Abstract
Background
Bronchoscopy with bronchoalveolar lavage (BAL) during the SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) pandemic should be reserved to a limited number of clinical indications. The yield of BAL for the diagnosis of suspected or confirmed pulmonary SARS-CoV-2 infection is still unknown.
Objectives
We aimed to evaluate the diagnostic ratio of BAL in detecting SARS-CoV-2 pulmonary infection in patients undergoing bronchoscopy for different indications as well as describe the clinical, radiological, and endoscopic characteristics of patients with SARS-CoV-2 on BAL.
Method
We conducted a multicenter retrospective study including all patients who underwent bronchoscopy for the detection of SARS-CoV-2 on BAL. Clinical, computed tomography (CT), endoscopic, and microbiologic data were gathered from March 16th to May 27th, 2020.
Results
131 patients were included. Bronchoscopy was performed for suspected SARS-CoV-2 infection (65.5%), alternative diagnosis (12.9%), suspected superinfections (19.8%), and lung atelectasis (1.5%). SARS-CoV-2 was isolated on BAL 43 times (32.8%) and the highest isolation rate was in patients with suspected SARS-CoV-2 infection (74.4%); 76% of positive patients had a double-negative nasopharyngeal swab. Peripheral, posterior and multilobar CT opacities were more frequent in SARS-CoV-2 patients, and the number of CT findings was higher in positive patients, particularly those with suspected SARS-CoV-2 infection. We recorded a progressive reduction of SARS-CoV-2 isolation during the observation period.
Conclusions
In our centers, the rate of detection of SARS-CoV-2 on BAL in patients with suspected infection was 37.2%. The agreement of BAL with nasopharyngeal swabs was high; CT alterations could predict the pretest probability of SARS-CoV-2 infection, but suspicion of viral infection should be always considered.
Keywords: COVID-19, SARS-CoV-2, Bronchoscopy, Bronchoalveolar lavage
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the virus responsible for the Coronavirus disease 2019 (COVID-19) pandemic affecting millions of people worldwide [1]. The diagnosis of a suspected case is confirmed by the detection of the SARS-CoV-2 in real-time reverse-transcriptase-polymerase chain reaction (rRT-PCR) on biologic samples obtained from nasopharyngeal swabs [2]. However, this method, even if considered the gold standard, has some limitations due to the high rate of false-negative results [3]. It has been demonstrated that, when performed at the initial evaluation of COVID-19 patients, the sensitivity of computed tomography (CT) is significantly higher than that of rRT-PCR (respectively, 97.2 and 83.3%), thus reducing the number of false-negatives related to oropharyngeal swabs tests [4]. To date, no algorithms based on the integration of clinical, radiological, and laboratory data to define the diagnosis of SARS-CoV-2 infection without a microbiological positive test have been developed.
Many international societies have published documents and guidelines to define the role of bronchoscopy during the COVID-19 pandemic; these documents specify procedures, indications, setting, protection for health-care workers and patients, and postprocedural disinfection recommendations [5]. Bronchoalveolar lavage (BAL), bronchial wash, as well as other diagnostic sampling procedures that provide fewer respiratory samples are not routinely indicated for COVID-19 diagnosis [6]. However, in a case of severe or progressive disease potentially requiring intubation, if additional specimens would be needed to establish a definitive diagnosis of COVID-19, or to rule out other diagnoses that could change patients' management, a bronchoscopy with BAL can be performed [7]. Recently, Torrego et al. [8] published a case series of 101 bronchoscopies performed on COVID-19 patients with severe acute hypoxemic respiratory failure requiring mechanical ventilation; the most common finding was the presence of a thick hypersecretion, and with guided mini-BAL, clinical suspicion of superinfection could be confirmed [8]. However, no study has reported the diagnostic yield of BAL in patients with suspected or confirmed pulmonary SARS-CoV-2. The aims of our study were to: (i) evaluate the diagnostic rate of BAL in detecting SARS-CoV-2 pulmonary infection in patients undergoing bronchoscopy for different indications during COVID-19 pandemic, and (ii) describe CT-radiological and endoscopic findings and the clinical characteristics of patients with a virological diagnosis of SARS-CoV-2 on BAL.
Materials and Methods
Study Design
This multicenter, retrospective, observational study was conducted in accordance with the STROBE (Strengthening the Reporting of Observational studies in Epidemiology) statement for observational studies [9]. We included all consecutive patients who underwent bronchoscopy with BAL for different indications as part of the research into SARS-CoV-2 between March 16th and May 27th, 2020. We retrospectively gathered data from 3 study centers in northwestern Italy who were actively involved in the emergency care of COVID-19 patients. We excluded cases with incomplete or nonretrievable data.
For each patient, demographics (age and gender), in-hospital stay, number of days from the onset of symptoms, indications for bronchoscopy (an inconclusive noninvasive COVID-19 diagnosis, concerns about an alternative etiology of respiratory disease which would alter the management, suspicion of superinfection, or mucus plug-related atelectasis), nasopharyngeal swab result (positive or negative and the number of days before the bronchoscopy), radiological CT characteristics (bilateral, posterior, or multilobar involvement, peripheral distribution, ground-glass opacities, and consolidations), microbiological results of BAL (SARS-CoV-2 positivity/negativity, other respiratory viruses, or bacteria and fungi detected), endoscopic findings (secretions, bronchial inflammation, or lesions), technical procedure (the site where the bronchoscopy was performed, the length of the procedure, sedation, and anesthesia), and laboratory data on peripheral blood (white blood cell count, C-reactive protein level, and procalcitonin level) were collected. For each patient, the number of CT alterations was also calculated.
Statistical Analysis
In the descriptive analysis, n (%) were reported for categorical variables, and mean (SD) or medians (IQR) were used for numerical variables, based on the assumption of normality.
To better understand the diagnostic result of BAL and compare the demographic and clinical characteristics between subjects in whom SARS-CoV-2 was or was not identified, Student's t test, Mann-Whitney U test, χ2 test, or Fisher's exact test were used as appropriate.
Further analysis was performed to examine the association between the number of CT alterations and indications on bronchoscopy. A one-way ANOVA model was used and box plots were reported.
To establish if sampling conducted at different time points can influence the rate of detection of SARS-CoV-2, firstly, the time was split into 6 intervals of 14 days each from March 16th to May 27th. Then, for each time interval, the proportion of patients diagnosed with SARS-CoV-2 was calculated in all subjects as well as separately in those suspected of having COVID and all others. Finally, Pearson's correlation was estimated, and linear regression models were fitted to better interpret the trend in time.
A two-sided α value <0.05 was considered statistically significant. Statistical analyses were done using SAS software v9.4.
Results
Study Population
We included 131 consecutive patients in our study. Most of them were males (n = 93, 70.9%) and were hospitalized in an internal medicine ward (n = 83, 63.3%). All procedures were performed in accordance with the most recent recommendations [6]. Indications for bronchoscopy were: 65.5% (n = 86) suspected to have SARS-CoV-2 infection, 12.9% (n = 17) alternative diagnosis (i.e., hemoptysis or lung consolidations), 19.8% (n = 26) suspected superinfections, and 1.5% (n = 2) lung atelectasis. Most patients had previously had a double-negative nasopharyngeal swab for SARS-CoV-2 (n = 120, 91.6%) and bronchoscopy was performed at a median of 1 day (IQR 1–3) after the last nasopharyngeal swab. Bronchoscopies were mainly performed in a bronchoscopic suite (n = 60, 45.8%) with the patient conscious and under sedation (with midazolam or midazolam plus fentanyl, n = 116, 88.5%). Endobronchial secretions were reported only 46 times (36.22%) and 76.0% (n = 35) of them were nonpurulent. Endobronchial erythematous mucosa was observed 31 times (23.6%). In 4 cases (3.1%), a hemorrhagic BAL fluid recovery was recorded (Table 1).
Table 1.
Demographic and clinical data
| All subjects (n = 131) | SARS-CoV-2 not identified (n = 88) | SARS-CoV-2 identified (n = 43) | p value | |
|---|---|---|---|---|
| Demographics | ||||
| Gender | 0.846 | |||
| Female | 38 (29.01) | 26 (29.55) | 12 (27.91) | |
| Male | 93 (70.99) | 62 (70.45) | 31 (72.09) | |
| Age | ||||
| <65 years | 68 (51.91) | 38 (43.18) | 30 (69.77) | 0.009 |
| 65–75 years | 34 (25.95) | 25 (28.41) | 9 (20.93) | |
| >75 years | 39 (22.14) | 25 (28.41) | 4 (9.30) | |
| Age, years | 64.65 (53.71–73.98) | 67.01 (56.14–78.56) | 55.85 (51.20–66.87) | 0.004 |
| Medical center | 0.218 | |||
| Novara | 48 (36.64) | 28 (31.82) | 20 (46.51) | |
| Torino | 77 (58.78) | 55 (62.50) | 22 (51.16) | |
| Vercelli | 6 (4.58) | 5 (5.68) | 1 (2.33) | |
| Hospital unit | ||||
| Intern ward | 83 (63.36) | 64 (72.73) | 19 (44.19) | 0.0008 |
| Subintensive | 36 (27.48) | 21 (23.86) | 15 (34.88) | |
| Intensive care unit | 12 (9.16) | 3 (3.41) | 9 (20.93) | |
| Clinical data | ||||
| Number of days | ||||
| From nasopharyngeal swab to bronchoscopy | 1 (1–3) | 1 (1–2) | 2 (1–4) | 0.0062 |
| From symptoms' onset to bronchoscopy | 15 (8–31) | 20 (9.5–33.5) | 12 (7–20) | 0.0221 |
| Indications for bronchoscopy | ||||
| Suspected SARS-CoV-2 infection | 86 (65.65) | 54 (61.36) | 32 (74.42) | 0.034 |
| Alternative diagnosis | 17 (12.89) | 15 (17.05) | 2 (4.65) | |
| Suspected superinfection | 26 (19.85) | 19 (21.59) | 7 (16.28) | |
| Lung atelectasis | 2 (1.53) | 0 (0) | 2 (4.65) | |
| Nasopharyngeal swab | ||||
| Double-negative | 120 (91.60) | 87 (98.86) | 33 (76.74) | <0.0001 |
| At least 1 positive | 11 (8.40) | 1 (1.14) | 10 (23.26) | |
| Blood analysis | ||||
| White blood cell count, ×103/µL | 8.11 (5.98–13.27) | 8.44 (6.48–13.40) | 7.15 (5.88–10.90) | 0.3364 |
| C-reactive protein, mg/dL | 7.40 (2–12.30) | 6.80 (1.36–12.00) | 8.74 (4.05–15.80) | 0.0208 |
| Procalcitonin, ng/mL | 0.22 (0.08–0.90) | 0.28 (0.08–0.80) | 0.16 (0.08–0.90) | 0.6675 |
Values express n (%) or median (IQR).
SARS-CoV-2 Isolation
SARS-CoV-2 was isolated on BAL 43 times (32.8%). We did not find a statistically significant gender prevalence (p = 0.846) of SARS-CoV-2 positivity, but the positive patients were younger than the negative ones (55.85 vs. 67.01 years, p = 0.004) and the prevalence was higher in those younger than 65 years (p = 0.009). Of 120 patients with 2 negative swabs, we isolated SARS-CoV-2 33 times (27.5%), and 76% of these BAL-positive patients had a double-negative swab. For completeness, we note that, in 98.9% of the double-negative swabs, negativity was confirmed even on BAL (p < 0.0001). Interestingly, in 1 case with a positive swab, BAL did not detect the virus infection. The number of days from symptoms' onset to bronchoscopy was lower in patients with SARS-CoV-2 infection (p = 0.022). C-reactive protein was higher in SARS-CoV-2 positive patients (p = 0.020) (Table 1).
Significant differences (p = 0.034) in the identification of SARS-CoV-2 were observed among the indications of bronchoscopy: in patients with successful virus isolation, the number of suspected COVID-19-positive patients was higher (74.4 vs. 61.36%), but the proportion of patients with suspected superinfections or an alternative diagnosis were lower (16.28 vs. 21.59%, and 4.65 vs. 17.05%) (p = 0.034). Remarkably, the virus was identified in only 32/86 (37.2%) patients with suspected SARS-CoV-2. In our cohort, the research of SARS-CoV-2 after a disobstructive bronchoscopy for mucus plugging was only performed twice, and we found the virus in both cases (2/2, 100%).
CT Scan Findings
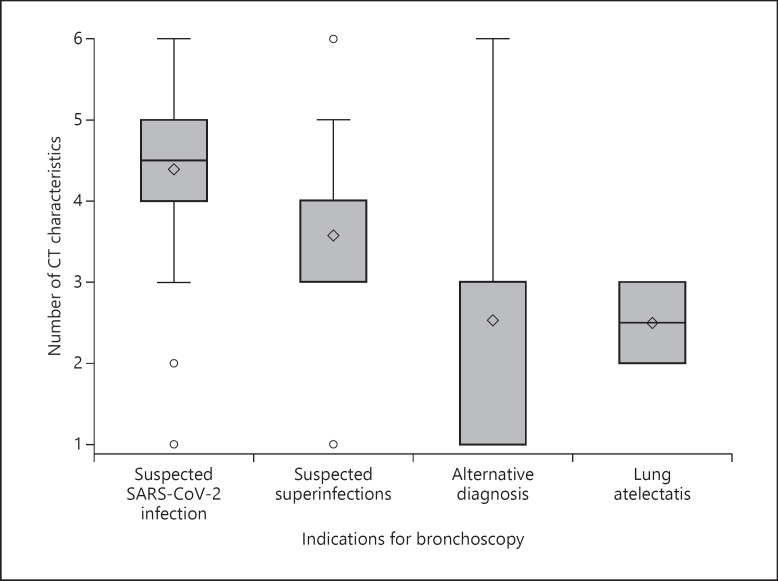
We observed that, in patients with SARS-CoV-2 infection, the most frequent CT alterations were diffuse ground-glass opacities (n = 38, 88.3%) followed by posterior and peripheral ones (respectively, n = 37, 86.0% and n = 34, 79.0%). Peripheral, posterior, and multilobar alterations were most frequent in SARS-CoV-2-positive patients (respectively, p = 0.009, <0.001, and 0.028). Moreover, SARS-CoV-2 patients presented with a higher number of CT alterations than patients without SARS-CoV-2 infection (median 5 vs. 4, p = 0.0001) (Table 2). Finally, when we considered 4 different indications on bronchoscopy, we observed that the group suspected of having SARS-CoV-2 presented a higher number of radiological alterations (p = 0.0001) (Fig. 1).
Table 2.
Radiologic, bronchoscopic, and microbiological data
| All n = 131 | SARS-CoV-2 not identified n = 88 | SARS-CoV-2 identified n = 43 | p value | |
|---|---|---|---|---|
| Radiologic data | ||||
| Type of CT characteristic | ||||
| Bilateral | 95 (75.52) | 62 (70.45) | 33 (76.74) | 0.4489 |
| Peripheral | 83 (63.36) | 49 (55.68) | 34 (79.07) | 0.0091 |
| Posterior | 77 (58.78) | 40 (45.45) | 37 (86.05) | <0.001 |
| Ground-glass opacities | 108 (102.44) | 70 (79.55) | 38 (88.37) | 0.2124 |
| Consolidation | 76 (58.02) | 49 (55.68) | 27 (62.79) | 0.4388 |
| Multilobar | 80 (61.07) | 48 (54.55) | 32 (74.42) | 0.0285 |
| CT characteristics per patient | ||||
| 1 | 12 (9.16) | 10 (11.36) | 2 (4.65) | 0.002 |
| 2 | 9 (6.87) | 7 (7.95) | 2 (4.65) | |
| 3 | 23 (17.56) | 19 (21.59) | 4 (9.30) | |
| 4 | 38 (29.01) | 32 (36.36) | 6 (13.95) | |
| 5 | 26 (19.85) | 11 (12.50) | 15 (34.88) | |
| 6 | 23 (17.56) | 9 (10.23) | 14 (32.56) | |
| Number of CT characteristics | 4 (3–5) | 4 (3–4) | 5 (4–6) | <0.0001 |
| Bronchoscopic data | ||||
| Setting | ||||
| The patient's bedside | 57 (43.51) | 37 (42.05) | 20 (46.51) | 0.0017 |
| Bronchoscopic suite | 60 (45.80) | 47 (53.41) | 13 (30.23) | |
| Subintensive/ICU | 14 (10.69) | 4 (4.55) | 10 (23.26) | |
| Sedation | ||||
| Conscious | 116 (88.55) | 84 (94.32) | 33 (76.74) | 0.0030 |
| General anesthesia | 15 (11.45) | 5 (5.68) | 10 (23.26) | |
| Length of procedure, min | 8 (7–9) | 8 (8–9.5) | 8 (7–9) | 0.0468 |
| Endobronchial secretions | 46 (36.22) | 33 (39.29) | 13 (30.23) | 0.3151 |
| Erythematous mucosa | 31 (23.85) | 21 (24.14) | 10 (23.26) | >0.9999 |
| Microbiological data | ||||
| At least 1 pathogen* | 46 (35.11) | 33 (37.50) | 13 (30.23) | 0.4132 |
| Virus (non-SARS-CoV-2) | 10 (7.75) | 7 (8.14) | 3 (6.98) | >0.9999 |
| Bacteria | 30 (22.90) | 20 (22.73) | 10 (23.26) | >0.9999 |
| Fungi | 19 (14.50) | 16 (18.18) | 3 (6.98) | 0.0507 |
Values are expressed as n (%) or median (IQR). CT, computed tomography; ICU, intensive care unit; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2.
Fig. 1.
Mean number of CT characteristics in different indications for bronchoscopy.
Time Course
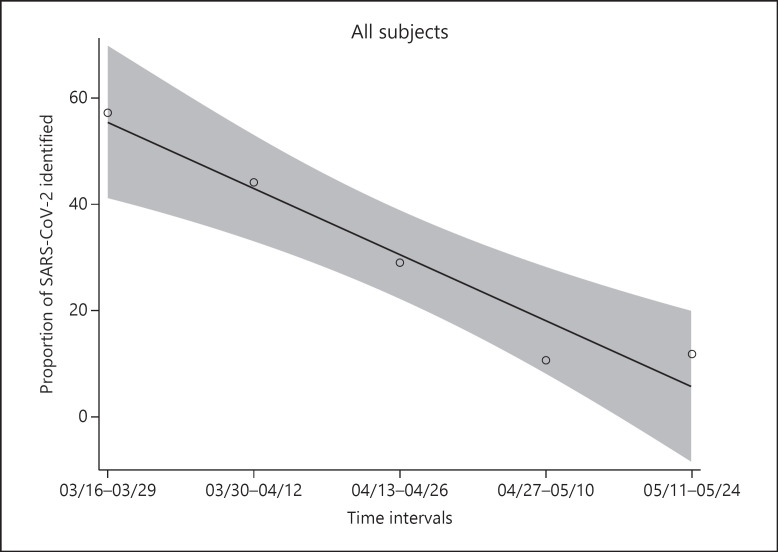
When we divided all bronchoscopies performed during the observation period into intervals of 14 days, we observed that the proportion of bronchoscopies with SARS-CoV-2 isolation decreased over time (correlation −0.9687; p = 0.0066) (Fig. 2).
Fig. 2.
Proportion of SARS-CoV-2 isolation during the observation period divided into intervals of 14 days.
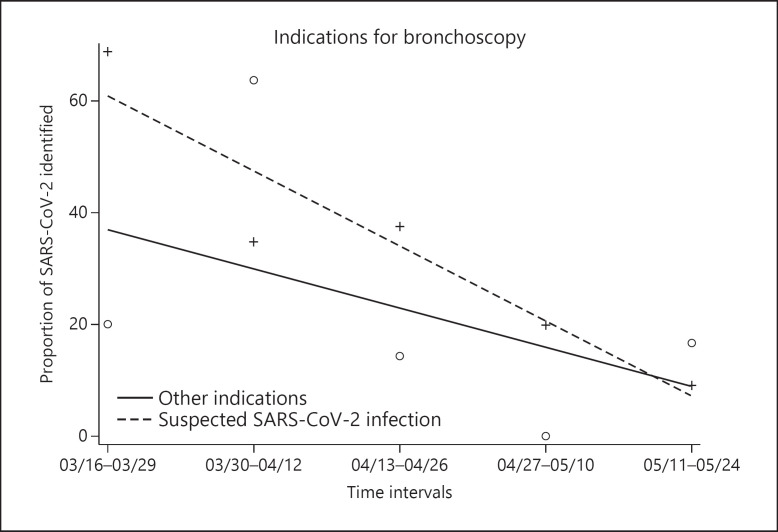
We observed the same trend when splitting patients into 2 groups, i.e., bronchoscopy for suspected SARS-CoV-2 infection and all other indications (Fig. 3). In the subjects with suspected infection, a higher proportion of SARS-CoV-2 was isolated, but this was nearly zero at the end of the study. The last 2 observations (on May 26th and 27th) were excluded from this analysis due to their sparse data.
Fig. 3.
Proportion of SARS-CoV-2 isolation and the detection of other indications during the observation period divided into intervals of 14 days.
Other Microbiological Findings
Among the patients who underwent bronchoscopy for suspected SARS-CoV-2 infection, 26 other microbiological isolations were observed and 32 SARS-CoV-2-positives (i.e., 67%). Herpesviruses were the other most common viruses isolated (10/57,17.5%); of these, Cytomegalovirus and Human herpesvirus 6 were the most frequent (4× each). Among 30 isolated bacteria, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa were the most frequent. Fungi were identified 17 times (Candida albicans was isolated 11 times, i.e., 64.7%). No correlation was found between isolated bacteria, virus, or fungi and SARS-CoV-2-positive or SARS-CoV-2-negative patients (Table 3).
Table 3.
Microbiological isolations on bronchoalveolar lavage
| N | % | |
|---|---|---|
| Patients | 131 | |
| Viruses | 57 | |
| SARS-CoV-2 | 43 | 75.44 |
| HHV-6 | 4 | 7.02 |
| HSV-1 | 2 | 3.51 |
| CMV | 4 | 7.02 |
| EBV | 2 | 3.51 |
| RSV | 1 | 1.75 |
| Metapneumovirus | 1 | 1.75 |
| Bacteria | 30 | |
| Staphylococcus aureus | 9 | 30 |
| Escherichia coli | 5 | 16.68 |
| Klebsiella pneumoniae | 4 | 13.33 |
| Pseudomonas aeruginosa | 4 | 13.33 |
| Chlamydia pneumoniae | 1 | 3.33 |
| Mycoplasma pneumoniae | 1 | 3.33 |
| Others | 6 | 20 |
| Fungi | 17 | |
| Candida albicans | 11 | 64.70 |
| Aspergillus spp. | 4 | 23.54 |
| Others | 2 | 11.77 |
CMV, Cytomegalovirus; EBV, Epstein-Barr virus; HHV-6, Human herpesvirus 6; HSV-1, herpes simplex virus 1; RSV, respiratory syncytial virus; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2.
Definitive Alternative Diagnosis
For completeness, a definitive alternative diagnosis was achieved in 15 cases. We diagnosed 8 primitive lung cancers, 4 alveolar hemorrhages, 2 cases of cryptogenic organizing pneumonia, and 1 of vasculitis. All cases of alveolar hemorrhage were SARS-CoV-2-negative with a sterile BAL recovery.
Discussion
During the COVID-19 pandemic, routine bronchoscopy with BAL to detect SARS-CoV-2 was not suggested as the first step of the diagnostic procedure, limiting the indications only to few cases [5]. In our cohort, the most frequent indication in the observation period was a suspected SARS-CoV-2 infection (65.5%) in patients with a double-negative nasopharyngeal swab. Interestingly, we confirmed a diagnosis of SARS-CoV-2 in only 37.2% of these patients. This result was probably influenced by several factors. First, the prevalence of SARS-CoV-2 infection changed over time. In fact, we observed that the overall proportion of positive bronchoscopies decreased during the weeks of observation, with a maximum in the first 3 weeks (16th March to 5th April). In Italy, in this lockdown period, we observed the highest incidence of COVID-19 infections (the peak was on the 20th March) [10]. Second, the knowledge about and the perception of the viral infection also changed over time, with several surveys demonstrating that Italian health workers had a good level of knowledge about SARS-CoV-2 and its clinical presentation [11, 12]. However, this was likely in contrast to the increased availability of diagnostic tools, with the fear of clinical and epidemiological implications due to a missed diagnosis [13]. Finally, we were asked to confirm or definitively exclude SARS-CoV-2 infection so as to isolate the infected patients. In cases with double-negative nasopharyngeal swabs, when the CT scan produced uncertain results, we decided to perform a bronchoscopy with BAL to confirm the diagnosis. This is probably the reason for the increased number of bronchoscopies with a negative SARS-CoV-2 outcome, lowering the positivity rate of this indication.
In this study population, the overall diagnostic rate of SARS-CoV-2 detection was 32.8% this diagnostic yield is better than that reported in the literature in patients affected by viral community-acquired pneumonia, where BAL provided a specific diagnosis in 15% of patients [14, 15]. Up to now, only Wang et al. [16] reported the results of BAL in severely ill COVID-19 patients whose diagnosis was based on symptoms, radiology, and SARS-CoV-2 detection. SARS-CoV-2 was detected in specimens from multiple anatomic sites. They reported a SARS-CoV-2 diagnosis in 14/15 patients who underwent bronchoscopy with BAL (93%). These data suggest that, in patients with COVID-19, the viral load in cases of lower respiratory tract infection was higher and this could explain the high positivity rates for BAL [16]. There are various possible explanations for our different diagnostic rates. First, we mainly investigated patients with a suspected SARS-CoV-2 infection with 2 negative nasopharyngeal swabs. When we considered patients with at least 1 positive swab, we identified SARS-CoV-2 in 90.9% of cases (10/11 patients). Second, in our cohort, we observed significant differences as the days elapsed after symptoms' onset and bronchoscopy among patients with and without infection. It has been demonstrated that the viral load, and consecutively the BAL diagnostic rate, decrease gradually from symptoms' onset, with a maximum of positive swabs after 5 days [17]. In our cohort, positive patients underwent bronchoscopy a median of 12 days after the development of symptoms. In the patients that tested negative, we cannot be absolutely certain about the absence of infection because more time had elapsed from symptoms' onset. Finally, when BAL results were negative for SARS-CoV-2 in 98% of the cases (87/88), we had a double-negative nasopharyngeal swab which confirmed the previously reported data [18]. On the other hand, when the BAL result was positive for SARS-CoV-2 in 76% of cases (33/43), we also had a double-negative nasopharyngeal swab. When we considered only those patients who underwent bronchoscopy for suspected SARS-CoV-2 infection, in 26 cases, we isolated other pathogens that could have influenced the suspicion of a viral infection. With these isolations, the diagnostic rate of BAL in clinical cases suspected for interstitial acute infectious diseases rose to 67% (58/86 bronchoscopies), which led to a correct therapeutic indication (COVID-19 or non-COVID-19) and admission to an appropriate setting. It must be noted that some isolated pathogens (viruses, in particular) can be innocent bystanders not representing an infectant agent. Among the noninfective diagnoses, we found 2 of cryptogenic organizing pneumonia [19]. In both these cases, BAL was negative for SARS-CoV-2; however, we can't completely rule out a previous viral infection because symptoms started 17 days before we performed the bronchoscopy.
There are several CT features that have been reported in COVID-19 patients, e.g., ground glass opacities, with or without consolidations in the peripheral and posterior lung zones. Their frequency and characteristics depend on when the patient undergoes the CT, and it has been demonstrated that the nature of the alterations can change during the course of an infection [20]. The number of CT findings has been used to predict SARS-CoV-2 infection in patients with moderate to severe symptoms, with a substantial interobserver agreement [21]. In our cohort, we confirmed that patients with SARS-CoV-2 infection presented with a higher number of CT alterations than the SARS-CoV-2-negative patients; moreover, peripheral, posterior, and multilobar alterations were observed most frequently. The patients who underwent bronchoscopy for a suspected SARS-CoV-2 infection presented with a higher number of CT alterations than patients with other indications. These results were likely biased by the variable indications used to perform the procedure; the higher the number of CT alterations, the higher the pretest probability of infection [21]. Nevertheless, we also tested for SARS-CoV-2 in patients with only 1 or 2 CT alterations and we could isolate the pathogen.
Our study has some limitations. First, we conducted a retrospective study; during the COVID-19 pandemic, due to the tumultuous course of the spread of the infection, once international guidelines become available, we gathered data about indications for bronchoscopy but with all the limitations of a retrospective study. Second, we did not collect data about the cycle threshold values of RT-PCR; such data would provide more information about the viral load in the BAL fluid. Wang et al. [16]demonstrated that BAL and nasal swabs had a lower cycle threshold, corresponding to the higher viral copy numbers. The final diagnosis of COVID-19 is difficult, particularly in patients with contrasting diagnostic test results, and so actually requires a multidisciplinary approach and discussion [22]. For this reason, we limited our study to the report of SARS-CoV-2 isolation in BAL fluid, and how these data influence the diagnostic rate of infection. Finally, regarding CT alterations, we calculated a score based on the sum of each CT finding by weighting each single alteration with the same value. Some features are more typical of SARS-CoV-2 infection (i.e., ground-glass opacities) and so they should probably have a specific single score.
In conclusion, bronchoscopy with BAL should be reserved for cases that match the internationally suggested and widely accepted indications. In our multicenter experience, we reported a high number of bronchoscopies with BAL for suspected SARS-CoV-2 infection, in patients with 2 negative swabs, with a viral detection rate of 37.2%. Nevertheless, BAL led to a final microbiological diagnosis in 67% of the patients. The agreement of BAL with nasopharyngeal swabs was high, accounting for 90 and 98% positive and negative cases, respectively. CT alterations could predict the pretest probability of SARS-CoV-2 infection, but clinical suspicion of viral infection should always be considered due to the mutability of CT patterns during infection evolution and seasonal epidemiologic burn.
Statement of Ethics
The study protocol was approved by the institute's committee on human research (study protocol CE 97/20).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There was no funding.
Author Contributions
Conception and design: F. Patrucco, P. Solidoro, P.E. Balbo, C. Albera. Data acquisition and analysis: F. Patrucco, M. Bellocchia, V. Foci. Interpretation: F. Patrucco, P. Solidoro, C. Airoldi, L. Castello, M. Bellan, P.P. Sainaghi. Drafting the work: F. Patrucco, P. Solidoro, F. Gavelli. Critical revision of the work: P. Balbo, C. Albera.
Acknowledgement
We extended our gratitude and appreciation to all health-care workers who provided and cared for the study subjects during the COVID-19 pandemic.
References
- 1.Luo F, Darwiche K, Singh S, Torrego A, Steinfort DP, Gasparini S, et al. Performing Bronchoscopy in Times of the COVID-19 Pandemic: Practice Statement from an International Expert Panel. Respiration. 2020;99((5)):417–22. doi: 10.1159/000507898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020 Jan;25((3)):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woloshin S, Patel N, Kesselheim AS. False Negative Tests for SARS-CoV-2 Infection - Challenges and Implications. N Engl J Med. 2020 Aug;383((6)):e38. doi: 10.1056/NEJMp2015897. [DOI] [PubMed] [Google Scholar]
- 4.Long C, Xu H, Shen Q, Zhang X, Fan B, Wang C, et al. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020 May;126:108961. doi: 10.1016/j.ejrad.2020.108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lentz RJ, Colt H. Summarizing societal guidelines regarding bronchoscopy during the COVID-19 pandemic. Respirology. 2020 Jun;25((6)):574–7. doi: 10.1111/resp.13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahidi MM, Lamb C, Murgu S, Musani A, Shojaee S, Sachdeva A, et al. American Association for Bronchology and Interventional Pulmonology (AABIP) Statement on the Use of Bronchoscopy and Respiratory Specimen Collection in Patients With Suspected or Confirmed COVID-19 Infection. J Bronchology Interv Pulmonol. 2020 Oct;27((4)):e52–4. doi: 10.1097/LBR.0000000000000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahidi MM, Shojaee S, Lamb CR, Ost D, Maldonado F, Eapen G, et al. The Use of Bronchoscopy during the COVID-19 Pandemic: CHEST/AABIP Guideline and Expert Panel Report. Chest. 2020. DOI: org/10.1016/j.chest.2020.04.036. [DOI] [PMC free article] [PubMed]
- 8.Torrego A, Pajares V, Fernández-Arias C, Vera P, Mancebo J. Bronchoscopy in COVID-19 Patients With Invasive Mechanical Ventilation: A Center Experience. Am J Respir Crit Care Med. 2020 Jul;202((2)):284–7. doi: 10.1164/rccm.202004-0945LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008 Apr;61((4)):344–9. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Ministero della salute Covid-19 - Situazione in Italia. [cited 2020 June 08] Available from: http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?area=nuovoCoronavirus&id=5351&lingua=italiano&menu=vuoto.
- 11.Moro M, Vigezzi GP, Capraro M, Biancardi A, Nizzero P, Signorelli C, et al. 2019-novel coronavirus survey: knowledge and attitudes of hospital staff of a large Italian teaching hospital. Acta Biomed. 2020 Apr;91(3-S):29–34. doi: 10.23750/abm.v91i3-S.9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Lorenzo G, Di Trolio R. Coronavirus Disease (COVID-19) in Italy: Analysis of Risk Factors and Proposed Remedial Measures. Front Med (Lausanne) 2020 Apr;7:140. doi: 10.3389/fmed.2020.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbaum L. Facing Covid-19 in Italy - Ethics, Logistics, and Therapeutics on the Epidemic's Front Line. N Engl J Med. 2020 May;382((20)):1873–5. doi: 10.1056/NEJMp2005492. [DOI] [PubMed] [Google Scholar]
- 14.Garbino J, Soccal PM, Aubert JD, Rochat T, Meylan P, Thomas Y, et al. Respiratory viruses in bronchoalveolar lavage: a hospital-based cohort study in adults. Thorax. 2009 May;64((5)):399–404. doi: 10.1136/thx.2008.105155. [DOI] [PubMed] [Google Scholar]
- 15.Oren I, Hardak E, Zuckerman T, Geffen Y, Hoffman R, Yigla M, et al. Does molecular analysis increase the efficacy of bronchoalveolar lavage in the diagnosis and management of respiratory infections in hemato-oncological patients? Int J Infect Dis. 2016 Sep;50:48–53. doi: 10.1016/j.ijid.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020 May;323((18)):1843–4. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure. Ann Intern Med. 2020 Aug;173((4)):262–7. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geri P, Salton F, Zuccatosta L, Tamburrini M, Biolo M, Busca A, et al. Limited role for bronchoalveolar lavage to exclude Covid-19 after negative upper respiratory tract swabs: a multicenter study. Eur Respir J. 2020 Aug;56((4)):2001733. doi: 10.1183/13993003.01733-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pogatchnik BP, Swenson KE, Sharifi H, Bedi H, Berry GJ, Guo HH. Radiology-pathology Correlation in Recovered COVID-19, Demonstrating Organizing Pneumonia. Am J Respir Crit Care Med. 2020 Jul;202((4)):598–9. doi: 10.1164/rccm.202004-1278IM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - Secondary Publication. J Thorac Imaging. 2020 Jul;35((4)):219–27. doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prokop M, van Everdingen W, van Rees Vellinga T, Quarles van Ufford H, Stöger L, Beenen L, et al. COVID-19 Standardized Reporting Working Group of the Dutch Radiological Society CO-RADS − A categorical CT assessment scheme for patients with suspected COVID-19: definition and evaluation. Radiology. 2020 Aug;296((2)):E97–104. doi: 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ora J, Puxeddu E, Cavalli F, Giorgino FM, Girolami A, Chiocchi M, et al. Does bronchoscopy help the diagnosis in COVID-19 infection? Eur Respir J. 2020 Aug;56((2)):2001619. doi: 10.1183/13993003.01619-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]