Abstract
Aim:
To investigate clinical characteristics and identify risk factors for severity of coronavirus disease 2019 (COVID-19) pneumonia outside of Wuhan, China.
Materials and methods:
We included 213 patients with confirmed COVID-19 who had been discharged or died by 15 March 2020. We retrospectively collected epidemiological, clinical, laboratory, computed tomography imaging and outcome data. Clinical characteristics were described and relative risk factors were compared.
Results:
Most clinical characteristics of this study were similar to those from studies in Wuhan, but there were lower mortality rate and milder severity. The median time from onset of symptoms to confirmation and hospitalization was 4 and 5 days, respectively. The median virus clearance and shedding times were 10 and 15 days, respectively. When the severe/critical group was compared with the mild/moderate group, significant risk factors included: older age; dyspnea; hypertension; poor appetite; fatigue; higher white cell count, neutrophil count, prothrombin time, creatine kinase, creatine kinase-MB, D-dimer, alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH) and C-reactive protein; and lower lymphocyte count and albumin (p < 0.05). In the intensive care unit (ICU) group compared with the non-ICU group, risk factors included: older age; chronic obstructive pulmonary disease (COPD); dyspnea; poor appetite; higher white cell count, D-dimer, ALT, AST and LDH; and lower lymphocyte count and albumin (p < 0.05). Independent risk factors associated with the severe/critical group were dyspnea [odds ratio (OR) = 19.48], ALT (OR = 6.02) and albumin (OR = 3.36). Independent risk factors associated with the ICU group were dyspnea (OR = 8.88), COPD (OR = 31.80), D-dimer (OR = 8.37), ALT (OR = 28.76) and LDH (OR = 9.95) (p < 0.05).
Conclusion:
The severity of COVID-19 outside Wuhan, China was milder than that within Wuhan. The clinical infective period was long, and the longest virus shedding time was 35 days. The most important risk factors were dyspnea, COPD, D-dimer, ALT, LDH and albumin.
The reviews of this paper are available via the supplemental material section.
Keywords: coronavirus disease 2019 (COVID-19), critical, intensive care unit (ICU), risk factors, severe
Introduction
In December 2019, a new unknown pneumonia emerged in Wuhan, China, and this disease was subsequently named coronavirus disease 2019 (COVID-19).1 COVID-19 spread rapidly throughout every province of China. Hunan Province, whose location is closest to Wuhan, became the second most affected area. One month later, COVID-19 broke out in Korea, Japan, Europe and America, and by 17 April 2020, it had spread to >200 countries, causing >2 million infections and nearly 150,000 deaths, and these figures are still soaring.
In the reports that described the clinical characteristics of COVID-19, we found obvious differences between Wuhan and elsewhere, with the former having higher mortality rate, acute respiratory distress syndrome (ARDS) rate and disease severity.2–4 Reports about the clinical infective period of COVID-19 were rare, such as time from onset of symptoms to confirmation or hospitalization, virus clearance and virus shedding. Furthermore, although Guan5 reported a nationwide study of clinical characteristics of COVID-19, that study classified severity according to community-acquired pneumonia criteria, and it did not investigate risk factors for severity of COVID-19, and final follow-up time was not reached (discharged rate 5%). Other studies outside Wuhan that analyzed risk factors for disease severity were also scarce4,6 and, more importantly, cut-off dates for follow-up had still not been reached at publication.4,7
In this study, we described the characteristics of COVID-19 outside Wuhan, especially the clinical infective period and the time needed for development of different clinical features. We also identified the risk factors for severity of COVID-19 pneumonia.
Patients and methods
Patients
Patients came from two medical centers, Changsha Public Health Treatment Center and Xiangtan Central Hospital, which were the main treatment centers for COVID-19 in Hunan Province. Inclusion criteria were: (1) laboratory confirmed inpatients with COVID-19; and (2) available epidemiological, clinical and outcomes data. This study was approved by the Institutional Review Board and Ethics Commission of The Second Xiangya Hospital (2020-017), and written informed consent was waived by the Ethics Commission because it was a retrospective analysis on a newly emerging infectious disease.
Data collection
We retrospectively collected the patient data from the two medical centers. The first date of patient admission was 24 January 2020 and the last date was 16 February 2020. The first discharge date was 4 February 2020 and the last date (final follow-up) was 15 March 2020. We included basic demographic, epidemiological, clinical, laboratory, imaging, therapy, and outcomes data.
Diagnosis of patients
COVID-19 was diagnosed on the basis of “Diagnosis and treatment protocol for novel coronavirus infection-induced pneumonia version 7.”8 Confirmation was based on real-time reverse transcription polymerase chain reaction (RT-PCR), nucleic-acid-positive test of respiratory or blood specimens and high-throughput gene sequencing of highly homologous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in respiratory or blood specimens. RT-PCR assays were performed in accordance with the protocol established by the World Health Organization.9
Outcomes and definitions
The primary outcome was patients diagnosed with severe/critical COVID-19, or admission to the intensive care unit (ICU). The secondary outcome was discharge from hospital or death. The incubation period was defined as from exposure to onset of symptoms.5 Viral clearance time was defined as negative detection of SARS-CoV-2 by RT-PCR at least twice at intervals of 24 h. Clinical viral shedding time was defined as from onset of symptoms to viral clearance.2 The severity of COVID-19 was classified according to the “Diagnosis and treatment protocol for novel coronavirus infection-induced pneumonia version 7.”8
Statistical analysis
Continuous variables were expressed as median (interquartile range) and compared with the Mann–Whitney U test. Categorical variables were expressed as number (%) and compared by χ2 test or Fisher’s exact test. Significant variables from univariate analysis were entered into logistic univariate regression analysis for calculating odds ratios (ORs) and statistical significance. Screened out significant variables were entered into logistic multivariate regression analysis for calculating independent risk factors. The statistical analyses were performed by SPSS 25.0 (IBM), and p < 0.05 was considered to be statistically significant.
Results
Epidemiological and clinical manifestations
We included 213 patients with COVID-19 (52.1% female, 47.8% male; median age 44 years). They had a low smoking rate (6.4%) and low exposure history of Huanan Seafood Market in Wuhan (0.7%), but 35% had visited Wuhan City. A total of 41.4% of patients had imported cases and 72.5% had a clustered exposure history. The median incubation period was 7 days.
The most common comorbidity was hypertension (14.0%), followed by diabetes (6.6%) and heart disease (4.2%). The most common clinical manifestations were cough (72.8%) and fever (60.6%), followed by expectoration (36.7%), fatigue (36.4%) and dyspnea (19.7%). The lung signs of dry/moist rales were rare (7.6%). Mild COVID-19 accounted for 4.2% of cases, moderate type for 77.9%, severe type for 13.1%, critical type for 4.7% and ICU admission for 9.4%. Epidemiological and clinical manifestations are shown in Table 1.
Table 1.
Clinical characteristics and risk factors of severity.
| Variables |
All patients N = 213 |
Disease severity |
p
|
Presence in ICU/death |
p
|
||
|---|---|---|---|---|---|---|---|
| Epidemiological characteristics | Mild/moderate n = 175 |
Sever/critical n = 38 |
Yes n = 20 |
No n = 193 |
|||
| Ages, years | 44 (34–58) | 41 (32–55) | 53 (43.8–64.0) | 0.003 | 57.5 (45.5–64.0) | 41 (33.5–56.5) | 0.007 |
| Sex | |||||||
| Male | 102/213 (47.8%) | 80/175 (45.7%) | 22/38 (57.9%) | 0.173 | 12/20 (60.0%) | 90/193 (46.6%) | 0.255 |
| Female | 111/213 (52.1%) | 95/175 (54.3%) | 16/38 (42.1%) | 0.173 | 8/20 (40.0%) | 93/193 (48.2%) | 0.485 |
| BMI | |||||||
| <18.5 | 7/134 (5.2%) | 7/118 (6.0%) | 0/16 (0.0%) | 0.688 | 0/7 (0.0%) | 7/127 (5.5%) | 1.000 |
| >24 | 57/134 (42.5%) | 47/118 (39.8%) | 10/17 (62.5%) | 0.138 | 6/8 (75.0%) | 51/127 (40.2%) | 0.117 |
| Median incubation period | 7 (3.5–11.0) | 7 (4–11) | 7 (3.5–14.25) | 0.685 | 7 (4–13) | 7 (3–11) | 0.690 |
| Smoking | 11/172 (6.4%) | 9/149 (6.0%) | 2/23 (8.7%) | 0.979 | 2/20 (10.0%) | 9/152 (5.9%) | 0.830 |
| Exposure Huanan Seafood Market | 1/140 (0.7%) | 0/175 (0.0%) | 1/38 (2.6%) | 0.178 | 1/20 (5.0%) | 0/193 (0.0%) | 0.094 |
| Visited Wuhan | 49/140 (35%) | 39/119 (32.8%) | 10/21 (47.6%) | 0.188 | 4/9 (44.4%) | 45/131 (34.4%) | 0.800 |
| Travelling history | 14/115 (12.2%) | 12/95 (12.6%) | 2/20 (10.0%) | 1.000 | 1/9 (11.1%) | 13/131 (9.9%) | 1.000 |
| Cluster exposure history | 137/189 (72.5%) | 108/151 (71.5%) | 29/29 (100.0%) | 0.001 | 9/14 (64.3%) | 128/175 (73.1%) | 0.687 |
| Imported cases | 79/191 (41.4%) | 78/160 (48.8%) | 11/31 (33.3%) | 0.175 | 6/16 (37.5%) | 73/175 (41.7%) | 0.743 |
| Comorbidities | 59/213 (27.7%) | 40/175 (22.9%) | 19/38 (50.0%) | 0.001 | 10/20 (50.0%) | 49/193 (25.4%) | 0.019 |
| Cardiac disease | 9/213 (4.2%) | 5/175 (2.9%) | 4/38 (10.5%) | 0.092 | 3/20 (15.0%) | 6/193 (3.1%) | 0.053 |
| Hypertension | 30/213 (14.0%) | 20/175 (11.4%) | 10/38 (26.3%) | 0.017 | 4/20 (20.0%) | 26/193 (13.5%) | 0.645 |
| Diabetes | 14/213 (6.6%) | 11/175 (6.3%) | 3/38 (7.9%) | 0.999 | 1/20 (5.0%) | 13/193 (6.7%) | 1.000 |
| COPD | 4/213 (1.9%) | 2/175 (1.1%) | 2/38 (5.3%) | 0.147* | 2/20 (10.0%) | 2/193 (1.0%) | 0.045* |
| Chronic liver disease | 7/213 (3.3%) | 5/175 (2.9%) | 2/38 (5.3%) | 0.801 | 2/20 (10.0%) | 5/193 (2.6%) | 0.267 |
| Cancer | 4/213 (1.9%) | 4/175 (2.3%) | 0/38 (0.0%) | 1.000* | 0/20 (0.0%) | 4/193 (2.1%) | 1.000* |
| Cerebrovascular | 7/213 (3.3%) | 3/175 (1.7%) | 4/38 (10.5%) | 0.024 | 1/20 (5.0%) | 6/193 (3.1%) | 0.504 |
| Immunodeficiency disease | 2/213 (0.9%) | 2/175 (1.1%) | 0/38 (0.0%) | 1.000* | 0/20 (0.0%) | 2/193 (1.0%) | 1.000* |
| Symptoms and complications | All patients N = 213 |
Disease severity |
p
|
Presence of ICU/death |
p
|
||
| Mild/moderate n = 175 |
Severe/critical n = 38 |
Yes n = 20 |
No n = 193 |
||||
| Fever | |||||||
| 37.5–38.0°C | 50/208 (24.0%) | 39/174 (22.4%) | 11/34 (32.4%) | 0.215 | 7/16 (43.8%) | 43/192 (22.4%) | 0.106 |
| 38.1–39.0°C | 65/208 (31.3%) | 52/174 (29.9%) | 13/34 (38.2%) | 0.337 | 4/16 (25.0%) | 59/192 (30.7%) | 0.845 |
| >39.0°C | 11/208 (5.3%) | 7/174 (4.0%) | 4/34 (11.8%) | 0.154 | 2/16 (12.5%) | 9/192 (4.7%) | 0.447 |
| Chill | 20/210 (9.5%) | 14/175 (8.0%) | 6/35 (17.1%) | 0.172 | 4/17 (23.5%) | 16/193 (8.3%) | 0.105 |
| Coughing | 155/213 (72.8%) | 126/175 (72.0%) | 29/38 (76.3%) | 0.588 | 13/20 (65.0%) | 142/193 (73.6%) | 0.412 |
| Expectoration | 78/213 (36.7%) | 63/175 (36.0%) | 15/38 (39.5%) | 0.687 | 10/20 (50.0%) | 68/193 (35.2%) | 0.192 |
| Dyspnea | 42/213 (19.7%) | 21/175 (12.0%) | 21/38 (55.3%) | <0.001 | 11/20 (55.0%) | 31/193 (16.1%) | 0.001 |
| Diarrhea | 28/209 (13.4%) | 25/175 (14.3%) | 3/34 (8.9%) | 0.562 | 1/16 (6.3%) | 27/193 (14.0%) | 0.623 |
| Nausea | 17/209 (8.1%) | 14/180 (7.8%) | 3/34 (8.8%) | 1.000 | 1/16 (6.3%) | 16/193 (8.3%) | 1.000 |
| Vomit | 10/209 (4.8%) | 8/175 (4.6%) | 2/34 (5.9%) | 1.000 | 0/16 (0.0%) | 10/193 (5.2%) | 0.364 |
| Poor appetite | 23/209 (11.0%) | 15/175 (8.6%) | 8/34 (23.5%) | 0.024 | 5/16 (31.3%) | 17/193 (8.8%) | 0.017 |
| Sore throat | 20/209 (9.6%) | 18/175 (10.3%) | 2/34 (5.9%) | 0.631 | 0/16 (0.0%) | 20/193 (10.4%) | 0.362 |
| Dizziness | 12/209 (5.7%) | 8/175 (4.6%) | 4/34 (11.8%) | 0.212 | 1/16 (6.3%) | 11/193 (5.7%) | 1.000 |
| Headache | 20/209 (9.6%) | 15/175 (8.6%) | 5/34 (14.7%) | 0.427 | 3/16 (18.8%) | 17/195 (8.7%) | 0.392 |
| Muscular soreness | 15/209 (7.2%) | 11/175 (6.3%) | 4/34 (11.8%) | 0.442 | 2/14 (14.3%) | 13/195 (6.7%) | 0.596 |
| Fatigue | 76/209 (36.4%) | 56/175 (32.0%) | 20/34 (58.8%) | 0.003 | 8/16 (50.0%) | 68/191 (35.6%) | 0.238 |
| Dry/moist rale | 13/170 (7.6%) | 8/143 (5.6%) | 5/27 (18.5%) | 0.054 | 2/12 (16.7%) | 11/158 (6.9%) | 0.230 |
| Complications | |||||||
| ARDS | 11/195 (5.6%) | 0/168 (0.0%) | 11/27 (40.7%) | <0.001 | 10/15 (66.7%) | 1/180 (0.6%) | <0.001 |
| Shock | 3/195 (1.5%) | 0/168 (0.0%) | 3/27 (11.1%) | <0.001 | 3/15 (20.0%) | 0/180 (0.0%) | <0.001 |
| Acute kidney injury | 7/193 (3.6%) | 4/168 (2.4%) | 3/27 (11.1%) | 0.091 | 3/15 (20.0%) | 4/178 (2.2%) | 0.005 |
| Acute liver injury | 18/164 (10.9%) | 10/148 (6.8%) | 8/16 (50.0%) | <0.001 | 7/15 (46.7%) | 11/149 (7.4%) | <0.001 |
| Cardiac injury | 2/164 (1.2%) | 0/148 (0.0%) | 2/16 (12.5%) | 0.009 | 2/15 (13.3%) | 0/149 (0.0%) | 0.008 |
| DIC | 2/164 (1.2%) | 0/168 (0.0%) | 2/16 (12.5%) | 0.009 | 2/15 (13.3%) | 0/149 (0.0%) | 0.008 |
| MODS | 5/195 (2.6%) | 0/168 (0.0%) | 5/27 (18.5%) | <0.001 | 5/15 (33.3%) | 0/149 (0.0%) | <0.001 |
| Laboratory and CT findings | All patients N = 213 |
Disease severity |
p
|
Presence in ICU/death |
p
|
||
| Mild/moderate n = 175 |
Severe/critical n = 38 |
Yes n = 20 |
No n = 193 |
||||
| White cell | 4.75 (3.44–6.00) | 4.94 (3.34–7.06) | 4.70 (3.44–5.90) | 0.347 | 4.75 (3.44–5.88) | 5.27 (2.93–7.59) | 0.470 |
| <4 × 109/L | 75/213 (35%) | 63/175 (36.0%) | 12/38 (31.6%) | 0.605 | 7/20 (35.0%) | 68/193 (35.2%) | 0.983 |
| >10 × 109/L | 9/213 (4.22%) | 3/175 (1.7%) | 6/38 (15.8%) | 0.001 | 3/20 (15.0%) | 6/193 (3.1%) | 0.041* |
| Neutrophil | 2.91 (2.20–3.68) | 2.86 (2.13–3.57) | 3.44 (2.33–4.44) | 0.027 | 3.49 (1.99–5.59) | 2.89 (2.22–3.60) | 0.112 |
| <2 × 109/L | 43/213 (20.2%) | 37/175 (21.1%) | 6/38 (15.8%) | 0.456 | 5/20 (25.0%) | 38/193 (19.7%) | 0.787 |
| >7 × 109/L | 7/213 (3.3%) | 4/175 (2.3%) | 3/38 (7.9%) | 0.209 | 2/20 (10.0%) | 5/193 (2.6%) | 0.267 |
| Lymphocyte | 1.20 (0.8–1.67) | 1.31 (0.91–1.75) | 0.79 (0.62–1.11) | <0.001 | 0.73 (0.61–0.91) | 1.21 (0.87–1.70) | <0.001 |
| <0.8 × 109/L | 52/213 (24.4%) | 33/175 (18.9%) | 19/38 (50.0%) | <0.001 | 12/20 (60.0%) | 40/193 (20.7%) | <0.001 |
| >4 × 109/L | 3/213 (1.4%) | 3/175 (1.7%) | 0/38 (0.0%) | 1.000 | 0/20 (0.0%) | 3/193 (1.6%) | 1.000 |
| Hemoglobin | 130 (119–141) | 130 (120–141) | 129 (115–136) | 0.339 | 128 (114–140) | 130 (120–141) | 0.54 |
| <110 g/L | 18/209 (8.6%) | 15/175 (8.6%) | 3/34 (8.8%) | 1.000 | 2/16 (12.5%) | 16/177 (9.0%) | 0.910 |
| Blood platelet <100 × 109/L | 178 (139–229) | 182 (139–232) | 163 (137–205) | 0.301 | 153 (126–183) | 182 (139–231) | 0.125 |
| <100 × 109/L | 12/209 (5.7%) | 9/175 (5.1%) | 3/34 (8.8%) | 0.659 | 2/16 (12.5%) | 10/193 (5.2%) | 0.516 |
| PT | 11.6 (11–12.4) | 11.5 (10.9–12.2) | 12.2 (11.0–12.8) | 0.029 | 12.3 (10.9–12.8) | 11.6 (11.1–12.3) | 0.393 |
| >16 s | 2/213 (0.9%) | 1/175 (0.6%) | 1/38 (2.6%) | 0.326 | 1/20 (5.0%) | 1/193 (0.5%) | 0.179 |
| APTT | 32.4 (29.9–35.0) | 32.4 (30.2–35.0) | 31.9 (28.7–35.1) | 0.443 | 29.6 (26.2–34.8) | 32.4 (30.3–35.1) | 0.068 |
| <22 s | 2/213 (0.9%) | 2/175 (1.1%) | 1/38 (2.6%) | 0.397 | 1/20 (5.0%) | 2/193 (1.0%) | 0.257 |
| >53 s | 5/213 (2.3%) | 0/175 (0.0%) | 1/38 (2.6%) | 0.178 | 1/20 (5.0%) | 0/193 (0.0%) | 0.094 |
| CK | 64.3 (42.7–100.0) | 63.7 (42.1–90.4) | 83.3 (45.7–169.1) | 0.053 | 120.9 (47–386) | 63.9 (42.1–91.9) | 0.063 |
| >200 U/L | 19/187 (10.1%) | 9/174 (5.2%) | 10/32 (31.3%) | 0.009 | 4/15 (26.7%) | 15/191 (7.9%) | 0.050 |
| CK-MB | 9.2 (6.1–12.2) | 8.8 (5.9–11.9) | 10.8 (7.8–14.9) | 0.074 | 11.2 (7.9–26.8) | 9.1 (5.9–12.1) | 0.111 |
| >23 U/L | 15/206 (7.3%) | 9/173 (5.2%) | 6/33 (18.2%) | 0.024 | 3/15 (20.0%) | 12/191 (6.3%) | 0.146 |
| D-dimer | 0.27 (0.13–0.58) | 0.22 (0.12–0.53) | 0.53 (0.26–1.34) | <0.001 | 1.26 (0.27–5.18) | 0.26 (0.12–0.53) | <0.001 |
| >0.5 mg/L | 55/176 (31.3%) | 39/144 (27.1%) | 16/32 (50.0%) | 0.011 | 13/17 (76.5%) | 42/159 (26.4%) | <0.001 |
| Albumin | 38.2 (35.3–40.9) | 38.8 (36.4–41.9) | 32.9 (29.1–36.0) | <0.001 | 30.7 (28.0–35.6) | 38.5 (35.7–41.5) | <0.001 |
| ALT | 20.1 (14.2–30.0) | 19.7 (14.0–28.1) | 29.3 (19.7–25.6) | <0.001 | 41.0 (19.2–77.2) | 19.7 (14.0–28.3) | 0.001 |
| >40 U/L | 33/213 (15.5%) | 21/175 (12.0%) | 12/38 (31.6%) | 0.002 | 9/20 (45.0%) | 24/193 (12.4%) | <0.001 |
| AST | 23.7 (19.2–31.3) | 22.9 (18.2–25.8) | 30.5 (23.9–43.7) | <0.001 | 33.4 (24.2–64.1) | 23.3 (19.0–29.5) | 0.001 |
| >40 U/L | 27/213 (12.7%) | 17/175 (9.7%) | 10/38 (26.3%) | 0.012 | 8/20 (40.0%) | 19/193 (9.8%) | <0.001 |
| Total bilirubin | 10.8 (7.9–15.3) | 10.8 (7.9–15.3) | 11.6 (7.8–15.0) | 0.568 | 11.6 (7.8–15.9) | 10.8 (7.9–15.3) | 0.498 |
| >17.1 µmol/L | 44/213 (20.7%) | 35/175 (20.0%) | 9/38 (23.7%) | 0.611 | 4/20 (20.0%) | 40/193 (20.7%) | 1.000 |
| Creatinine | 51.1 (40.3–64.5) | 51.4 (40.8–66.6) | 48.1 (39.3–57.1) | 0.139 | 48.4 (38.9–66.9) | 51.3 (40.4–64.5) | 0.714 |
| >133 µmol/L | 5/212 (2.4%) | 3/175 (1.7%) | 2/27 (7.4%) | 0.133 | 2/19 (10.5%) | 3/191 (1.6%) | 0.066 |
| LDH | 163 (138–209) | 154 (132–205) | 197 (162–314) | <0.001 | 270 (184–362) | 158 (134–203) | <0.001 |
| >250 U/L | 27/212 (12.7%) | 14/175 (8.0%) | 13/37 (35.1%) | <0.001 | 10/20 (50.0%) | 17/192 (8.8%) | <0.001 |
| CRP | 12.7 (3.5–27.3) | 11.6 (3.0–24.5) | 26.1 (11.0–49.9) | <0.001 | 44.0 (10.2–71.5) | 12.3 (3.3–25.2) | 0.003 |
| >10 mg/L | 111/198 (56.1%) | 86/166 (51.8%) | 25/32 (78.1%) | 0.006 | 12/15 (80.0%) | 99/173 (57.2%) | 0.052 |
| Procalcitonin >0.5 ug/L | 5/210 (2.4%) | 3/174 (1.7%) | 2/36 (5.6%) | 0.204 | 2/17 (11.8%) | 3/188 (1.6%) | 0.066 |
| CT imaging | |||||||
| Single lung | 37/182 (20.3%) | 34/146 (23.3%) | 3/36 (7.7%) | 0.046 | 1/20 (5.0%) | 36/162 (22.2%) | 0.131 |
| Bilateral lung | 145/182 (79.7%) | 112/146 (76.7%) | 33/36 (91.7%) | 19/20 (95.0%) | 126/162 (77.3%) | ||
| Normal | 9/179 (5.0%) | 9/147 (6.1%) | 0/32 (0.0%) | 0.322 | 0/19 (0.0%) | 9/160 (5.6%) | 0.600* |
| Ground glass opacities | 161/172 (93.6%) | 139/146 (95.2%) | 22/24 (91.7%) | 0.426 | 10/13 (76.9%) | 151/159 (95.0%) | 0.172 |
| Consolidative/mixed opacities | 11/172 (6.4%) | 8/148 (5.4%) | 3/24 (12.5%) | 2/13 (15.4%) | 9/159 (5.7%) | ||
| Two left lobe | 62/159 (39.0%) | 48/136 (35.3%) | 14/23 (60.9%) | 0.066 | 6/11 (27.3%) | 56/148 (37.8%) | 0.538 |
| Three right lobe | 54/159 (34.0%) | 41/136 (30.1%) | 13/23 (56.5%) | 0.058 | 6/11 (27.3%) | 48/148 (32.4%) | 0.557 |
| Treatment | |||||||
| Antiviral therapy | 171/172 (99.4%) | 139/140 (96.3%) | 32/32 (100.0%) | 1.000* | 15/15 (100.0%) | 156/157 (96.7%) | 1.000* |
| Antibiotic therapy | 78/171 (45.6%) | 51/135 (37.8%) | 27/36 (75.0%) | <0.001 | 16/19 (84.2%) | 62/152 (40.8%) | <0.001 |
| Antifungal drug | 1/171 (0.6%) | 0/135 (0.0%) | 1/36 (2.8%) | 0.211* | 1/19 (5.3%) | 0/152 (0.0%) | 0.111* |
| Invasive mechanical ventilation | 2/213 (0.9%) | 0/175 (0.0%) | 2/38 (5.3%) | 0.031* | 2/20 (10.0%) | 0/193 (0.0%) | 0.008* |
| Continuous renal replacement therapy | 1/213 (0.5%) | 0/175 (0.0%) | 1/38 (2.6%) | 0.178* | 1/20 (5.0%) | 0/193 (0.0%) | 0.094* |
| Gamma globulin | 40/213 (18.8%) | 22/175 (12.6%) | 18/38 (47.4%) | <0.001 | 8/20 (40.0%) | 32/193 (16.6%) | 0.024 |
| Immunoplasma therapy | 7/213 (3.3%) | 3/175 (1.7%) | 4/38 (10.5%) | 0.024 | 4/20 (20.0%) | 3/193 (1.6%) | 0.002* |
| Death | 3/213 (1.4%) | 0/175 (0.0%) | 3/38 (7.9%) | 0.005* | 3/20 (15.0%) | 0/193 (0.0%) | 0.001* |
Using Fisher exact test.
ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; BMI, body mass index; CK, creatine kinase; CK-MB, creatine kinase-MB; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; CT, computed tomography; DIC, disseminated intravascular coagulation; ICU, intensive care unit; LDH, lactate dehydrogenase; MODS, multiple organ dysfunction syndrome; PT, prothrombin time.
Laboratory and computed tomography findings
In all patients, the most common abnormal laboratory examinations were higher C-reactive protein (56.1%) and D-dimer (31.3%), and lower white cell count (35.0%), monocyte count (24.4%), albumin (24.0%) and neutrophil count (20.2%). Elevation of creatine kinase (CK) (10.1%), alanine aminotransferase (ALT) (15.5%), aspartate aminotransferase (AST) (12.7%) and total bilirubin (20.7%) were also found in some patients. Laboratory findings are shown in Table 1.
In computed tomography (CT) imaging, the most common presentations were ground-glass opacities (93.6%) and bilateral lung involvement (79.7%), but consolidation was rare (6.4%) (Table 1; Figures 1–5). Involvement of two left lobes (39.0%) and three right lobes (34.0%) was also common. Five percent of patients had normal CT imaging.
Figure 1.
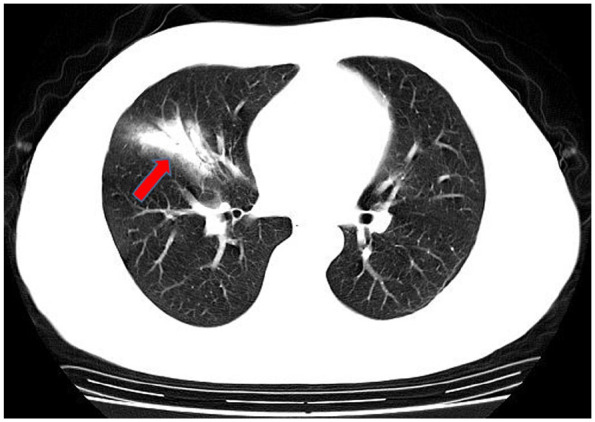
Unilateral lung consolidation opacities.
Figure 2.
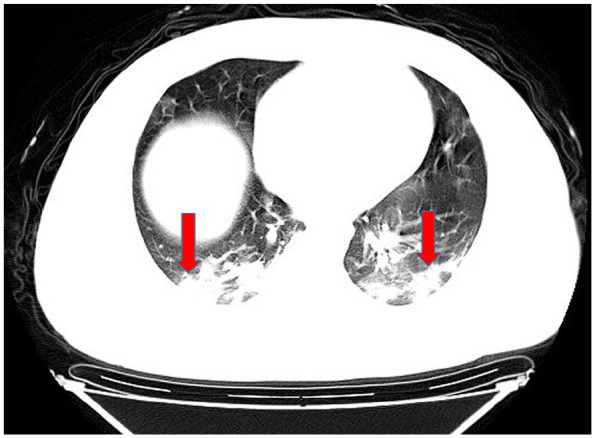
Bilateral lung consolidation opacities.
Figure 3.
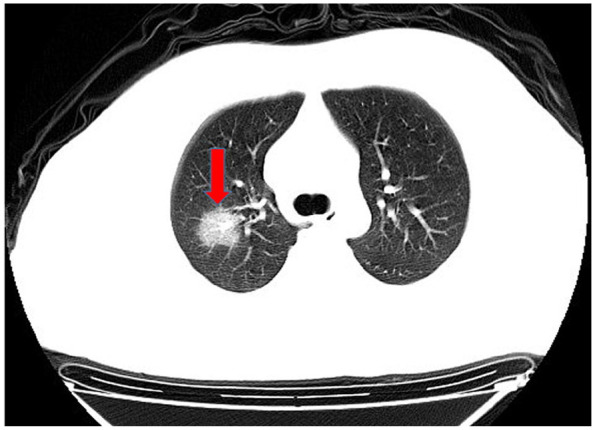
Unilateral lung ground-glass opacities.
Figure 4.
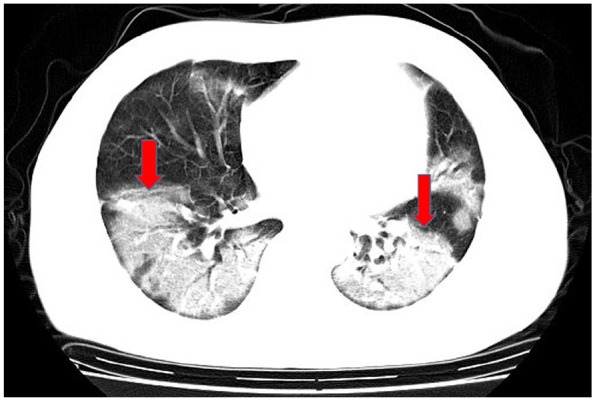
Bilateral lung ground-glass opacities.
Figure 5.
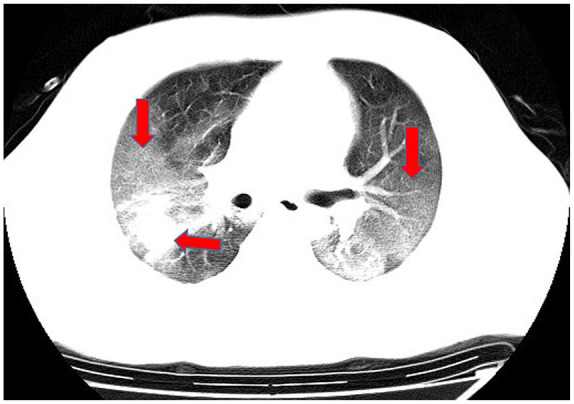
Ground-glass and consolidation opacities.
Clinical infective period, time to acute respiratory distress syndrome and ICU admission, and length of hospitalization
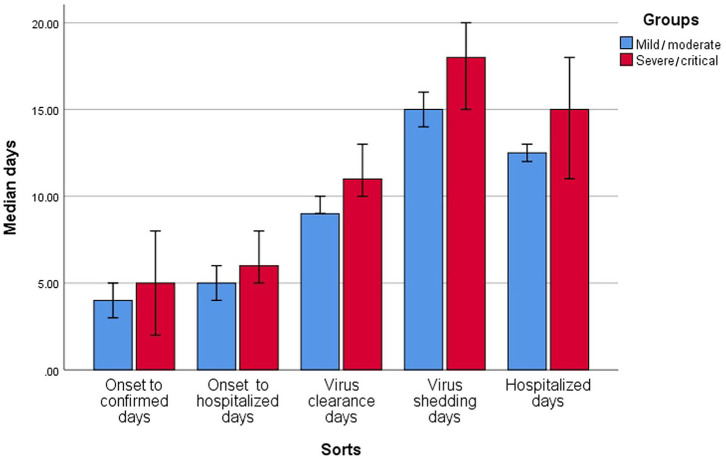
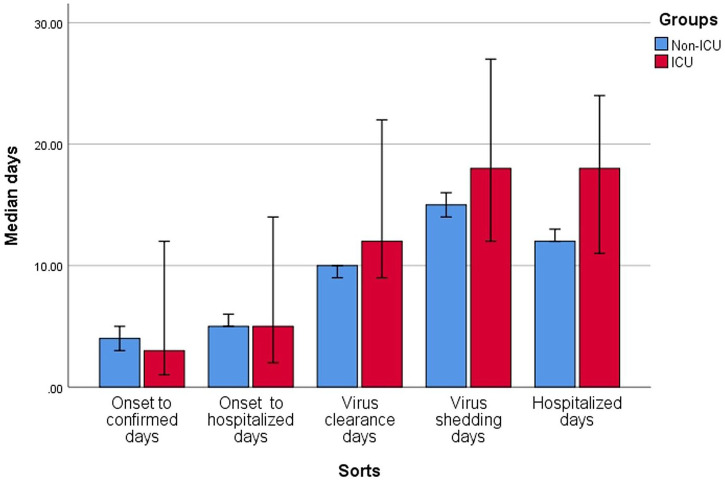
In all patients: the time from onset of symptoms to confirmation was 4 (2–8) days (longest 18 days); time from onset of symptoms to hospitalization was 5 (3–8) days (longest 21 days); viral clearance time was 10 (8–12) days (longest 30 days); and viral shedding time was 15 (11–19) days (longest 34 days). Time from hospital admission to occurrence of ARDS or admission to ICU was 1 (0–4) days and 2 (2–4) days, respectively, and median length of hospitalization was 13 (10–17) days (longest 32 days).
The severe/critical group compared with mild/moderate group had longer viral clearance time (median: 11 versus 9 days, p = 0.040) and viral shedding time (median: 18 versus 15 days, p = 0.015). The ICU group compared with non-ICU group had longer hospitalization (median: 18 versus 12 days, p = 0.003). Results are presented in Table 2 and Figures 6 and 7.
Table 2.
Clinical infective period, time to ARDS/ICU and hospitalized time.
| Variables | All | Mild/moderate | Severe/critical | p | ICU | Non-ICU | p |
|---|---|---|---|---|---|---|---|
| Onset of symptoms to confirmed days | 4 (2–8) | 4 (1–7) | 5 (2–8) | 0.587 | 3 (2–8) | 4 (2–8) | 0.939 |
| Onset of symptoms to hospitalized days | 5 (3–8) | 5 (3–8) | 6 (4–9.5) | 0.101 | 5 (3–10) | 5 (3–8) | 0.827 |
| Virus clearance days | 10 (8–12) | 9 (8–12) | 11 (9.5–15) | 0.040 | 12 (10–18) | 10 (8–12) | 0.050 |
| Virus shedding days | 15 (11–19) | 15 (11–18) | 18 (13–21) | 0.015 | 18 (13–21) | 15 (11–19) | 0.147 |
| Hospitalized days | 13 (10–17) | 12.5 (10–16) | 15 (11–20) | 0.169 | 18 (11–24) | 12 (10–16) | 0.003 |
| Hospitalization to ARDS days | 1 (0–4) | – | – | – | – | – | – |
| Hospitalization to ICU days | – | – | – | – | 2 (2–4) | – | – |
ARDS, acute respiratory distress syndrome; ICU, intensive care unit.
Figure 6.
Clinical infective and development time in severe/critical and mild/moderate groups.
Figure 7.
Clinical infective and development time in intensive care unit (ICU) and non-ICU groups.
Treatment and outcomes
A total of 99.4% of patients received antiviral therapy, and the most commonly used drugs were arbidol, lopinavir/ritonavir, interferon-α, novaferon and their different combined regimens; 45.6% of patients received antibiotic therapy; 27.5% received corticosteroid therapy (median time was 5 days); and 18.8% received gammaglobulin therapy. Four critical patients received convalescent plasma therapy. Three patients were treated with non-invasive ventilation, two with invasive ventilation and one with continuous renal replacement therapy.
Until the final follow-up date, 15 March 2020, the incidence of complications of ARDS was 5.6%, acute liver injury rate 10.9%, and other complications such as shock (1.5%), disseminated intravascular coagulation (DIC) (1.2%) and multiorgan dysfunction syndrome (2.6%) were rare. All complications (except acute kidney injury) were significantly higher in severe/critical or ICU groups than their matched groups (p < 0.05). Three patients died and all other patients were discharged.
Risk factors associated with severe/critical COVID-19 and ICU admission
Compared with the mild/moderate COVID-19 groups, the severe/critical groups were significantly older (53 versus 41 years); had higher cluster exposure history (100% versus 71.5%), hypertension (26.3% versus 11.4%), dyspnea (55.3% versus 12.0%), poor appetite (23.5% versus 8.6%), fatigue (58.8% versus 32.0%), and white cell count, neutrophil count, CK, CK-MB, D-dimer, prolonged prothrombin time (PT), ALT, AST, lactate dehydrogenase (LDH), C-reactive protein (CRP); lower monocyte count and albumin; and more bilateral lung involvement (100.0% versus 76.7%) (p < 0.05).
The ICU group compared with non-ICU group had significantly older age (57.5 versus 41.0 years); more chronic obstructive pulmonary disease (COPD) (10.0% versus 1.0%), dyspnea (55.0% versus 16.1%) and poor appetite (31.3% versus 8.8%); higher leukocyte count, D-dimer, ALT, AST, LDH, and CRP; and lower monocyte count and albumin (p < 0.05). Results are presented in Table 1.
Logistic regression analysis for OR of risk factors
We entered the above significant risk factors into logistic univariate analysis to calculate each OR and statistical significance. Except for clustered exposure history (p = 0.836) and involvement of both lungs in CT (p = 0.204), all the other factors were associated with the risk of severe/critical COVID-19: age (OR = 1.03), dyspnea (OR = 12.57), hypertension (OR = 2.77), poor appetite (OR = 3.16), fatigue (OR = 3.04), higher white cell count (OR = 10.75), neutrophil count (OR = 1.30), PT (OR = 1.32), CK (OR = 4.42), CK-MB (OR = 4.23), D-dimer (OR = 2.69), ALT (OR = 3.39), AST (OR = 3.32), LDH (OR = 6.75), CRP (OR = 3.28), and lower lymphocyte count (OR = 4.30) and albumin (OR = 9.86) (p < 0.05). Except CRP (p = 0.068), all the other risk factors were significantly associated with ICU admission: age (OR = 1.04), COPD (OR = 5.50), dyspnea (OR = 12.19), poor appetite (OR = 4.05), higher white cell count (OR = 5.50), D-dimer (OR = 7.18), ALT (OR = 7.39), AST (OR = 6.11), LDH (OR = 12.29), and lower lymphocyte (OR = 5.74) and albumin (OR = 11.35) (p < 0.05).
Multivariate logistic analysis showed that independent risk factors associated with the severe/critical group were dyspnea (OR = 19.48), ALT (OR = 6.02) and albumin (OR = 3.36) (p < 0.05), and independent risk factors associated with ICU admission were dyspnea (OR = 8.88), COPD (OR = 31.80), D-dimer (OR = 8.37), ALT (OR = 28.76) and LDH (OR = 9.95) (p < 0.05). The logistic regression results are presented in Table 3.
Table 3.
Logistics regression analysis for OR value.
| Univariate analysis |
Severe/critical versus mild/moderate |
ICU versus non-ICU |
||
|---|---|---|---|---|
| Variables | OR | p | OR | p |
| Age | 1.03 (1.01–1.06) | 0.015 | 1.04 (1.01–1.07) | 0.022 |
| Dyspnea | 12.57 (5.64–28.02) | <0.001 | 12.19 (4.35–34.18) | <0.001 |
| Cluster exposure history | 0.91 (0.39–2.14) | 0.836 | – | – |
| Hypertension | 2.77 (1.17–6.54) | 0.020 | – | – |
| COPD | – | – | 10.61 (1.41–78.88) 0.022 | 0.022 |
| Poor appetite | 3.16 (1.22–8.17) | 0.018 | 4.05 (1.28–12.80) | 0.017 |
| Fatigue | 3.04 (1.43–6.45) | 0.004 | – | – |
| Two lung involvement | 2.27 (0.64–8.01) | 0.204 | – | – |
| WBC >10 × 109/L | 10.75 (2.56–42.21) | 0.001 | 5.50 (1.26–23.97) | 0.023 |
| Neutrophil | 1.30 (1.06–1.59) | 0.011 | ||
| Lymphopenia <0.8 × 109/L | 4.30 (0.25–9.02) | <0.001 | 5.74 (2.20–14.98) | <0.001 |
| PT | 1.32 (1.02–1.72) | 0.037 | ||
| CK >200 U/L | 4.42 (1.31–14.93) | 0.017 | – | – |
| CK-MB >23 U/L | 4.23 (1.39–12.87) | 0.011 | – | – |
| D-dimer >0.5 mg/L | 2.69 (1.23–5.90) | 0.013 | 7.18 (2.41–21.36) | <0.001 |
| Albumin <35 g/L | 9.86 (4.25–22.89) | <0.001 | 11.35 (3.76–34.28) | <0.001 |
| ALT >40 U/L | 3.39 (1.49–7.70) | 0.004 | 7.39 (2.78–19.67) | <0.001 |
| AST >40 U/L | 3.32 (1.38–7.99) | 0.007 | 6.11 (2.22–16.80) | <0.001 |
| LDH >250 U/L | 6.75 (2.80–16.28) | <0.001 | 12.29 (4.36–34.63) | <0.001 |
| CRP >10 mg/L | 3.28 (1.35–8.01) | 0.009 | 3.35 (0.92–12.29) | 0.068 |
| Multivariate analysis | ||||
| Dyspnea | 19.48 (5.65–67.19) | <0.001 | 8.88 (1.54–51.07) | 0.014 |
| COPD | – | – | 31.80 (2.21–457.65) | 0.011 |
| D-dimer | – | – | 8.37 (1.33–52.86) | 0.024 |
| Albumin | 3.36 (1.06–10.67) | 0.040 | – | – |
| ALT | 6.02 (1.50–24.19) | 0.011 | 28.76 (4.19–197.24) | 0.001 |
| LDH | – | – | 9.95 (1.83–54.09) | 0.008 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; CK-MB, creatine kinase-MB; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; ICU, intensive care unit; LDH, lactate dehydrogenase; OR, odds ratio; PT, prothrombin time; WBC, white blood cell.
Discussion
Our study described the epidemiological and clinical characteristics of COVID-19 and identified the risk factors for disease severity. Patients in this study had a low exposure to Huanan Seafood Market (0.7%) compared with patients in Wuhan (49–66%).10,11 History of smoking was low, although clustered exposure history, visiting Wuhan, and imported disease all accounted for some cases, which agree with the strong infectious characteristics of COVID-19.5,7
Most of the patients’ clinical manifestations were similar to those in Wuhan studies:10,12,13 fever (80–98%), cough (76–82%), shortness of breath (31%), and fatigue (32.1–44%). However, for laboratory examination results, compared with studies from Wuhan, we found a lower rate of leukocytosis (4.22% versus 24–30%), lymphopenia (24.4% versus 35–63%),2,12 mortality rate (1.4% versus 11–28.3%)2,10 and ARDS (5.6% versus 17–41.8%).3,10 These characteristics indicated milder severity of COVID-19 outside of Wuhan. Why should this be? One report14 showed that, from 22 January to 2 March 2020, the mortality rates in Wuhan declined continuously, while those outside Wuhan remained constant. This was due to lack of medical workers and material resources in the early stage of the epidemic. During the outbreak, a large number of health workers from all over the country were dispatched to Wuhan, and acute care beds, temporary hospitals and medical resources were provided, so the mortality curve in Wuhan declined. Our results were consistent with other studies outside of Wuhan.4,7 In Chen’s study, the discharge rate was 87.2%, mortality rate 0.8%, ARDS rate 3.2%, and ICU admission rate 8.8%.4 In Yang’s study, the discharged rate was 49% and mortality, ARDS, and ICU admission rates were all 0%.7
The median time from onset of symptoms to confirmation in our study was 4 days, which was shorter than Cao’s study in Wuhan (9 days).15 The median time from onset of symptoms to hospitalization was 5 days, which was shorter than studies in Wuhan (6–7 days).12,15 These features indicate that, after the outbreak of COVID-19 in Wuhan, awareness of seeing a doctor had increased and government management had been effective. However, we found that the longest time to confirmation and hospitalization was 18 and 21 days, respectively, and these patients were “superspreaders.”
Previous studies reported that time from onset of symptoms to virus clearance was 11 and 14 days.4,16 We found that the time from confirmation to virus clearance was 10 days, and 14 days if we added the time from symptom onset to confirmation. All three studies showed that virus clearance time in severe/critical patients was longer than in non-severe/critical patients. One study reported that virus shedding time in Wuhan was a median 20 days (longest 37 days).2 In our study, the time was 15 days (longest 35 days), with 18 days in the severe/critical group versus 15 days in the mild/moderate group, which suggested that patients in Wuhan had more serious COVID-19 than those outside Wuhan. We found that virus shedding time was associated with the longest clinical infective period, which might bring trouble to the control of virus transmission.
Some patients with severe COVID-19 may rapidly develop ARDS or require ICU admission, and timely intervention is important. The median time from hospital admission to ARDS and ICU admission was 1 (0–4) and 2 (2–4) days, respectively, compared with the time from hospital admission to ICU admission in Wuhan of 1 (0–3) days.17
Older age and comorbidities were poor predictors of COVID-19. Older age was proved to be associated not only with severity of COVID-19,2,16 but also with Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome.18,19 In previous studies of COVID-19, the median of age in severe/critical, ICU and non-survivor groups was higher than mild/moderate, non-ICU and survivor groups (53.7 years versus 41.9 years, 66 years versus 51 years and 69 years versus 52 years, all p < 0.001).2,6,17 Besides, in our study, if age was calculated as a categorical variable (>50 years versus <50 years), the age >50 years increased risk of severe/critical admission 2.27 times (p = 0.025) and ICU admission 3.75 times (p = 0.01) (Supplemental Material Table online). One of the molecular mechanisms may be that older animals have stronger innate inflammation responses to viral infection than younger animals.20 Another reason was that older people generally combined more comorbidities, however, the latter were associated with the severity of COVID-19, even death, such as hypertension, cardiac disease, COPD or diabetes.2,6,16,17 As reported, the total incidence of comorbidities in severe/critical, ICU and non-survivor groups was higher than in their comparative groups [44% (11/25) versus 6.9% (4/58), 72.2% (26/36) versus 37.3% (38/102) and 67% (36/54) versus 40% (55/137), all p ⩽ 0.001].2,6,17 The incidence of hypertension in severe, ICU group and non-survivor groups was higher than in their comparative groups [37.9% (22/58) versus 10.4% (25/240), 58.3% (21/36) versus 21.6% (22/102) and 48% (26/54) versus 23% (32/137), all p < 0.001].2,16,17 Hypertension significantly increased the risk of death 3.05 times in Zhou’s study;2 in the current study it increased the risk of severe/critical admission 2.77 times. COVID-19 exacerbated the burden of heart through producing massive inflammation in the lung; hypertension combined with COVID-19 may display a synergistic effect on cardiovascular events. As to whether angiotensin-converting enzyme (ACE) inhibitors of hypertension patients altered ACE2 expression and influenced the virulence of COVID-19 infection, there was no definitive evidence.21 Cardiac disease was also related to severity of COVID-19; its incidence was higher in severe, ICU and non-survivor groups than in their comparative groups.2,16,17 In our study, cardiac disease rate was numerically higher in severe/critical and ICU groups; it nearly reached statistical significance in ICU versus non-ICU groups (p = 0.053). Besides cardiovascular diseases, COPD also was another important risk factor of COVID-19, as shown in the literature; its incidences in severe/critical and non-survivor groups were higher than in their comparative groups [16.0% (4/25) versus 1.7% (1/58) and 7% (4/54) versus 1% (2/137), all p < 0.05].2,6 In this study, COPD increased the risk of ICU admission by 5.50 times; most importantly, it was an independent predictor of ICU admission (adjusted OR = 31.80). Obstructive ventilatory disorder was the main feature of COPD. COVID-19, through producing massive inflammation, causes lung consolidation and induces restrictive and diffusive function defects. Therefore, comorbid COPD might have an additive effect and cause deterioration in COVID-19.
Dyspnea is the most obvious manifestation and primary driver of COVID-19, with an incidence of 17.8–55%,5,12 and severe dyspnea is associated with severity of COVID-19.6,12,17 In Li’s study (N = 83) it significantly increased the risk of severe/critical admission by 10.9 times;6 in the current study, dyspnea was one of the most distinct risk factors, being independently associated with both severe/critical and ICU admission (adjusted OR = 19.48 and 8.88).
There were several important laboratory risk factors for severe/critical or ICU admission. Elevation of leukocytes and reduction of lymphocytes are associated with severe or fatal COVID-19.2,6,12,17 In Zhou’s study higher white cell count increased the risk of death by 6.6 times,2 in Li’s study lower lymphocyte count increased risk of severe/critical admission by 12.0 times.6 A high leukocyte count is related to the cytokine storm induced by virus invasion, and low lymphocyte count is related to coronavirus-mediated, severe immune injury.22 Low albumin level is also a result of immune consumption, which is an equally important poor prognosis factor.2,12 In the present study, all of the factors higher white cell, lower lymphocyte and albumin increased the risk of severe/critical and ICU admission, and albumin was an independent predictor of severe/critical group (adjusted OR = 3.36). The cytokine storm activates the coagulation system, which causes elevation of D-dimer and prolonged PT. High D-dimer might cause small thromboses and ischemia in lung capillaries, which trigger the occurrence of dyspnea/ARDS or DIC,23 and it is also associated with ICU admission or death.2,12,17 LDH is a tissue injury indicator that is commonly found in all kinds of tissue, including the lungs, so its elevation indicates a more serious inflammatory response in COVID-19, which is associated with ICU admission or death.2,12,16 CRP, an inflammatory factor, is more common than procalcitonin in COVID-19, and it has similar clinical importance to D-dimer and LDH in severe or critical patients.3,4,16 Although elevation of ALT is not more common than that of D-dimer, LDH and CRP, they are more likely to occur in severe or critical patients, and even patients who die,2,17,24 it increased risk of death by 2.87 times.2 In the current study, D-dimer and LDH were independently associated with ICU admission, ALT was independently associated with both severe/critical and ICU admission (adjusted OR = 6.02 and 28.76).
Our study had some limitations. First, we included a small number of cases in the critical group (n = 10) and severe group (n = 28), and we did not carry out a comparison between the critical and severe groups. The number of patients in the ICU group was also limited (n = 20) so more cases and multicenter studies are needed outside of Wuhan. Second, the mortality rate was low in our study, so we could not identify the mortality risk factors. Third, we need to investigate more inflammatory factors such as interleukin-2 and -6.
In conclusion, this study described some different characteristics of COVID-19 patients outside of Wuhan, such as fewer complications, lower mortality rate and milder disease severity. We described some important features of the clinical infective period, which may be useful to hospital and government administration. There were many risk factors associated with the severity of COVID-19, among which the most frequent were dyspnea, higher ALT, D-dimer and LDH, and lower albumin. Other factors, such as older age, comorbidity, higher leukocyte count and CRP and lower lymphocyte count, were also important. COVID-19 is prevalent worldwide, and these characteristics may provide references for clinical judgment and early intervention, and may be beneficial to our overall understanding of COVID-19.
Supplemental Material
Supplemental material, Author_Response_1 for Clinical characteristics and risk factors for severity of COVID-19 outside Wuhan: a double-center retrospective cohort study of 213 cases in Hunan, China by Xingsheng Hu, Chunhong Hu, Yong Yang, Juan Chen, Ping Zhong, Yajing Wen and Xiangyu Chen in Therapeutic Advances in Respiratory Disease
Supplemental material, Author_Response_2 for Clinical characteristics and risk factors for severity of COVID-19 outside Wuhan: a double-center retrospective cohort study of 213 cases in Hunan, China by Xingsheng Hu, Chunhong Hu, Yong Yang, Juan Chen, Ping Zhong, Yajing Wen and Xiangyu Chen in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Clinical characteristics and risk factors for severity of COVID-19 outside Wuhan: a double-center retrospective cohort study of 213 cases in Hunan, China by Xingsheng Hu, Chunhong Hu, Yong Yang, Juan Chen, Ping Zhong, Yajing Wen and Xiangyu Chen in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Clinical characteristics and risk factors for severity of COVID-19 outside Wuhan: a double-center retrospective cohort study of 213 cases in Hunan, China by Xingsheng Hu, Chunhong Hu, Yong Yang, Juan Chen, Ping Zhong, Yajing Wen and Xiangyu Chen in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.2 for Clinical characteristics and risk factors for severity of COVID-19 outside Wuhan: a double-center retrospective cohort study of 213 cases in Hunan, China by Xingsheng Hu, Chunhong Hu, Yong Yang, Juan Chen, Ping Zhong, Yajing Wen and Xiangyu Chen in Therapeutic Advances in Respiratory Disease
Supplemental material, Supplementary_table for Clinical characteristics and risk factors for severity of COVID-19 outside Wuhan: a double-center retrospective cohort study of 213 cases in Hunan, China by Xingsheng Hu, Chunhong Hu, Yong Yang, Juan Chen, Ping Zhong, Yajing Wen and Xiangyu Chen in Therapeutic Advances in Respiratory Disease
Footnotes
Author contribution(s): Xingsheng Hu: Conceptualization; Data curation; Formal analysis; Methodology; Writing-original draft.
Chunhong Hu: Data curation; Investigation; Methodology; Writing-original draft.
Yong Yang: Data curation; Formal analysis; Investigation; Resources; Writing-review & editing.
Juan Chen: Data curation; Investigation; Resources; Writing-review & editing.
Ping Zhong: Data curation; Investigation; Writing-original draft.
Yajing Wen: Investigation; Resources; Writing-review & editing.
Xiangyu Chen: Conceptualization; Supervision; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: Natural Science Foundation of Hunan Province, NO. 2019JJ40435.
ORCID iD: Xiangyu Chen  https://orcid.org/0000-0002-4233-8822
https://orcid.org/0000-0002-4233-8822
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Xingsheng Hu, Department of Oncology, The Second Xiangya Hospital of Central South University, Changsha, Hunan, P.R. China.
Chunhong Hu, Department of Oncology, The Second Xiangya Hospital of Central South University, Changsha, Hunan, P.R. China.
Yong Yang, Department of Intensive care unit, Changsha Central Hospital of Nanhua University, Changsha, Hunan, P.R. China.
Juan Chen, Department of Radiology, The Central Hospital of Xiangtan, Hunan, Xiangtan, Hunan, P.R. China.
Ping Zhong, Department of Dermatology, Nanchong Central Hospital, Nanchong, Sichuan, P.R. China.
Yajing Wen, Department of Clinical Medicine, Chengdu Medical College, Chengdu, Sichuan, P.R. China.
Xiangyu Chen, Department of Radiology, The Second Xiangya Hospital of Central South University, No.139 People Road of Changsha City, 410011 Changsha, Hunan, P.R. China; Department of Radiology, The Second Xiangya Hospital of Central South University, Changsha, Hunan, P.R. China.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180: 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen J, Qi T, Liu L, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect 2020; 80: e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol 2020; 55: 327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect 2020; 80: 388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Health Commission of the People’s Republic of China. Diagnosis and treatment protocol for novel coronavirus pneumonia. 7th interim ed., http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. (accessed 20 March 2020).
- 9. World Health Organization. Coronavirus disease (COVID-19) technical guidance: laboratory testing for 2019-nCoV in humans, https://www.who.int/publications/m/item/molecular-assays-to-diagnose-covid-19-summary-table-of-available-protocols
- 10. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu K, Fang Y-Y, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020; 133: 1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Z, Yao W, Wang Y, et al. Wuhan and Hubei COVID-19 mortality analysis reveals the critical role of timely supply of medical resources. J Infect 2020; 81: 147–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cao J, Tu W-J, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis 2020; 71: 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy 2020; 75: 1742–1752. [DOI] [PubMed] [Google Scholar]
- 17. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi KW, Chau TN, Tsang O, et al. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med 2003; 139: 715–723. [DOI] [PubMed] [Google Scholar]
- 19. Hong K-H, Choi J-P, Hong S-H, et al. Predictors of mortality in Middle East respiratory syndrome (MERS). Thorax 2018; 73: 286–289. [DOI] [PubMed] [Google Scholar]
- 20. Smits SL, de Lang A, van den Brand JMA, et al. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog 2010; 6: e1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Drager LF, Pio-Abreu A, Lopes RD, et al. Is hypertension a real risk factor for poor prognosis in the COVID-19 pandemic? Curr Hypertens Rep 2020; 22: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu W, Tao ZW, Lei W, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020; 133: 1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang G, Zhang J, Wang B, et al. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res 2020; 21: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_1 for Clinical characteristics and risk factors for severity of COVID-19 outside Wuhan: a double-center retrospective cohort study of 213 cases in Hunan, China by Xingsheng Hu, Chunhong Hu, Yong Yang, Juan Chen, Ping Zhong, Yajing Wen and Xiangyu Chen in Therapeutic Advances in Respiratory Disease
Supplemental material, Author_Response_2 for Clinical characteristics and risk factors for severity of COVID-19 outside Wuhan: a double-center retrospective cohort study of 213 cases in Hunan, China by Xingsheng Hu, Chunhong Hu, Yong Yang, Juan Chen, Ping Zhong, Yajing Wen and Xiangyu Chen in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Clinical characteristics and risk factors for severity of COVID-19 outside Wuhan: a double-center retrospective cohort study of 213 cases in Hunan, China by Xingsheng Hu, Chunhong Hu, Yong Yang, Juan Chen, Ping Zhong, Yajing Wen and Xiangyu Chen in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Clinical characteristics and risk factors for severity of COVID-19 outside Wuhan: a double-center retrospective cohort study of 213 cases in Hunan, China by Xingsheng Hu, Chunhong Hu, Yong Yang, Juan Chen, Ping Zhong, Yajing Wen and Xiangyu Chen in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.2 for Clinical characteristics and risk factors for severity of COVID-19 outside Wuhan: a double-center retrospective cohort study of 213 cases in Hunan, China by Xingsheng Hu, Chunhong Hu, Yong Yang, Juan Chen, Ping Zhong, Yajing Wen and Xiangyu Chen in Therapeutic Advances in Respiratory Disease
Supplemental material, Supplementary_table for Clinical characteristics and risk factors for severity of COVID-19 outside Wuhan: a double-center retrospective cohort study of 213 cases in Hunan, China by Xingsheng Hu, Chunhong Hu, Yong Yang, Juan Chen, Ping Zhong, Yajing Wen and Xiangyu Chen in Therapeutic Advances in Respiratory Disease