Abstract
Pausing of RNA polymerase II (RNAP II) by backtracking on the DNA is a major regulatory mechanism in control of eukaryotic transcription. Backtracking occurs by extrusion of the 3’ end of the RNA from the active center after bond formation and before translocation of RNAP II on DNA. In several documented cases, backtracking requires a special signal such as A/T-rich sequences forming an unstable RNA-DNA hybrid in the elongation complex. However, other sequence-dependent backtracking signals and conformations of RNAP II leading to backtracking remain unknown. Here, we demonstrate with S. cerevisiae RNAP II that a cleavage-deficient elongation factor TFIIS (TFIISAA) enhances backtracked pauses during regular transcription. This is due to increased efficiency of formation of an intermediate that leads to backtracking. This intermediate may involve mis-alignment at the 3’ end of the nascent RNA in the active center of the yeast RNAP II and TFIISAA promotes formation of this intermediate at the DNA sequences presenting high-energy barrier to translocation. We proposed a three-step mechanism for RNAP II pausing in which a prolonged dwell time in the pre-translocated state increases the likelihood of the 3’ RNA end misalignment facilitating a backtrack pausing. These results demonstrate an important role of the intrinsic blocks to forward translocation in pausing by RNAP II.
Keywords: backtracking, pausing, RNA polymerase II, transcription elongation, translocation
Graphical Abstract

Introduction
The rate of cellular mRNA production by RNA polymerase II (RNAP II) is regulated over a broad range by targeting the transcription elongation step. During elongation, RNAP cycles continuously through several steps: NTP binding, active site closure, phosphodiester bond formation, pyrophosphate release and translocation forward on the DNA (reviewed in). The translocation clears the active center of the NMP at the 3’ end of the transcript in preparation for the next cycle. Theoretical studies proposed that certain DNA sequences provide a relatively higher free energy of post-translocated state of the enzyme, which causes a pre-translocated pause due to disfavored forward translocation. However, this type of pausing has not yet been experimentally proven for either the bacterial or eukaryotic polymerase.
One of the well-established mechanisms of transcription pausing is known as backtracking. During backtracking, RNAP moves backwards on the DNA relative to the 3’ end of the RNA by one or more base pairs leading to the extrusion of the 3’ end of the RNA from the active center and causing catalytic inactivation of the enzyme. Backtracking a short distance is reversible enabling escape from the pause as opposed to backtracking at a long distance, which can cause an irreversible transcription arrest. GreB for bacterial RNAP and TFIIS (also called S-II) for eukaryotic RNAP II rescue their backtracked complexes by promoting an endonucleolytic cleavage of the extruded RNA. The catalytic loop of TFIIS juxtaposes two catalytic D290 and E291 residues with the acidic residues of the RNAP II active center. In this complex, Mg2+ ion is positioned for hydrolytic cleavage of the backtracked RNA.19 The hydrolysis generates a shortened RNA with its 3’ OH end in the active center, allowing resumption of transcription. A recent report demonstrated that inactivation of the catalytic residues of TFIIS by a double D290A/E291A substitution (hereafter TFIISAA) converts the cleavage factor from an elongation activator into a strong transcription inhibitor.20 Physiologically, TFIISAA exhibits a dominant lethal phenotype in yeast cells by inhibiting transcription elongation.20
An X-ray crystallographic analysis of RNAPII-TFIISAA complex revealed a shift in positions of the 3’ RNA strand due to interaction of the mutant TFIISAA with the 3’ end of the RNA-DNA hybrid in the non-backtracked elongation complex of RNAP II.21 A crystal structure of the backtracked complex of yeast RNAP II with TFIISAA also showed an open active center structure, where the helix-loop-helix module of the Rpb1 subunit called the trigger loop was away from the active center.22 The opening and closing motions of the trigger loop are necessary to allow NTP entry and translocation by RNAP. A Rpb1 mutant has been isolated that favors closure of the trigger loop.23 We speculate that TFIISAA might counteract the effect of this mutation by impeding trigger loop closing.
In this work, we found that TFIISAA increased efficiency of formation of a pre-backtracking intermediate, allowing investigation of the initial step in backtracking by yeast RNAP II. The chance of backtracking is initially increased by an intrinsic barrier to translocation, which the enzyme frequently faces. Increasing dwell time in the pre-translocated state stimulates backtracking by providing additional time for formation of the pre-backtracking intermediate in which the 3’ end of the nascent transcript may be not accurately aligned with the DNA template.
Results
TFIISAA induces sequence-specific pausing of RNAP II
We evaluated the effect of TFIISAA on bulk transcription elongation using the ternary elongation complex (TEC) reconstituted on Template 1 with the 9-nt RNA (TEC9G, here and hereafter the capital letter, following the number indicating RNA length, indicates the nature of the residue of 3’ RNA end, Fig. 1a). This sequence was previously analyzed for the inhibitory effects of TFIISAA on intrinsic transcript cleavage by yeast RNAP II.20 On this template, TFIISAA significantly decreased the rate of run-off RNA formation at both low (1 μM) and high (100 μM) NTP concentrations, mostly by inducing RNAP II pausing and arrest at a unique 12A position (Fig. 1b). This inhibitory activity appeared to be distinct from the reported ability of TFIISAA to suppress the intrinsic RNA cleavage.20 We discuss this issue in supplementary information.
Fig. 1.
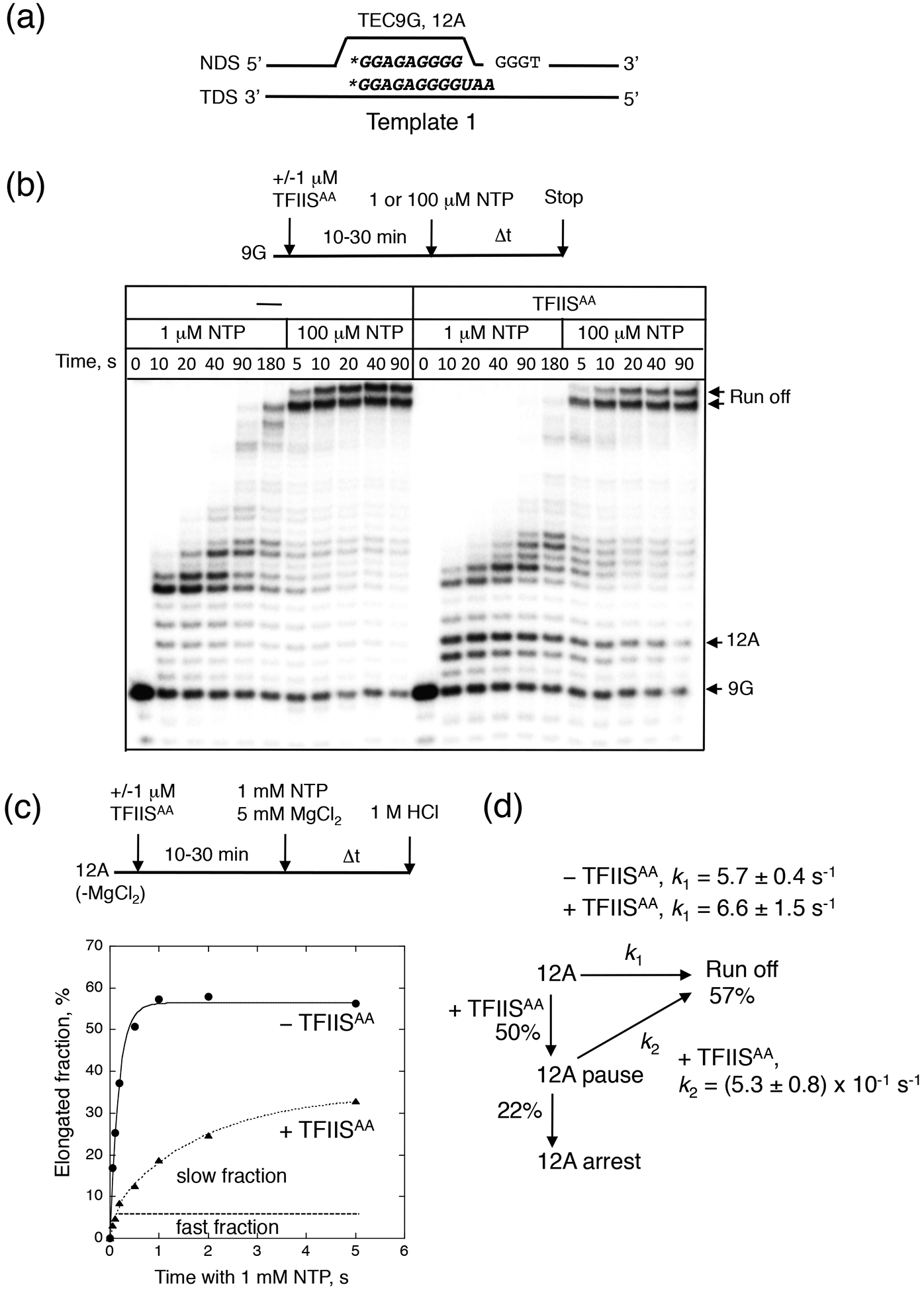
Stimulation of RNAP II pausing by TFIISAA. (a) The RNA and the non-template (NDS) and template (TDS) DNA strands in TEC9G and TEC12A are displayed. The asterisk indicates 5’-labeleing of the RNA. (b) Bulk elongation starting from TEC9G at low and high NTP concentration. The reaction scheme is shown on the top of the gel. The arrows show the starting RNA (9G), the 12A pausing/arrest and the runoff product. (c) Addition of TFIISAA causes partition of TEC12A into the three fractions with different elongation rates. The reaction was quenched with 1 M HCl after different times. The curves represent single- and double exponential fits of the data. The fast and slow fractions of TEC12A generated by TFIISAA are indicated. (d) Kinetic scheme describing the off-pathway pausing of TEC12A. The apparent rate constants for the fast and slow fractions (k1 and k2) and the efficiency of pausing and arrest by TFIISAA are indicated.
To characterize the pausing, we performed a pre-steady-state kinetic analysis of RNAP II escape from the 12A position at a physiological concentration of NTPs by using a pre-formed TEC12A (Fig. 1c). The escape kinetics revealed three distinct properties of TEC12A (Figs. 1c and 1d): (1) even in the absence of TFIISAA, TEC12A resumed transcription about 5–10 times slower than many other RNAP II elongation complexes described in a literature (k ≥ 25 s−1, 27). This indicates that there is a short-lived pause at this position. On the other hand, the pause duration in the absence of TFIISAA was shorter than that shown for an elemental or ubiquitous pauses in single molecule elongation studies of E. coli RNAP at saturating NTP concentration (1s,28,29), (2) In the presence of TFIISAA, TEC12A had an additional ~10-fold reduction in the rate for the major fraction of the complex, but leaving a small active fraction unaffected. While pre-incubation with TFIISAA may affect the quantity of the arrested fraction, about 40% of the major fraction was kept permanently inactive at the 12A position. Thus, TFIISAA induced differential fractionation of TEC12A into active, paused and arrested fractions.
TFIISAA inhibits RNAP II translocation and promotes 1-bp backtracking
Next, we examined if pausing at the 12A sequence in the presence of TFIISAA was caused by backtracking. DNA footprinting by Exonuclease III (ExoIII) has been broadly used for the time-resolved detection of the rear-end boundaries of RNAP II on DNA to detect reversible translocation and backtracking of TECs deprived NTP substrates. Typically, if polymerase is not backtracking, ExoIII produces two distinct footprints of the polymerase on the DNA one base pair apart. These footprints correspond to the boundaries of the pre-translocated and post-translocated states of that complex.23 Upon prolonged incubation with ExoIII, the pre-translocated position shifts to the post-translocated position. This occurs because there is transient forward translocation of RNAP II, which allows the exonuclease to degrade an extra base in DNA (Fig. 2a). Thus, the rate of the footprint transition from the pre-translocated to the post-translocated boundary during incubation with ExoIII reflects the native bias of translocation equilibrium for a single TEC.
Fig. 2.
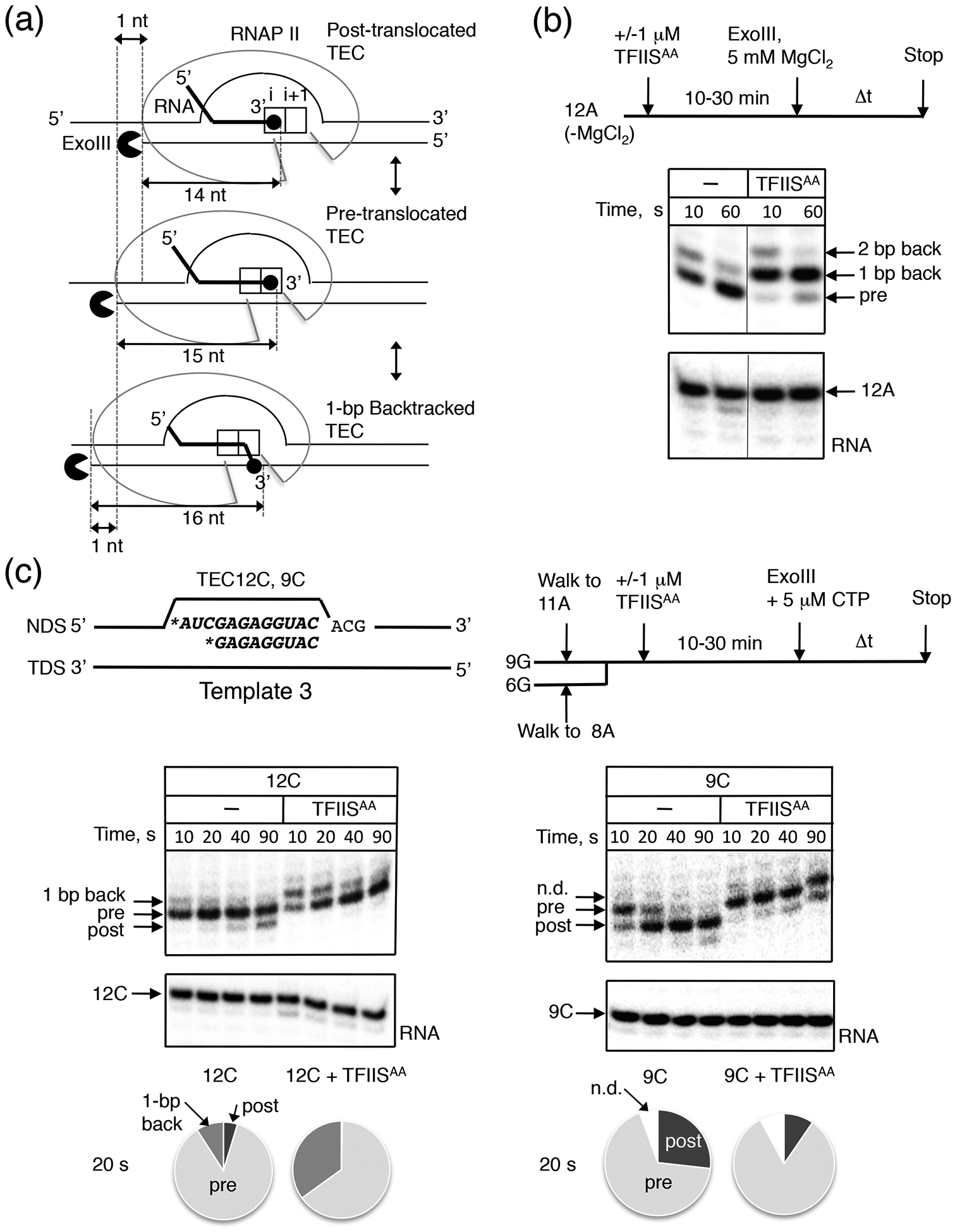
Effect of TFIISAA on translocation equilibrium in the stalled TECs. (a) Schematic representation of the rear-end ExoIII DNA footprinting of the post-translocated (top), pre-translocated (middle), and 1-bp backtracked (bottom) states of RNAP II. The lengths (nt) from the 3’ RNA end to the rear-end boundary of the TEC was shown.23 (b) The translocation equilibrium in TEC12A (Template 1) monitored by dynamics of the RNAP II rear-end boundaries. The arrows indicate the corresponding states of RNAP II. The bottom panel shows the 12A RNA. (c) ExoIII footprinting of TEC12C and TEC9C (Template 3, shown on the top), which were obtained from TEC11A and TEC8A by incubation with 5 pM CTP followed by addition of ExoIII. The presence of CTP does not affect the translocation equilibria in TEC12C and TEC9C. Non-defined boundaries (n.d.) correspond to the rear-end boundaries of 1-bp backtracked RNAP II but were resistant to the ExoIII degradation within 90 sec. A diagram at the bottom shows the estimated fractions of the translocation states for 20 sec incubation of different Complexes with ExoIII.
The ExoIII footprinting revealed that TEC12A was primarily pre-translocated having as well a minor 1-bp backtracked fraction (Fig. 2b). This property of TEC12A was distinguished from the majority of other TECs analyzed previously, in which the post-translocated footprint was generated after about 1 min with ExoIII. The increased dwell time in the pre-translocated state was consistent with the low catalytic activity of TEC12A observed in the absence of TFIISAA (Fig. 1b and 1c). Remarkably, addition of TFIISAA induced a shift of the TEC12A footprint to the 1-bp backtracked state. This result indicates that 1-bp backtracking was responsible for the TFIISAA-dependent pausing at the 12A site. We also noted that a fraction of TEC12A backtracked at the longer 2-bp distance in the presence of TFIISAA. The 2-bp backtracked complex could also explain the irreversible inactivation by TFIISAA of a fraction of TEC12A detected by the kinetic analysis (Fig. 1c, the lower plateau in the graph). Interestingly, the transcript length in TEC12A allowed backtracking at a longer 3–4-bp distance, yet the effect of TFIISAA on this complex was mostly limited backtracking to 1-bp. To test if enhancement of the 1-bp backtracking is the main pausing mechanism in the presence of TFIISAA, we replaced the 3’ two nucleotides of RNA sequence in TEC12A with two uridines (Supplementary Fig. S1a, TEC12U: A11A12->U11U12). Uridine-rich sequences near 3’ ends in nascent RNA have been shown to stimulate greater backtracking because the RNA base pairing with template DNA is weakened.33 As expected, TEC12U backtracked by 2–3-bp as opposed to 1-bp of TEC12A (Supplementary Fig. S1b). Accordingly, the intrinsic catalytic activity of TEC12U was reduced compared to TEC12A (Supplementary Fig. S1c). Nevertheless, TFIISAA induced more 1-bp backtracking of TEC12U than the longer 2–3 bp (Supplementary Fig. S1b). We concluded that initiation of 1-bp backtracking rather than backtracking at the longer distance was the primary cause of pausing in the presence of TFIISAA. A minor increase in the >1-bp backtracking, observed at some DNA sites (Fig. S1b), likely derived from TFIISAA–independent conversion of the highly populated 1-bp backtracked TEC to the >1-bp backtracked states.
We hypothesized that a sequence-dependent energy barrier to translocation made TEC12A a preferred target for pausing by TFIISAA. Since TEC12A is primarily in the pre-translocated position on Template 1, we could not distinguish whether TFIISAA affects pre-translocated complex or stabilizes backtracked complex. We assembled a different pair of TECs on a new DNA sequence (Fig. 2c, Template 3)34 with RNAs of two different lengths (TEC9C/12C, Fig. 2c) in order to find a TEC in which backtracking is eliminated. Using an RNA length of 9 nucleotides was proposed previously to prevent backtracking by shifting the translocation equilibrium to the post-translocated state in a number of TECs.35 In fact, TEC9C was more often in the post-translocated state as compared to TEC12C, and TEC9C generated no backtracked state defined by the Exo III footprint (Fig. 2c). The addition of TFIISAA shifted TEC9C to the pre-translocated state without causing backtracking (Fig. 2c). In contrast, pre-translocated TEC12C was shifted by TFIISAA to a 1-bp backtracked state, similarly to the effect observed for TEC12A on Template 1 (Fig. 2c). We conclude that TFIISAA stabilizes pre-translocated complexes with a modification of the active site in RNAPII that can facilitate 1-bp backtrack pausing at DNA sites where backtracking of RNAP II is allowed. This conclusion is fully consistent with the effect of TFIISAA on translocation of TEC12A and TEC12U on Templates 1 and 2 (Fig. 2b and Supplementary Fig. S1b).
Pausing of RNAP II by TFIISAA requires backtracking
TFIISAA appeared to have two distinct effects on the assembled TECs. In the complexes capable of backtracking, TFIISAA enhanced 1-bp backtracking. When backtracking capacity was eliminated by using a 9-bp hybrid instead of 12-bp hybrid where 3’ end was the same, TFIISAA hindered forward translocation of the enzyme (Fig. 2c). To distinguish between reduced translocation effect and enhanced backtracking effect on pausing, we monitored AMP incorporation and the reverse reaction of pyrophosphorolysis in TEC9C and TEC12C with TFIISAA (Fig. 3a). These complexes were different in their translocation equilibria and backtracking potentials due to the aforementioned difference in the length of their transcripts (Fig. 2c). We measured single bond formation and bond pyrophosphorolysis in both complexes as a function of ATP and pyrophosphate concentrations, respectively (Fig. 3b, c). Notably, TEC12C responded to TFIISAA in two ways. In the forward reaction, TFIISAA increased about 1.6-fold the inactivated (paused) fractions of the complex without a significant effect on the AMP incorporation in the remaining active fraction. TFIISAA also decreased more than 4-fold the susceptibility of TEC12C to pyrophosphate. At the saturated substrate concentrations, the maximal rates of the incorporation were decreased >10-fold by TFIISAA (Fig. 3d). Based on the biphasic incorporation curve, TFIISAA caused a minor ~2-fold decrease of the rate in a “fast” fraction of the complex (Fig. 3d). This might be due to a slight inhibition of forward translocation by TFIISAA in this sequence (see discussion for the minor role of TFIISAA). TFIISAA also caused a >10-fold decrease in rate of pyrophosphorolysis (Fig. 3d). Importantly, these effects of TFIISAA are not manifest in the non-backtracking TEC9C (Fig. 3b) even though TFIISAA causes a clear shift of this complex to the pre-translocated state (Fig. 2c). We conclude that ability to backtrack by TEC12C is essential for the effect on pausing by TFIISAA. Backtracking was responsible for depleting both, the pyrophosphate sensitive pre-translocated fraction and the catalytically competent post-translocated fraction of the complex.
Fig. 3.

Effect of TFIISAA on phosphodiester bond formation and pyrophosphorolysis in TEC12C and TEC9C. (a) Reaction schemes for AMP incorporation (left) and pyrophosphorolysis (right) in the 5’-labeled TECs on Template 3 (The reactions for panel d are described below). (b and c) AMP-incorporated fractions and pyrophosphorolyzed fractions (% of initial TECs) were plotted against ATP and PPi concentrations, respectively. The curves represent Michaelis-Menten-type hyperbolic fits of the data. ATP and PPi concentrations at half saturation (C1/2) in the absence and presence of TFIISAA are indicated in each panel. (d) AMP-CMP-incorporated fractions (+ 1 mM ATP and CTP) and pyrophosphorolyzed fractions (+ 2.5 mM PPi) in the absence and presence of μM TFIISAA were plotted against time, respectively. The reactions were quenched with 1 M HCl after different times. The curves represent single-exponential fits of the data except for the incorporation in the presence of TFIISAA, which is fitted to double-exponential curve. The apparent rate constants (k) are indicated.
We also observed that TFIISAA slightly inhibited pyrophosphorolysis in the pre-translocated TEC9C (Fig. 3b), which was surprising because the pre-translocated TECs are typically more Sensitive to pyrophosphorolysis than their post-translocated counterparts.3 This observation suggests that, in addition to the inhibition of forward translocation, TFIISAA directly inhibited phosphoryl transfer or pyrophosphate binding to the pre-translocated RNAP II. We discuss the mechanism below.
The Rpb1 trigger loop closure suppresses all defects in transcription by TFIISAA
The catalytic domain of TFIIS enters RNAP II active center through the secondary pore to stimulate intrinsic RNA cleavage.22 The trigger loop domain in the Rpb1 subunit also transiently enters the active center via the same pore to participate in NTP sequestration and phosphodiester bond formation. In RNAP II/TFIISAA co-crystals, the catalytic domain of TFIISAA plugs the secondary pore, interfering with the trigger loop closure, suggesting that the the TFIIS loop and the trigger loop must not enter the pore simultaneously. We previously proposed that the E1103 residue of the loop affects its mobility by stabilizing the opened state, and by interacting with T1095 residue located in the trigger loop hinge region.23
We employed the rpb1-T1095G mutation to test if facilitated loop closure counteracts modification of the active center by TFIISAA in vitro and in vivo, because rpb1-E1103G is known to affect translocation whereas the T1095G does not (shown below). affects RNAP II translocation.23 First, we probed the loop dynamics in the mutant enzyme with the previously developed NTP sequestration assay employing rapid quenching the bond formation with EDTA as opposed to HCl.27 HCl rapidly denatures proteins, whereas EDTA deprives Mg2+ ions from a pool of free NTPs without affecting the NTP-Mg2+ complex bound to the active site of RNAP II. Because the sequestration occurs substantially faster than the subsequent bond formation, the chemical step with the bound NTP-Mg2+ proceeds to completion in the presence of EDTA added with a shortest possible delay after the substrate (2 ms, as allowed by the current protocol). Therefore, a substantially larger faction of TEC appears to form a single bond at the 2-ms quench with EDTA compared to the quench with HCl. This phenomenon is defined as a “burst” of bond formation in the presence of EDTA, and an amplitude of the burst serves as the quantitative measure of the NTP sequestration.27 Using this assay, we observed that the T1095G mutant sequestered the CTP in TEC11A more efficiently than the WT enzyme (Fig. 4a). Note, that the mutation did not affect the rate of a phosphodiester bond formation in the reaction quenched with HCl (Fig. 4a). This result demonstrated that the T1095G mutation facilitated the NTP sequestration most likely by facilitating the trigger loop closure without affecting the subsequent catalysis.
Fig. 4.

Rpb1-T1095G trigger loop mutation enhances NTP sequestration in RNAP II and Counteracts TFIISAA in vitro and in vivo. (a) A rapid quench-flow analysis of a single NMP addition in TEC11A made by the wild type, rpb1-T1095, and rpb1-E1103G mutant RNAP II. The reaction was quenched with EDTA or HCl after 0.002s incubation with 1 mM CTP and ATP. The averaged elongated fractions (n = 3) are shown in the graph. The elongated RNA fraction in the EDTA quench subtracted by that of HCl quench corresponded to the NTP substrate, which was sequestered in the active center prior to its incorporation to the RNA at 0.002 s. (b) The effect of TFIISAA on translocation in TEC11A and TEC12C formed with rpb1-T1095G RNAP II. The experimental design and the diagram at the bottom is the same as in Fig. 2c. (c) Effect of TFIISAA on the rate of escape of rpb1-T1095G RNAP II from the +12A position. The other details are the same as in Fig. 1c. (d) A synthetic phenotype of TFIISAA and the rpb1-T1095G mutant. The dst1D290A, E291A gene encoding TFIISAA was expressed under control of the WT and the three mutant variants of GAL1 promoters in the presence of glucose (repressed) or galactose (induced). 10-fold dilution series of yeast strains (~3 × 105 cells before dilution) were plated on yeast minimal medium plates without uracil and containing 3% glucose (upper) or galactose (lower). The distances from a transcription start site (bp) to the upstream junction of the cloned sequence of the GAL1 promoters are indicated with the relative activities on the top of pictures. The full length contains the intact upstream activating sequence.37
Next, we examined the effect of TFIISAA on translocation of TEC11A and TEC12C reconstituted with the T1095G-RNAP II on Template 3. Although the mutation did not affect the intrinsic translocation equilibrium in these TECs, it almost completely relieved the translocation block by TFIISAA in both complexes (compare Fig. 4b with Fig. 2c, and Supplementary Fig. S2b). Similarly, the mutation suppressed the TFIISAA-induced backtracked pause of TEC12A on Template 1, which is in sharp contrast to the 10-fold catalytic inhibition of the WT RNAP II in the same complex (compare Figs. 4c and 1c, and Supplementary Fig. S3a and Fig. 2b).
The competitive binding between the trigger loop and TFIIS to the active center was further examined by using the backtracked TEC12U as a substrate for TFIIS-induced transcript cleavage (Supplementary Fig. S1a, Template 2). TEC12U with T1095G-RNAP II showed the decreased sensitivity to transcript cleavage by wild-type TFIIS (TFIISWT, Supplementary Fig. S3b).
In good agreement with the in vitro results, the T1095G mutation suppressed the dominant lethality of TFIISAA expression in vivo (Fig. 4d).20 Suppression by the T1095G mutant occurred at several levels of TFIISAA expression from the GAL1 promoter fusion.37 At all levels of TFIISAA expression, rpb1-T1095G mutant cells consistently showed better growth compared to the RPB1-WT cells (Fig. 4d). Induction of the GAL1 promoter on the vector plasmid lacking TFIISAA did not affect growth of the rpb1-T1095G cells (Supplementary Fig. S3c). These results suggest that competition of the catalytic domain of TFIISAA with the trigger loop for the active site of RNAP II is the source of the lethal phenotype in vivo.
Structural flexibility of the catalytic loop of TFIIS is important for pausing
In contrast to the dst1-D290A/E291A mutation, another previously characterized cleavage-deficient TFIIS variant Δ290–291 (TFIISΔDE) showed no effect on transcription in vitro.20 The idea about the loop flexibility derived from a comparison of the X-ray structures of RNAP II elongation complexes with the two different catalytic loop mutants of TFIIS (D290A/E291A and E291H), which identified a very different position of the loops near the active center of RNAPII.12 We hypothesized that the flexibility of the catalytic loop of TFIIS was increased by the D290A/E291A substitutions compared WT and the Δ290–291 mutation. This increase was essential for pausing. In addition to the Δ290–291, the E291H single substitution (TFIISH) has also been reported as a catalytically inactive. The bulky side chain of H291 and the Δ290–291 deletion are likely to decrease loop flexibility. We purified TFIISΔDE, TFIISH and TFIISWT proteins and analyzed their effects on pausing on Template 4 (Fig. 5a, also see Discussion and Supplementary Information for the effect of TFIISWT on the pausing). This template was chosen because TFIISAA had the most dramatic effect on pausing at the 26A position relative to other templates used here. For example, resumption of elongation at 26A is two orders of magnitude slower than that of 12A and 12C positions on Template 1 and 3, respectively (Fig. 1d, 3d, and 5c). TFIISΔDE was completely inactive in stimulation of the 26A pausing and TFIISH showed a ten-fold decrease in the duration of pausing relative to TFIISAA on this template (Fig. 5b and 5c). Interestingly, the duration of the 26A pause, but not the size of the paused fraction (~70%) was affected by the TFIISH mutant (Fig. 5c). Like TFIISAA, TFIISH promoted backtracking of TEC26A probed by the ExoIII footprinting (Supplementary Fig. S4). These results are consistent with the idea that the relative structural flexibility of the catalytic loop of TFIIS determines the pausing duration.
Fig. 5.

The effect of different TFIIS mutations on RNAP II pausing. (a) The RNA sequence surrounding the +25U/+26A pausing sites is shown on the top. TEC24A was obtained from TEC9G by walking on Template 4 and chased with 0.5 mM NTPs. (b) A time-course of the run-off RNA formation in the presence of the TFIIS mutants. The curves represent single- (for -TFIIS and TFIISΔDE) and double (for TFIISAA and TFIISH) exponential fits of the data. (c) Kinetic scheme of elongation and pausing at A24 position. The apparent rate constants of the fast and slow fractions (k1 and k2) and the efficiencies of pausing by the mutants of TFIIS (TFIISmut) are shown.
Discussion
Kinetic model of pausing
We propose a kinetic model of pausing that is consistent with our results (Fig. 6). In a majority of DNA sequences, forward translocation of RNAP II occurs significantly faster than backtracking, which assures processive elongation.8 At a pause site, RNAP II encounters an energy barrier to translocation that increases dwell time of the pre-translocated state, allowing a conformational change to a transient backtracking intermediate (Pre* state, here and hereafter) (k2 ≥ k1, Fig. 6). In our experiments, this transition is revealed by the mutant TFIISAA. The catalytic loop of TFIISAA may increase the efficiency for Pre* state formation [increase k2 /(k1+k2), Fig. 6] without limiting any rates in the off-pathway pausing. The Pre* state has a lower propensity for forward translocation and a higher propensity for 1-bp backtracking compared to the regular pre-translocated conformation, which leads to pausing by 1-bp backtracking. Pausing becomes less likely as the rate of forward translocation increases relative to the rate of Pre* state formation (k1 > k2, Fig. 6). Thus, the core concept of the model is a kinetic competition between forward translocation and transition to the Pre* state (see Supplementary Information how the model describes elongation properties of individual TECs).
Fig. 6.
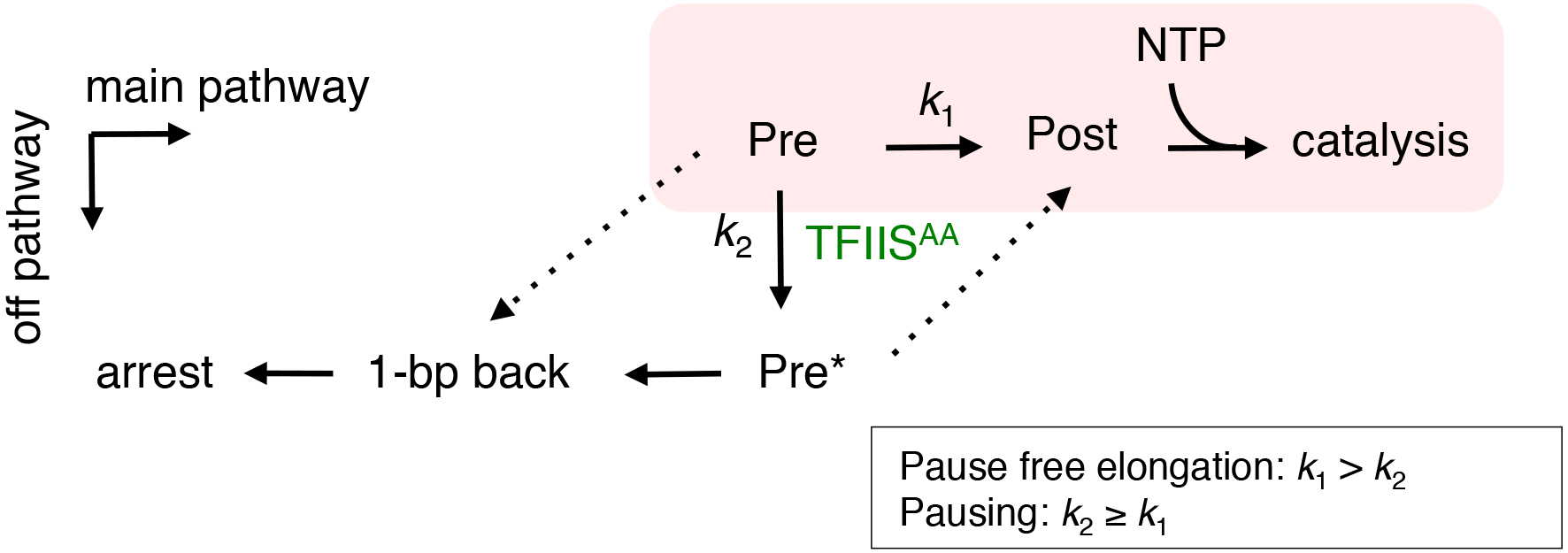
A kinetic model for entry into off-pathway backtrack pausing of RNAP II and the stimulation by TFIISAA. The discrete RNAP II translocation states are shown and the main (online) pathway is shaded. The text in the box shows fundamental assumptions of the model. The dashed lines indicate the potential minimal pathways for the forward translocation from the Pre* state and for the 1-bp backtracking from the regular pre-translocation state. The apparent rate constant k1 is for the forward translocation from the pre-translocated state. k2 is for the conformational change associated with Pre->Pre* transition. TFIISAA increases the efficiency of Pre->Pre*. The forward translocation from the Pre* state of TEC is not allowed or is too slow to compete with backtracking. All pathways shown in the scheme are reversible and allows escape routes due to the reversibility but unidirectional arrows indicate only those pathways that affect the kinetic competition.
At pause-free sequences, our model seems to be compatible with the thermal (or Brownian) ratchet model, where the translocation does not require any energy input other than thermal energy.39 The thermal ratchet model implies that the pre- and post-translocated states cannot be defined as homogeneous kinetic states due to the lack of an energy barrier between these states that exceeds the thermal energy. Therefore, the concentrations of these states and their corresponding affinities to pyrophosphate cannot be principally determined. This notion can explain an apparent poor correlation between sensitivity to pyrophosphorolysis in seemingly pre-translocated TECs such as TEC9C (Fig. 3b) and their post-translocated counterparts analyzed by us. The recent finding of a lack of correlation between translocation bias and the ability to bind pyrophosphate in bacterial RNAP is consistent with this view.40
A model linking hindered translocation with transcription pausing has been also proposed by Bai, Shundrovsky, and Wang.6 In their model, TEC stability at a particular DNA site was determined as a function of free energy changes involved in formation of the DNA bubble and the RNA hybrid, and RNAP binding, as described by Yager and von Hippel.41 At a pause site, the post-translocated state of TEC is less stable than the pre-translocated state, which limits availability of the post-translocated state for NTP binding. Consequently, RNAP pauses when transcription is performed at limiting concentration of NTP.6 The RNAP II pausing described in our work remained even at a saturating 1 mM concentration of NTP (Figs. 1 and 5), suggesting that the pausing is different from the one of Bai et al.6
As far as a branching pathway starting from the pre-translocated state (off-pathway) is concerned, our model for pausing by RNAP II is the same as the elemental pause model previously established for bacterial RNAP.42 This model explains ubiquitous short-lived pausing events frequently observed in bulk transcription study or in a single molecule analysis. The elemental pause model postulates formation of a special inactivated pre-translocated state (closely related to the Pre* state) that resists both, forward translocation and pyrophosphorolysis due to fraying of the 3’ RNA residue from the template DNA during pausing. An idea that a delay of forward translocation increases the probability of the off-pathway entry has been also proposed by this model.40 The Pre* state of RNAP II appears to be a rapidly formed pre-backtracking intermediate converted to backtrack pauses as opposed to the bacterial RNAP where this intermediate is a direct source of pausing. Interestingly, we observed the minor TFIISAA-dependent pauses of non-backtracked TEC9G on the two templates of this work (Fig. S1) suggesting that the Pre* state of RNAP II may be a source of weak pauses at some DNA sequences. Our data argue that an energy barrier to 1-bp backtracking seems to be lower for RNAP II compared to its bacterial counterpart, which leads to the more rapid backtracking of the eukaryotic enzyme from the Pre* state. This idea is supported by single molecule studies showing that an opposing force applied to prevent recovery from backtracked state was three times lower for the yeast RNAP II compared to E. coli RNAP. A recent biochemical study identidied DNA sequence where translocation of E. coli RNAP occurs slower than nucleotide incorporation47 suggesting that, similarly to RNAP II, the E. coli enzyme also faces a high translocation barrier at some DNA sequences. It is possible that bacterial Gre factors may induce backtrack pauses at these sequence by stabilization of the backtracked TEC as was previously suggested.48
Structural model for the backtrack intermediate and the origin of translocation barrier
An alignment of X-ray crystal structures of pre-translocated TEC and RNAP II/TFIISAA complexes identified a potential reason for the abnormal Pre* conformation depicted in Figure 7a,b [also see]. Notably, the analysis showed that binding of the catalytic loop of TFIISAA next to the 3’ end of the RNA causes a strong distortion (mis-alignment or fraying) of the 3’ end of the nascent RNA from the template by tilting it toward the secondary pore in RNAP II. This distortion may guide the 3’ RNA to the pore and promote 1-bp backtracking. The 3’ fraying may also obstruct forward translocation by interfering with transfer of the 3’ RNA end from the i+1 site to the more spatially constrained i site of the active center. In addition, TFIISAA may indirectly inhibit translocation by making an extensive contact with the bridge helix as it was observed in the crystal structures of RNAP II-TFIISAA binary and in the ternary TEC-TFIISAA complexes containing the opened trigger loop. The bridge helix, a long α-helix in the Rpb1 subunit implicated in catalysis and translocation (for review, see3). The 3’ RNA fraying interferes with the phosphoryl transfer, which may explain resistance of the Pre* conformation to pyrophosphorolysis as was observed with TEC9C (Fig. 3b). The mis-aligned or frayed 3’ RNA occupies variable locations in different crystals of the yeast RNAP II (Fig. 7b) making it difficult to determine which of these intermediates corresponds to the Pre* state characterized here. A RNAP II/TFIISWT co-crystal is not available to test if the wild type protein induces a similar conformation.
Fig. 7.

(a) A structural model for the off-pathway pausing. The RNA transcript (red), the 3’-terminal NMP (blue), DNA (gray), the catalytic loop of TFIISAA (dark green), the trigger loop (light green with rpb1-T1095 “hinge”, yellow dot) and i and i+1 parts of the active center are shown in the pre-translocated TEC. The pre-translocation (i+1 aligned), the Pre*-translocation (i+1 misaligned), and the 1-bp backtracked conformations are displayed. A competition between the trigger loop and TFIISAA for binding to the active center is shown on the top. (b) A superposition of the X-ray structures of the pre-translocated TEC containing the properly aligned 3’RNA end (structure #1, PDB: 1I6H,49) and the 1-bp backtracked TEC/TFIISAA containing partially mis-aligned RNA residue in the i+1 site (structure #2, PDB: 3PO3,22). Structure #3 (PDB: 3I4M,51) depicts the most severe mis-alignment (fraying) of the 3’RNA observed in the TEC stalled after incorporation of the complementary UMP immediately downstream from the 8-oxoG lesion in the template DNA strand.51 The similar configuration was observed in the absence of the lesion (PDB coordinates are not available51). The alignment reveals that binding of TFIISAA causes a tilt of the 3’ RNA residue leading to its partial displacement from pairing with the i+1 template base. The similar (but more dramatic) transition was detected in the absence of TFIIS in the TECs carrying the complementary 3’UMP (PDB: 3I4M) and the 3’-mismatched RNA (not shown). We proposed that evolution of the structure #1 to #2 (or #3) corresponds the Pre->Pre* state transition (shown by arrow). In RNAP II, this transition is induced by TFIISAA. The color scheme is the same as in the panel A. (c) ExoIII rear-end footprinting of TEC12U (Scaffold D and E51), which were obtained from TEC11C by incubation with 30 μM UTP followed by addition of ExoIII. The Scaffold D corresponds to PDB: 3I4M of the panel (b). In the original crystal structure51, the scaffolds D and E contained 8-oxoguanine (8-oxoG) in the template DNA strand base paired with the penultimate 3’ RNA residue. The scaffolds used in our work, contained the regular guanine or thymine residue at this position.
A 3’ RNA distortion was first observed in the crystal structures of the yeast RNAP II with the RNA primers carrying a 3’ mismatched base followed by a penultimate T-U mismatch.50 More recently, a similar abnormality was disclosed in the regular pre-translocated TEC of RNAP II containing the complementary 3’ end of the RNA indicating that the 3’ fraying can occur spontaneously during regular elongation in the absence of TFIIS.51 We validated the proposed role of the 3’ RNA fraying in hindering translocation by direct assembly of yeast RNAP II on the same RNA/DNA sequences that was used for the crystallographic detection of the 3’-frayed RNA in the pre-translocated TEC.51 As expected, the rear-end ExoIII footprinting showed that translocation equilibrium in this TEC was shifted to the pre-translocated state; TFIISAA made this complex fully pre-translocated with a minor 1-bp backtracked fraction (Fig. 7c). This pattern matched the properties of the other paused TECs described in our work. This sequence induced pausing of RNAP II in the presence of TFIISAA (data not shown), which was consistent with the prediction that the 3’ RNA fraying hinders forward translocation and makes the TEC a plausible target for TFIISAA.
We have previously shown that structurally stable 8–9-bp RNA-DNA hybrid and a front-end DNA duplex are essential for binding of RNAP to template during processive elongation. Our present work did not determine what part of the RNA/DNA sequence in TEC is responsible for the translocation barrier. Backtrack pauses described here, appeared not to be specifically associated with weak RNA-DNA hybrids. We noted that if anything the RNA strand at these sequences is enriched with purine nucleotides that make the hybrids adopt a non-canonical conformation potentially impeding translocation.55 This odd conformation may impede translocation of the hybrid through the catalytic cleft of the enzyme. Alternatively, an intrinsic bend of the DNA duplex at the downstream boundary of the RNA/DNA hybrid may hinder translocation of the TEC by obstructing sliding of RNAP II clamped to the DNA. Importantly, such non-canonical structures may promote backtracking of RNAP II to a more uniform upstream DNA. These barriers may also have a synergistic effect with additional physical barriers like nucleosome or the other protein-DNA complexes in the cell. Indeed, recent studies showed that backtracking is a major cause of nucleosomal barrier to transcription in vivo and in vitro (reviewed by Luse and Studitsky60).
More work is needed to determine how frequently the intrinsic translocation barriers emerge during elongation by bacterial and eukaryotic RNAPs and what fraction of these events gives rise to pausing. Future studies will attempt to estimate the energy input, which is required to overcome translocation barriers at certain DNA sequences and to determine whether RNAP employs thermal ratchet translocation during elongation on the pause-free sequences.
Materials and methods
Reagents.
NTPs, oligonucleotides, and [γ−32P]ATP were purchased from GE Healthcare, Integrated DNA Technologies, and MP Biomedicals, respectively.
Proteins.
Wild-type and rpb1-T1095G RNAP II of yeast Saccharomyces cerevisiae containing a histidine-tagged Rpb3 subunit were purified as described previously. The histidine-tagged TFIIS expression plasmid14 was a kind gift from Dr. Caroline Kane (U.C. Berkeley). By using the plasmid as a template, the TFIISAA (D290A/E291A) expression plasmid was made by PCR-based mutagenesis. The recombinant TFIISAA was purified according to,14 with addition of Mono-S column chromatography (GE Healthcare).
In vitro transcription.
The elongation complexes carrying 5’-labelled 8–12-nt RNAs on the Templates 1–5 (see Supplementary Table S1) were assembled and immobilized on Ni2+-NTA agarose (QIAGEN) in transcription buffer (TB; 20 mM Tris-HCl, pH 7.9, 5 mM MgCl2, 1 mM 2-mercaptoehanol, 40 mM KCl, 0.1 mg/ml BSA) as described.61 5–10 pmol of RNAP II was incubated with 7.5–15 pmols of the pre- annealed RNA-DNA hybrid in 25–50-μl volume for 10 min at room temperature. Next, 15–50 pmols of the non-template DNA strand were added for 10 min. The immobilized TECs were washed with TB containing 1M KCl to remove the free RNA and DNA. The TECs were eluted from Ni2+-NTA agarose by 100 mM imidazole as described previously23 and diluted with TB + 10 mM 2-mercaptoehanol to bring the imidazole concentration below 10 mM and to maintain TFIIS activity. The efficiency of the TEC assembly is ~10% in the optimal condition, and the typical concentration of TEC is approximately 1 nM. For TEC12A on Template 1, TB without MgCl2 was used for the above process to avoid cleavage of the transcript. All reactions were performed in TB + 10 mM 2-mercaptoehanol at 25°C. The reactions were stopped by gel-loading buffer (5 M urea; 25 mM EDTA final concentrations). The reaction for shorter than 5 s were obtained using RQF3 rapid quench flow instruments (KinTek Corporation) as described previously.23 The RNA products were analyzed as described previously.23
Exonuclease III (ExoIII) footprinting.
The ExoIII footprinting was performed as described previously.23 For the rear-end footprinting, TECs were assembled on the 5’ end-labeled template DNA strand (TDS) and the unlabeled non-template DNA strand (NDS) (Supplementary Table SI). The labeled DNA strand is opposite for the front-end footprinting. The concentrations of TECs were similar to those of in vitro transcription. The reaction was started by mixing 15 μl TB + 10 mM 2-mercaptoehanol containing 10 units of Exonuclease III (New England Biolabs) with 15 μl of the elongation complex at 25°C. To prevent digestion of the NDS by ExoIII for the rear-end footprinting, the NDS carries phosphorothioate bond at the 3’ end. The 3’ modified strand is opposite for the front-end footprinting. The relative states of translocation of TECs were determined by shifting the boundaries of RNAP II due to stepwise extensions of RNA of TECs.23
Yeast phenotypic analysis.
Genotypes of S. cerevisiae strains used in this study were shown in Table SII. dst1D290A/E291A gene in the expression plasmid was cloned to pYES2.1 plasmid, and transformed to GRY2915 and GRY2769 harboring RPB1 and rpb1-T1095G genes, respectively. The dilution series of the strains carrying the plasmid was performed as described in the Figure legend.
Supplementary Material
Backtracking on DNA is a major mechanism for pausing of RNAP II.
Backtracking occurs at DNA sequences with high translocation barrier to RNAP II.
TFIISAA induces pauses by promoting formation of the pre-backtracking intermediate.
The intermediate is likely due to fraying of the 3’ RNA end from DNA template.
Acknowledgements
We thank Dr. Donald Court for critical reading of the manuscript. This research was supported by the Intramural Research Program at the NIH. The contents of this publication do not necessarily reveal the views of the policy of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Abbreviations used:
- RNAP II
RNA polymerase II
- TEC
ternary elongation complex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maiuri P, Knezevich A, De Marco A, Mazza D, Kula A, McNally JG & Marcello A (2011). Fast transcription rates of RNA polymerase II in human cells. EMBO reports 12, 1280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardehali MB & Lis JT (2009). Tracking rates of transcription and splicing in vivo. Nature structural & molecular biology 16, 1123–4. [DOI] [PubMed] [Google Scholar]
- 3.Kireeva M, Kashlev M & Burton ZF (2010). Translocation by multi-subunit RNA polymerases. Biochim Biophys Acta. [DOI] [PubMed] [Google Scholar]
- 4.Gelles J & Landick R (1998). RNA polymerase as a molecular motor. Cell 93, 13–6. [DOI] [PubMed] [Google Scholar]
- 5.Uptain SM, Kane CM & Chamberlin MJ (1997). Basic mechanisms of transcript elongation and its regulation. Annu Rev Biochem 66, 117–72. [DOI] [PubMed] [Google Scholar]
- 6.Bai L, Shundrovsky A & Wang MD (2004). Sequence-dependent kinetic model for transcription elongation by RNA polymerase. J Mol Biol 344, 335–49. [DOI] [PubMed] [Google Scholar]
- 7.Julicher F & Bruinsma R (1998). Motion of RNA polymerase along DNA: a stochastic model. Biophys J 74, 1169–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galburt EA, Grill SW, Wiedmann A, Lubkowska L, Choy J, Nogales E, Kashlev M & Bustamante C (2007). Backtracking determines the force sensitivity of RNAP II in a factor-dependent manner. Nature 446, 820–3. [DOI] [PubMed] [Google Scholar]
- 9.Shaevitz JW, Abbondanzieri EA, Landick R & Block SM (2003). Backtracking by single RNA polymerase molecules observed at near-base-pair resolution. Nature 426, 684–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komissarova N & Kashlev M (1997). Transcriptional arrest: Escherichia coli RNA polymerase translocates backward, leaving the 3’ end of the RNA intact and extruded. Proc. Natl. Acad. Sci 94, 1755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palangat M & Landick R (2001). Roles of RNA:DNA hybrid stability, RNA structure, and active site conformation in pausing by human RNA polymerase II. J Mol Biol 311, 265–82. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Bushnell DA, Huang X, Westover KD, Levitt M & Kornberg RD (2009). Structural basis of transcription: backtracked RNA polymerase II at 3.4 angstrom resolution. Science 324, 1203–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keene RG, Mueller A, Landick R & London L (1999). Transcriptional pause, arrest and termination sites for RNA polymerase II in mammalian N- and c-myc genes. Nucleic Acids Res 27, 3173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Awrey DE, Weilbaecher RG, Hemming SA, Orlicky SM, Kane CM & Edwards AM (1997). Transcription elongation through DNA arrest sites. A multistep process involving both RNA polymerase II subunit RPB9 and TFIIS. J Biol Chem 272, 14747–54. [DOI] [PubMed] [Google Scholar]
- 15.Borukhov S, Sagitov V & Goldfarb A (1993). Transcript cleavage factors from E. coli. Cell. 72, 459–66. [DOI] [PubMed] [Google Scholar]
- 16.Izban MG & Luse DS (1992). The RNA polymerase II ternary complex cleaves the nascent transcript in a 3’----5’ direction in the presence of elongation factor SII. Genes Dev 6, 1342–56. [DOI] [PubMed] [Google Scholar]
- 17.Reines D, Ghanouni P, Li QQ & Mote J Jr. (1992). The RNA polymerase II elongation complex. Factor-dependent transcription elongation involves nascent RNA cleavage. J Biol Chem 267, 15516–22. [PMC free article] [PubMed] [Google Scholar]
- 18.Komissarova N & Kashlev M (1997). RNA polymerase switches between inactivated and activated states By translocating back and forth along the DNA and the RNA. J Biol Chem 272, 15329–38. [DOI] [PubMed] [Google Scholar]
- 19.Kettenberger H, Armache KJ & Cramer P (2003). Architecture of the RNA polymerase II-TFIIS complex and implications for mRNA cleavage. Cell 114, 347–57. [DOI] [PubMed] [Google Scholar]
- 20.Sigurdsson S, Dirac-Svejstrup AB & Svejstrup JQ (2010). Evidence that transcript cleavage is essential for RNA polymerase II transcription and cell viability. Mol Cell 38, 202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kettenberger H, Armache KJ & Cramer P (2004). Complete RNA polymerase II elongation complex structure and its interactions with NTP and TFIIS. Mol Cell 16, 955–65. [DOI] [PubMed] [Google Scholar]
- 22.Cheung AC & Cramer P (2011). Structural basis of RNA polymerase II backtracking, arrest and reactivation. Nature 471, 249–53. [DOI] [PubMed] [Google Scholar]
- 23.Kireeva ML, Nedialkov YA, Cremona GH, Purtov YA, Lubkowska L, Malagon F, Burton ZF, Strathern JN & Kashlev M (2008). Transient reversal of RNA polymerase II active site closing controls fidelity of transcription elongation. Mol Cell 30, 557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Bushnell DA, Westover KD, Kaplan CD & Kornberg RD (2006). Structural basis of transcription: role of the trigger loop in substrate specificity and catalysis. Cell. 127, 941–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bar-Nahum G, Epshtein V, Ruckenstein AE, Rafikov R, Mustaev A & Nudler E (2005). A ratchet mechanism of transcription elongation and its control. Cell. 120, 183–93. [DOI] [PubMed] [Google Scholar]
- 26.Vassylyev DG, Vassylyeva MN, Zhang J, Palangat M, Artsimovitch I & Landick R (2007). Structural basis for substrate loading in bacterial RNA polymerase. Nature. 448, 163–8. [DOI] [PubMed] [Google Scholar]
- 27.Kireeva M, Nedialkov YA, Gong XQ, Zhang C, Xiong Y, Moon W, Burton ZF & Kashlev M (2009). Millisecond phase kinetic analysis of elongation catalyzed by human, yeast, and Escherichia coli RNA polymerase. Methods 48, 333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuman KC, Abbondanzieri EA, Landick R, Gelles J & Block SM (2003). Ubiquitous transcriptional pausing is independent of RNA polymerase backtracking. Cell 115, 437–47. [DOI] [PubMed] [Google Scholar]
- 29.Herbert KM, La Porta A, Wong BJ, Mooney RA, Neuman KC, Landick R & Block SM (2006). Sequence-resolved detection of pausing by single RNA polymerase molecules. Cell 125, 1083–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Artsimovitch I, Chu C, Lynch AS & Landick R (2003). A new class of bacterial RNA polymerase inhibitor affects nucleotide addition. Science 302, 650–4. [DOI] [PubMed] [Google Scholar]
- 31.Walmacq C, Cheung AC, Kireeva ML, Lubkowska L, Ye C, Gotte D, Strathern JN, Carell T, Cramer P & Kashlev M (2012). Mechanism of translesion transcription by RNA polymerase II and its role in cellular resistance to DNA damage. Mol Cell 46, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kireeva ML, Domecq C, Coulombe B, Burton ZF & Kashlev M (2011). Interaction of RNA polymerase II fork loop 2 with downstream non-template DNA regulates transcription elongation. The Journal of biological chemistry 286, 30898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komissarova N, Becker J, Solter S, Kireeva M & Kashlev M (2002). Shortening of RNA:DNA hybrid in the elongation complex of RNA polymerase is a prerequisite for transcription termination. Mol Cell 10, 1151–62. [DOI] [PubMed] [Google Scholar]
- 34.Johnson RS, Strausbauch M, Cooper R & Register JK (2008). Rapid kinetic analysis of transcription elongation by Escherichia coli RNA polymerase. J Mol Biol 381, 1106–13. [DOI] [PubMed] [Google Scholar]
- 35.Kashkina E, Anikin M, Tahirov TH, Kochetkov SN, Vassylyev DG & Temiakov D (2006). Elongation complexes of Thermus thermophilus RNA polymerase that possess distinct translocation conformations. Nucleic Acids Res 34, 4036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaplan CD, Larsson KM & Kornberg RD (2008). The RNA polymerase II trigger loop functions in substrate selection and is directly targeted by alpha-amanitin. Mol Cell 30, 547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West RW Jr., Yocum RR & Ptashne M (1984). Saccharomyces cerevisiae GAL1-GAL10 divergent promoter region: location and function of the upstream activating sequence UASG. Mol Cell Biol 4, 2467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Awrey DE, Shimasaki N, Koth C, Weilbaecher R, Olmsted V, Kazanis S, Shan X, Arellano J, Arrowsmith CH, Kane CM & Edwards AM (1998). Yeast transcript elongation factor (TFIIS), structure and function. II: RNA polymerase binding, transcript cleavage, and read-through. The Journal of biological chemistry 273, 22595–605. [DOI] [PubMed] [Google Scholar]
- 39.Vale RD & Oosawa F (1990). Protein motors and Maxwell’s demons: does mechanochemical transduction involve a thermal ratchet? Advances in biophysics 26, 97–134. [DOI] [PubMed] [Google Scholar]
- 40.Hein PP, Palangat M & Landick R (2011). RNA transcript 3’-proximal sequence affects translocation bias of RNA polymerase. Biochemistry 50, 7002–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yager TD & von Hippel PH (1991). A thermodynamic analysis of RNA transcript elongation and termination in Escherichia coli. Biochemistry 30, 1097–118. [DOI] [PubMed] [Google Scholar]
- 42.Landick R (2006). The regulatory roles and mechanism of transcriptional pausing. Biochem Soc Trans 34, 1062–6. [DOI] [PubMed] [Google Scholar]
- 43.Artsimovitch I & Landick R (2000). Pausing by bacterial RNA polymerase is mediated by mechanistically distinct classes of signals. Proc Natl Acad Sci U S A 97, 7090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Artsimovitch I & Landick R (1998). Interaction of a nascent RNA structure with RNA polymerase is required for hairpin-dependent transcriptional pausing but not for transcript release. Genes Dev 12, 3110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toulokhonov I, Zhang J, Palangat M & Landick R (2007). A central role of the RNA polymerase trigger loop in active-site rearrangement during transcriptional pausing. Mol Cell 27, 406–19. [DOI] [PubMed] [Google Scholar]
- 46.Davenport RJ, Wuite GJ, Landick R & Bustamante C (2000). Single-molecule study of transcriptional pausing and arrest by E. coli RNA polymerase. Science 287, 2497–500. [DOI] [PubMed] [Google Scholar]
- 47.Malinen AM, Turtola M, Parthiban M, Vainonen L, Johnson MS & Belogurov GA (2012). Active site opening and closure control translocation of multisubunit RNA polymerase. Nucleic acids research 40, 7442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laptenko O, Lee J, Lomakin I & Borukhov S (2003). Transcript cleavage factors GreA and GreB act as transient catalytic components of RNA polymerase. Embo J 22, 6322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gnatt AL, Cramer P, Fu J, Bushnell DA & Kornberg RD (2001). Structural basis of transcription: an RNA polymerase II elongation complex at 3.3 A resolution. Science. 292, 1876–82. [DOI] [PubMed] [Google Scholar]
- 50.Sydow JF, Brueckner F, Cheung AC, Damsma GE, Dengl S, Lehmann E, Vassylyev D & Cramer P (2009). Structural basis of transcription: mismatch-specific fidelity mechanisms and paused RNA polymerase II with frayed RNA. Mol Cell 34, 710–21. [DOI] [PubMed] [Google Scholar]
- 51.Damsma GE & Cramer P (2009). Molecular basis of transcriptional mutagenesis at 8-oxoguanine. The Journal of biological chemistry 284, 31658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sidorenkov I, Komissarova N & Kashlev M (1998). Crucial role of the RNA:DNA hybrid in the processivity of transcription. Mol Cell 2, 55–64. [DOI] [PubMed] [Google Scholar]
- 53.Kireeva ML, Komissarova N, Waugh DS & Kashlev M (2000). The 8-nucleotide-long RNA:DNA hybrid is a primary stability determinant of the RNA polymerase II elongation complex. J Biol Chem 275, 6530–6. [DOI] [PubMed] [Google Scholar]
- 54.Komissarova N, Kireeva ML, Becker J, Sidorenkov I & Kashlev M (2003). Engineering of elongation complexes of bacterial and yeast RNA polymerases. Methods Enzymol 371, 233–51. [DOI] [PubMed] [Google Scholar]
- 55.Shaw NN & Arya DP (2008). Recognition of the unique structure of DNA:RNA hybrids. Biochimie 90, 1026–39. [DOI] [PubMed] [Google Scholar]
- 56.Toulme F, Guerin M, Robichon N, Leng M & Rahmouni AR (1999). In vivo evidence for back and forth oscillations of the transcription elongation complex. Embo J 18, 5052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Churchman LS & Weissman JS (2011). Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature 469, 368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bintu L, Kopaczynska M, Hodges C, Lubkowska L, Kashlev M & Bustamante C (2011). The elongation rate of RNA polymerase determines the fate of transcribed nucleosomes. Nature structural & molecular biology 18, 1394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kireeva ML, Hancock B, Cremona GH, Walter W, Studitsky VM & Kashlev M (2005). Nature of the nucleosomal barrier to RNA polymerase II. Mol Cell 18, 97–108. [DOI] [PubMed] [Google Scholar]
- 60.Luse DS & Studitsky VM (2011). The mechanism of nucleosome traversal by RNA polymerase II: roles for template uncoiling and transcript elongation factors. RNA biology 8, 581–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kireeva ML, Lubkowska L, Komissarova N & Kashlev M (2003). Assays and affinity purification of biotinylated and nonbiotinylated forms of double-tagged core RNA polymerase II from Saccharomyces cerevisiae. Methods Enzymol 370, 138–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


