Highlights
-
•
In CD34 mobilization of Multiple Myeloma patients, Cyclophosphamide at the dose of 4 gr/m2 is usually administered. A lower dose of Cyclophosphamide (1.5–2.0 gr/m2) has a lower mobilizing effect and, for this reason, this dose is not widely used in CD34+ cells mobilization.
-
•
The use of Plerixafor on demand, however, could have changed these conclusions. We hypothesized that when used in conjunction with on-demand Plerixafor, low lose CTX is more advantageous than the higher dose.
-
•
The results of this prospective trial support, indeed, the view that low dose Cyclophosphamide in association to on-demand PLX allows the reaching efficacy and low toxicity.
Abstract
In CD34+ cells mobilization of patients with multiple myeloma (MM), the use of Cyclophosphamide (CTX) at a dose of 2 g/m2 has low efficacy although also lower toxicity. The suboptimal mobilizing effect of low-dose CTX, however, may be overcome by plerixafor (PLX) on demand.
We conducted a prospective multicenter study in 138 patients with MM to evaluate CTX 2 g/m2 in association with granulocyte-colony stimulating factor (G-CSF) and on-demand PLX. We compared results with a historical group of MM patients (n = 138) mobilized using CTX at a dose of 4 g/m2.
CD34+ cells greater than 2 × 106/kg in max three aphereses were harvested in 98.6% of patients in the on-demand PLX study group while in 84.0% in the historical group, (p = 0.0001). In the on-demand-PLX study group, a successful harvest greater than 5 × 106/kg in max three aphereses was observed in 85.5% of patients versus 62.3% of patients in the historical control group, (p=0.0001). In the on-demand-PLX study group, 4.3% (6/138) of patients had febrile complications. Salvage mobilization in the on-demand PLX study group was 1.4%.
In conclusions, on-demand PLX + CTX 2 g/m2 + G-CSF 10 μg/kg has higher efficacy and lower toxicity compared with CTX 4 g/m2 + G-CSF. An analysis of costs is presented.
1. Introduction
Cyclophosphamide (CTX) is widely employed in peripheral blood hematopoietic stem cell (PBSC) mobilization of patients with multiple myeloma (MM), usually at a dose of 3 to 4 g/m2. When used at this dose, it is effective in 85% to 95% of all patients [1], [2], [3], [4]; however, its use is associated with some toxicity [5], [6], [7].
The administration of a lower dose of CTX (1.5–2.0 g/m2) in patients with MM can decrease toxicity, transfusion needs, and hospitalization [8]. This dose, however, has a decreased CD34+ cell mobilizing effect [6,9,10].
In the present study, in PBSC mobilization of MM patients, we used a low dose of CTX (2 g/m2) in association with granulocyte-colony stimulating factor (G-CSF) and plerixafor (PLX) on-demand. The rationale for combining a decreased dose of CTX with on-demand PLX was, first, the evidence that PLX on-demand could substantially improve the mobilizing action of CTX [11,12]. Second, the evidence, as reported in published studies, that CTX does not add any antineoplastic effects after a first-line treatment that incorporates new anti-myeloma agents such as proteasome inhibitors or thalidomide [5,13,14].
Despite that on-demand PLX in association with chemotherapy is widely used [15], very few prospective studies have been conducted [16,17], and only very few have used reproducible criteria for the use of this agent after chemotherapy [12,18]. In the present study, to obtain reproducible results and to reduce the need for PLX use, the criteria for on-demand administration of PLX were described by an algorithm designed according to a previously reported methodology [19].
2. Patients and methods
2.1. Design of the study
This prospective study was aimed to determine the effectiveness and the toxicity of a mobilization strategy based on low-dose CTX (2 g/m2) and G-CSF in conjunction with PLX on-demand in patients with MM. One hundred thirty-eight patients with MM were enrolled from three Italian centres, from October 30, 2014, to June 18, 2018. We compared results with a historical control group (n = 138) in which CTX was administered at the dose of 4 g/m2 along with G-CSF at the dose of 5 to 10 μg/kg from day 3 to the end of collections. The study was approved, as an observational study, by the Ethical Committee of the coordinating center (study code 32/2014/PO) on October 13, 2014. The study was offered, on November 21, 2014, to all Italian hematopoietic transplantation centres affiliated to GITMO. It was conducted in accordance with the declaration of Helsinki.
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows: diagnosis of MM; eligibility for autologous transplantation; first mobilization attempt; adequate liver, cardiac, and lung function; age between 18 and 70 years. The exclusion criteria were as follows: heart ejection fraction less than 45% or presence of tachyarrhythmia; impaired lung function with FEV1 < 60%; chronic active hepatitis or bilirubin greater than 2 mg/dL; pregnancy; psychiatric disorders; peripheral vascular disease; a myocardial infarction during the previous six months; a previous stroke; the presence of active systemic infections; MM progressive disease (PD) after the first line and salvage treatment. Patients were required to give informed consent to the study.
The patients of the control group (n = 138) were selected from an existing database of 184 patients with MM, selection criteria were: age 18–70 years; first mobilization attempt; response to first line treatment. The control group received the mobilization treatment during the years 2000 to 2009. The results of PBSC mobilization in this whole database have been published previously [11].
2.3. Endpoints
The primary endpoint of the study was the proportion of patients collecting CD34+ higher than 2 × 106/kg in max three aphereses. The secondary endpoints of the study were the proportion of patients reaching a count of CD34+ in peripheral blood (PB) greater than 20 × 106/L; the proportion of patients who had a harvest of CD34+ higher than 5 × 106/kg in max three aphereses; the proportion of patients experiencing neutropenic fever after CTX administration (days 4 to 16); the proportion of patients needing readmission for neutropenic fever after CTX administration (days 4 to 16).
2.4. Study size
The proportion of patients reaching the primary endpoint (i.e., a successful harvest of CD34+ cells > 2 × 106/kg) in our whole retrospective database using CTX 4 g/m2 + G-CSF without the use of PLX was 83.6%. A clinical benefit would occur if this proportion increased with the use of CTX 2.0 g/m2 + G-CSF 10 μg/kg + PLX on demand by 10%, that is to say from 85% (H0, null hypothesis) to 95% (H1, alternative hypothesis). The calculated sample size is 138 patients in each treatment group [power (1-beta): 80%; significance (alpha): 5%, two-sided].
2.5. Study treatment
CTX 2.0 g/m2 was administered on day 1 in a single intravenous dose, in dextrose 5%, in 60 min, followed by G-CSF 10 μg/kg from day 3 to the end of apheresis. Mesna was used at 60% of CTX dose (1200 mg/m2) in two daily fractions of 200 mg/m2 for three days. Intravenous hydration will be administered on day +1 and +2 at the total volume of 2000 ml/m2/24 h.
CD34+ progenitor cells were quantified by flow cytometry using the International Society of Hemotherapy and Graft Engineering (ISHAGE) protocol [19], daily CD34+ counts started from day +8. The flow cytometry laboratories involved in this study regularly participated in external quality control on CD34+ cell count (UKNEQUAS).
Apheresis (2 x blood volume+/-10%) began on day 9th-12th when CD34+ count was >20 × 106/L and continued daily for up to 5 days or until more than or equal to 5 × 106 CD34+ cells/kg were collected. We used cell separators validated for PBSC collection (Fresenius Com.Tec and SpectraOptia) throughout the study.
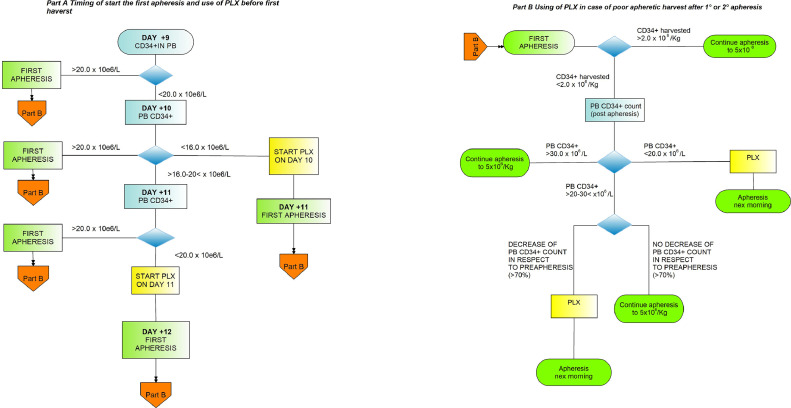
The use of PLX on demand was applied according to an algorithm designed in part A and part B (Fig. 1A and B). Part A algorithm applied to start PLX in patients predicted to fail mobilization of CD34+ cells in PB while Part B algorithm applied to use of PLX when the first apheresis resulted in CD34+ cells less than 2 × 106/Kg. In a previous study, we identified day 10th as the time point at which a CD34+ below the threshold of 16 × 106/L had a 95% rate of positive predictive value for failure in mobilization [20]. In poor mobilizing patients, the first dose of PLX was generally administered on the evening of day 10th. In patients started on PLX according to criteria described in Fig. 1A and 1B, a second and third dose of PLX could be administered in order to reach the target of CD34+ >5 × 106/Kg. PLX was administered by subcutaneous injection at a dose of 0.24 μg/kg, in the evening (h 22.00), 10 hours before the start of apheresis the following day. In patients having a decrease of creatinine clearance 20‐50 ml/min, PLX dose was reduced to 0.16 mcg/kg.
Fig. 1.
Algorithm to use PLX on demand in patients predicted to fail PBSC mobilization (part A) or poor PBSC harvest (part B).
Day 10th was planned to be on Tuesday and, in the majority of patients, the apheresis session started on the following day, on Wednesday. In such a manner, in most patients, the session finished within Friday.
2.6. Statistical analysis
We performed the final data analysis on October 1, 2019. Continuous values were reported using medians and interquartile ranges, and comparisons were evaluated using the Mann-Whitney test or the t-test, as appropriate. Primary and secondary endpoints were compared in the study group and the control group by the chi-square test or Fisher test, as appropriate. To control for differences in the distribution of factors important for PBSC mobilization in the two study groups, we used multivariable logistic regression. Mobilization outcomes were entered as dependent variables, and the study group characteristics and factors found unbalanced in the distribution in the two groups were entered as independent variables together with the mobilization strategy used. Cumulative incidence of engraftment was compared using Gray test. All analyses were two-sided, and p<0.05 was considered significant. The statistical software used were StatView v 5 (Cary NC, US) and NCSS 2007 (Kaysville, Utah, US).
2.7. Patient features and comparison of the two groups
Patient characteristics of the two groups (controls and study group) are reported in Table 1. Time of administration of mobilizing treatment was years 2000 to 2009 in the control group, whereas it was years 2014 to 2018 in the study group. In the control group, only 40.0% received G-CSF at the dose of 10 μg/kg; whereas in the study group, 100% received this dose (p = 0.0001). The mean patient age was higher in the study group: 55.5 years in the control group versus 59.4 years in the study group (p=0.0001). The white blood cell (WBC) count at the start of CTX administration was higher in the study group compared with the control group: 5.960 × 106/mL versus 5.380 × 106/mL (p=0.003). The proportion of patients reaching a complete remission/very good partial remission was higher in the on-demand study group compared with the control group (42.5% vs 23.1%, p=0.0001).
Table. 1.
Comparison of the two groups in demographic and in disease-related factors.
| Control Group CTX 4 g/m2 + G-CSF |
PLX on-demand Study Group | P | |
|---|---|---|---|
| N | 138 | 138 | |
| Gender: male, n (%) | 91 (66%) | 87 (63%) | 0.53 |
| Median Age, years | 55.5 | 59.4 | 0.0001 |
| (IQR) | (10) | (11) | |
| IgG Type, n (%) | NA | 102/138 (73.9%) | – |
| IgA Type, n (%) | 22/138 (15.9%) | ||
| light chain, n (%) | 14/138 (10.1%) | ||
| Stage | NA | I°: 12/138 (8.6%) | – |
| (Durie and Salmon), n (%) | II°: 32/138 (23.1%) | ||
| III°:94/138 (68.2%) | |||
| First-line treatment containing bortezomib: | |||
| VTD or VD, n (%) | NA | 132 (96%) | – |
| other schemes, n (%) | 6 (4%) | ||
| Response to induction | |||
| CR/VGPR, n (%) | 32 (23.1%) | 59 (42.5%) | 0.0001 |
| PR/SD, n (%) | 106 (76.9%) | 79 (57.5%) | |
| WBC in PB at the start of mobilization | |||
| Median, cells × 106/mL | 5,380 | 5,960 | 0.003 |
| (IQR) | (2,290) | (2,375) | |
| Platelets in PB at the start of mobilization | |||
| Median, cells × 106/mL | 214,000 | 220,000 | 0.25 |
| (IQR) | (69,000) | (79,000) | |
| Dose of G-CSF | |||
| 10 μg/kg, n (%) | 55/138 (39.8%) | 138/138 (100%) | 0.0001 |
| Previous | |||
| Radiotherapy, n (%) | 125/138 (8%) | 29/138 (21%) | 0,64 |
3. Results
3.1. Success in harvesting a minimal CD34+ dose of 2 × 106/kg
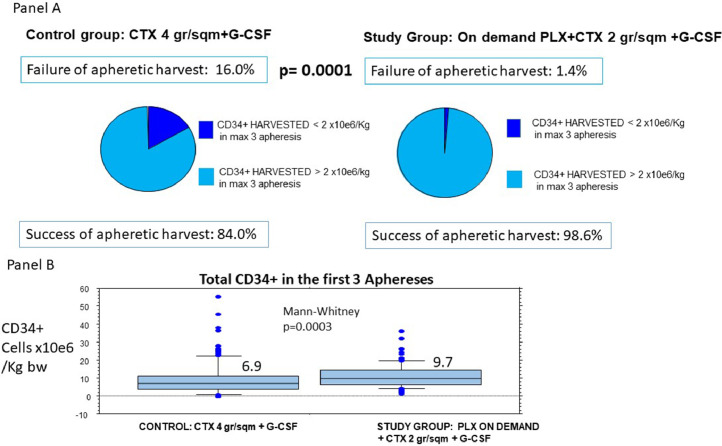
An amount of CD34+ cells higher than 2 × 106/kg in no more than three aphereses was reached in 98.6% of patients in the prospective study group (136/138), whereas this proportion was 84.0% (116/138) in the control group (p= 0.0001) (Fig. 2).
Fig. 2.
Percentage of Success in Minimal Harvest (CD34+>2×10e6/kg) in max 3 apheresis in the two groups (panel A) and distribution of the harvested CD34+ (panel B). Legend: Panel A: rate of failure in minimal aphaeretic harvest is significantly lower in on-demand PLX study group. Panel B: Harvested CD34+ cells are significantly higher in on-demand PLX study group.
To confirm the importance of the strategy adopted in the prospective on-demand PLX group and since differences were found in the comparison of the two groups (Table 1), we performed a multivariate logistic regression analysis to account for these differences (Table 2). In this analysis, the PLX on-demand plus low-dose CTX strategy maintained its importance in the achievement of the minimum harvest success [odds ratio (OR): 8.540; p=0.01]; the other predictive factor was the WBC count at the start of mobilization (OR: 1.470; p=0.02).
Table. 2.
Factors important for success in harvesting CD34+ cells >2 × 106/kg evaluated in multivariable logistic regression analysis.
| Success in harvest of CD34+ cells > 2×106/kg in max 3 apheresis | |||
|---|---|---|---|
| Odd Ratio | 95% Confidence Interval | P | |
| PLX on demand study group versus control group | 8.540 | 1.471-49.689 | 0.01 |
| Age (in decades) | 1.100 | 0.608-2.147 | 0.67 |
| Dose of G-CSF (5 μg/kg versus 10 μg/kg) | 0.710 | 0.224-2.254 | 0.56 |
| Response to induction treatment (PR/SD versus CR/VGPR) | 0.890 | 0.301-2.656 | 0.83 |
| WBC at start of conditioning (thousands WBC /μl) | 1.470 | 1.057-2.065 | 0.02 |
3.2. Proportion of patients reaching a peak in CD34+ in PB > 20 × 106/L
Evaluation of this secondary endpoint resulted in a 99.3% success rate (137/138) in the group PLX on-demand plus low-dose CTX versus 89.1% (123/138) in the control group, and the difference was statistically significant (p=0.0004) (Supplementary figure 1). As a consequence of the higher number of patients reaching a CD34+ threshold > 20 × 106/L in PB, the proportion of patients who started apheresis was higher in the on-demand PLX study group (100% vs 90.5%, p=0.0002). In contrast, apheresis resources were comparable in the two groups (Table 3). The median number of apheresis was 1.0 in the two groups while the mean was 1.40 (range 1–5) and 1.59 (range 1–5) in the control group and the PLX on-demand study group, respectively (p=0.42).
Table. 3.
Aphaeretic resources in the two groups.
| Control Group CTX 4 g/m2 + G-CSF group: | PLX on-demand Study Group | P | |
|---|---|---|---|
| Percentage of patients who started apheresis | 90.5% | 100% | 0.0002 |
| Blood volume processed in the first apheresis (mL) Median (range) |
10,695 (1,100-19,000) |
10,616 (3,000-29,600) |
0.62 |
| Blood volume processed in a single apheresis (mL) Median (range) |
10,319 (0-19900) |
10,687 (0-33,300) |
0.47 |
| Number of apheresis per patient Mean (range) |
1.40 (1-5) |
1.59 (1-5) |
0.42 |
3.3. Proportion of patients reaching a harvest CD34+cells higher than 5 × 106/kg, in max three apheresis
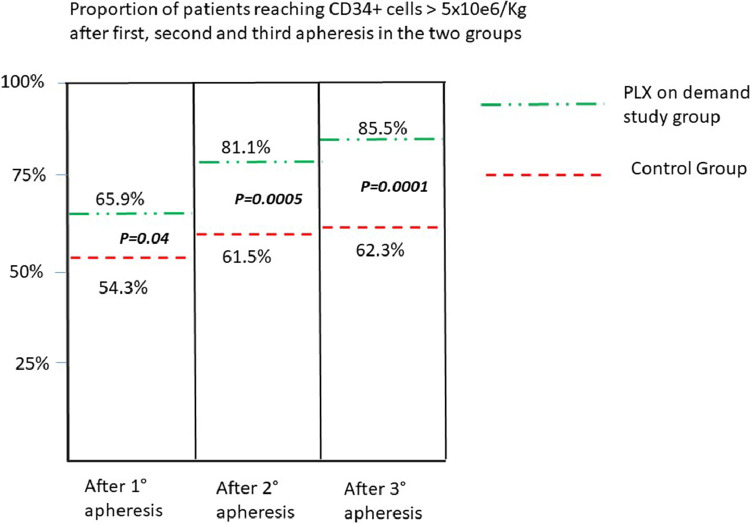
The median total of CD34+ cells collected in the first three aphereses was 9.7 × 106/kg in the PLX on demand plus low-dose CTX group versus 6.9 × 106/kg in the control group (CTX 4 g/m2) (p=0.0003) (Fig. 2). Success in harvesting CD34+ cells higher than 5 × 106/kg in max three aphereses was reached in 85.5% of patients (118/138) in the PLX on-demand plus low-dose CTX group versus 62.3% of patients (86/138) in the control group, and the difference was statistically significant (p=0.0001) (Fig. 3).
Fig. 3.
The percentage of patients reaching a harvest containing CD34+>5×106/kg after first, second, and third apheresis, in the two groups. Legend: The percentage of patients reaching a CD34+ harvest >5×106/kg, at each time point, is significantly higher in the on-demand study group.
3.4. Kinetics of mobilization in the two groups
Maximum CD34+ in PB before any PLX administration was lower in patients receiving CTX at the dose of 2 g/m2 compared with those treated with 4 g/m2 (median CD34+ peaks were 77.0 × 106/L vs 104.0 × 106/L, p=0.07) (Supplementary figure 2, panel A). However, when the comparison is made after PLX administration, the peaks of CD34+ cells in the PLX on-demand group treated with a low dose of CTX were superimposable on the peak obtained in the control group treated with CTX 4 g/m2 (median CD34+ peaks were 100 cells × 106/L vs 104 cells × 106/L, p=0.46) (Supplementary figure 2, panel A).
The kinetics of CD34+ cells in PB in patients treated by low-dose CTX has been studied by grouping patients from the on-demand PLX study group according to the presence or not of signs of poor mobilization. In the control group, the peak of CD34+ cells was reached on day 13th, whereas the peak was registered on days 10th-11th in patients treated with low-dose CTX and having good mobilization (Supplementary figure 2, panel B).
3.5. Proportion of patients experiencing neutropenic fever after 2 g/m2 CTX administration
In patients treated according to the on-demand PLX strategy, 4.3% (6/138) of patients had a fever. The duration of fever in four patients was 1 day, in one patient was 3 days, and in one patient was 5 days. All episodes were categorized as fever of unknown origin (FUO) and treated by oral antibiotics. No readmission was needed. For comparison, in a previous prospective study of our group [12], a higher dose of CTX (4 g/m2) was associated to an 11.1% rate of fever during mobilization in patients with MM (p=0.12).
3.6. Need for salvage mobilization because of insufficient harvest (<2.0 × 106/kg) within three months after treatment
A second mobilization because an insufficient harvest was undertaken in 17/133 (12.7%) patients in the control group and 2/138 (1.4%) in the on-demand PLX group (p=0.0001).
3.7. Proportion of patients treated by PLX in the present study and response to PLX
The proportion of patients who received PLX was 15.2% (21/138). The mean number of administered vials of PLX was 1.42 vial/patient [interquartile range (IQR):1].
In 14/21 (66%) patients treated with PLX, the reason to employ the agent was a CD34+ count in PB on day 10th below the CD34+ threshold of 16 cells × 106/L, as this is the threshold planned in part A of the algorithm.
CD34+ counts in PB increased after PLX, from a median of 11 × 106/L (IQR 11) to a median of 64 × 106/L (IQR 75). The median increase in CD34+ after PLX was equal to a 5.0-fold increase.
3.8. Negative predictive value of part A of the algorithm
A failed mobilization was defined as when a patient failed to reach a CD34+ count greater than 20 × 106/L during mobilization. Failed mobilization was considered a “disease status” and a test predicting it has a positive prediction and a negative predictive value. Since patients received PLX before the end of the mobilization course, the positive predictive value for a failed mobilization of our algorithm was not assessable in this set of patients. We were, instead, able to measure negative predicting value (NPV) of part A of the algorithm.
According to part A criteria of the algorithm, if CD34+ in PB is less than 16 cells × 106/L at day 10, the mobilization is predicted to fail, and therefore this dictates the use of PLX. One hundred twelve patients had a count equal or greater than 16 × 106/L on day ten and were not treated by PLX. For these patients, the test was not predictive for a failed mobilization (test was negative). In this group, the rate of failed mobilization was 0 since all 112 patients had a successful mobilization (number of false-negatives was 0). In contrast, the number of true negatives was 112 because all 112 had a successful mobilization. Thus, the negative predictive value of the algorithm part A was 100% (true negative/true negative + false negative).
3.9. Requirement for blood products transfusion
Only 1/138 patients (0.7%) required red blood cell transfusion during CD34+ cell mobilization. Two patients (1.4%) required platelet transfusions.
3.10. Time to engraftment in the two groups
The times to neutrophil engraftment (N>0.5 × 106/mL) after high-dose chemotherapy and autologous transplantation were available in 176 patients, 56 of the control group and 120 of the PLX on-demand study group. In all patients who received high dose chemotherapy, the schedule was Alkeran 200 mg/m2, and G-CSF was administered to hasten neutrophil recovery from day +3 to engraftment.
The median time to neutrophil engraftment (N>0.5 × 106/mL) was 11.0 (IQR 1) days in the PLX on-demand study group and 11.0 (IOR 3) in the control group; the cumulative incidence of engraftment times was not different (Gray test, p=0.38) (Supplementary figure 3).
The time to platelet engraftment (PLT>50 × 106/mL) was available in 129 patients. In the PLX on-demand study group, platelet engraftment was reached at a median of 14.0 days (IQR 7) versus a median time of 15.0 (IQR 5) in the control group; the difference in the cumulative incidence of engraftment time was not significant (Gray test, p=0.71) (Supplementary figure 3).
3.11. Estimate of economic cost for a set of 100 patients
From data collected in the present study, we have obtained, in both groups, the frequencies of use of some economically relevant items. Thus, using these frequencies, we calculated the mobilization costs for a set of 100 patients (Table 4).
Table. 4.
Estimates of costs of the first mobilization, of salvage mobilization, and overall mobilization costs in the two groups.
| Unit cost | Control Group CTX 4 g/m2 + G-CSF | Plx on-demand Study Group | |
|---|---|---|---|
| Proportion of patients who received PLX during mobilization | – | 0% | 15.2 % |
| Proportion of patients who had not received PLX during mobilization | – | 100% | 84.8% |
| (A) Cost of first mobilization done without PLXPLX (A) | 3,354 euro | 100 × 3,354=335,400 euro | 84.8 × 3,354=284,419 euro |
| (B) Cost of first mobilization done using PLX (1.42 vials/treated patient) | 10,854 euro | 0 | 15.2 × 10,854= 164,980 euro |
| G-CSF used in at 10 μg/kg | 1 vial G-CSF= 50 euro | 2 vials day for 11 days= 22 vials/patient in 40 pts=880 vials × 50 euro = 44,000 euro | 2 vials for 8 days=16 vials/patient in 100 pts=1,600 vials × 50 euro = 80,000 euro |
| G-CSF used at 5 μg/kg | – | In 60% of patients, 1 vial day for 11 days= 11 vials × 60 pts= 660 vials ×50 euro=33,000 euro | |
| (C) Total cost for G-CSF | – | 77,000 euro | 80,000 euro |
| (D) Infectious episode cost | w/o hospital admission= 517 euro |
11.1% 11.1× 517=5,738 euro 5,738 euro |
4.3% 4.3×517=2,223 euro 2,223 euro |
| E) Total cost for the first mobilization (A+B+C+D) |
335,400 + 0 + 77,000 + 5,738= 418,138 euro |
248,419+164,980 + 80,000 + 2,223= 495,622 euro |
|
| F) Cost for salvage mobilizations | 10,854 euro | 12.7% in 100 pts × 10,854=137,845 euro | 1.49% in 100 pts ×10,854= 16,172 euro |
| Overall cost first mobilization and salvage mobilization in 100 patients (E+F) | – | 418,138+137,845= 555,983 euro | 495,622+16,172= 511,794 euro |
A mobilization based on CTX+G-CSF was evaluated as 3,354 euro [21]. A mobilization based on PLX was evaluated as 10,854 euro. The second mobilization after a failure in the first attempt was evaluated 10.854 euro. G-CSF vials required in patients mobilized with PLX on demand plus low-dose CTX strategy (10 μg/kg) was two vials/day for 8 days (from days 3 to 10). The G-CSF dose administered in the control group mobilized with CTX 4 g/m2 varied. In 125 patients (60%), G-CSF dose was 5 μg/kg, and these patients needed 1 vial of G-CSF daily for 11 days (from days 3 to 12). While in 55 patients (40%) of the control group, the dose of G-CSF was 10 μg/kg and vials required was two vials/day for 11 days (from days 3 to 12). A G-CSF vial (biosimilar) was evaluated at 50 euro. The cost of an infectious episode managed as an outpatient was evaluated as 517 euro [22].
The overall cost, which was the sum of the cost for the first attempt of mobilization plus cost for any salvage mobilization, in 100 patients, was calculated (Table 4). The cost for the first mobilization attempt for the control group was 418,138 euro, whereas it was 495,622 euro in the PLX on-demand study group (Table 4). However, if we consider the costs of first and salvage mobilizations, the on-demand strategy leads to a decrease in the overall costs: 555,983 euro for control group versus 511,794 euro for the PLX on-demand study group (Table 4).
4. Discussion
The introduction of the second generation of novel agents and consolidation and maintenance therapy have widened the therapeutic armamentarium for MM. Nevertheless, high-dose chemotherapy, either upfront or at relapse, maintains its pivotal role in obtaining prolonged progression-free survival (PFS) in patients with MM. Therefore, a successful mobilization strategy is essential for optimizing patient outcome.
In this study, the mobilizing strategy of low-dose CTX 2 g/m2 + G-CSF 10 μg/kg + PLX on-demand obtained results statistically superior to those obtained using CTX 4 g/m2 plus G-CSF, a scheme that until now has been considered the standard in this field.
The limit of our study is that the two groups were not comparable for some features, a common problem when a historical group is used as a comparator. Indeed, the group of MM patients treated with high dose Cyclophosphamide without on-demand PLX were composed by older patients and also received a lower dose of G-CSF (5 mcg/Kg) while the group treated with a lower dose of Cyclophosphamide and on-demand PLX were less old, and all received full dose G-CSF (10 mcg/Kg). The proportion of patients who after induction were in CR/VGPR was higher in the group lower dose of Cyclophosphamide and on-demand PLX.
Age [23], previous treatment with melphalan [24] or agents like lenalidomide [25] are clinical factors recognized as relevant for determining results of mobilization and apheretic harvest in MM.
Thus, the better results obtained using low dose Cyclophosphamide plus on-demand PLX could be in part ascribed to the higher dose of G-CSF administered in this group or to the higher proportion of patients reaching CR/VGPR. However, the better PBSC mobilization and harvest results of the group low dose Cyclophosphamide plus on-demand PLX was also confirmed in a multivariable logistic regression analysis. In this analysis, the differences in age, dose of G-CSF, rate of response to induction and WCB level in PB, were taken into account.
Furthermore, the better mobilizing effect of low-dose CTX plus PLX on-demand, compared to conventional-dose CTX was evident also when compared to results available in the literature.
Rate of harvest failure and success in harvesting CD34+ cells enough for two high dose treatments are among the best reported. The failure to reach the minimum amount of CD34+ cells (2 × 106/kg) in our study was only 1.4%, whereas it was 1.6% in the study published by Di Persio using upfront PLX in conjunction with G-CSF [26]. The superiority of the results obtained also holds when we compare it to those obtained using the schedule CTX+G-CSF, in published trials of adequate size. A rate of failure of 12.2% has been reported in a retrospective study that included a wide number of patients mobilized using CTX 3 to 5 g/m2 [4]. A failure rate of 5.9% has been registered in the study of Pusic [1], whereas Awan reported a failure of 12.7% in reaching this minimum amount of CD34+ cells [7], and a 17% failure rate is reported by Afifi [27].
The other endpoint of clinical importance in the treatment of MM patients is the proportion of patients reaching a harvest enough for two rounds of high-dose chemotherapy. Comparison among different studies in respect of this endpoint is, however, difficult since it is influenced by the amount of harvested CD34+ cells chosen as the threshold to perform the two rounds of high-dose chemotherapy and by the volume of blood processed. Some studies have set a limit on the number of aphereses to reach the harvest threshold.
In the present study, an amount of CD34+ cells >5 × 106/kg has been harvested in 85.5% of patients. The amount of CD34+ cells sufficient for two rounds has been reached in 71.6% of patients who received PLX upfront plus G-CSF [26] and in 34.4% of patients mobilized with G-CSF alone [22]. This endpoint can be reached in 76.5% of cases presented by Pusic after CTX +G-CSF [1]; in 79.2% of patients receiving CTX 3-5 g/m2 and studied by Musto [4]; and in 84% in the study of Afifi using intermediate-dose CTX [27].
The toxicity of low-dose CTX (2 g/m2) is manageable, and the rate of infections (FUO) is limited, in our study, to 4.3% of patients. Febrile episodes were of brief length, responded promptly to oral antibiotics, and no readmission due to infections was registered. Published data indicates, indeed, that after CTX at a dose of 3 to 4 g/m2, the infection rate is higher and may affect 15% to 20% of all patients [5], [6], [7]. Thus, the strategy of on-demand PLX plus low-dose CTX seems an ideal mobilization schedule, since it couples high efficacy and low toxicity.
The schedule is also noteworthy for cost. With respect to CTX 4 g/m2, the use of on-demand PLX strategy leads to an improvement in the minimum harvest success of 14.6%. This increase in success rate is obtained with an increase in the costs for the first mobilization, in 100 patients, of 77,484 euro. The ICER calculation (77.484 euro/14.6%) is 5,307 euro for each point of increase in the rate of success in reaching the minimum aphaeretic harvest for 100 patients. This calculation corresponds to an increase of cost of 53 euro per patient for each point of increase in the rate of first mobilization success, indeed an affordable cost. In this regard, it should be underlined the absence of readmission due to infectious complications that we have registered in the PLX on-demand study group. In fact, the costs for readmission and G-CSF administration represent 85% of the expenses determined by infectious complications [28]. In on-demand PLX study group, the cost for the first mobilization is higher due to PLX; however, the costs for the salvage mobilizations are reduced so that if the costs of first and of salvage mobilizations are considered, the on-demand strategy leads to a decrease of the overall costs (Table 4). Our economic estimate is conservative and, indeed, the economic advantage of this schedule may be higher. We have not considered the cost for blood transfusions, an issue that can be expected to increase costs in patients receiving CTX 4 g/m2 compared with those receiving CTX at a lower dose.
Our schedule offers an efficacy comparable to the chemo-free mobilization based on PLX plus G-CSF. However, chemo-free programs provide excellent results when used in conjunction with large volume apheresis, in which the blood processed is more than three volumes [26,29,30], a practice that is not the standard in Europe. Thus, the schedule proposed in this study may be especially apt to European centres and, in a broader sense, where apheretic resources are a limiting issue.
We have used an algorithm specifically designed for detecting the failure of mobilization in patients treated with 2 g of CTX, and the algorithm has high positive and negative predictive values. The reliability of our algorithm explains why the results obtained are better than those obtained in another study using PLX on demand [18]. In the latter study, a different algorithm leads to the use of PLX of only 5% (2/35 pts), but with inferior mobilization results.
Further improvement could be obtained by adopting a modified algorithm aimed to reach not only success in minimum harvest but also an increase in the probability of an optimal harvest.
In conclusion, in the mobilization of PBSC of MM patients, CTX 2 g/m2 in conjunction with PLX on demand is more advantageous from all points of view compared with CTX 4 g/m2 + G- CSF, which has been considered until now the standard for chemotherapy-based mobilization.
Declaration of Competing Interest
Giuseppe Milone and Riccardo Saccardi received speaker or consultancy fees from Sanofi. As also reported in the manuscript text, Giuseppe Milone received funding from the Company Sanofi to support some activities needed to complete this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lrr.2020.100227.
Appendix. Supplementary materials
References
- 1.Pusic I, Jiang Shi Y, Landua S, Uy GL, Rettig MP, Cashen AF. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14:1045–1056. doi: 10.1016/j.bbmt.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Wuchter P, Ran D, Bruckner T, Schmitt T, Witzens-Harig M, Kai Neben, Goldschmidt H. Poor mobilization of hematopoietic stem cells— definitions, incidence, risk factors, and impact on outcome of autologous transplantation. Biol Blood Marrow Transplant. 2010;16:490–499. doi: 10.1016/j.bbmt.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Gertz MA, Kumar SK, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, Dingli D, Gastineau DA, Winters JL, Litzow MR. Comparison of high-dose CY and growth factor with growth factor alone for mobilization of stem cells for transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2009;43(8):619–625. doi: 10.1038/bmt.2008.369. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musto P, Simeon V, Grossi A, Gay F, Bringhen S, Larocca A, Guariglia R, Pietrantuono G, Villani O, D'Arena G, Cuomo C, Musto C, Morabito F, Petrucci MT, Offidani M, Zamagni E, Tacchetti P, Conticello C, Milone G, Palumbo A, Cavo M, Boccadoro M. Predicting poor peripheral blood stem cell collection in patients with multiple myeloma receiving pre-transplant induction therapy with novel agents and mobilized with Cyclophosphamide plus granulocyte- colony-stimulating factor: results from a Gruppo Italiano Malattie EMatologiche dell'Adulto Multiple Myeloma. Stem Cell Res Ther. 2015;17(6):64. doi: 10.1186/s13287-015-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuchman SA, Bacon WA, Huang LW, Long G, Rizzieri D, Horwitz M, Chute JP, Sullivan K, Morris Engemann A, Yopp A, Li Z, Corbet K, Chao N, Gasparetto C. Cyclophosphamide-based hematopoietic stem cell mobilization before autologous stem cell transplantation in newly diagnosed multiple myeloma. J Clin Apher. 2015;30(3):176–182. doi: 10.1002/jca.21360. Epub 2014 Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamadani M, Kochuparambil ST, Osman S, Cumpston A, Leadmon S, Bunner P, Watkins K, Morrison D, Speir E, Deremer D, Kota V, Jillella A, Craig M, Awan F. Intermediate-dose versus low-dose cyclophosphamide and granulocyte colony-stimulating factor for peripheral blood stem cell mobilization in patients with multiple myeloma treated with novel induction therapies. Biol Blood Marrow Transplant. 2012;18(7):1128–1135. doi: 10.1016/j.bbmt.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Awan F, Kochuparambil ST, Falconer DE, Cumpston A, Leadmon S, Watkins K, Deremer D, Jillella A, Craig M, Hamadani M. Comparable efficacy and lower cost of PBSC mobilization with intermediate-dose Cyclophosphamide and G-CSF compared with plerixafor and G-CSF in patients with multiple myeloma treated with novel therapies. Bone Marrow Transplant. 2013;48(10):1279–1284. doi: 10.1038/bmt.2013.52. [DOI] [PubMed] [Google Scholar]
- 8.Jantunen E, Putkonen M, Nousiainen T, Pelliniemi TT, Mahlamaki E, Remes K. Low-dose or intermediate-dose cyclophosphamide plus granulocyte colony-stimulating factor for progenitor cell mobilization in patients with multiple myeloma. Bone Marrow Transplant. 2003;31(5):347–351. doi: 10.1038/sj.bmt.1703840. [DOI] [PubMed] [Google Scholar]
- 9.Bellido M, Sureda A, Martino R, Madoz P, Garcia J. Brunet S Collection of peripheral blood progenitor cells for autografting with low-dose Cyclophosphamide plus granulocyte colony-stimulating factor. Haematologica. 1998;83(5):428–431. [PubMed] [Google Scholar]
- 10.Hiwase DK, Bollard G, Hiwase S, Bailey M, Muirhead J, Schwarer AP. Intermediate-dose CY and G-CSF more efficiently mobilize adequate numbers of PBSC for tandem autologous PBSC transplantation compared with low-dose CY in patients with multiple myeloma. Cytotherapy. 2007;9(6):539–547. doi: 10.1080/14653240701452800. [DOI] [PubMed] [Google Scholar]
- 11.D'Addio, A., Curti, A., Worel, N., Douglas, K., Motta, M.R., Rizzi, S., Dan, E., Taioli, The addition of plerixafor is safe and allows adequate PBSC collection in multiple myeloma and lymphoma patients poor mobilizers after chemotherapy and G-CSF. Bone Marrow Transplantation. 2011; 46(3): 356–363. [DOI] [PubMed]
- 12.Milone G, Martino M, Spadaro A, Leotta S, Di Marco A, Scalzulli P, Cupri A, Di Martina V, Schinocca E, Spina E, Tripepi G. Plerixafor on-demand combined with chemotherapy and granulocyte colony-stimulating factor: significant improvement in peripheral blood stem cells mobilization and harvest with no increase in costs. Br J Haematol. 2014;164(1):113–123. doi: 10.1111/bjh.12606. [DOI] [PubMed] [Google Scholar]
- 13.Uy GL, Costa LJ, Hari PN, Zhang MJ, Huang JX, Anderson KC, Bredeson CN, Callander NS, Cornell RF, Perez MA. Contribution of chemotherapy mobilization to disease control in multiple myeloma treated with autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50:1513–1518. doi: 10.1038/bmt.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman S, Litzow MR, Gastineau DA, Gertz MA. Cyclophosphamide mobilization does not improve outcome in patients receiving stem cell transplantation for multiple myeloma. Clin Lymphoma Myeloma. 2006;6:384–388. doi: 10.3816/CLM.2006.n.014. [DOI] [PubMed] [Google Scholar]
- 15.Chabannon C, Bijou F, Miclea JM, Milpied N, Grouin JM, Mohty M. A nationwide survey of the use of plerixafor in patients with lymphoid malignancies who mobilize poorly demonstrates the predominant use of the "on-demand" scheme of administration at French autologous hematopoietic stem cell transplant programs. Transfusion. 2015;55(9):2149–2157. doi: 10.1111/trf.13141. [DOI] [PubMed] [Google Scholar]
- 16.Chow E, Rao KV, Wood WA, Covington D, Armistead PM, Coghill J, Serody JS, Gabriel DA, Jamieson KJ, Park YA, Raval JS, Shea TC. Effectiveness of an algorithm-based approach to the utilization of plerixafor in patients undergoing chemotherapy-based stem cell mobilization. Biol Blood Marrow Transplant. 2014;20(7):1064–1068. doi: 10.1016/j.bbmt.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Yuan S, Nademanee A, Kaniewski M, Palmer J, Shayani S, Wang S. Efficacy of just-in-time plerixafor rescue for Hodgkin's lymphoma patients with poor peripheral blood stem cell mobilization. Transfusion. 2014;54(8):2015–2021. doi: 10.1111/trf.12594. [DOI] [PubMed] [Google Scholar]
- 18.Silvennoinen R, Anttila P, Saily M, Lundan T, Heiskanen J, Siitonen TM, Kakko S, Putkonen M, Ollikainen H, Terava V, Kutila A, Launonen K, Rasanen A, Sikio A, Suominen M, Bazia P, Kananen K, Selander T, Kuittinen T, Remes K, Jantunen E. A randomized phase II study of stem cell mobilization with cyclophosphamide+G-CSF or G-CSF alone after lenalidomide- based induction in multiple myeloma. Bone Marrow Transplant. 2016;51(3):372–376. doi: 10.1038/bmt.2015.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutherland D.R., Anderson L., Keeney M., Nayar R., Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5(3):213–226. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 20.Milone G, Tripepi G, Martino M, Ancora F, Bartolozzi B. Spadaro A et al Early measurement of CD34+ cells in peripheral blood after cyclophosphamide and granulocyte colony-stimulating factor treatment predicts later CD34+ mobilization failure and is a possible criterion for guiding "on demand" use of plerixafor. Blood Transfus. 2013;11(1):94–101. doi: 10.2450/2012.0004-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chabannon C., Le Corroller A.G., Viret F., Eillen C., Faucher C., Moatti J.P., Viens P., Vey N., Braud A.C., Novakovitch G., Ladaique P., Stoppa A.M., Camerlo J., Genre D., Maraninchi D., Blaise D. Cost-effectiveness of repeated aphereses in poor mobilizers undergoing high-dose chemotherapy and autologous hematopoietic cell transplantation. Leukemia. 2003;17(4):811–813. doi: 10.1038/sj.leu.2402867. [DOI] [PubMed] [Google Scholar]
- 22.Wong W, Yim YM, Kim A, Cloutier M, Gauthier-Loiselle M, Gagnon-Sanschagrin P. Guerin A Assessment of costs associated with adverse events in patients with cancer. PLOS one. 2018;13(4) doi: 10.1371/journal.pone.0196007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richa E, Papari M, Allen J, Martinez G, Wickrema A, Anastasi J, Van Besien K, Artz A. Older age but not donor health impairs allogeneic granulocyte colony-stimulating factor (G-CSF) peripheral blood stem cell mobilization. Biol Blood Marrow Transplant. 2009;15(11):1394–1399. doi: 10.1016/j.bbmt.2009.07.005. NovEpub 2009 Sep 8. PMID: 19822298. [DOI] [PubMed] [Google Scholar]
- 24.Wuchter P, Ran D, Bruckner T, Schmitt T, Witzens-Harig M, Neben K, Goldschmidt H, Ho AD. Poor mobilization of hematopoietic stem cells-definitions, incidence, risk factors, and impact on outcome of autologous transplantation. Biol Blood Marrow Transplant. 2010;16(4):490–499. doi: 10.1016/j.bbmt.2009.11.012. AprEpub 2009 Nov 17. PMID: 19925876. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA, Litzow MR, Fonseca R, Roy V, Rajkumar SV, Gertz MA. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21(9):2035–2042. doi: 10.1038/sj.leu.2404801. SepEpub 2007 Jun 21. PMID: 17581613. [DOI] [PubMed] [Google Scholar]
- 26.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef INM, Stiff PJ, Kaufman JL. et al for the 3102 Investigators. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 27.Afifi S, Adel NG, Devlin S, Duck E, Vanak J, Landau H, Chung DJ, Lendvai N, Lesokhin A, Korde N, Reich L, Landgren O, Giralt S, Hassoun H. Upfront plerixafor plus G-CSF versus Cyclophosphamide plus G-CSF for stem cell mobilization in multiple myeloma: efficacy and cost analysis study. Bone Marrow Transplant. 2016;51(4):546–552. doi: 10.1038/bmt.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayordomo JI, Lopez A, Vinolas N, Castellanos J, Pernas S, Domingo Alonso J, Frau A, Layola M, Antonio Gasquet J, Sanchez J. ENIA Study Group. Retrospective cost analysis of management of febrile neutropenia in cancer patients in Spain. Curr Med Res Opin. 2009;25(10):2533–2542. doi: 10.1185/03007990903209563. [DOI] [PubMed] [Google Scholar]
- 29.Awan F, Kochuparambil ST, Falconer DE. Comparable efficacy and lower cost of PBSC mobilization with intermediate-dose Cyclophosphamide and G-CSF compared with plerixafor and G-CSF in patients with multiple myeloma treated with novel therapies. Bone Marrow Transplant. 2013;48(10):1279–1284. doi: 10.1038/bmt.2013.52. [DOI] [PubMed] [Google Scholar]
- 30.Abhyankar S, DeJarnette S, Aljitawi O, Ganguly S, Merkel D, McGuirk J. A risk-based approach to optimize autologous hematopoietic stem cell (HSC) collection with the use of plerixafor. Bone Marrow Transplant. 2012;47(4):483–487. doi: 10.1038/bmt.2011.133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.