Abstract
Background
Coronavirus disease 2019 (COVID-19) has emerged as a major global health threat with a great number of deaths worldwide. Acute kidney injury (AKI) is a common complication in patients admitted to the intensive care unit. We aimed to assess the incidence, risk factors and in-hospital outcomes of AKI in COVID-19 patients admitted to the intensive care unit.
Methods
We conducted a retrospective observational study in the intensive care unit of Tongji Hospital, which was assigned responsibility for the treatments of severe COVID-19 patients by the Wuhan government. AKI was defined and staged based on Kidney Disease: Improving Global Outcomes (KDIGO) criteria. Mild AKI was defined as stage 1, and severe AKI was defined as stage 2 or stage 3. Logistic regression analysis was used to evaluate AKI risk factors, and Cox proportional hazards model was used to assess the association between AKI and in-hospital mortality.
Results
A total of 119 patients with COVID-19 were included in our study. The median patient age was 70 years (interquartile range, 59–77) and 61.3% were male. Fifty-one (42.8%) patients developed AKI during hospitalization, corresponding to 14.3% in stage 1, 28.6% in stage 2 and 18.5% in stage 3, respectively. Compared to patients without AKI, patients with AKI had a higher proportion of mechanical ventilation mortality and higher in-hospital mortality. A total of 97.1% of patients with severe AKI received mechanical ventilation and in-hospital mortality was up to 79.4%. Severe AKI was independently associated with high in-hospital mortality (OR: 1.82; 95% CI: 1.06–3.13). Logistic regression analysis demonstrated that high serum interleukin-8 (OR: 4.21; 95% CI: 1.23–14.38), interleukin-10 (OR: 3.32; 95% CI: 1.04–10.59) and interleukin-2 receptor (OR: 4.50; 95% CI: 0.73–6.78) were risk factors for severe AKI development.
Conclusions
Severe AKI was associated with high in-hospital mortality, and inflammatory response may play a role in AKI development in critically ill patients with COVID-19.
Keywords: Coronavirus disease 2019, Acute kidney injury, Mortality, Risk factor
Introduction
Coronavirus disease 2019 (COVID-19) is a respiratory tract infection caused by a newly emergent coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; previously known as 2019-nCoV) [1, 2]. COVID-19 has swept into more than 100 countries and more than 2 million cases have been confirmed within a few months. While most patients with COVID-19 develop mild or uncomplicated illness, it is reported that 14–16% develop severe disease requiring hospitalization and 5–27% hospitalized patients required admission to an intensive care unit [3, 4, 5, 6]. Thus, the COVID-19 pandemic significantly increased the burden of critical illness globally.
Studies showed that SARS-CoV-2 uses angiotensin-converting enzyme II (ACE2) as a cell entry receptor [7], which is highly expressed in lung tissues but also in human kidneys [8]. This suggests that lungs were not the only organ involved and the kidney would also be a possible target in COVID-19. Acute kidney injury (AKI) is a common, serious complication and is associated with poor outcome in critically ill patients [9, 10]. Previous studies reported that AKI developed in 0.5–5.1% of patients with COVID-19 [4, 11, 12]. However, details of the epidemiological characteristics and outcome of AKI in critically ill patients with COVID-19 have not yet been well described.
We conduct a retrospective study of COVID-19 patients admitted to the intensive care unit to assess the incidence and risk factors of AKI and its impact on in-hospital mortality.
Materials and Methods
Participants
This retrospective observational study was done at Tongji Hospital, which is located in Wuhan, Hubei Province, the major endemic area of COVID-19. Tongji Hospital was assigned responsibility for the treatments of severe COVID-19 patients by Wuhan government on January 31, 2020. From January 28 to March 29, 2020, we retrospectively analyzed patients who had been diagnosed with COVID-19 according to the guidance provided by the Chinese National Health Commission (version 7.0) [13], and who were admitted to the intensive care unit.
We excluded patients without serum creatinine on admission. Pediatric patients and patients with a history of maintenance dialysis or kidney transplantation were also excluded from the study. To avoid the inclusion of patients with ongoing AKI, we excluded patients with elevated serum creatinine on admission (online suppl. Fig. 1; see www.karger.com/doi/10.1159/000512270 for all online suppl. material). Clinical outcomes were monitored up to April 23, 2020, the final date of follow-up.
Data Collection
The demographic characteristics, laboratory data and outcome were extracted from electronic medical records. The admission data of these patients were collected. Laboratory data consisted of complete blood count, liver and kidney function, D-dimer, high-sensitivity C-reactive protein, procalcitonin, lactate dehydrogenase and cytokines. The data were reviewed by a trained team of physicians. eGFR was calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [14]. The Sequential Organ Failure Assessment (SOFA) score [15] and Acute Physiologic and Chronic Health Evaluation (APACHE) II score [16] were assessed on admission.
Definition
AKI was defined as an increase in serum creatinine by 0.3 mg/dL within 48 h or a 50% increase in serum creatinine from baseline within 7 days according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria [17]. Urine output criteria were not used to define AKI. Baseline serum creatinine was defined as the serum creatinine value on admission. The date of AKI onset was defined as the earliest day of a serum creatinine change meeting KDIGO criteria. The maximum stage of AKI was determined using the peak serum creatinine level after AKI detection, with increases of 1.5–1.9, 2.0–2.9 and ≥3 times of the baseline being defined as AKI stage 1, 2, and 3, respectively. Mild AKI was defined as stage 1, and severe AKI was defined as stage 2 or stage 3. Recovery from AKI was defined as a serum creatinine decrease below the level determined in the definition of stage 1 AKI [18].
Statistical Analysis
Categorical variables were summarized as n (%), and continuous variables were expressed as median with interquartile range (IQR). Mann-Whitney U test was used for continuous variables, and χ2 test or Fisher's exact test for categorical variables as appropriate. Kaplan-Meier survival curves and log-rank tests were used to describe the effect of the stage of AKI on in-hospital mortality. The association of AKI and in-hospital mortality was assessed with Cox proportional hazards model analysis. Adjusted variables were chosen on the basis of previous findings and significant risk factors (p < 0.05) for in-hospital mortality in univariable Cox proportional hazards model. Age and SOFA score were reported to be associated with in-hospital mortality [6, 19, 20]. In addition, previous studies have shown that the mortality was higher in male patients [21]. Univariable Cox proportional hazards model showed that SOFA score, platelet count and lactose dehydrogenase were associated with high in-hospital mortality (online suppl. Table 1). To explore the risk factors associated with severe AKI, univariable logistic regression models were used. We excluded variables if the number of events was too small to estimate odds ratios. No imputation was made for missing data. Categorization was performed for continuous variables, as it is easier to interpret and also for the simplicity of reporting results. For common laboratory values, we used the cutoff points which were widely recognized and adopted in clinical practice. The median was used as cutoff points for inflammation-related cytokines. Statistical analyses were performed using SPSS, version 22.0, with statistical significance set at 2-sided p < 0.05.
Results
Patient Characteristics
A total of 119 patients with COVID-19 admitted to the intensive care unit were included in our study. Demographic and clinical characteristics are summarized in Table 1. The median age of the patients was 70 years (IQR, 59–77) and most were male (61.3%). A total of 58.0% of patients had at least one comorbidity, the most common were hypertension (41.2%) and diabetes (25.2%). The median APACHE II score of all patients was 9 (IQR, 6–13), and the median of the SOFA score was 3 (IQR, 2–4).
Table 1.
Demographic characteristics, laboratory data and outcomes of critically ill COVID-19 patients
| All patients (n = 119) |
Non-AKI (n = 68) |
Mild AKI (n = 17) |
Severe AKI (n = 34) |
|||||
|---|---|---|---|---|---|---|---|---|
| n | summary | n | summary | n | summary | n | summary | |
| Clinical characteristics | ||||||||
| Age, years | 119 | 70 (59–77) | 68 | 70(55–80) | 17 | 66 (57–74) | 34 | 71 (65–78) |
| Male patients, % | 119 | 73 (61.3) | 68 | 40 (58.8) | 17 | 11 (64.7) | 34 | 22 (64.7) |
| Any comorbidity, % | 119 | 69 (58.0) | 68 | 36 (52.9) | 17 | 12 (70.6) | 34 | 21 (61.8) |
| Chronic lung disease, % | 119 | 10 (8.4) | 68 | 4 (5.9) | 17 | 3 (17.6) | 34 | 3 (8.8) |
| Diabetes, % | 119 | 30 (25.2) | 68 | 15 (22.1) | 17 | 6 (35.3) | 34 | 9 (26.5) |
| Hypertension, % | 119 | 49 (41.2) | 68 | 26 (38.2) | 17 | 8 (47.1) | 34 | 15 (44.1) |
| Tumor, % | 119 | 7 (5.9) | 68 | 5 (7.4) | 17 | 0 (0) | 34 | 2 (5.9) |
| APACHE II score | 119 | 9 (6–13) | 68 | 9 (6–15) | 17 | 9 (6–12) | 34 | 7 (6–12) |
| SOFA score | 119 | 3 (2–4) | 68 | 3 (2–4) | 17 | 3 (1–4) | 34 | 3 (2–5) |
|
| ||||||||
| Laboratory data | ||||||||
| Leukocyte count, ×109/L | 119 | 8.5 (6.2–13) | 68 | 8.8 (6.2–13.2) | 17 | 9.1 (5.5–16.4) | 34 | 8.1 (7.1–11.0) |
| Lymphocyte count, ×109/L | 119 | 0.7 (0.5–0.9) | 68 | 0.7 (0.5–0.9) | 17 | 0.7 (0.4–0.9) | 34 | 0.7 (0.4–0.9) |
| Platelet count, ×109/L | 119 | 170 (125–263) | 68 | 172 (127–271) | 17 | 150 (123–279) | 34 | 173 (118–252) |
| Total bilirubin, µmol/L | 119 | 11.8 (8.6–18.6) | 68 | 11.8 (8.5–18.9) | 17 | 10.4 (7.1–15.5) | 34 | 12.3 (10.2–17.1) |
| d-dimer, mg/L | 113 | 2.8 (1.3–15.8) | 66 | 2.7 (1.4–14.8) | 17 | 4.6 (1.1–21.0) | 30 | 3.1 (1.4–15.8) |
| Procalcitonin, ng/mL | 98 | 0.2 (0.1–0.3) | 59 | 0.2 (0.1–0.4) | 14 | 0.2 (0.1–0.3) | 25 | 0.1 (0.1–0.3) |
| hs-CRP, mg/L | 110 | 74 (38–129) | 62 | 74 (30–149) | 17 | 65 (35–111) | 31 | 93 (63–121) |
| Lactose dehydrogenase, U/L | 119 | 456 (307–570) | 68 | 431 (305–538) | 17 | 461 (292–704) | 34 | 466 (348–592) |
| Serum creatinine, µmol/L | 119 | 69 (54–85) | 68 | 68 (50–85) | 17 | 74 (58–89) | 34 | 68 (56–82) |
| Interleukin-6, pg/mL | 79 | 40 (14–99) | 51 | 37 (14–115) | 11 | 19 (9–43) | 17 | 62 (31–119) |
| Interleukin-8, pg/mL | 79 | 24 (15–48) | 51 | 23 (14–42) | 11 | 17 (10–28) | 17 | 55 (24–93) |
| Interleukin-10, pg/mL | 79 | 8.6 (5–13.5) | 51 | 8.2 (5.0–11.1) | 11 | 5.0 (5.0–13.5) | 17 | 10.5 (7.2–15.3) |
| Interleukin-2 receptor, U/mL | 79 | 994 (644–1,266) | 51 | 944 (496–1,431) | 11 | 866 (378–1,079) | 17 | 1,087 (976–1,317) |
| Tumor necrosis factor α, pg/mL | 79 | 9.5 (7.1–12.6) | 51 | 9.3 (7.2–13.2) | 11 | 8.3 (6.7–9.6) | 17 | 10.7 (7.3–13.3) |
|
| ||||||||
| Outcomes | ||||||||
| Mechanical ventilation, % | 119 | 106 (89.1) | 68 | 56 (82.4) | 17 | 17 (100) | 34 | 33 (97.1) |
| Non-invasive, % | 119 | 94 (79.0) | 68 | 49 (72.1) | 17 | 16 (94.1) | 34 | 29 (85.3) |
| Invasive, % | 119 | 63 (52.9) | 68 | 30 (44.1) | 17 | 10 (58.8) | 34 | 23 (67.6) |
| Hospital length of stay, days | 119 | 22 (13–42) | 68 | 21 (9–38) | 17 | 38 (18–55) | 34 | 20 (13–33) |
| 14-day mortality, % | 119 | 35 (29.4) | 68 | 20 (29.4) | 17 | 3 (17.6) | 34 | 12 (35.3) |
| 28-day mortality, % | 119 | 57 (47.9) | 68 | 27 (39.7) | 17 | 8 (47.1) | 34 | 22 (64.7) |
| In-hospital mortality, % | 119 | 67 (56.3) | 68 | 31 (45.6) | 17 | 9 (52.9) | 34 | 27 (79.4) |
AKI, acute kidney injury; COVID-19, coronavirus disease 2019; APACHE, acute physiologic and chronic health evaluation; SOFA, sequential organ failure assessment; hs-CRP, high-sensitivity C-reactive protein.
AKI and In-Hospital Outcomes
During hospitalization, AKI developed in 51 (42.8%) patients, corresponding to 14.3% of mild AKI (stage 1) and 28.6% severe AKI (10.1% in stage 2 and 18.5% in stage 3, respectively). The median duration from admission to AKI occurrence was 17 days (IQR, 9–28). During hospitalization, 41.2% of patients with AKI received kidney replacement therapy. The 28-day mortality and in-hospital mortality of critically ill patients with COVID-19 were 47.9 and 56.3%, respectively. The median hospital length of stay was 22 days (IQR, 13–42). Of the 15 patients who developed AKI and survived to discharge, 13 (86.7%) patients recovered from AKI.
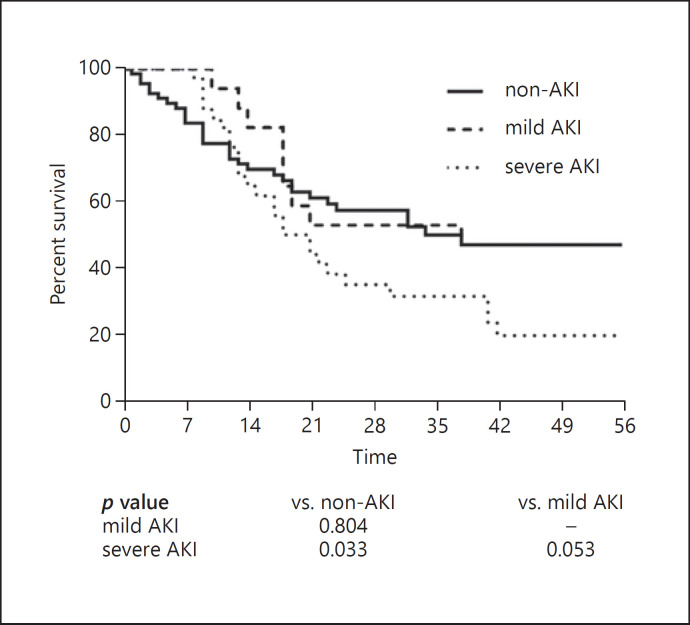
Compared to patients without AKI, patients with AKI had a higher proportion of receiving mechanical ventilation, higher in-hospital mortality and longer hospital length of stay. Notably, 97.1% of patients with severe AKI received mechanical ventilation and the in-hospital mortality was up to 79.4%. The Kaplan-Meier curve showed a diversion of the survival rates between severe AKI and non-AKI, while no significant difference was observed in mild AKI and non-AKI (Fig. 1). Likewise, Cox proportional hazards model demonstrated that only severe AKI was associated with in-hospital mortality, and the association remained significant after adjustment for confounders (OR: 1.82; 95% CI: 1.06–3.13) (Table 2).
Fig. 1.
Survival curves according to severity of AKI in critically ill COVID-19 patients.
Table 2.
Association of AKI and in-hospital mortality in critically ill COVID-19 patients
| Unadjusted |
Adjusted |
||||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| 28-Days mortality | |||||
| Non-AKI Mild AKI Severe AKI |
1.00 (reference) 1.35 (0.46–3.93) 2.78 (l.18–6.55) |
0.582 0.019 |
1.00 (reference) 0.87 (0.38–1.99) 1.68 (0.93–3.02) |
0.745 0.086 |
|
|
| |||||
| In-hospital mortality | |||||
| Non-AKI Mild AKI Severe AKI |
1.00 (reference) 1.34 (0.46–3.90) 4.60 (1.77–12.01) |
0.588 0.002 |
1.00 (reference) 0.89 (0.41–1.91) 1.82 (1.06–3.13) |
0.761 0.029 |
|
AKI, acute kidney injury; COVID-19, coronavirus disease 2019; HR, hazard ratio; 95% CI, 95% confidence interval. HRs were obtained in multivariate Cox proportional hazards model after adjustment for age, sex, hypertension, sequential organ failure assessment (SOFA) score, platelet count and lactose dehydrogenase.
Risk Factors for AKI
Compared to patients without AKI, patients with AKI had a higher proportion of comorbidities, including diabetes and hypertension. In addition, patients with AKI showed higher D-dimer, high-sensitivity C-reactive protein and lactose dehydrogenase (Table 1). We further analyzed the level of serum cytokines, which was available in 82 patients in our study. Moreover, nearly all clinical characteristics, laboratory data and in-hospital mortality in 82 patients with available data on cytokines were similar to those of the 37 patients with missing data on cytokines (online suppl. Table 2). We found that the cytokines on admission were similar between mild AKI and non-AKI, while interleukin (IL)-6, IL8, IL10 and IL2R on admission was notably higher in patients with severe AKI.
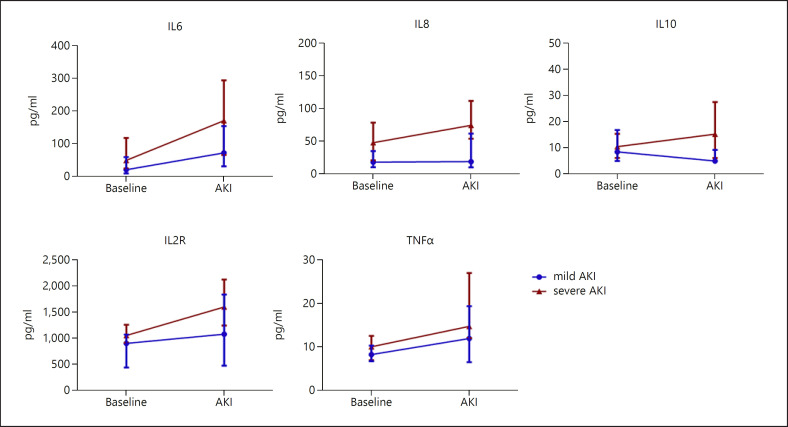
Given that the in-hospital mortality was remarkable increased in patients with severe AKI but not in mild AKI, we further to explore the risk factors for severe AKI in patients with COVID-19 and admitted to the intensive care unit. Among the clinical characteristics, common laboratory data and cytokines, we found that only higher IL8 (OR: 4.21; 95% CI: 1.23–14.38), IL10 (OR: 3.32; 95% CI: 1.04–10.59) and IL2R (OR: 4.50, 95% CI: 0.73–6.78) were associated with severe AKI development in univariate logistic regression (Table 3). In addition, we described the trends of cytokines in patients with AKI. We found that cytokines were markedly increased, and patients with severe AKI tended to have a higher level of IL6, IL10 and IL2R than patients with mild AKI when AKI occurred (Fig. 2).
Table 3.
Univariable logistic regression analysis of risk factors for severe AKI in critically ill COVID-19 patients
| Variables | OR | 95% CI | p value |
|---|---|---|---|
| Age (≥65 vs. <65 years) | 1.94 | 0.81–4.67 | 0.137 |
| Sex (male vs. female) | 1.22 | 0.54–2.79 | 0.634 |
| Diabetes (yes vs. no) | 1.10 | 0.44–2.72 | 0.841 |
| Hypertension (yes vs. no) | 1.18 | 0.53–2.65 | 0.680 |
| APACHE II score | 0.95 | 0.88–1.02 | 0.162 |
| SOFA score | 1.01 | 0.86–1.18 | 0.907 |
| Leukocyte count × 109/L (>10 vs. ≤10) | 0.74 | 0.32–1.72 | 0.481 |
| Lymphocyte count × 109/L (<1.0 vs. ≥1.0) | 0.87 | 0.30–2.51 | 0.792 |
| Platelet count × 109/L (<100 vs. ≥100) | 0.85 | 0.28–2.58 | 0.774 |
| Total bilirubin, µmol/L (>20 vs. ≤20) | 0.92 | 0.33–2.60 | 0.881 |
| D-dimer, mg/L (≥0.5 vs. <0.5) | 0.46 | 0.10–2.17 | 0.323 |
| Procalcitonin, ng/mL (≥0.5 vs. <0.5) | 0.49 | 0.10–2.38 | 0.377 |
| Lactose dehydrogenase, U/L (>245 vs. ≤245) | 1.48 | 0.45–4.86 | 0.519 |
| hs-CRP, mg/L (>74 vs. ≤74) | 1.31 | 0.57–3.02 | 0.525 |
| Interleukin-6, pg/mL (>44 vs. ≤44) | 2.91 | 0.92–9.27 | 0.070 |
| Interleukin-8, pg/mL (>24 vs. ≤24) | 4.21 | 1.23–14.38 | 0.022 |
| Interleukin-10, pg/mL (>9 vs. ≤9) | 3.32 | 1.04–10.59 | 0.042 |
| Interleukin-2 receptor, U/mL (>1,049 vs. ≤1,049) | 4.50 | 1.32–15.38 | 0.016 |
| Tumor necrosis factor α, pg/mL (>10 vs. ≤10) | 2.23 | 0.73–6.78 | 0.159 |
AKI, acute kidney injury; COVID-19, coronavirus disease 2019; OR, odds ratio; 95% CI, 95% confidence interval. APACHE, acute physiologic and chronic health evaluation; SOFA, sequential organ failure assessment, hs-CRP, high-sensitivity C-reactive protein.
Fig. 2.
Trends of serum cytokines in critically ill COVID-19 patients with AKI. Data were expressed as median with interquartile range.
Medications
We summarized the medications during hospitalization in Table 4. Most critically ill patients with COVID-19 received treatment with antibiotics (92.4%), antivirus (73.9%) and glucocorticoid (74.8%). The proportion of treatment with anti-diabetes and diuretics was higher in patients with AKI. In addition, few patients (5.0%) received angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker.
Table 4.
Treatment during hospitalization in critically ill COVID-19 patients
| All patients n = 119 | Non-AKI n = 68 | Mild AKI n = 17 | Severe AKI n = 34 | |
|---|---|---|---|---|
| ACEI or ARB, n (%) | 6 (5) | 4 (5.9) | 2 (11.8) | 0 |
| Antibiotics, n (%) | 110 (92.4) | 62 (91.2) | 16 (94.1) | 32 (94.1) |
| Antivirus, n (%) | 88 (73.9) | 51 (75) | 13 (76.5) | 24 (70.6) |
| Antidiabetic, n (%) | 41 (34.5) | 19 (27.9) | 6 (35.3) | 16 (47.1) |
| Diuretic, n (%) | 78 (65.5) | 38 (55.9) | 13 (76.5) | 27 (79.4) |
| Glucocorticoid, n (%) | 89 (74.8) | 50 (73.5) | 10 (58.8) | 29 (85.3) |
AKI, acute kidney injury; COVID-19, coronavirus disease 2019; ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin-receptor blocker.
Discussion
In this cohort of 150 critically ill patients with COVID-19, about half (42.8%) of the patients developed AKI during hospitalization, and most of whom (66.7%) had severe AKI. Patients with severe AKI had extremely high in-hospital mortality (79.4%). After adjustment for confounders, severe AKI was independently associated with in-hospital mortality. In addition, patients with a high level of serum cytokines were more likely to develop severe AKI during hospitalization.
AKI occurred frequently in critically ill patients with COVID-19. However, previous studies reported that the incidence of AKI varies from 8.9–28.8% [12, 22, 23], which was much lower than 42.8% reported in our study. This variation probably results from differences in the case numbers, illness severity, and coexisting conditions. Another major reason was that we retrospectively recorded the AKI occurrence during the entire hospitalization, and the previous studies were cross-sectional [23] or had a short follow-up duration [12, 22]. In addition, it is reported that the incidence of AKI in critically ill patients was 58.0% in MERS [24] and 61% in septic shock patients [25]. Therefore, the actual incidence might be higher considering that some AKI might be missed in our study. In accordance with the recent reports on the outcomes of COVID-19 patients, 5–26% of patients will be admitted to the intensive care unit during hospitalization [4, 5, 6]. Given that the number of COVID-19 cases is enormous and growing rapidly, there would be a substantial burden of medical care from AKI during the treatment of COVID-19. Therefore, plans should be made at local and regional levels for how to prepare and manage the potential surge in the need for critical care resources for AKI, including kidney replacement treatment.
Our result demonstrated that the median of hospital length of stay was 22 days and the median duration from admission to AKI occurrence was 17 days, which suggested that most AKI cases occurred in the late stage of hospitalization. Studies showed that ACE2, as a receptor for SARS-CoV-2, was highly expressed in renal tubular epithelium [8]. In addition, SARS viral particles were detected in the epithelium of the renal distal tubules [26]. Thus, AKI development in the early stage may be associated with SARS-COV-2 infection. However, AKI in the late stage may be due to the aggravation of respiratory failure and consequently sepsis, cytokine storm, shock and acidosis.
In our study, patients with AKI demonstrated an extremely poor prognosis. Of note, the mortality of severe AKI was 79.5%, which was much higher than those who developed ARDS [19]. Likewise, the reported mortality of AKI in patients with SARS was up to 91.7% [27]. One possible explanation is that the development of AKI may indicate the involvement of multiple organ dysfunction. In addition, AKI may worsen lung injury via several mechanisms including impaired fluid excretion, direct capillary endothelial injury, and exacerbating inflammatory response [28]. Hence, in order to detect the AKI timely and reduce the deaths, closer monitoring of serum creatinine and urine output is needed in the management of critically ill patients with COVID-19. Moreover, our findings showed that only severe AKI but not mild AKI was associated with in-hospital mortality. Therefore, a clear escalation plan on individualized treatment should be made depending on the severity of AKI.
The increased cytokines might be a predictor of severe AKI development in critically ill patients with COVID-19. Accumulating evidence suggests that cytokine release syndrome plays a role in severe COVID-19 [29]. It is reported that an inflammation-related cytokine profile, characterized by IL2, IL2R, IL-8, IL-10 and tumor necrosis factor-α (TNFα), was associated with COVID-19 disease severity [30, 31]. Our study was the first to confirm the high levels of IL8, IL10 and IL2R were associated with a high risk of severe AKI development in critically ill patients with COVID-19. In addition, we found that cytokines were increased remarkably when AKI occurred. Although kidney tissues from postmortems showed that AKI might be attributed to reduced kidney perfusion and widespread tubular necrosis [32], our results suggested that more complex and subtle mechanisms of immune-mediated microvascular and tubular dysfunction were involved in AKI in critically ill patients with COVID-19. Moreover, inhibiting the excessive inflammatory response in its early stage through such means as immunomodulators and cytokine antagonists, as well as continuous kidney replacement therapy, might be breakthroughs in the treatment of critically ill patients with COVID-19.
Some conventional risk factors for AKI in critically ill patients, including age and severity of the disease assessed by SOFA score, were not identified in our study. One possible explanation is that the Tongji Hospital was one of the designated hospitals for COVID-19 patients and the study population was relatively homogeneous with respect to age and disease severity. Most patients included in our study were the elderly, with a median age of 70 years. Furthermore, almost all patients were admitted to the intensive care unit due to the respiratory failure; thus, the SOFA score on admission was lower than in studies that included patients with different causes and multiple organ dysfunction [33]. In addition, our study was performed in a single center and the generalizability of the study is limited by the small sample size. Further study in a larger cohort is needed to gain a better understanding of risk factors for AKI in critically ill COVID-19 patients.
We also summarized the medications during the hospitalization in critically ill patients with COVID-19. Our result showed that most patients have been treated with antibiotics and antivirus, which might induce and worsen AKI. Thus, the appropriate dosage of antibiotics and antivirus should be considered based on kidney function in clinical practice. In addition, we found that patients with AKI had a high proportion of treatment with diuretics, which are often applied to control hypervolemia and prevent pulmonary edema in critically ill patients [34]. Previous studies showed that the use of diuretics in critically ill patients with AKI was associated with an increased risk of death and nonrecovery of kidney function [35]. Therefore, diuretics should be used with caution in the management of critically ill patients with COVID-19. However, due to the small number of patients and the bias in a different therapy of COVID-19 patients, the causal relationship between medications and AKI in critically ill COVID-19 patients remains undetermined.
Our study has several limitations. First, an accurate baseline serum creatinine and urine output was not available, which may have led to an underestimation of AKI or erroneous associations. Second, our study has a retrospective observational design with its inherent biases and potential for unmeasured confounding variables. Finally, this investigation was conducted in a medical intensive care unit of a tertiary referral center, potentially limiting the generalizability of our study results.
In conclusion, our study involving critically ill patients with COVID-19 showed that AKI is common and carries extremely high in-hospital mortality. Inflammatory response may play a role in AKI development. Clinicians should increase their awareness of AKI in critically ill patients with COVID-19.
Statement of Ethics
The study protocol and waived written informed consent were approved by the Medical Ethics Committee of Tongji Hospital (No. TJ-C20200132).
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
Funding Sources
This work was financially supported by the International (regional) Cooperation and Exchange Projects, (NSFC-DFG, Grant No. 81761138041); National Natural Science Foundation of China (Grants 81570667, 81470948, 81670633); Major Research Plan of the National Natural Science Foundation of China (Grant No. 91742204); the National Key R&D Program of China (Grants 2018YFC1314003–1, 2015BAI12B07), and the National Key Research and Development Program (Grant 2016YFC0906103).
Author Contributions
G.X., S.G. designed the study. Y.C., N.Z., R.L., M.Z., Z.W., L.D., J.L., R.Z., and Y.Y. collected the data, prepared the figures and tables. Y.C. and S.G. contributed the analytical tools. Y.C. and S.G. wrote the paper. S.G. and G.X. conceived the project and supervised and coordinated all the work.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. China Novel Coronavirus Investigating and Research Team A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020 Feb;382((8)):727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 Feb;395((10224)):P565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Team TN. the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) − China. China CDC Weekly. 2020;2((8)):113–22. [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China Medical Treatment Expert Group for Covid-19 Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr;382((18)):1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. China Medical Treatment Expert Group for COVID-19 Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020 May;55((5)):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar;395((10229)):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Mar;579((7798)):270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020 Apr;14((2)):185–92. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandelbaum T, Scott DJ, Lee J, Mark RG, Malhotra A, Waikar SS, et al. Outcome of critically ill patients with acute kidney injury using the Acute Kidney Injury Network criteria. Crit Care Med. 2011 Dec;39((12)):2659–64. doi: 10.1097/CCM.0b013e3182281f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009 Sep;37((9)):2552–8. doi: 10.1097/CCM.0b013e3181a5906f. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020 May;97((5)):829–38. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. China: Jama; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.China NHCotPsRo: Chinese management guideline for COVID-19 (version 7.0) 2020. ( http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf)
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May;150((9)):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016 Feb;315((8)):801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985 Oct;13((10)):818–29. [PubMed] [Google Scholar]
- 17.Outcomes KD, Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;(Suppl 2):1–138. [Google Scholar]
- 18.Forni LG, Darmon M, Ostermann M, Oudemans-van Straaten HM, Pettilä V, Prowle JR, et al. Renal recovery after acute kidney injury. Intensive Care Med. 2017 Jun;43((6)):855–66. doi: 10.1007/s00134-017-4809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 Jul;180((7)):934–43. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen R, Liang W, Jiang M, Guan W, Zhan C, Wang T, et al. Medical Treatment Expert Group for COVID-19 Risk Factors of Fatal Outcome in Hospitalized Subjects With Coronavirus Disease 2019 From a Nationwide Analysis in China. Chest. 2020 Jul;158((1)):97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jixiang Zhang, Xiaoli Wang, Xuemei Jia, Jiao Li, Ke Hu, Guozhong Chen, Jie Wei, Zuojiong Gong, Chenliang Zhou, Hongang Yu, et al. Risk Factors for Disease Severity, Unimprovement, and Mortality of COVID-19 Patients in Wuhan, China. Clin Microbiol Infect. 2020 Jun;26((6)):767–72. doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 May;8((5)):475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2019 doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arabi YM, Arifi AA, Balkhy HH, Najm H, Aldawood AS, Ghabashi A, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014 Mar;160((6)):389–97. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 25.Plataki M, Kashani K, Cabello-Garza J, Maldonado F, Kashyap R, Kor DJ, et al. Predictors of acute kidney injury in septic shock patients: an observational cohort study. Clin J Am Soc Nephrol. 2011 Jul;6((7)):1744–51. doi: 10.2215/CJN.05480610. [DOI] [PubMed] [Google Scholar]
- 26.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020 Jul;98((1)):219–27. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM, To KF, et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005 Feb;67((2)):698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faubel S, Edelstein CL. Mechanisms and mediators of lung injury after acute kidney injury. Nat Rev Nephrol. 2016 Jan;12((1)):48–60. doi: 10.1038/nrneph.2015.158. [DOI] [PubMed] [Google Scholar]
- 29.Ye Q, Wang B, Mao J. Cytokine Storm in COVID-19 and Treatment. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong J, Dong H, Xia SQ, Huang YZ, Wang D, Zhao Y, Liu W, Tu S, Zhang M, Wang Q, et al. Correlation Analysis Between Disease Severity and Inflammation-related Parameters in Patients with COVID-19 Pneumonia. medRxiv. 2020;02(25):20025643. doi: 10.1101/2020.02.25.20025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb;395((10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diao B, Wang C, Wang R, Feng Z, Tan Y, Wang H, et al. Human Kidney is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Medrxiv. 2020 doi: 10.1101/2020.03.04.20031120. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Md Ralib A, Mat Nor MB. Acute kidney injury in a Malaysian intensive care unit: assessment of incidence, risk factors, and outcome. J Crit Care. 2015 Jun;30((3)):636–42. doi: 10.1016/j.jcrc.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Patschan D, Patschan S, Buschmann I, Ritter O. Loop Diuretics in Acute Kidney Injury Prevention, Therapy, and Risk Stratification. Kidney Blood Press Res. 2019;44((4)):457–64. doi: 10.1159/000501315. [DOI] [PubMed] [Google Scholar]
- 35.Mehta RL, Pascual MT, Soroko S, Chertow GM, PICARD Study Group Association between furosemide administration and outcomes in critically ill patients with acute kidney injury. JAMA. 2002 Nov;288((20)):2547–53. doi: 10.1001/jama.288.20.2547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data