Graphical abstract
Keywords: Resting-state functional connectivity, Neurodevelopmental disorder, Autism, ADHD, OCD
Highlights
-
•
Resting-state connectivity did not differ across neurodevelopmental disorders.
-
•
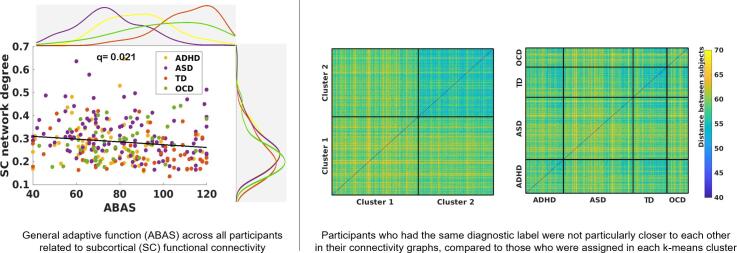
General adaptive function across all participants related to subcortical connectivity.
-
•
Participants in the same data-driven clusters were highly heterogeneous in diagnosis.
-
•
Neurobiological similarity and dissimilarity may be seen in beyond-diagnosis categories.
Abstract
Children with neurodevelopmental disorders (NDDs) share common behavioural manifestations despite distinct categorical diagnostic criteria. Here, we examined canonical resting-state network connectivity in three diagnostic groups (autism spectrum disorder, attention-deficit/hyperactivity disorder and paediatric obsessive–compulsive disorder) and typically developing controls (TD) in a large single-site sample (N = 407), applying diagnosis-based and dimensional approaches to understand underlying neurobiology across NDDs. Each participant’s functional network graphs were computed using five graph metrics. In diagnosis-based comparisons, an analysis of covariance was performed to compare all NDDs to TD, followed by pairwise comparisons between NDDs. In the dimensional approach, participants’ functional network graphs were correlated with continuous behavioural measures, and a data-driven k-means clustering analysis was applied to determine if subgroups of participants were seen, without diagnostic information having been included. In the diagnosis-based comparisons, children with NDDs did not differ significantly from the TD group and the NDD categorical groups also did not differ significantly from each other, across all graph metrics. In the dimensional, diagnostic-independent approach, however, subcortical functional connectivity was significantly correlated with participants’ general adaptive functioning across all participants. The clustering analysis identified an optimal solution of two clusters, and participants assigned in the same data-driven cluster were highly heterogeneous in diagnosis. Neither cluster exclusively contained a specific diagnostic group, nor did NDDs separate cleanly from TDs. Each participant’s distance ratio between the two clusters was significantly correlated with general adaptive functioning, social deficits and attentional problems. Our results suggest the neurobiological similarity and dissimilarity between NDDs need to be investigated beyond DSM/ICD-based, behaviourally-defined diagnostic categories.
1. Introduction
Autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD) and obsessive–compulsive disorder (OCD) are common neurodevelopmental disorders (NDDs) present in childhood (De Felice et al., 2015). Their behavioural manifestations range widely, resulting in specific limitations and global impairments, and crucially overlap in some domains across NDDs despite distinct behaviour-based criteria in the current diagnostic systems (e.g. DSM-5 and ICD-10/11) (Hollingdale et al., 2019, Kashyap et al., 2012, Lai et al., 2019, Ruzzano et al., 2014). The overlapping phenotypes across NDDs have led to inquiries about shared versus non-shared neurobiological correlates underlying these diagnoses. Recent investigations tackling this question have demonstrated the value of measuring resting-state functional connectivity (rs-fc), which allows the study of intrinsic large-scale brain networks that underlie a range of sensory and cognitive processes. Although emerging evidence from genetic studies support shared genetic susceptibility across NDD groups (Lionel et al., 2014, Martin et al., 2014, Zarrei et al., 2019), studies of rs-fc have remained largely inconsistent, in findings both in independent analyses of each NDD (Castellanos and Aoki, 2016, Gürsel et al., 2018a, Hull et al., 2017) and in direct comparisons between NDDs (Akkermans et al., 2018, Dajani et al., 2019, Di Martino et al., 2013, Jung et al., 2019, Ray et al., 2014). Most studies have relied on case-control designs using diagnostic labels for comparisons, but have not explored heterogeneity within and across the groups. Demographic variability, differences in data acquisition protocols, a variety of metrics representing different aspects of connectivity features, and generally small sample sizes have also led to conflicting reports. Furthermore, the majority of the previous findings from diagnosis-based comparisons across NDDs are mostly available between children with ASD and ADHD due their high comorbidity, but the large genomic data have indicated that genetic vulnerability is more commonly shared across NDDs, including OCD and schizophrenia, which have yet to be examined in rs-fc studies (Zarrei et al., 2019). All these factors suggest the need for studies including multiple disorders on well-powered cohorts, and the use of more integrative and data-driven approaches on local and global levels.
Recent efforts to overcome large inter-subject variability existing in NDDs and to reconcile conflicting findings have initiated data exchange platforms, such as ABIDE (Di Martino et al., 2014) and ADHD-200 (Consortium et al., 2012). With this effort, the rs-fc findings seem to be somewhat more convergent than before; for example, decreased default-mode and visual networks connectivity, and increased subcortical connectivity, were found more often in large ASD samples (Boedhoe et al., 2017, Borràs-Ferrís et al., 2019, Delbruck et al., 2019, Harlalka et al., 2019, Sen et al., 2018). There are still many conflicting findings, however, even in these large datasets. A recent study directly compared a number of rs-fc features in ABIDE I, II, and combined ABIDE, as well as across data acquisition sites, to see if any of those features could be generalized as ASD-related markers. The study demonstrated extensive variability and limited reproducibility in rs-fc features across sites, including in the combined samples (ABIDE I, II, and combined ABIDE) (King et al., 2019). Other studies that have applied data-driven approaches in ABIDE are showing similar results. A recent examination of functional alterations in ASD and ADHD versus a TD sample, found shared and diagnoses-related features in default-mode network coupling with dorsal attentional and salience networks (Kernbach et al., 2018). Another study demonstrated that the diagnostic labels of ASD and ADHD were mapped onto diffusive brain regions with both overlapping and distinct patterns of connectivity, while dimensional behavioural measures such as Social Responsiveness Scale and ADHD Rating Scale were defined on more distinct brain circuitry (Lake et al., 2019). Although studies, which applied machine learning strategies, were able to successfully classify ASD versus TD with a peak accuracy of between 60 and 90% (Chen et al., 2015, Fredo et al., 2018, Kazeminejad and Sotero, 2019), there was little consistency in the connectivity features classifying ASD versus TD across the studies, conducted at different sites. This suggests that studying NDDs in a large single-site cohort would be a benefit in reducing the noise originating from inter-site variability, for understanding similarity and dissimilarity among diagnostic categories. A recent report, on a sub-sample of our study, demonstrated the value of machine learning-based clustering work in discovering biologically homogeneous subgroups in children with ASD, ADHD and OCD, by integrating cortical thickness data and continuous core-symptom measures. This work revealed that clusters with neuroanatomical homogeneity did not correspond to diagnostic labels (Kushki et al., 2019).
Thus, in the present study, we investigated canonical resting-state functional networks in three diagnostic groups (ASD, ADHD and OCD) and a TD sample, in a large well-characterized single cohort, using both diagnosis-based and dimensional/data-driven approaches, to explore shared and distinct functional brain markers at a large-scale network level.
2. Material and methods
2.1. Participants
A total of 486 participants (ASD = 221, ADHD = 115, OCD = 58, TD = 92) were recruited through the Province of Ontario Neurodevelopmental Disorders (POND) Network, a large single-cohort multicentre research collaboration across Ontario, Canada between July 2012 and March 2019. Clinical participants who had one of the primary diagnoses of ASD, ADHD or OCD and no contraindications for MRI were included in the study. Diagnoses for the clinical groups were based on DSM-IV (American Psychiatric Association, 1994) or DSM-V (American Psychological Association, 2013), and confirmed using the Autism Diagnostic Observation Schedule–2 (ADOS) (Lord et al., 2000) and the Autism Diagnostic Interview–Revised (ADI-R) (Lord et al., 1994) for ASD, the Parent Interview for Child Symptoms (PICS) (Ickowicz et al., 2006) for ADHD, and the K-SADS and the Children’s Yale–Brown Obsessive Compulsive Scale (CY-BOCS) (Scahill et al., 1997) for OCD. The presence of psychiatric comorbidities in participants with NDDs were noted in Supplementary Table 1. TD participants were recruited via advertisements and had no personal or first degree family history of neurodevelopmental, psychiatric or neurological diagnoses, nor history of prematurity. Research ethics board approval was obtained at participating institutions. Written, informed consent/assent from primary caregivers/study participants was obtained after a complete description of the study and according to the Declaration of Helsinki.
2.2. Behavioural assessment
To investigate associations between participants’ graph measures and behavioural manifestations, we examined dimensional information about general adaptive function (Adaptive Behavior Assessment System-II: ABAS-II) (Voets et al., 2001), social deficits (Social communication questionnaire: SCQ) (Rutter et al., 2003), ADHD related symptoms (Attention-deficit/hyperactivity problem subscale from the Child Behavior Checklist: CBCL) (Achenbach and Rescorla, 2000, Achenbach and Rescorla, 2001) and obsessive–compulsive symptoms (Toronto Obsessive-Compulsive Scale: TOCS) (Park et al., 2016). These symptoms may be characteristic of one NDD but also are expressed in varying degrees across the other NDDs.
2.3. Imaging acquisition and processing
We collected each participant’s MRI data on a 3 T Siemen’s MAGNETOM Trio with a 12 channel head coil or a 3 T Siemen’s PrismaFIT with a 20 channel head and neck coil, as the research scanner was upgraded once at our institution during this study. Acquisitions included anatomical T1-weighted images (Trio: TR/TE: 2300/2.96 ms; FA: 9°; FOV: 192 × 240 × 256mm; 1.0 mm isotropic voxels; Prisma: TR/TE: 1870/3.14 ms, FA: 9°, FOV: 192 × 240 × 256mm, 0.8 mm isotropic voxels; both scan times: 5 min) and resting-state fMRI scans (Trio: TR/TE: 2340/30 ms; FA: 70°; FOV: 224 × 224 × 140mm; 3.5 mm isotropic voxels; Prisma: TR/TE: 1500/30 ms, FA: 70°, FOV: 222 × 222 × 150, 3.0 mm isotropic voxels, both scan times: 5 min). During the resting-state scans, participants scanned pre-upgrade were presented with a movie they had chosen (movie) and post-upgrade participants were presented with a naturalistic movie paradigm (Inscapes) (Vanderwal et al., 2015); the resting-state viewing condition version was added as a covariate in all subsequent analyses.
Resting-state data preprocessing was performed using AFNI (http://afni.nimh.nih.gov/afni/), FMRIB Software Library (https://fsl.fmrib.ox.ac.uk/) and locally developed tools. All resting-state volumes were corrected for slice-timing and head motion (extracting the six rigid body parameters), smoothed by a 2D 7 mm full-width-half-maximum Gaussian kernel, and bandpass filtered between 0.01 and 0.2 Hz. Framewise displacement was calculated from the six rigid body parameters. Volumes were censored based on framewise displacement (FD) (Power et al., 2012) exceeding 0.5 mm or the root mean square change in BOLD signal across the whole brain (DVARS) (Smyser et al., 2010) exceeding 5%. Participants who lost more than 1/3 of the volumes were excluded from the analyses (see Supplementary Table 2 for head motion in each diagnostic group). The 36 nuisance signals from whole brain, white matter, cerebrospinal fluid, six rigid body motion parameters (total 9) and their derivatives (9 × 2) and quadratic terms (18 × 2) were regressed out. Data were further cleaned of motion and physiological artefacts using FMRIB’s ICA-based Xnoiseifier (FIX) to denoise residual artefact not handled by nuisance regression and scrubbing process (Salimi-Khorshidi et al., 2014). FIX was trained by hand-classifying 20 datasets equally distributed across diagnosis and acquisition scanner, and used to automatically classify the rest of the data.
2.4. Functional network construction
CIVET (http://www.bic.mni.mcgill.ca/ServicesSoftware/CIVET) was used to parcellate each participant’s T1-weighted image into the 76 ROIs of the Desikan-Killiany-Tourville atlas (Supplementary Table 3). The ROIs were mapped to the Yeo et al. (2011) eight functional networks (VIS, visual network; SMOT, sensory motor network; DAN, dorsal attentional network; VAN, ventral attentional network; LIM, limbic network; FP, fronto-parietal network; DMN, default mode network; SC, subcortical network). To construct each participant’s functional network, mean time series were extracted for each participant for each ROI (node) and pairwise Pearson’s correlation coefficients were calculated between all nodes and converted to a z-value using Fisher’s r-to-z transform to better approximate a normal distribution. Graph theory-based measures were used to characterize and summarize each individual’s connectivity features (Bullmore and Sporns, 2009). Participants’ graphs were thresholded at a range of connection densities (0.5%–10%, 0.5% intervals) and binarized. For each threshold t, the top t% of connections were kept, except if this threshold disconnected a node, in which case its highest edge was retained. The following measures were then computed over the range of thresholds: degree (the number of links connected to the node), betweenness centrality (a measure of “hubness” in each functional network), local efficiency (the averaged inverse shortest path length) and clustering coefficient (a measure of a node’s neighbourhood connectivity); for more details for each measure see the reference (Bullmore and Sporns, 2009). Each of the functional networks (subgraphs, e.g., nodes belonging to VIS or to SMOT) were also extracted and subgraph density (the fraction of present connections to possible connections) was also calculated between each functional network pair. Finally, for each participant we calculated the area under the curve (AUC) for each measure, providing a summarized metric independent of threshold selection, and averaged across functional networks.
2.5. Statistical analyses
To investigate functional network features in NDDs and TDs, we performed two sets of analyses: (1) group comparisons based on participants’ diagnoses using an analysis of covariance (ANCOVA), and (2) diagnosis-independent correlation analysis with behavioural measures and clustering analysis using the k-means clustering algorithm.
2.5.1. Analysis 1
ANCOVAs were employed to evaluate differences in each network graph measure AUC between groups. We compared all children with NDDs (as a group) to TDs, with age, sex and the resting-state viewing condition (movie or inscapes) as covariates to examine shared functional alterations across all NDDs, followed by pairwise comparisons between NDDs (ASD vs. ADHD, ASD vs. OCD, ADHD vs. OCD) with the same covariates to explore overlapping and distinct functional network characteristics in each diagnostic group. For each group comparison, group labels were permuted 10,000 times and the ANCOVA t-statistic was recalculated, creating a null distribution of t-statistics. We assigned the group difference a p-value by determining the percentage of the computed null distributions that exceeded the observed t-statistic and considered the resulting values significant at p < 0.05. For each pairwise comparison and graph measure, FDR-corrections were performed using Holm-Bonferroni (Holm, 1979), q < 0.05, to correct for comparisons across multiple networks.
2.5.2. Analysis 2
We examined the relation between the functional graph measures and behavioural measures (SCQ, ABAS, CBCL-ADHD subscale, TOCS) across all individuals with the same covariates, but without diagnostic labels. As we removed group as a factor in the ANCOVAs, the beta weights (β) were calculated to indicate the associations between the variables and the same corrections for multiple comparisons were applied (q < 0.05). Lastly, the data-driven cluster analysis was applied for all participants without diagnostic information using the k-means clustering algorithm. We vectorized each participant’s binarized graphs over the range of thresholds (0.5% −10%, 0.5% intervals) and ran k-means clustering to partition the N observations (i.e. each participant’s vectorized data) into k clusters, where each observation belonged to the cluster with the nearest mean according to the Hamming distance. We used the silhouette method to test for the optimal number of clusters, which was determined to be two (k = 2) (Supplementary Fig. 1). Then for the purpose of clinical validation of data-driven clusters, we examined the association between each participant’s distance to each cluster and behavioural outcomes, by a permutation test (10,000 times). In addition, regression analyses applied to examine the relation between the data-driven clusters and behavioural measures with the same covariates.
3. Results
3.1. Participant demographics
After excluding participants whose T1-w images failed QC (reviewed on a case-by-case basis) or whose resting-state data failed the motion criteria (n = 79), a total of 407 participants (ASD = 175, ADHD = 93, OCD = 55, TD = 84) remained. The characteristics of those who were excluded from the final analysis are presented in Supplementary Table 4. The descriptive statistics for age, sex and four behavioural measures are summarized in Table 1. The ASD and ADHD groups demonstrated a higher proportion of males.
Table 1.
Demographics and behavioural indices of participants.
| characteristic | Group |
Analysis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. ASD (N = 175) |
2. ADHD (N = 93) |
3. OCD (N = 55) |
4. TD (N = 84) |
|||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | df | p | Post hoc analysis | |
| Age (years) | 11.79 | 4.46 | 12.04 | 2.81 | 12.94 | 2.84 | 12.21 | 5.02 | 1.15 | 3,403 | 0.33 | – |
| N | % | N | % | N | % | N | % | χ2 | df | p | Post hoc analysis | |
| Gender (male) | 140 | 80.0 | 74 | 79.6 | 35 | 63.6 | 48 | 57.1 | 19.54 | 3 | 0.001 | – |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | df | p | Post hoc analysis | |
| Full-scale IQ | 96.82 | 19.90 | 102.53 | 13.91 | 109.10 | 16.06 | 112.31 | 11.67 | 14.26 | 3,270 | <0.001 | TD > ADHD,ASD |
| SCQ (total score) |
19.70 | 7.47 | 7.08 | 5.83 | 6.22 | 5.62 | 2.19 | 2.29 | 174.45 | 3,346 | <0.001 | ASD > ADHD,OCD > TD |
| ABAS-II (GAC score) |
67.69 | 15.37 | 81.58 | 15.10 | 93.97 | 20.02 | 104.79 | 12.86 | 106.48 | 3,340 | <0.001 | TD > OCD > ADHD > ASD |
| TOCS (total score) |
−7.66 | 22.57 | −24.90 | 25.41 | 19.27 | 19.88 | −34.69 | 25.27 | 74.56 | 3,333 | <0.001 | OCD > ASD > ADHD,TD |
| CBCL (ADHD score) |
62.60 | 8.54 | 67.03 | 7.36 | 58.47 | 8.52 | 51.29 | 2.53 | 59.16 | 3,341 | <0.001 | ADHD > ASD > OCD > TD |
SCQ: The Social Communication Questionnaire, ABAS-II: The Adaptive Behavior Assessment System-II, TOCS: The Toronto Obsessive-Compulsive Scale, CBCL-ADHD: Attention-Deficit/Hyperactivity Problems subscale from the Child Behavior Checklist.
3.2. Analysis 1
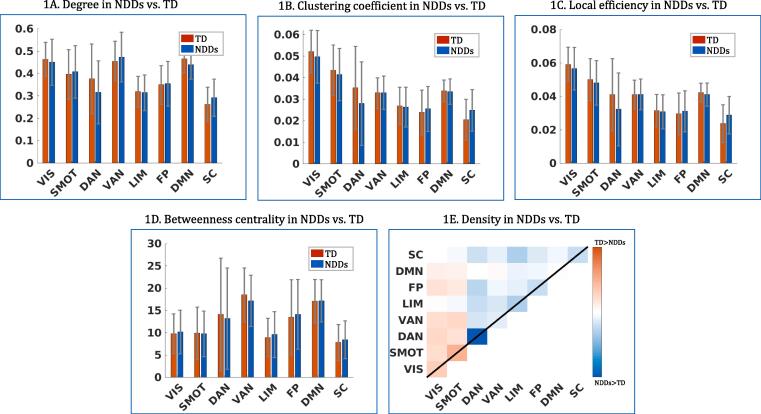
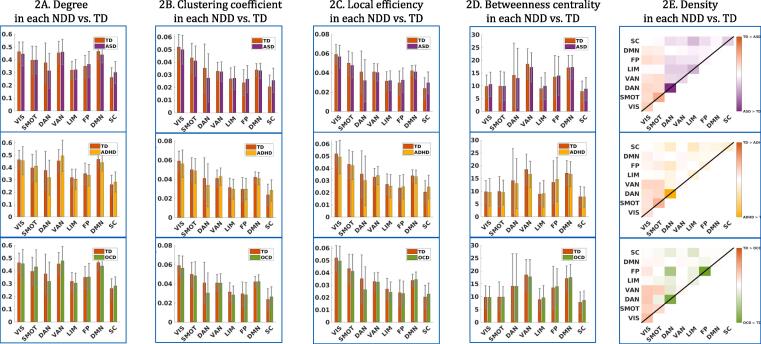
In diagnosis-based comparisons, children with NDDs did not significantly differ from TD children and each NDD did not differ significantly from the other diagnostic groups across all five graph metrics (Fig. 1A–E and Supplementary Table 5). For exploratory purposes, the overall connectivity patterns between each NDD and TD were visualized each within the same graph metrics, and the connectivity patterns were seen to be very similar across three diagnostic groups, compared to TDs (Fig. 2). Furthermore, the two resting-state viewing conditions were separated and the ANCOVA analyses were performed again within each condition, to examine the possible effect of the different resting-state viewing conditions on the results. Demographic and clinical distributions in each viewing condition are presented in Supplementary Table 6. Although the Inscapes condition had a higher portion of typically developing children and the mean age was also slightly higher in this condition, children with NDDs did not differ significantly from the TDs on any graph metrics in either resting-state viewing condition (Supplementary Fig. 2).
Fig. 1.
Comparisons between NDDs and TD in graph metrics Each panel represents group differences in degree, clustering coefficient, lobal efficiency, betweenness centrality and density across 8 networks (VIS, SMOT, DAN, VAN, LIM, FP, DMN, SC) from ANCOVA in comparison of all NDDs vs TD (significance: FDR-corrections were performed using Holm-Bonferroni, q < 0.05; errors bars are SDs). The incresed subcortical connectivity and decreased dorsal attentional connectivity were observed in NDDs compared to TD, but were not statistically significant. (VIS: visual network, SMOT: sensorimotor network, DAN: dorsal attentional network, VAN: ventral attentional network, FP: frontoparietal network, DMN: default-mode network, SC: subcortical network).
Fig. 2.
Exploratory comparisons between each NDD and TD. In an exploratory purpose, each graph metrics were visualized between each NDD and TD. Each NDD group showed similar patterns of fucntional connectivity compared to TD.
3.3. Analysis 2
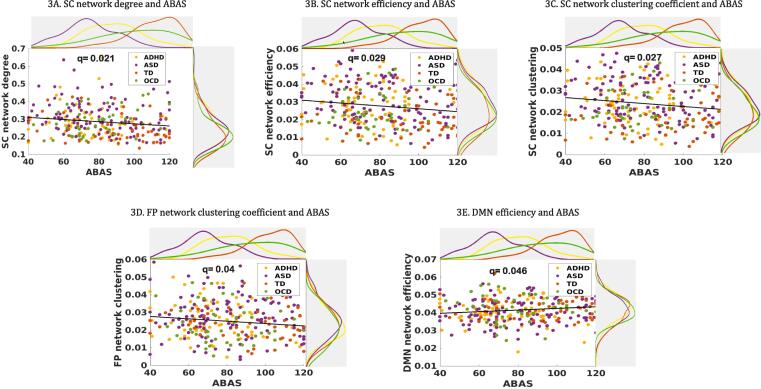
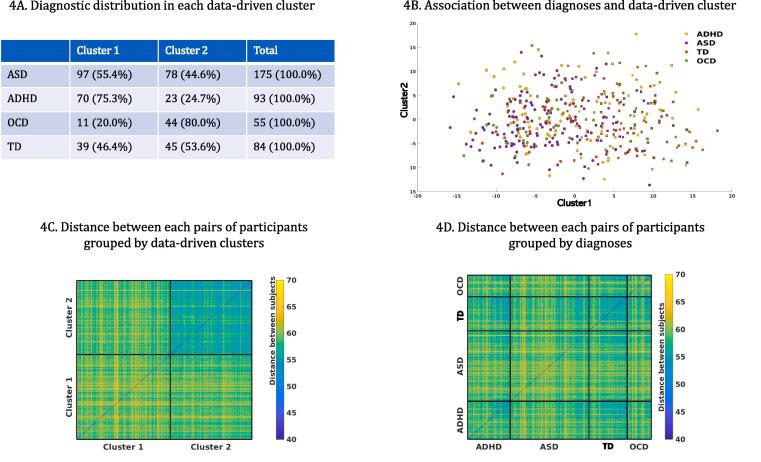
In the dimensional approach which employed behavioural measures across all individuals regardless of diagnosis, subcortical network connectivity was significantly correlated with participants’ general adaptive functioning in degree (β = −0.01, q = 0.02), clustering (β = −0.002, q = 0.03) and local efficiency (β = −0.002, q = 0.03) (Fig. 3A–C), but not with other NDD symptom-based behavioural measures (SCQ, ADHD subscale from CBCL, TOCS). Participants’ general adaptive functioning was also significantly correlated with the frontoparietal network in clustering (β = −0.002, q = 0.04) and the DMN in local efficiency (β = 0.001, q = 0.05), but not in other graph metrics (degree, betweenness centrality and density) (Fig. 3D and E). The correlations between behavioural measures are presented in Supplementary Table 7. The data-driven analysis grouped participants into two clusters based on participants’ connectivity graphs without diagnostic information, and no cluster had one diagnostic group exclusively, including the TD group. The TD and the ASD participants were split between the two clusters, while approximately 80% of the OCD and the ADHD participants were each loaded on different clusters, respectively (Fig. 4A). The distance to the data-driven clusters as well as between each pair of participants were calculated (Fig. 4B) and the participants grouped by either diagnosis or their assigned k-means cluster (Fig. 4C and 4D). None of diagnostic categories presented as a group; those who had the same diagnostic label were not particularly closer or more similar to each other in terms of their connectivity graphs, compared to those who were assigned in each k-means cluster. In the subsequent analysis with behavioural outcomes, we found each participant’s distance ratio (cluster 1: cluster 2) was significantly correlated with participants’ general adaptive function (β = 31.20, q = 0.001), social deficits (β = −18.29, q = 0.001) and inattention problems (β = −10.87, q = 0.001). The regression analyses also revealed that the data-driven clusters were significantly explained by participants’ general adaptive function (β = 0.11, p = 0.060) and CBCL-ADHD subscales (β = −0.125, p = 0.021) while controlling for age, sex and the scanner effects. Additionally, we grouped participants by the viewing condition. About half of movie condition (55.6%) was grouped into cluster 2, while half of Inscapes condition (60.9%) was grouped into cluster 1. When these effects were examined in each diagnosis, children with ASD looked to be more affected by the viewing condition, while no effects were observed in children with ADHD and OCD. Although the TD group seemed to show effects of the viewing condition, opposite to the ASD group, the overall number in the movie condition was too small to compare (Supplementary Fig. 3).
Fig. 3.
Behavioural correlations with graph metrics Subjects’ general adaptive function scores (ABAS) were significantly correlated with three subcortical network indices (degree, local efficiency, clustering coefficient; q = 0.02, 0.03, 0.03, respectively), the FP network clustering coefficient (q = 0.04), DMN efficiency (q = 0.05). SC, Subcortical network; FP, Fronto-parietal network; DMN, Default-mode network.
Fig. 4.
k-means clustering results based on each subject’s graph measure without diagnostic information. 4A. This panel depicts how all participants in each diagnostic group can be subgrouped into k-means clusters (k = 2) only based on their graph metrics similarity. No cluster had exclusively any specific diagnostic group. We calculated distance of each participant to the data-driven clusters (4B) and of each pair of participancts based on their graph metrics similarity and grouped them by either data-driven cluster (4C) or diagnosis (4D).
4. Discussion
In this study, we sought to identify cross-diagnostic rs-fc features underlying three common NDDs to determine homogeneous subgroups based on rs-fc similarity. Our results demonstrated that the categorical diagnoses did not represent distinct neurobiological features in canonical resting-state networks, nor were they matched with data-driven clusters, which grouped participants based on connectivity similarity. Instead, participants’ general adaptive functioning, a diagnosis-free continuous measure, was associated with connectivity features across the entire sample.
In our diagnosis-based comparisons, which used five graph metrics summarized according to eight canonical resting-state networks, we did not find any significant group-specific rs-fc features across the three diagnostic groups and the TD controls. These findings suggest that diagnosis-based approach is not as useful in identifying distinctions in rs-fc between the behaviourally defined, DSM/ICD-based NDD groups. This extends studies which have assessed functional connectivity, typically comparing two groups of children with NDDs based on their diagnoses, finding contradictory results even in a large multi-site sample (Chen et al., 2015, Fredo et al., 2018, King et al., 2019). A recent study applied both categorical and dimensional approaches to compare rs-fc features driven by each approach in an ASD group and controls. Notably, a strong difference was found between the data-driven clusters (i.e., site effects), but not between the diagnostic groups, supporting the discrepancy between the behaviourally based diagnoses and the underling neurobiological presentation (Easson et al., 2019). Another recent report also found that the overall connectivity patterns were very similar amongst those with ASD, ADHD, schizophrenia and even TDs (Spronk et al., 2018), while a third study, assessed frontostriatal rs-fc in children with ASD and OCD, and found no diagnosis-specific differences but an association between increased nucleus accumbens connectivity with the frontal region and symptom severity in repetitive behaviours (Akkermans et al., 2018). Our findings and those of the recent literature suggest that a categorical approach may not be useful even to define typically developing controls. We need to reconsider neurobiological presentations along a continuum beyond categorical clinical diagnoses and dichotomized typical-atypical development.
In contrast to the diagnosis-based comparisons, the dimensional approach, in which we examined the associations between connectivity indices and behavioural measures across all participants independent of diagnosis, revealed that participants’ general adaptive functioning was significantly correlated with subcortical functional connectivity across three graph metrics. Subcortical network function has been implicated in individual differences in TD individuals, such as reward-based learning and decision-making (Yin and Knowlton, 2006), and structural and functional abnormalities in subcortical regions have been reported in NDDs (Boedhoe et al., 2017, Hoogman et al., 2015, Shepherd, 2013, Van Rooij et al., 2018). Atypical subcortical connectivity has been noted in interactions with sensory-motor cortices in ASD (Cerliani et al., 2015), and connectivity with frontal regions was related to atypical reward processing or attention problems in ADHD (Cubillo et al., 2012). In OCD, patterns of both increased and decreased frontostriatal and within-striatal connectivity have also been reported (Posner et al., 2014, Vaghi et al., 2017). Although the majority of studies using the ABIDE samples did not report subcortical findings, increased functional connectivity in subcortical regions was one of the frequent findings in ASD from other samples, in particular when they were defined and assessed at a network-level, rather than a node-level (Cerliani et al., 2015, Chen et al., 2015, Di Martino et al., 2014, Fredo et al., 2018, King et al., 2018, Maximo and Kana, 2019, Smith et al., 2018). We also found that DMN connectivity in local efficiency and fronto-parietal network connectivity in clustering coefficient were significantly correlated with participants’ general adaptive functioning. Atypical DMN activity is one of the most frequent findings in rs-fc studies characterizing ASD (Hull et al., 2017), ADHD (Castellanos and Aoki, 2016) and OCD (Stern et al., 2012), and a few studies have reported the posterior DMN as a shared brain marker between ASD and ADHD (Di Martino et al., 2013, Kernbach et al., 2018, Ray et al., 2014) and between ADHD and OCD (Norman et al., 2017). The fronto-parietal network is known to play a critical role in cognitive control, particularly to maintain control and provide flexibility by adjusting control in response to feedback (Marek and Dosenbach, 2018). Although atypical fronto-parietal network function has been linked to cognitive difficulties in NDDs (Castellanos and Proal, 2012, Gürsel et al., 2018b, Uddin, 2020) and in large multi-site samples in ASD (Chen et al., 2019), less has been reported in comparisons between NDDs. This may be partly attributed to high heterogeneity from mild to severe deficits in cognitive control in each diagnosis (Gruner and Pittenger, 2018, Luo et al., 2019, McTeague et al., 2016, Mottron and Bzdok, 2020). Our findings demonstrated that cognitive control issues exist in NDDs along a continuum, which does not appear to be diagnosis-specific but be related to adaptive functioning across NDDs.
Notably, general adaptive functioning was found to be associated with graph metrics across specific networks, but not other NDD symptom-related measures (SCQ, CBCL-ADHD subscale, TOCS), although diagnostic groups have shown significant differences in these measures. The previous literature has revealed that some standardized behavioural tests, such as the Reading the Mind in the Eyes Test (RMET) (Baron-Cohen et al., 2001), which was designed to detect diagnostically specific individual differences in social sensitivity, have highlighted a large amount of overlap in cognitive and behavioural manifestations across disorders (Baribeau et al., 2015, Lipszyc and Schachar, 2010, Van Hulst et al., 2018). Our result, that only the general adaptive functioning was significantly associated with the functional connectivity features, would be related to the fact that general adaptive functioning was designed in a diagnosis-free manner, while other symptom measures were designed to confirm (or support) DSM-based diagnostic criteria. In addition, this also mirrors the group comparison results in that the diagnostic criteria have failed to identify biological differences in the NDDs. We also previously reported in a subsample that investigated structural connectivity across NDDs that only general adaptive functioning was positively correlated with fractional anisotropy in major interhemispheric and cortico-cortical connections across three disorders (Ameis et al., 2016). Together these findings establish that adaptive functions are critical trans-diagnostic behavioural constructs in NDDs, closely associated with underlying neurobiology and are key to understanding the heterogeneity within NDDs. Taken together, even when using a dimensional approach, a non-diagnosis-specific measure may better capture the functional variability and underlying neurobiological heterogeneity across individuals with NDDs.
Lastly, our data-driven clustering results support the current model that categorical approaches have significant limitations in understanding the intrinsic functional brain organization and related mechanisms in NDDs. In our study, the optimal number of clusters across all participants was two, supporting lumping more than splitting based on behaviourally defined categorical diagnoses, as highlighted in the long debates of diagnostic classifications in neuropsychiatric conditions (McKusick, 1969). Notably, no cluster exclusively mapped onto any diagnostic category, suggesting a lack of evidence of specificity of rs-fc differences for any of the NDD diagnostic categories. The patterns of diagnostic distribution show that a dimensional approach identifies typical and atypical brain development along a continuum, suggesting that the NDD and TD groups share similar connectivity patterns overall and other factors, such as age and sex, may further contribute to different connectivity patterns (Easson et al., 2019, Easson and McIntosh, 2019, Rashid et al., 2018, Spronk et al., 2018). This idea was supported by our analyses for clinical validation of the data-driven clusters. The closer a participant was to the cluster that included more OCD and TD participants, the better general adaptive function, fewer social deficits and fewer attentional problems. When these associations were explored more specifically on the general measures, the data-driven clusters were significantly explained by participants’ general adaptive function and attention and hyperactivity. These associations demonstrate that the interactions between rs-fc features and the multiple levels of human cognition need to be understood beyond specific diagnostic labels. In addition, non-diagnosis-specific symptom measures would be useful to explain the clinical validation for data-driven clusters. Observations from the distance matrices, which calculated distances between each pair of participants and grouped them either as data-driven clusters (Fig. 4B) or by diagnoses (Fig. 4C), revealed that diagnosis-based grouping was less successful in subgrouping participants having similar functional connectivity features than the data-driven clusters.
The functional connectivity features in NDDs are complex and our results are on large canonical resting-state networks, and have more of a focus on within-network features since we have used graph theory metrics. Some recent studies have attempted to understand functional alterations in NDDs by examining not only over- or under-connectivity but also investigating various types of connectivity features such as temporal dynamics (Harlalka et al., 2019), imbalance in intra- and inter-network connectivity (Smith et al., 2018), sustained connectivity (King et al., 2018) or HRF dynamics in underlying neurochemical mechanism (Yan et al., 2018). A transient and activation-dependent functional connectivity as well as more focused networks which targeted specific regions or functions have also been investigated in NDDs. A deep-and-big data approach by the combination of different types of biological information in a single large cohort (Lombardo et al., 2019), such as genomics, imaging and other –omics, combined with information on cognition and behaviour will be necessary to understand the complexity of shared biology from genes to the full expression of signs/symptoms, strengths and weakness, across the NDDs. Finally, the scanner upgrade in our study needs to be considered in terms of its potential impact on the results. Although we demonstrated that the NDD groups did not differ significantly from the TD group in either viewing condition and that the viewing condition was not the main factor in clustering the participants’ functional connectivity features, we did not have enough power to address this issue. This can be achieved in a future, larger study.
5. Conclusions
In summary, by investigating three NDD diagnostic groups together with a control group in a single large cohort, we demonstrated that the diagnosis-based approach has substantial limitations in understanding the neurobiology of NDDs. Our results determined that the dimensional or data-driven approach beyond specific diagnoses is a useful alternative to understand both neuroanatomical similarity and heterogeneity in NDDs.
CRediT authorship contribution statement
EJC: Investigation, Formal analysis, Writing - original draft. MMV: Investigation, Formal analysis, Software, Data curation, Writing - review & editing. MJT: Investigation, Resources, Data curation, Supervision, Writing - review & editing. PDA: Investigation, Writing - review. JB: Investigation, Writing – review. JC: Investigation, Writing - review. EK: Investigation, Writing - review. MCL: Investigation, Resources, Writing - review & editing. XL: Investigation, Writing – review. RS: Investigation, Writing - review & editing. JPL: Conceptualization, Funding acquisition, Investigation, Supervision, Writing - review & editing. EA: Conceptualization, Funding acquisition, Investigation, Supervision, Writing - review & editing.
Conflict of interest
E.A. has served as a consultant to Roche and quadrant therapeutics. She has received in kind support from AMO pharma, royalties from APPI and Springer, and editorial honoraria from Wiley. She also holds a patent for the device, “Anxiety Meter.” The remaining authors declare no competing interests.
Acknowledgements
This research was conducted with the support of the Ontario Brain Institute (POND, PIs: Anagnostou/Lerch), funded in part by the Government of Ontario. The opinions, results and conclusions are those of the authors and no endorsement by the Ontario Brain Institute is intended or should be inferred. We thank the following individuals for research support and data collection: Tara Goodale, M.Sc., Reva Schachter, M.Sc., Mithula Sriskandarajah, B.Sc., Marlena Colasanto, M.Sc., Jennifer Gomez, M.A., Laura Park, M.Sc, Susan Day Fragiadakis, M.A., Naomi Peleg, M.Sc., Leanne Ristic, B.A., Richa Mehta, B.A., Christina Sommerdyk, M.Sc., Carolyn Russell, B.Sc., Alessia Greco, M.A., Mike Chalupka, B.A., B.Sc., Christina Chrysler, B.A., and Irene O’Connor, M.Ed. Psych., Amy McNaughton, Ph.D., and Melissa Hudson, B.Sc.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102476.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Achenbach T., Rescorla L. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2000. Manual for the ASEBA preschool forms and profiles. [Google Scholar]
- Achenbach T.M., Rescorla L.A. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2001. Manual for the ASEBA School-Age Forms & Profiles. [Google Scholar]
- Akkermans S.E.A., Rheinheimer N., Bruchhage M.M.K., Durston S., Brandeis D., Banaschewski T., Boecker- R., Wolf I., Williams S.C.R., Buitelaar J.K., Van D., Oldehinkel M. Frontostriatal functional connectivity correlates with repetitive behaviour across autism spectrum disorder and obsessive-compulsive disorder. Psychol. Med. 2018:1–9. doi: 10.1017/S0033291718003136. [DOI] [PubMed] [Google Scholar]
- Ameis S.H., Lerch J.P., Taylor M.J., Lee W., Viviano J.D., Pipitone J., Nazeri A., Croarkin P.E., Voineskos A.N., Lai M.C., Crosbie J., Brian J., Soreni N., Schachar R., Szatmari P., Arnold P.D., Anagnostou E. A Diffusion Tensor Imaging Study in Children With ADHD, Autism Spectrum Disorder, OCD, and Matched Controls: Distinct and Non-Distinct White Matter Disruption and Dimensional Brain-Behavior Relationships. Am J Psychiatry. 2016;173:1213–1222. doi: 10.1176/appi.ajp.2016.15111435. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 1994. Diagnostic and Statistical Manual of Mental Disorders (4th ed.), Author. American Medical Association (AMA), Washington DC. https://doi.org/10.1001/jama.1994.03520100096046.
- American Psychological Association, 2013. Diagnostic and Statistical Manual of Mental Disorders: Depressive Disorders, Diagnostic and Statistical Manual of Mental Disorders,. American Psychiatric Publishing, Inc. https://doi.org/10.1176/appi.books.9780890425596.dsm04.
- Baribeau D.A., Doyle-Thomas K.A., Dupuis A., Iaboni A., Crosbie J., McGinn H., Arnold P.D., Brian J., Kushki A., Nicolson R., Schachar R.J., Soreni N., Szatmari P., Anagnostou E. Examining and comparing social perception abilities across childhood-onset neurodevelopmental disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54(479–86) doi: 10.1016/j.jaac.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Hill J., Raste Y., Plumb I. The “Reading the Mind in the Eyes” Test revised version: A study with normal adults, and adults with Asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry Allied Discip. 2001;42:241–251. doi: 10.1017/S0021963001006643. [DOI] [PubMed] [Google Scholar]
- Boedhoe P.S.W., Schmaal L., Abe Y., Ameis S.H., Arnold P.D., Batistuzzo M.C., Benedetti F., Beucke J.C., Bollettini I., Bose A., Brem S., Calvo A., Cheng Y.Q., Cho K.I.K., Dallaspezia S., Denys D., Fitzgerald K.D., Fouche J.P., Gimenez M., Gruner P., Hanna G.L., Hibar D.P., Hoexter M.Q., Hu H., Huyser C., Ikari K., Jahanshad N., Kathmann N., Kaufmann C., Koch K., Kwon J.S., Lazaro L., Liu Y., Lochner C., Marsh R., Martinez-Zalacain I., Mataix-Cols D., Menchon J.M., Minuzzi L., Nakamae T., Nakao T., Narayanaswamy J.C., Piras F., Piras F., Pittenger C., Reddy Y.C.J., Sato J.R., Simpson H.B., Soreni N., Soriano-Mas C., Spalletta G., Stevens M.C., Szeszko P.R., Tolin D.F., Venkatasubramanian G., Walitza S., Wang Z., van Wingen G.A., Xu J., Xu X.F., Yun J.Y., Zhao Q., Thompson P.M., Stein D.J., van den Heuvel O.A., Grp E.O.C.D.W. Distinct Subcortical Volume Alterations in Pediatric and Adult OCD: A Worldwide Meta- and Mega-Analysis. Am. J. Psychiatry. 2017;174:60–69. doi: 10.1176/appi.ajp.2016.16020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borràs-Ferrís L., Pérez-Ramírez Ú., Moratal D. Link-Level Functional Connectivity Neuroalterations in Autism Spectrum Disorder: A Developmental Resting-State fMRI Study. Diagnostics (Basel, Switzerland) 2019;9:32. doi: 10.3390/diagnostics9010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems (vol 10, pg 186, 2009) Nat. Rev. Neurosci. 2009;10 doi: 10.1038/nrn2618. [DOI] [PubMed] [Google Scholar]
- Castellanos, F.X., Aoki, Y., 2016. Intrinsic Functional Connectivity in Attention-Deficit/Hyperactivity Disorder: A Science in Development. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. https://doi.org/10.1016/j.bpsc.2016.03.004. [DOI] [PMC free article] [PubMed]
- Castellanos, F.X., Proal, E., 2012. Large-scale brain systems in ADHD: Beyond the prefrontal-striatal model. Trends Cogn. Sci. https://doi.org/10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed]
- Cerliani L., Mennes M., Thomas R.M., Di Martino A., Thioux M., Keysers C. Increased functional connectivity between subcortical and cortical resting-state networks in Autism spectrum disorder. JAMA Psychiatry. 2015;72:767–777. doi: 10.1001/jamapsychiatry.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.M., Yang P., Wu M.T., Chuang T.C., Huang T.Y. Deriving and validating biomarkers associated with autism spectrum disorders from a large-scale resting-state database. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-45465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.P., Keown C.L., Jahedi A., Nair A., Pflieger M.E., Bailey B.A., Müller R.-A. Diagnostic classification of intrinsic functional connectivity highlights somatosensory, default mode, and visual regions in autism. NeuroImage Clin. 2015;8:238–245. doi: 10.1016/j.nicl.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium, T., Fair, Damien PA-C, P.., Mennes, M.P.D., Mostofsky, S.H.M.D., 2012. The ADHD-200 Consortium: a model to advance the translational potential of neuroimaging in clinical neuroscience. Front. Syst. Neurosci. 6, 62. https://doi.org/10.3389/fnsys.2012.00062. [DOI] [PMC free article] [PubMed]
- Cubillo A., Halari R., Smith A., Taylor E., Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012 doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Dajani D.R., Burrows C.A., Nebel M.B., Mostofsky S.H., Gates K.M., Uddin L.Q. Parsing Heterogeneity in Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder with Individual Connectome Mapping. Brain Connect. 2019;9:673–691. doi: 10.1089/brain.2019.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice A., Ricceri L., Venerosi A., Chiarotti F., Calamandrei G. Multifactorial Origin of Neurodevelopmental Disorders: Approaches to Understanding Complex Etiologies. Toxics. 2015;3:89–129. doi: 10.3390/toxics3010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbruck E., Yang M., Yassine A., Grossman E.D. Functional connectivity in ASD: Atypical pathways in brain networks supporting action observation and joint attention. Brain Res. 2019;1706:157–165. doi: 10.1016/j.brainres.2018.10.029. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Yan C., Li Q., Denio E., Castellanos F.X., Alaerts K., Anderson J.S., Assaf M., Bookheimer S.Y., Dapretto M., Deen B., Delmonte S., Dinstein I., Ertl-Wagner B., Fair D.A., Gallagher L., Kennedy D.P., Keown C.L., Keysers C., Lainhart J.E., Lord C., Luna B., Menon V., Minshew N.J., Monk C.S., Mueller S., Müller R.-A., Nebel M.B., Nigg J.T., O’Hearn K., Pelphrey K.A., Peltier S.J., Rudie J.D., Sunaert S., Thioux M., Tyszka J.M., Uddin L.Q., Verhoeven J.S., Wenderoth N., Wiggins J.L., Mostofsky S.H., Milham M.P. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry. 2014;19:659–667. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Zuo X.N., Kelly C., Grzadzinski R., Mennes M., Schvarcz A., Rodman J., Lord C., Castellanos F.X., Milham M.P. Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2013;74:623–632. doi: 10.1016/j.biopsych.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easson A.K., Fatima Z., McIntosh A.R. Functional connectivity-based subtypes of individuals with and without autism spectrum disorder. Netw. Neurosci. 2019;3:344–362. doi: 10.1162/netn_a_00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easson A.K., McIntosh A.R. BOLD signal variability and complexity in children and adolescents with and without autism spectrum disorder. Dev. Cogn. Neurosci. 2019;36 doi: 10.1016/J.DCN.2019.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredo, J., Jahedi, A., Reiter, M.A., Müller, R.-A., Jac Fredo, A.R., Reiter, M., 2018. Diagnostic Classification of Autism Using Resting-State Fmri Data and Conditional Random Forest Analysis of EMG signals View project Data Processing Methods View project Diagnostic Classification of Autism using Resting-State fMRI Data and Conditional Ran.
- Gruner P., Pittenger C. Cognitive inflexibility in Obsessive-Compulsive Disorder. Neuroscience. 2018;345:243–255. doi: 10.1016/j.neuroscience.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürsel D.A., Avram M., Sorg C., Brandl F., Koch K. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci. Biobehav. Rev. 2018;87:151–160. doi: 10.1016/j.neubiorev.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Gürsel, D.A., Avram, M., Sorg, C., Brandl, F., Koch, K., 2018b. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci. Biobehav. Rev. https://doi.org/10.1016/j.neubiorev.2018.01.016. [DOI] [PubMed]
- Harlalka V., Bapi R.S., Vinod P.K., Roy D. Atypical Flexibility in Dynamic Functional Connectivity Quantifies the Severity in Autism Spectrum Disorder. Front. Hum. Neurosci. 2019;13:6. doi: 10.3389/fnhum.2019.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingdale J., Woodhouse E., Young S., Fridman A., Mandy W. Autistic spectrum disorder symptoms in children and adolescents with attention-deficit/hyperactivity disorder: a meta-analytical review. Psychol. Med. 2019:1–14. doi: 10.1017/S0033291719002368. [DOI] [PubMed] [Google Scholar]
- Holm, S., 1979. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. https://doi.org/10.2307/4615733.
- Hoogman, M., Bralten, J., Mennes, M., Zwiers, M., K, V.H., Schweren, L., Hibar, D., Thompson, P., Franke, B., 2015. Subcortical volumes across the life span in ADHD: An ENIGMA collaboration. Eur. Neuropsychopharmacol. 25, S189. https://doi.org/10.1016/S0924-977X(15)30184-X.
- Hull, J. V., Jacokes, Z.J., Torgerson, C.M., Irimia, A., Van Horn, J.D., Aylward, E., Bernier, R., Bookheimer, S., Dapretto, M., Gaab, N., Geschwind, D., Jack, A., Nelson, C., Pelphrey, K., State, M., Ventola, P., Webb, S.J., 2017. Resting-state functional connectivity in autism spectrum disorders: A review. Front. Psychiatry. https://doi.org/10.3389/fpsyt.2016.00205.
- Ickowicz A., Schachar R.J., Sugarman R., Chen S.X., Millette C., Cook L. The parent interview for child symptoms: A situation-specific clinical research interview for attention-deficit hyperactivity and related disorders. Can. J. Psychiatry. 2006;51:325–328. doi: 10.1177/070674370605100508. [DOI] [PubMed] [Google Scholar]
- Jung M., Tu Y., Park J., Jorgenson K., Lang C., Song W., Kong J. Surface-based shared and distinct resting functional connectivity in attention-deficit hyperactivity disorder and autism spectrum disorder. Br. J. Psychiatry. 2019;214:339–344. doi: 10.1192/bjp.2018.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap H., Fontenelle L.F., Miguel E.C., Ferrão Y.A., Torres A.R., Shavitt R.G., Ferreira-Garcia R., Do Rosário M.C., Yücel M. “Impulsive compulsivity” in obsessive-compulsive disorder: A phenotypic marker of patients with poor clinical outcome. J. Psychiatr. Res. 2012;46:1146–1152. doi: 10.1016/j.jpsychires.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Kazeminejad A., Sotero R.C. Topological Properties of Resting-State fMRI Functional Networks Improve Machine Learning-Based Autism Classification. Front. Neurosci. 2019;12 doi: 10.3389/fnins.2018.01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernbach J.M., Satterthwaite T.D., Bassett D.S., Smallwood J., Margulies D., Krall S., Shaw P., Varoquaux G., Thirion B., Konrad K., Bzdok D. Shared endo-phenotypes of default mode dsfunction in attention deficit/hyperactivity disorder and autism spectrum disorder. Transl. Psychiatry. 2018;8 doi: 10.1038/s41398-018-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J.B., Prigge M.B.D., King C.K., Morgan J., Dean D.C., Freeman A., Villaruz J.A.M., Kane K.L., Bigler E.D., Alexander A.L., Lange N., Zielinski B.A., Lainhart J.E., Anderson J.S. Evaluation of Differences in Temporal Synchrony Between Brain Regions in Individuals With Autism and Typical Development. JAMA Netw. Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J.B., Prigge M.B.D., King C.K., Morgan J., Weathersby F., Fox J.C., Dean D.C., Freeman A., Villaruz J.A.M., Kane K.L., Bigler E.D., Alexander A.L., Lange N., Zielinski B., Lainhart J.E., Anderson J.S. Generalizability and reproducibility of functional connectivity in autism. Mol. Autism. 2019;10:27. doi: 10.1186/s13229-019-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushki A., Anagnostou E., Hammill C., Duez P., Brian J., Iaboni A., Schachar R., Crosbie J., Arnold P., Lerch J.P. Examining overlap and homogeneity in ASD, ADHD, and OCD: a data-driven, diagnosis-agnostic approach. Transl. Psychiatry. 2019;9:318. doi: 10.1038/s41398-019-0631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.-C., Kassee C., Besney R., Bonato S., Hull L., Mandy W., Szatmari P., Ameis S.H. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. The Lancet Psychiatry. 2019;6:819–829. doi: 10.1016/S2215-0366(19)30289-5. [DOI] [PubMed] [Google Scholar]
- Lake E.M.R., Finn E.S., Noble S.M., Vanderwal T., Shen X., Rosenberg M.D., Spann M.N., Chun M.M., Scheinost D., Constable R.T. The Functional Brain Organization of an Individual Allows Prediction of Measures of Social Abilities Transdiagnostically in Autism and Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry. 2019;86:315–326. doi: 10.1016/j.biopsych.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionel A.C., Tammimies K., Vaags A.K., Rosenfeld J.A., Ahn J.W., Merico D., Noor A., Runke C.K., Pillalamarri V.K., Carter M.T., Gazzellone M.J., Thiruvahindrapuram B., Fagerberg C., Laulund L.W., Pellecchia G., Lamoureux S., Deshpande C., Clayton-Smith J., White A.C., Leather S., Trounce J., Bedford H.M., Hatchwell E., Eis P.S., Yuen R.K.C., Walker S., Uddin M., Geraghty M.T., Nikkel S.M., Tomiak E.M., Fernandez B.A., Soreni N., Crosbie J., Arnold P.D., Schachar R.J., Roberts W., Paterson A.D., So J., Szatmari P., Chrysler C., Woodbury-Smith M., Lowry R.B., Zwaigenbaum L., Mandyam D., Wei J., MacDonald J.R., Howe J.L., Nalpathamkalam T., Wang Z., Tolson D., Cobb D.S., Wilks T.M., Sorensen M.J., Bader P.I., An Y., Wu B.L., Musumeci S.A., Romano C., Postorivo D., Nardone A.M., Monica M. Della, Scarano G., Zoccante L., Novara F., Zuffardi O., Ciccone R., Antona V., Carella M., Zelante L., Cavalli P., Poggiani C., Cavallari U., Argiropoulos B., Chernos J., Brasch-Andersen C., Speevak M., Fichera M., Ogilvie C.M., Shen Y., Hodge J.C., Talkowski M.E., Stavropoulos D.J., Marshall C.R., Scherer S.W. Disruption of the ASTN2/TRIM32 locus at 9q33.1 is a risk factor in males for autism spectrum disorders, ADHD and other neurodevelopmental phenotypes. Hum. Mol. Genet. 2014;23:2752–2768. doi: 10.1093/hmg/ddt669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipszyc J., Schachar R. Inhibitory control and psychopathology: A meta-analysis of studies using the stop signal task. J. Int. Neuropsychol. Soc. 2010;16:1064–1076. doi: 10.1017/S1355617710000895. [DOI] [PubMed] [Google Scholar]
- Lombardo M.V., Lai M.-C., Baron-Cohen S. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol. Psychiatry. 2019;24:1435–1450. doi: 10.1038/s41380-018-0321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Leventhal B.L., Dilavore P.C., Pickles A., Rutter M. The Autism Diagnostic Observation Schedule (ADOS) J. Autism Dev. Disord. 2000;30:205–223. doi: 10.1023/A:1005592401947. [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism Diagnostic Interview-Revised: A Revised Version of a Diagnostic Interview for Caregivers of Individuals with Possible Pervasive Developmental Disorders. J. Autism Dev. Disord. 1994 doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luo Y., Weibman D., Halperin J.M., Li X. A review of heterogeneity in attention deficit/hyperactivity disorder (ADHD) Front. Hum. Neurosci. 2019;13:42. doi: 10.3389/fnhum.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S., Dosenbach N.U.F. The frontoparietal network: Function, electrophysiology, and importance of individual precision mapping. Dialogues Clin Neurosci. 2018 doi: 10.31887/DCNS.2018.20.2/smarek. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J., Cooper M., Hamshere M.L., Pocklington A., Scherer S.W., Kent L., Gill M., Owen M.J., Williams N., O’Donovan M.C., Thapar A., Holmans P. Biological overlap of attention-deficit/hyperactivity disorder and autism spectrum disorder: Evidence from copy number variants. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53 doi: 10.1016/j.jaac.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximo J.O., Kana R.K. Aberrant “deep connectivity” in autism: A cortico-subcortical functional connectivity magnetic resonance imaging study. Autism Res. 2019;12:384–400. doi: 10.1002/aur.2058. [DOI] [PubMed] [Google Scholar]
- McKusick V.A. On lumpers and splitters, or the nosology of genetic disease. Perspect. Biol. Med. 1969;12:298–312. doi: 10.1353/pbm.1969.0039. [DOI] [PubMed] [Google Scholar]
- McTeague L.M., Goodkind M.S., Etkin A. Transdiagnostic impairment of cognitive control in mental illness. J. Psychiatr.Res. 2016;83:37–46. doi: 10.1016/j.jpsychires.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L., Bzdok D. Autism Spectrum heterogeneity: fact or artifact? Mol. Psychiatry. 2020:1–8. doi: 10.1038/s41380-020-0748-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman L.J., Carlisi C.O., Christakou A., Cubillo A., Murphy C.M., Chantiluke K., Simmons A., Giampietro V., Brammer M., Mataix-Cols D., Rubia K. Shared and disorder-specific task-positive and default mode network dysfunctions during sustained attention in paediatric Attention-Deficit/Hyperactivity Disorder and obsessive/compulsive disorder. NeuroImage Clin. 2017;15:181–193. doi: 10.1016/j.nicl.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, L., Burton, C., Dupuis, A., … J.S.-J. of the A., 2016, U., 2016. The Toronto Obsessive-Compulsive Scale: psychometrics of a dimensional measure of obsessive-compulsive traits. J. Am. Acad. Child Adolesc. Psychiatry 55, 310–318. [DOI] [PubMed]
- Posner J., Marsh R., Maia T.V., Peterson B.S., Gruber A., Simpson H.B. Reduced functional connectivity within the limbic cortico-striato-thalamo-cortical loop in unmedicated adults with obsessive-compulsive disorder. Hum. Brain Mapp. 2014;35:2852–2860. doi: 10.1002/hbm.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/J.NEUROIMAGE.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid B., Blanken L.M.E., Muetzel R.L., Miller R., Damaraju E., Arbabshirani M.R., Erhardt E.B., Verhulst F.C., van der Lugt A., Jaddoe V.W.V., Tiemeier H., White T., Calhoun V. Connectivity dynamics in typical development and its relationship to autistic traits and autism spectrum disorder. Hum. Brain Mapp. 2018;39:3127–3142. doi: 10.1002/hbm.24064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S., Miller M., Karalunas S., Robertson C., Grayson D.S., Cary R.P., Hawkey E., Painter J.G., Kriz D., Fombonne E., Nigg J.T., Fair D.A. Structural and functional connectivity of the human brain in autism spectrum disorders and attention-deficit/hyperactivity disorder: A rich club-organization study. Hum. Brain Mapp. 2014;35:6032–6048. doi: 10.1002/hbm.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M., Bailey A., Lord C. Western Psychological Services; CA: 2003. The Social Communication Questionnaire (SCQ) Manual. [Google Scholar]
- Ruzzano L., Borsboom D., Geurts H.M. Repetitive Behaviors in Autism and Obsessive-Compulsive Disorder: New Perspectives from a Network Analysis. J. Autism Dev. Disord. 2014;45:192–202. doi: 10.1007/s10803-014-2204-9. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G., Douaud G., Beckmann C.F., Glasser M.F., Griffanti L., Smith S.M. Automatic denoising of functional MRI data: Combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 2014;90:449–468. doi: 10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L., Riddle M.A., McSwiggin-Hardin M., Ort S.I., King R.A., Goodman W.K., Cicchetti D., Leckman J.F. Children’s Yale-Brown Obsessive Compulsive Scale: Reliability and validity. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Sen B., Borle N.C., Greiner R., Brown M.R.G. A general prediction model for the detection of ADHD and Autism using structural and functional MRI. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0194856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd, G.M.G., 2013. Corticostriatal connectivity and its role in disease. Nat. Rev. Neurosci. https://doi.org/10.1038/nrn3469. [DOI] [PMC free article] [PubMed]
- Smith R.X., Jann K., Dapretto M., Wang D.J.J. Imbalance of Functional Connectivity and Temporal Entropy in Resting-State Networks in Autism Spectrum Disorder: A Machine Learning Approach. Front. Neurosci. 2018;12:869. doi: 10.3389/fnins.2018.00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser C., Inder T., Shimony J., Hill J., Degnan A., Snyder A., Neil J. Longitudinal analysis of neural network development in preterm infants. Cereb. Cortex. 2010;20:2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk, M., Kulkarni, K., Ji, J.L., Keane, B.P., Anticevic, A., Cole, M.W., 2018. A whole-brain and cross-diagnostic perspective on functional brain network dysfunction. bioRxiv 326728. [DOI] [PMC free article] [PubMed]
- Stern E.R., Fitzgerald K.D., Welsh R.C., Abelson J.L., Taylor S.F. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0036356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin, L.Q., 2020. Brain mechanisms supporting flexible cognition and behavior in adolescents with autism spectrum disorder. Biol. Psychiatry. https://doi.org/10.1016/j.biopsych.2020.05.010. [DOI] [PMC free article] [PubMed]
- Vaghi M.M., Vértes P.E., Kitzbichler M.G., Apergis-Schoute A.M., van der Flier F.E., Fineberg N.A., Sule A., Zaman R., Voon V., Kundu P., Bullmore E.T., Robbins T.W. Specific Frontostriatal Circuits for Impaired Cognitive Flexibility and Goal-Directed Planning in Obsessive-Compulsive Disorder: Evidence From Resting-State Functional Connectivity. Biol. Psychiatry. 2017;81:708–717. doi: 10.1016/j.biopsych.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hulst B.M., De Zeeuw P., Vlaskamp C., Rijks Y., Zandbelt B.B., Durston S. Children with ADHD symptoms show deficits in reactive but not proactive inhibition, irrespective of their formal diagnosis. Psychol. Med. 2018;48:2508–2514. doi: 10.1017/S0033291718000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooij D., Anagnostou E., Arango C., Auzias G., Behrmann M., Busatto G.F., Calderoni S., Daly E., Deruelle C., Di Martino A., Dinstein I., Duran F.L.S., Durston S., Ecker C., Fair D., Fedor J., Fitzgerald J., Freitag C.M., Gallagher L., Gori I., Haar S., Hoekstra L., Jahanshad N., Jalbrzikowski M., Janssen J., Lerch J., Luna B., Martinho M.M., McGrath J., Muratori F., Murphy C.M., Murphy D.G.M., O’Hearn K., Oranje B., Parellada M., Retico A., Rosa P., Rubia K., Shook D., Taylor M., Thompson P.M., Tosetti M., Wallace G.L., Zhou F., Buitelaar J.K. Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: Results from the ENIGMA ASD working group. Am. J. Psychiatry. 2018;175:359–369. doi: 10.1176/appi.ajp.2017.17010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwal T., Kelly C., Eilbott J., Mayes L., Castellanos F. Inscapes: A movie paradigm to improve compliance in functional magnetic resonance imaging. Neuroimage. 2015;122:222–232. doi: 10.1016/j.neuroimage.2015.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets, T., Moser, T., Lund, P.-E., Chow, R.H., Geppert, M., Sudhof, T.C., Neher, E., 2001. Intracellular calcium dependence of large dense-core vesicle exocytosis in the absence of synaptotagmin I, Proceedings of the National Academy of Sciences. https://doi.org/10.1073/pnas.201398798. [DOI] [PMC free article] [PubMed]
- Yan W., Rangaprakash D., Deshpande G. Aberrant hemodynamic responses in autism: Implications for resting state fMRI functional connectivity studies. NeuroImage Clin. 2018;19:320–330. doi: 10.1016/j.nicl.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Roffman J.L., Smoller J.W., Zöllei L., Polimeni J.R., Fischl B., Liu H., Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H.H., Knowlton B.J. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Zarrei M., Burton C.L., Engchuan W., Young E.J., Higginbotham E.J., MacDonald J.R., Trost B., Chan A.J.S., Walker S., Lamoureux S., Heung T., Mojarad B.A., Kellam B., Paton T., Faheem M., Miron K., Lu C., Wang T., Samler K., Wang X., Costain G., Hoang N., Pellecchia G., Wei J., Patel R.V., Thiruvahindrapuram B., Roifman M., Merico D., Goodale T., Drmic I., Speevak M., Howe J.L., Yuen R.K.C., Buchanan J.A., Vorstman J.A.S., Marshall C.R., Wintle R.F., Rosenberg D.R., Hanna G.L., Woodbury-Smith M., Cytrynbaum C., Zwaigenbaum L., Elsabbagh M., Flanagan J., Fernandez B.A., Carter M.T., Szatmari P., Roberts W., Lerch J., Liu X., Nicolson R., Georgiades S., Weksberg R., Arnold P.D., Bassett A.S., Crosbie J., Schachar R., Stavropoulos D.J., Anagnostou E., Scherer S.W. A large data resource of genomic copy number variation across neurodevelopmental disorders. npj. Genomic Med. 2019;4 doi: 10.1038/s41525-019-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.