi: 10.1097/QAD.0b013e32835fab77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chandra P.K., Gerlach S.L., Wu C., Khurana N., Swientoniewski L.T., Abdel-Mageed A.B., Li J., Braun S.E., Mondal D. Mesenchymal stem cells are attracted to latent HIV-1-infected cells and enable virus reactivation via a non-canonical PI3K-NFkappaB signaling pathway. Scientific reports. 2018;8(1):14702. doi: 10.1038/s41598-018-32657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cotter E.J., Chew N., Powderly W.G., Doran P.P. HIV type 1 alters mesenchymal stem cell differentiation potential and cell phenotype ex vivo. AIDS Res Hum Retroviruses. 2011;27(2):187–199. doi: 10.1089/aid.2010.0114. [DOI] [PubMed] [Google Scholar]
- 62.Huang K., Ji F., Xie Z., Wu D., Xu X., Gao H., Ouyang X., Xiao L., Zhou M., Zhu D., Li L. Artificial liver support system therapy in acute-on-chronic hepatitis B liver failure: classification and regression tree analysis. Scientific reports. 2019;9(1):16462. doi: 10.1038/s41598-019-53029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie C., Zheng Y.B., Zhu H.P., Peng L., Gao Z.L. Human bone marrow mesenchymal stem cells are resistant to HBV infection during differentiation into hepatocytes in vivo and in vitro. Cell Biol Int. 2009;33(4):493–500. doi: 10.1016/j.cellbi.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 64.Ma R., Xing Q., Shao L., Wang D., Hao Q., Li X., Sai L., Ma L. Hepatitis B virus infection and replication in human bone marrow mesenchymal stem cells. Virol J. 2011;8:486. doi: 10.1186/1743-422X-8-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng L., Xie D.Y., Lin B.L., Liu J., Zhu H.P., Xie C., Zheng Y.B., Gao Z.L. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54(3):820–828. doi: 10.1002/hep.24434. [DOI] [PubMed] [Google Scholar]
- 66.Zhong Y.S., Lin N., Deng M.H., Zhang F.C., Tang Z.F., Xu R.Y. Deficient proliferation of bone marrow-derived mesenchymal stem cells in patients with chronic hepatitis B viral infections and cirrhosis of the liver. Dig Dis Sci. 2010;55(2):438–445. doi: 10.1007/s10620-009-0733-4. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y., Wang F., Zhao H., Zhang X., Chen H., Zhang K. Human adipose-derived mesenchymal stem cells are resistant to HBV infection during differentiation into hepatocytes in vitro. Int J Mol Sci. 2014;15(4):6096–6110. doi: 10.3390/ijms15046096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liang B., Chen J., Li T., Wu H., Yang W., Li Y. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. Medicine (Baltimore) 2020;99(31):e21429. doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., Fan J., Wang W., Deng L., Shi H., Li H., Hu Z., Zhang F., Gao J., Liu H., Li X., Zhao Y., Yin K., He X., Gao Z., Wang Y., Yang B., Jin R., Stambler I., Lim L.W., Su H., Moskalev A., Cano A., Chakrabarti S., Min K.J., Ellison-Hughes G., Caruso C., Jin K., Zhao R.C. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging and disease. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.B.B.C. Research, FDA approved mesenchymal stem cell (MSC) treatments as compassionate use in the very sickest COVID-19 patients., 2020. https://bbcrconsulting.com/clinical-trials/fda-approved-mesenchymal-stem-cell-msc-treatments-as-compassionate-use-in-the-very-sickest-covid-19-patients/. (Accessed 21 May 2020).
- 71.V. Rees, US researchers to study stem cell therapy in COVID-19 patients, 2020. https://www.europeanpharmaceuticalreview.com/news/116794/us-researchers-to-study-stem-cell-therapy-in-covid-19-patients/. (Accessed 21 May 2020).
- 72.D. Patel, GIOSTAR Announces FDA Approval Under Compassionate Use for a COVID-19 Clinical Trial with Stem Cells, 2020. https://www.prnewswire.com/news-releases/giostar-announces-fda-approval-under-compassionate-use-for-a-covid-19-clinical-trial-with-stem-cells-301051830.html (Accessed 21 May 2020).
- 73.B. Disptach, First patients dosed in Mesoblast COVID-19 trial, 2020. https://biotechdispatch.com.au/news/first-patients-dosed-in-mesoblast-covid-19-trial. (Accessed 23 May 2020).
- 74.J. Meldrum, Bothwell, K., First Patients Dosed in Phase 2/3 Randomized Controlled Trial of Mesoblast's Remestemcel-l for COVID-19 Acute Respiratory Distress Syndrome, 2020. https://www.globenewswire.com/news-release/2020/05/06/2028234/0/en/First-Patients-Dosed-in-Phase-2-3-Randomized-Controlled-Trial-of-Mesoblast-s-Remestemcel-l-for-COVID-19-Acute-Respiratory-Distress-Syndrome.html. (Accessed 23 May 2020).
- 75.L. Parsons, Mesoblast expands compassionate use COVID-19 programme, 2020. https://www.pmlive.com/pharma_news/mesoblast_expands_compassionate_use_covid-19_programme_1344461#:~:text=Will%20now%20include %20children%20with %20multisystem%20inflammatory%20syndrome&text=Australian%2 Dbased %20regenerative%20medicine%20company,children%20infected %20with%20 COVID%2D19. (Accessed 27 July 2020).
- 76.D. Rubin, U.S. FDA Clears Pluristem's IND Application for Phase II COVID-19 Study, 2020. https://www.globenewswire.com/news-release/2020/05/08/2030212/0/en/U-S-FDA-Clears-Pluristem-s-IND-Application-for-Phase-II-COVID-19-Study.html. (Accessed 25 May 2020).
- 77.R. Staff, Novel Stem Cell Therapy for COVID-19-related ARDS in Development, 2020. https://www.rtmagazine.com/products-treatment/pharmaceuticals/us-pharmaceuticals/stem-cells-covid-19-ards. (Accessed 26 May 2020).
- 78.R. Weermeijer, Athersys and UH Cleveland trial stem cell therapy for Covid-19, 2020. https://www.clinicaltrialsarena.com/news/athersys-stem-cell-therapy-covid-19/. (Accessed 25 May 2020).
- 79.A. Staff, COVID-19 and other Viral Induced ARDS, 2020. https://www.athersys.com/clinical-trials/ards/default.aspx. (Accessed 27 July 2020 2020).
- 80.R. Macdonald, LaCagnina, C., Cynata Receives Ethics Approval to Commence Clinical Trial in COVID-19 and Clinical Development Update, 2020. https://www.globenewswire.com/news-release/2020/05/08/2030332/0/en/Cynata-Receives-Ethics-Approval-to-Commence-Clinical-Trial-in-COVID-19-and-Clinical-Development-Update.html. (Accessed 27 May 2020).
- 81.Sanchez-Guijo F., Garcia-Arranz M., Lopez-Parra M., Monedero P., Mata-Martinez C., Santos A., Sagredo V., Alvarez-Avello J.M., Guerrero J.E., Perez-Calvo C., Sanchez-Hernandez M.V., Del-Pozo J.L., Andreu E.J., Fernandez-Santos M.E., Soria-Juan B., Hernandez-Blasco L.M., Andreu E., Sempere J.M., Zapata A.G., Moraleda J.M., Soria B., Fernandez-Aviles F., Garcia-Olmo D., Prosper F. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matthay M.A., Calfee C.S., Zhuo H., Thompson B.T., Wilson J.G., Levitt J.E., Rogers A.J., Gotts J.E., Wiener-Kronish J.P., Bajwa E.K., Donahoe M.P., McVerry B.J., Ortiz L.A., Exline M., Christman J.W., Abbott J., Delucchi K.L., Caballero L., McMillan M., McKenna D.H., Liu K.D. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. The Lancet. Respiratory medicine. 2019;7(2):154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bari E., Ferrarotti I., Saracino L., Perteghella S., Torre M.L., Corsico A.G. Mesenchymal Stromal Cell Secretome for Severe COVID-19 Infections: Premises for the Therapeutic Use. Cells. 2020;9(4):924. doi: 10.3390/cells9040924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sanap A, Bhonde R, Kharat A, Kheur S. Mesenchymal Stem Cells Secretome as a prospective therapeutic option for COVID-19 patients, Drug Target Review, Russell Publishing Ltd, Kent, United Kingdom 2020; 13 April:1-3
- 85.Ponte A.L., Marais E., Gallay N., Langonne A., Delorme B., Herault O., Charbord P., Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem cells. 2007;25(7):1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 86.Ruster B., Gottig S., Ludwig R.J., Bistrian R., Muller S., Seifried E., Gille J., Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108(12):3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 87.Segers V.F., Van Riet I., Andries L.J., Lemmens K., Demolder M.J., De Becker A.J., Kockx M.M., De Keulenaer G.W. Mesenchymal stem cell adhesion to cardiac microvascular endothelium: activators and mechanisms. American journal of physiology. Heart and circulatory physiology. 2006;290(4):H1370–H1377. doi: 10.1152/ajpheart.00523.2005. [DOI] [PubMed] [Google Scholar]
- 88.Shi Y., Su J., Roberts A.I., Shou P., Rabson A.B., Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends in immunology. 2012;33(3):136–143. doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meirelles Lda S., Fontes A.M., Covas D.T., Caplan A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine & growth factor reviews. 2009;20(5-6):419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 90.Mao F., Kang J.J., Cai X., Ding N.F., Wu Y.B., Yan Y.M., Qian H., Zhang X., Xu W.R. Crosstalk between mesenchymal stem cells and macrophages in inflammatory bowel disease and associated colorectal cancer. Contemporary oncology. 2017;21(2):91–97. doi: 10.5114/wo.2017.68616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang R., Liu Y., Yan K., Chen L., Chen X.R., Li P., Chen F.F., Jiang X.D. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. Journal of neuroinflammation. 2013;10:106. doi: 10.1186/1742-2094-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Le Blanc K., Rasmusson I., Sundberg B., Gotherstrom C., Hassan M., Uzunel M., Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 93.Stemberger S., Jamnig A., Stefanova N., Lepperdinger G., Reindl M., Wenning G.K. Mesenchymal stem cells in a transgenic mouse model of multiple system atrophy: immunomodulation and neuroprotection. PloS one. 2011;6(5):e19808. doi: 10.1371/journal.pone.0019808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lucas R., Verin A.D., Black S.M., Catravas J.D. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem Pharmacol. 2009;77(12):1763–1772. doi: 10.1016/j.bcp.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu H., Xiong Y., Xia Y., Zhang R., Tian D., Wang T., Dai J., Wang L., Yao H., Jiang H., Yang K., Liu E., Shi Y., Fu Z., Gao L., Zou L. Therapeutic Effects of Human Umbilical Cord-Derived Mesenchymal Stem Cells in Acute Lung Injury Mice. Scientific reports. 2017;7:39889. doi: 10.1038/srep39889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iyer S.S., Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert opinion on biological therapy. 2008;8(5):569–581. doi: 10.1517/14712598.8.5.569. [DOI] [PubMed] [Google Scholar]
- 97.Pedrazza L., Cunha A.A., Luft C., Nunes N.K., Schimitz F., Gassen R.B., Breda R.V., Donadio M.V., de Souza Wyse A.T., Pitrez P.M.C., Rosa J.L., de Oliveira J.R. Mesenchymal stem cells improves survival in LPS-induced acute lung injury acting through inhibition of NETs formation. Journal of cellular physiology. 2017;232(12):3552–3564. doi: 10.1002/jcp.25816. [DOI] [PubMed] [Google Scholar]
- 98.Hall S.R., Tsoyi K., Ith B., Padera R.F., Jr., Lederer J.A., Wang Z., Liu X., Perrella M.A. Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: the importance of neutrophils. Stem cells. 2013;31(2):397–407. doi: 10.1002/stem.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mei S.H., Haitsma J.J., Dos Santos C.C., Deng Y., Lai P.F., Slutsky A.S., Liles W.C., Stewart D.J. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. American journal of respiratory and critical care medicine. 2010;182(8):1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 100.Ye L.Z., Zhang A.D., Shi H.P., Zhao S.M., Luo H.Y., Yuan L.F., Ma S.W., Li S.H., Cheng Z.Y., Zhao Y. Analysis of 4628 cases in the genetic counselling clinic of PUMC Hospital, Proceedings of the Chinese Academy of Medical Sciences and the Peking Union Medical College = Chung-kuo i hsueh k'o hsueh yuan. Chung-kuo hsieh ho i k'o ta hsueh hsueh pao. 1989;4(3):126–130. [PubMed] [Google Scholar]
- 101.Belkaid Y., Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annual review of immunology. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 102.dos Santos C.C., Murthy S., Hu P., Shan Y., Haitsma J.J., Mei S.H., Stewart D.J., Liles W.C. Network analysis of transcriptional responses induced by mesenchymal stem cell treatment of experimental sepsis. The American journal of pathology. 2012;181(5):1681–1692. doi: 10.1016/j.ajpath.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 103.Islam M.N., Das S.R., Emin M.T., Wei M., Sun L., Westphalen K., Rowlands D.J., Quadri S.K., Bhattacharya S., Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nature medicine. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jackson M.V., Morrison T.J., Doherty D.F., McAuley D.F., Matthay M.A., Kissenpfennig A., O'Kane C.M., Krasnodembskaya A.D. Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem cells. 2016;34(8):2210–2223. doi: 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guery B.P., Mason C.M., Dobard E.P., Beaucaire G., Summer W.R., Nelson S. Keratinocyte growth factor increases transalveolar sodium reabsorption in normal and injured rat lungs. American journal of respiratory and critical care medicine. 1997;155(5):1777–1784. doi: 10.1164/ajrccm.155.5.9154891. [DOI] [PubMed] [Google Scholar]
- 106.Shyamsundar M., McAuley D.F., Ingram R.J., Gibson D.S., O'Kane D., McKeown S.T., Edwards A., Taggart C., Elborn J.S., Calfee C.S., Matthay M.A., O'Kane C.M. Keratinocyte growth factor promotes epithelial survival and resolution in a human model of lung injury. American journal of respiratory and critical care medicine. 2014;189(12):1520–1529. doi: 10.1164/rccm.201310-1892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang S., Mo M., Wang J., Sadia S., Shi B., Fu X., Yu L., Tredget E.E., Wu Y. Platelet-derived growth factor receptor beta identifies mesenchymal stem cells with enhanced engraftment to tissue injury and pro-angiogenic property. Cellular and molecular life sciences: CMLS. 2018;75(3):547–561. doi: 10.1007/s00018-017-2641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mei S.H., McCarter S.D., Deng Y., Parker C.H., Liles W.C., Stewart D.J. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS medicine. 2007;4(9):e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He H., Liu L., Chen Q., Liu A., Cai S., Yang Y., Lu X., Qiu H. Mesenchymal Stem Cells Overexpressing Angiotensin-Converting Enzyme 2 Rescue Lipopolysaccharide-Induced Lung Injury. Cell transplantation. 2015;24(9):1699–1715. doi: 10.3727/096368914X685087. [DOI] [PubMed] [Google Scholar]
- 110.Yang J.X., Zhang N., Wang H.W., Gao P., Yang Q.P., Wen Q.P. CXCR4 receptor overexpression in mesenchymal stem cells facilitates treatment of acute lung injury in rats. The Journal of biological chemistry. 2015;290(4):1994–2006. doi: 10.1074/jbc.M114.605063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang H., Yang Y.F., Zhao L., Xiao F.J., Zhang Q.W., Wen M.L., Wu C.T., Peng R.Y., Wang L.S. Hepatocyte growth factor gene-modified mesenchymal stem cells reduce radiation-induced lung injury. Human gene therapy. 2013;24(3):343–353. doi: 10.1089/hum.2012.177. [DOI] [PubMed] [Google Scholar]
- 112.Loy H., Kuok D.I.T., Hui K.P.Y., Choi M.H.L., Yuen W., Nicholls J.M., Peiris J.S.M., Chan M.C.W. Therapeutic Implications of Human Umbilical Cord Mesenchymal Stromal Cells in Attenuating Influenza A(H5N1) Virus-Associated Acute Lung Injury. The Journal of infectious diseases. 2019;219(2):186–196. doi: 10.1093/infdis/jiy478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu X., Dao Thi V.L., Huang Y., Billerbeck E., Saha D., Hoffmann H.H., Wang Y., Silva L.A.V., Sarbanes S., Sun T., Andrus L., Yu Y., Quirk C., Li M., MacDonald M.R., Schneider W.M., An X., Rosenberg B.R., Rice C.M. Intrinsic Immunity Shapes Viral Resistance of Stem Cells. Cell. 2018;172(3) doi: 10.1016/j.cell.2017.11.018. 423-438 e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang I.C., Bailey C.C., Weyer J.L., Radoshitzky S.R., Becker M.M., Chiang J.J., Brass A.L., Ahmed A.A., Chi X., Dong L., Longobardi L.E., Boltz D., Kuhn J.H., Elledge S.J., Bavari S., Denison M.R., Choe H., Farzan M. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS pathogens. 2011;7(1) doi: 10.1371/journal.ppat.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bailey C.C., Zhong G., Huang I.C., Farzan M. IFITM-Family Proteins: The Cell's First Line of Antiviral Defense. Annual review of virology. 2014;1:261–283. doi: 10.1146/annurev-virology-031413-085537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J., Muus C., Wadsworth M.H., 2nd, Kazer S.W., Hughes T.K., Doran B., Gatter G.J., Vukovic M., Taliaferro F., Mead B.E., Guo Z., Wang J.P., Gras D., Plaisant M., Ansari M., Angelidis I., Adler H., Sucre J.M.S., Taylor C.J., Lin B., Waghray A., Mitsialis V., Dwyer D.F., Buchheit K.M., Boyce J.A., Barrett N.A., Laidlaw T.M., Carroll S.L., Colonna L., Tkachev V., Peterson C.W., Yu A., Zheng H.B., Gideon H.P., Winchell C.G., Lin P.L., Bingle C.D., Snapper S.B., Kropski J.A., Theis F.J., Schiller H.B., Zaragosi L.E., Barbry P., Leslie A., Kiem H.P., Flynn J.L., Fortune S.M., Berger B., Finberg R.W., Kean L.S., Garber M., Schmidt A.G., Lingwood D., Shalek A.K., Ordovas-Montanes J. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181(5) doi: 10.1016/j.cell.2020.04.035. 1016-1035 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li X., Molina-Molina M., Abdul-Hafez A., Uhal V., Xaubet A., Uhal B.D. Angiotensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. American journal of physiology. Lung cellular and molecular physiology. 2008;295(1):L178–L185. doi: 10.1152/ajplung.00009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.He H.L., Liu L., Chen Q.H., Cai S.X., Han J.B., Hu S.L., Chun P., Yang Y., Guo F.M., Huang Y.Z., Qiu H.B. MSCs modified with ACE2 restore endothelial function following LPS challenge by inhibiting the activation of RAS. Journal of cellular physiology. 2015;230(3):691–701. doi: 10.1002/jcp.24794. [DOI] [PubMed] [Google Scholar]
- 119.Niu M.J., Yang J.K., Lin S.S., Ji X.J., Guo L.M. Loss of angiotensin-converting enzyme 2 leads to impaired glucose homeostasis in mice. Endocrine. 2008;34(1-3):56–61. doi: 10.1007/s12020-008-9110-x. [DOI] [PubMed] [Google Scholar]
- 120.Roberts M.A., Velkoska E., Ierino F.L., Burrell L.M. Angiotensin-converting enzyme 2 activity in patients with chronic kidney disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28(9):2287–2294. doi: 10.1093/ndt/gft038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhong J.C., Huang D.Y., Yang Y.M., Li Y.F., Liu G.F., Song X.H., Du K. Upregulation of angiotensin-converting enzyme 2 by all-trans retinoic acid in spontaneously hypertensive rats. Hypertension. 2004;44(6):907–912. doi: 10.1161/01.HYP.0000146400.57221.74. [DOI] [PubMed] [Google Scholar]