Abstract
Childhood uveitis comprises a collection of heterogenous ocular phenotypes which are associated with a diverse range of childhood autoimmune and autoinflammatory disorders. Of these genetic and/or acquired disorders, juvenile idiopathic arthritis is the most common, affecting 30-80% of children with uveitis. Up to a third of children with uveitis have ‘isolated’ idiopathic disease and do not have an associated systemic disease which manifests in childhood. However, uveitis may be the presenting manifestation of disease; thus, the apparently well child who presents with uveitis may have isolated idiopathic disease, but they may have an evolving systemic disorder. The diagnosis of most of the associated disorders is reliant on clinical features rather than serological or genetic investigations, necessitating detailed medical history taking and systemic examination. Adequate control of inflammation is key to good visual outcomes, and multidisciplinary care is key to good broader health outcomes.
Keywords: autoimmune, autoinflammation, child, uveitis
Introduction
Uveitis, a descriptive term, encompasses a heterogeneous group of inflammatory eye disorders which are classified by anatomy (anterior, intermediate, posterior or ‘pan’uveitis; Table 1), by cause (e.g. infectious versus noninfectious, idiopathic versus known disorder) or by the occurrence of an associated systemic disorder [e.g. with juvenile idiopathic arthritis (JIA)].1
Table 1.
| Type | Primary site of inflammation | Manifest conditions include |
|---|---|---|
| Anterior uveitis | Inflammation of the anterior chamber affecting the iris and anterior ciliary body | Iritis Iridocyclitis Anterior cyclitis |
| Intermediate uveitis | Inflammation of the vitreous | Pars planitis Posterior cyclitis Hyalitis |
| Posterior uveitis | Inflammation of the retina or choroid | Focal or diffuse choroiditis Chorioretinitis Retinochoroiditis Retinitis Neuroretinitis Retinal vasculitis |
| Panuveitis | Inflammation of the anterior chamber, vitreous and retina or choroid |
The Standardization of Uveitis Nomenclature (SUN) group have provided a framework for uveitis research and clinical practice.
The uvea is the highly vascularized middle tract of the eye and the primary intraocular site for resident immune-competent cells. Infection, trauma or as-yet poorly understood inflammatory and immune-mediated processes can trigger abnormal responses leading to intraocular inflammation.
Childhood onset of uveitis is uncommon and typically chronic, relapsing or recurrent, with prolonged disease activity resulting in irreversible ocular structural damage.2,3 There is a paucity of robust evidence on the prevalence or incidence of paediatric uveitis. An often-cited UK annual incidence of 5 new cases per 100,000 children is derived from a 2003 retrospective observational study across three primary care centres with noncoincident ascertainment periods (1993–1994 at one centre, 1995 at another, 1996–2001 at the other).2 This incidence is similar to that reported by another European population-based study, which estimated the Finnish national annual incidence at 4/100,000, and the prevalence at 28/100,000 [95% confidence interval (CI), 17.1–38.6].4 Such a prevalence would suggest an approximate 3–6000 UK children living with uveitis. However, both these population-based studies on disease frequency are limited by their retrospective nature and incomplete national coverage.
Adult uveitis differs from childhood-onset disease in several ways. The most common paediatric disease manifestation (up to 90% of cases) is chronic anterior uveitis, which is less common in adult-onset disease.5,6 Children with uveitis exhibit a different spectrum of disease association than that seen in the adult population.2,5,6 Inflammatory sequelae such as glaucoma and cataract are more challenging to manage in children.7–9 There are also the additional obstacles of amblyopia, managing a chronic disease in a developing child and the difficulties of effective transition to adult care services.
Immunopathogenesis of uveitis
The eye is thought to be an immune-privileged site, in that immune responses to apparently non-self-antigens are suppressed, preventing the collateral damage to ‘innocent bystander’ tissue that might occur in such a response. This offers protection for sites such as the brain, joint capsules and the eye, which have vital highly specialized roles in human function and limited capacity for regeneration.10 With immune privilege comes the absence of the full complement of cells and factors which have evolved to mitigate the inflammatory response. Consequently, breaches of the immune-privileged sites can overwhelm the resident protective systems, resulting in significant tissue damage.
In noninfectious uveitis, the cause of the breach of immune privilege is not yet understood, but what is clear is that it results in significant expansion of the intraocular expression of proinflammatory agents such as cytokines, chemokines and complement.11 The expression of these agents is mediated through either autoimmune (abnormal innate or immune response to self) or autoinflammatory (disordered adaptive or response to environmental signals) pathways.
Histopathological investigations have suggested different pathogenic pathways for childhood uveitis. Subsets of CD4+ and CD8+ T cells are thought to have either pathogenic (e.g. CD4+ Th17 cell lines) or protective (e.g. CD8+ Treg) roles, or in some cases both (Cd4+ γδT cells).12 Animal models of uveitis, particularly experimental autoimmune uveitis (EAU) in which a susceptible animal is immunized with retinal derived proteins, have provided valuable information on disease pathogenesis. This includes identifying tumour necrosis factor alpha (TNFα) as a therapeutic target, leading to the development of anti-TNFα monoclonal antibodies, such as adalimumab.13 No single animal model encapsulates the broad, heterogeneous spectrum of human disease. Plasma cells have been found to be the predominant cell type within ocular tissue samples of children with juvenile idiopathic arthritis–associated uveitis (JIAU). This finding suggests that the pathogenesis of JIAU may be antibody mediated and therefore more on the autoimmune, rather than autoinflammatory, end of the spectrum of diseases.14
Autoimmune versus autoinflammatory disorders associated with childhood uveitis
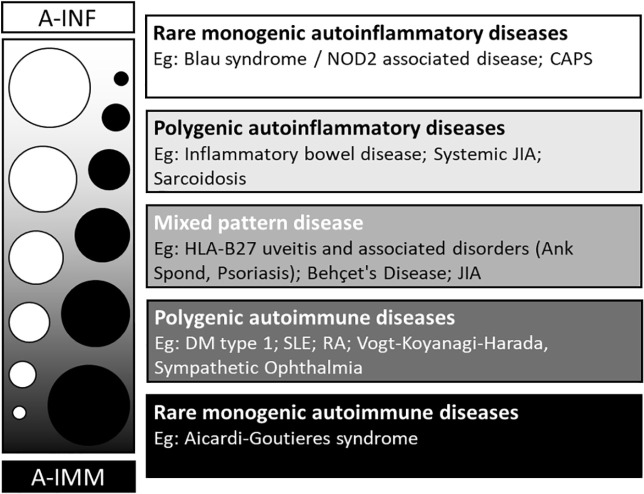
In autoimmune disorders, there is self-directed inflammation, involving aberrant dendritic, B and T cells in response to native antigens.15 Pure autoimmune disease can become organ specific as aberrant major histocompatibility complex (MHC) expression and autoantibody formation develops over a number of years towards the target organ(s). In contrast, the self-directed inflammatory processes in autoinflammatory diseases are in response to so-called ‘danger’ signals which activate the innate immune cells, including macrophages, natural killer cells and neutrophils. These ‘danger’ signals may come in the form of non-self-antigens or tissue microdamage. Mutations in inflammasome-related genes have been strongly associated with autoinflammatory disease and may also be involved in autoimmune disorders.16–20 Inflammasomes are a group of powerful intracellular protein complexes which sense a wide range of inflammatory ‘danger’ signals and stimulate the release of the pro-inflammatory interleukin (IL) cytokines, IL-1β and IL-18. The best characterized members of the inflammasome complex are the nucleotide-binding and oligomerization domain (Nod)-like receptors (NLRs: NLRP1, NLRP3, NLRC4) and the absent in melanoma 2 (AIM2)-like receptors (ALRs). Rather than there being a distinct division between autoinflammatory and autoimmune disorders, immunological disease can be classified using a continuous ‘spectrum’ model which runs between the two (Figure 1). Uveitis is a well-recognized feature of autoinflammatory and mixed disorders, but is less commonly seen in predominantly autoimmune disorders (Table 2).
Figure 1.
The spectrum of autoinflammation and autoimmunity.
Source: Adapted from McGonagle and colleagues.15
A-IMM, autoimmunity; A-INF, autoinflammation; Ank Spond, ankylosing spondylitis; CAPS, cryopyrin-associated periodic syndromes; DM, diabetes mellitus; HLA, human leukocyte antigen; JIA, juvenile idiopathic arthritis; NOD2, nucleotide-binding oligomerization domain containing 2; RA, rheumatoid arthritis; SLE, systemic lupus erythematous.
Table 2.
| Ocular manifestations of autoinflammatory and autoimmune disease. | Disease, % childhood uveitis casesa
(HLA/genetic association) |
Systemic involvement | Common ocular phenotypes | Cited prevalence of uveitis |
|---|---|---|---|---|
| Monogenic autoinflammatory disease(s) | Blau syndrome, <1% (NOD2/CARD15) |
Skin, joints | Panuveitis with multifocal chorioretinitis | 78% (n = 38/50)21
Early-onset disease |
| Cryopyrin-associated periodic syndromes, <0.1% (NLRP3/CIAS1) |
Skin, joints, liver, deafness, meningitis | Anterior uveitis | 55% (n = 17/31)22 | |
| Polygenic autoinflammatory disease(s) | Inflammatory bowel disease, <0.1% (NOD2/CARD15 HLA DRB1*1502, 0103) |
Bowel, arthritis | Anterior uveitis | 0.62–1.82% More prevalent in Crohn’s than ulcerative colitis23 |
| Sarcoid, 2–3% (HLA-DRB1*0301, *0401, DQB1*0301) |
Skin, joints, lung (less common than in adults), liver | Chronic anterior uveitis, panuveitis | 24–58%24–26 | |
| TINU syndrome, <1% (HLA-DRB1*0102) |
Kidney | Chronic anterior uveitis | <2% of all uveitis cases,27 median age of TINU onset is 15 years old28 | |
| Mixed pattern disease(s) | BD, 2–3% (HLA-B*5101, HLA-B*27) |
Mucosa, skin, vasculitis | Panuveitis, retinal vasculitis | 24–80% in childhood BD series29–31 |
| JIA, >60% (HLA DRB*0801, *1101, *1301, DPB1*02 (HLA-B*27, HLA-C*06, NOD/CARD15 in ERA and psoriatic) |
Joints (seven distinct disorders) | Anterior uveitis | 1–67%32,33
Dependent on JIA type34–37 Oligoarticular > enthesitis related, psoriatic, undifferentiated > polyarticular rheumatoid+ > polyarticular rheumatoid factor– > systemic |
|
| Polygenic autoimmune disease(s) | Vogt–Koyanagi–Harada syndrome, <1% (HLA-DRB1*04) | Skin, CNS | Panuveitis | 0.5–16%, higher percentage in Saudi Arabia38,39 |
| Multiple sclerosis, <0.1% (HLA-DRB1*1501) |
Central nervous system (CNS) | Optic neuritis, intermediate uveitis | Uveitis uncommon: 10–22% present with optic neuritis40 | |
| Monogenic autoimmune disease(s) | Aicardi Goutières syndrome, <0.1% | Skin, CNS, joints, lung | Glaucoma, episcleritis | Uveitis unknown41 |
BD, Behçet’s disease; CNS, central nervous system; ERA, enthesitis-related arthritis; HLA, human leukocyte antigen; JIA, juvenile idiopathic arthritis; TINU, tubulointerstitial nephritis and uveitis.
The commonest ‘cause’ of childhood uveitis is isolated idiopathic disease.
In childhood-onset uveitis, autoinflammatory and autoimmune conditions are more likely to present with certain manifestations of ocular inflammation (Table 2), but no single uveitic phenotype (Figure 2) is pathognomonic of a particular systemic inflammatory disease. For example, the majority of children with JIA develop chronic anterior uveitis (83%) with posterior synechiae, cataract and band keratopathy in uncontrolled disease, but panuveitis has also been noted in children with JIA.43
Figure 2.
Manifestations of childhood uveitis. (a) Anterior uveitis: chronic anterior uveitis, with posterior synechiae and cataract in a child with delayed diagnosis of juvenile idiopathic arthritis–related uveitis. (b) Intermediate uveitis: pars planitis at the slit lamp in a child with idiopathic disease. (c) Posterior uveitis: Retinal vasculitis: periphlebitis, midperipheral neovascularization with evidence of retinal ischaemia in a child with sarcoidosis. (d) Panuveitis: vitritis, optic disc oedema and multifocal chorioretinitis in a child with idiopathic disease. (e) Sequelae of inflammation: retinal photograph of an inflammatory choroidal neovascular membrane (CNVM) in a child with idiopathic panuveitis. (f) Sequelae of inflammation: cross-sectional optical coherence tomography (OCT) and OCT angiography (en face choriocapillaris window) images of the same CNVM noted in (e). Images (a), (b) and (d) courtesy of DSI Taylor.
Specific disorders associated with uveitis
Up to a third of children with uveitis have ‘isolated’ idiopathic disease and do not have an associated systemic disease which manifests in childhood. Other than JIA, which is itself an idiopathic disorder, this group is the largest ‘single’ group. Uveitis may be the presenting manifestation of disease; thus, the apparently well child who presents with uveitis may have isolated idiopathic disease, but they may have an evolving systemic disorder. We will now describe the autoimmune and autoinflammatory disorders associated with childhood uveitis, starting with the most prevalent disorders within paediatric uveitis clinics.
Juvenile idiopathic arthritis
Juvenile idiopathic arthritis, the descriptive term for arthropathy of unknown cause which has lasted for more than 6 weeks, is the most common chronic inflammatory rheumatological disorder seen in children.44 In turn, JIA is the most common systemic disorder seen in paediatric uveitis clinics, affecting between 30% and 80% of children with uveitis. Within the ‘umbrella term’ of JIA sit seven distinct but overlapping subtypes, with differing incidences of uveitis (Table 2): systemic, oligoarticular (up to four joints affected), rheumatoid factor–positive (RF+) polyarticular (more than five joints affected), RF– polyarticular, enthesitis-related (ERA), psoriatic (PsA) and undifferentiated arthritides.45 The estimated European annual incidence of JIA is 8.2 (95% CI, 7.5–9.0) per 100,000 with a significant variation between and across populations, occurring almost twice as often in girls (10.0/100,000) in comparison with boys (5.7/100,000).44 The pathogenesis of JIA is unknown, but the strong familial pattern of disease and human leukocyte antigen (HLA) associations indicate a complex genetic risk profile.46 There are also signals indicating that environmental risk factors, for example, a history of breastfeeding, may be at play.47
Uveitis occurs in up to a third of children with JIA across various countries,34 with most affected children developing a recurrent or chronic anterior uveitis.48 The median age of presentation is 5 years.49 Children with oligoarticular arthritis are at the highest risk (uveitis occurring in 16–25%) and those with polyarticular or systemic arthritis are at the lowest risk of JIAU (seen in 1–4%).50 Girls with early-onset oligoarticular arthritis who are seropositive for IgG antibodies recognizing nuclear antigens (antinuclear antibody (ANA) positive) are at the highest risk of developing chronic anterior uveitis.34,49,51,52 There are contrasting theories as to whether ANA positivity, gender or age at diagnosis confers the greatest risk. Recent studies indicate that the different genetic traits in JIA may confer differential uveitis risk in girls and boys.53 ANA positivity is also seen in cases of recurrent or chronic anterior uveitis in children without arthropathy. These children may have an atypical or attenuated form of JIAU.
The classical presentation of a delayed diagnosis of JIAU is the previously asymptomatic child who develops strabismus (unilateral disease) or evidence of poor vision (bilateral disease) with a ‘white eye’, band keratopathy, anterior chamber inflammation, posterior synechiae and cataract, with secondary disc and macular oedema.2 This is uncommon in modern practice, thanks to two developments: the wide adoption of national uveitis surveillance programmes, in which children with JIA are regularly checked for the onset of ocular inflammation,43,54,55 and the increasing use of systemic immunosuppressive therapies for children with JIA.56–58
Treatment for JIAU or ANA-positive chronic anterior uveitis (the latter thought to be a ‘forme fruste’ of JIAU) acts as a paradigm for the general management for noninfectious childhood uveitis. Inflammation which remains uncontrolled despite 3 months of topical corticosteroids is in most cases an appropriate indication for the use of a systemic disease-modifying steroid sparing agent.34 These agents may take up to 3 months to reach full efficacy, necessitating the use of temporizing oral steroids in cases of severe, sight-threatening levels of inflammation.
Methotrexate, an antimetabolite, is the first choice for chronic anterior uveitis, but can be associated with significant nausea, malaise or iatrogenic liver inflammation in a minority of children.56 Mycophenolate mofetil has been used for those children unable to tolerate methotrexate, and there is evidence of noninferiority in the management of most forms of uveitis when compared with methotrexate in adults.59 It is, however, a poorer therapeutic choice for JIA arthropathy.34 Insufficient response to these agents is an indication for a ‘step-up’ on the therapeutic ladder, with the introduction of a tumour necrosis factor inhibitor.
The use of adalimumab (an anti-TNFα agent) has improved disease control for adult and paediatric inflammatory eye disease,60 and following strong evidence of efficacy and safety in JIAU refractory to methotrexate,60 and in other uveitides,61–63 adalimumab has been commissioned in the United Kingdom for use in childhood-onset anterior and posterior uveitis. With regard to uveitis management, adalimumab has the advantage over other anti-TNFα agents such as etanercept and infliximab due to exhibiting greater disease control versus the former (in the form of fewer recurrences)64–67 and subcutaneous delivery rather than intravenous delivery for the latter.64 Infliximab has also been associated with greater rates of anaphylactic responses on administration due in part to the chimeric mouse–human nature of the molecule versus the fully human recombinant antibody of adalimumab. There is also evidence of effectiveness on switching to a second anti-TNFα in children with disease refractory to the first, specifically switching to adalimumab or to infliximab from etanercept.64
Newer agents targeted against inflammatory factors such as IL-6 (e.g. IL-6 monoclonal antibody, tocilizumab) and janus kinase (JAK; e.g. JAK-inhibitor baricitinib) have also emerged as possible therapeutic agents for childhood uveitis. Interleukin-6 has been postulated as a key mediator for inflammatory macular oedema, and tocilizumab appears to be of particular benefit in individuals with uveitic macular oedema.68–70 The janus-associated kinase pathways mediate cell responses to several inflammatory cytokines involved in uveitis including IL-2 and IL-6, and JAK inhibitors such as baricitinib and tofacitinib have been shown to control disease in individuals whose uveitis has failed to respond to other immunomodulators.71
Two of the JIA subtypes, PsA and ERA, are associated with HLA-B*27 positivity. HLA-B*27-positive acute anterior uveitis (AAU), presenting with a red painful eye, is one of the most common forms of adult uveitis.72 Many children with ERA or psoriatic JIAU present with a symptomatic uveitis, but the majority (57% in one population-based study)43 of these children present more insidiously. HLA-B*27 is also associated with spondyloarthropathy, reactive arthritis and inflammatory bowel disorders. There is growing evidence that HLA expression is altered in gastrointestinal (GI) disease and along with changes in the gut microbiome may suggest a mechanism of disease pathogenesis in these individuals73 and open avenues to new therapeutic options.
Although JIA is a disorder of childhood onset, activity often continues into adulthood, with an ongoing need for topical and systemic therapy and ocular surgery, and an ongoing negative impact on vision-related quality of life.74,75
Sarcoidosis and NOD2-associated autoinflammatory disease (Blau syndrome)
Sarcoidosis is a chronic inflammatory disorder characterized by a granulomatous response, which particularly manifests in the lungs and lymphatic system. It is a rare disorder in adults, and even more so in childhood, with a cumulative annual incidence of 0.3–0.8 per 100,000 children.76 Sarcoidosis typically presents in older children, with a mean age at diagnosis of 11–13.24 The proposed pathogenesis of disease involves a ‘two-hit model’, in which genetic predisposition (‘first hit’) is combined with environmental exposure to a triggering environmental agent. Hypothesized second hit agents include foreign antigen, tuberculosis77 and inorganic material such as silicone.78
Almost a half of children diagnosed with sarcoidosis develop ocular disease (Box 1), which may in some cases predate the systemic features.
Box 1.
International Workshop on Ocular Sarcoidosis (IWOS) criteria for the diagnosis of ocular sarcoidosis (OS).79
| Other causes of granulomatous uveitis must be ruled out. In children this includes NOD2 syndrome, tuberculosis or other mycobacteria, immune deficiency disorders, eosinophilic granuloma, Crohn’s disease, tumours, drug-induced granulomatosis |
|---|
| Intraocular clinical signs suggestive of OS |
| Mutton-fat keratic precipitates (large and small) and/or iris nodules at the pupillary margin (Koeppe) or in stroma (Busacca) Trabecular meshwork nodules and/or tent-shaped peripheral anterior synechia Snowballs/string of pearls vitreous opacities Multiple chorioretinal peripheral lesions (active and atrophic) Nodular and/or segmental periphlebitis (±candle wax drippings) and/or macroaneurysm in an inflamed eye Optic disc nodule(s)/granuloma(s) and/or solitary choroidal nodule Bilaterality |
| Systemic investigation results in suspected OS |
| Bilateral hilar lymphadenopathy (BHL) on chest X-ray and/or CT scan Negative tuberculin test or interferon-gamma assays Elevated: serum ACE OR serum lysozyme OR bronchoalveolar lavage fluid CD4/CD8 ratio (>3.5) Abnormal accumulation of gallium-67 scintigraphy or 18F-fluorodeoxyglucose positron emission tomography imaging Lymphopenia Parenchymal lung changes |
| Diagnostic criteria |
| Definite OS: diagnosis supported by biopsya with compatible uveitis Presumed OS: diagnosis not supported by biopsy, but BHL present with two intraocular signs Probable OS: diagnosis not supported by biopsy and BHL absent, but three intraocular signs and two systemic investigations are present |
ACE, angiotensin-converting enzyme; BHL, bilateral hilar lymphadenopathy; CT, computed tomography; IWOS, International Workshop on Ocular Sarcoidosis; OS, ocular sarcoidosis.
Corticosteroids can mask granuloma after only a few days of treatment.
Methotrexate and mycophenolate mofetil are first-line agents for childhood ocular sarcoidosis, with no consistent evidence of superiority of either agent, but continued preference for the use of methotrexate due to its status as the most studies immunomodulatory for chronic ocular inflammation. Anti-TNFα agents are typically used for those refractory to their first-line treatment, although clinicians may first ‘switch’ nonresponding patients from methotrexate to mycophenolate in cases of nonresponse and vice versa.76,80,81
Edward Blau82 described a family affected by early-onset arthritis, dermatitis and uveitis, with granulomatous skin lesions consistent with sarcoidosis. Blau’s syndrome, however, lacks the pulmonary features which are present in the majority of paediatric sarcoidosis cases, and has an earlier median age at uveitis onset (5 years, with median onset of arthritis at 2 years old).76 Multiple genotypes for Blau syndrome exist, and it is possible that the many different mutations of NOD2 confer different risks of ocular involvement. Panuveitis with chronic multifocal chorioretinitis is the most common ocular finding, although a third of children have an isolated chronic anterior uveitis.
Behçet’s disease
Behçet’s disease (BD) is a relapsing-remitting variable vessel vasculitis (i.e. affecting large and small veins and arteries), with a phenotype that differs depending on age at onset, gender, ethnicity and country of residence. Children appear to manifest ‘full-blown’ disease slower than adults (Box 2), leading to delays in diagnosis.86,87 The typical hallmark features of paediatric BD are painful, recurrent, oropharyngeal, perianal and genital ulceration (Box 2).85,88 In an echo of the later diagnosis of monogenic Blau syndrome in cases previously thought to be ‘early-onset sarcoid’, a monogenic mimic of early-onset paediatric BD, haploinsufficiency of TNFα induced protein 3 (TNFα-IP3), has been described.89
Box 2.
Diagnostic criteria sets for BD.
| Major diagnostic features GA: genital aphthosis (recurrent painful, sharply defined, round or ovaloid shallow ulceration, including perianal) OA: oral aphthosis OM: ocular manifestations Minor diagnostic features NM: neurologic manifestations (vascular or parenchymal involvement) SM: skin manifestations (necrotic folliculitis, acneiform lesions or erythema nodosum) VM: vascular manifestations (venous thrombosis, arterial thrombosis and arterial aneurysms) |
| Diagnostic criteria set for paediatric BD |
| Any three out of the six criteriaPEDBD |
| Diagnostic criteria set for adult BD |
| ⩾ 4 points (2 points for major, 1 point for minor and 1 point for a positive pathergy test (pustular response to blunt dermal needle prick) test if carried out in at least 90% of patients)ICBD OR OA + 2 out of remaining criteriaISG |
BD, Behçet’s disease; GA, genital aphthosis; ICBD, International Criteria for Behçet’s disease;83 ISG, International Study Group;84 NM, neurologic manifestations; OA, oral aphthosis; OM, ocular manifestations; PEDBD, paediatric Behçet’s disease; SM, skin manifestations;85 VM, vascular manifestations.
Ocular manifestations of BD also appear to be less common in childhood (45% of children with BD versus 70% in adult BD).85 Uveitis in BD has been described as ‘explosive’ with aggressive sight-threatening recurrences of inflammation and an associated vasculitis which can be necrotizing and obliterative, leading to ischaemic damage to the macular or optic nerve. The mobile sterile transient hypopyon seen in adult BD also occurs in paediatric disease, as do episcleritis and scleritis. Boys with BD are more likely to develop uveitis and more likely to have severe disease, sight-threatening retinal vasculitis.88
Treatment with anti-TNFα agents has been shown to be effective in BD, but there is significant heterogeneity in this disease population, and a significant number with disease refractory to anti-TNFαs. Azathioprine and cyclosporine A have been shown to be effective for the posterior uveitis seen in BD, as have biologic agents targeted against the IL-1 family of cytokines (such as anakinra and canakinumab) which are elevated in patients with BD.17,90,91
Inflammatory bowel disease
Ulcerative colitis (UC) and CD are chronic granulomatous GI diseases with extraintestinal manifestations such as arthritis and uveitis, which can occur many years before the onset of GI disease. There is a lower prevalence of ocular involvement in children (0.6–1.8%) than in adults (2–6%).23 Affected children can develop uveitis, which is usually a painful bilateral anterior uveitis, although studies in which entire populations of children with inflammatory bowel disease (IBD) have been examined report a uveitis prevalence of 9% in UC and 1% in CD. Children can also develop retinal vasculitis, and vascular occlusion, retrobulbar neuritis and keratopathy. Children with CD are much more likely to present with uveitis than those with UC.23,37
Tubulointerstitial nephritis and uveitis
Tubulointerstitial nephritis and uveitis (TINU), in which there is immune-mediated inflammation causing acute kidney injury, typically presents in older or mid-teenage children, with new-onset nonspecific symptoms including fever, weight loss, fatigue, nausea, anorexia, arthralgia and myalgia.92,93 As with many other immune-mediated disorders, such as Vogt–Koyanagi–Harada (VKH), girls are more likely to develop disease with a female-to-male ratio within TINU populations of 3:1.94 The uveitis can precede the TIN, but in most cases uveitis occurs with or follows the kidney injury, appearing within 6 months of TIN presentation.94 The majority of children develop a chronic anterior uveitis, although posterior involvement (retinal periphlebitis, haemorrhages, multifocal chorioretinitis) is also seen. As with other rare disorders, diagnosis can often be a challenge: tubulointerstitial nephritis also occurs in sarcoidosis, BD and IBD.
Vogt–Koyanagi–Harada
Vogt–Koyanagi–Harada is an idiopathic multisystem granulomatous disease, in which a dysfunctional immune response is directed against melanin-associated antigens within the eye, inner ear, meninges, hair and skin, leading to acute-onset meningoencephalitis, dysacusia and tinnitus, and later vitiligo and poliosis.95,96 Vogt–Koyanagi–Harada patients overwhelmingly present in adult life; however, one group in Turkey describes 15% of VKH cases developing in childhood in a retrospective review of seven tertiary centres.95 Children, like adults with VKH, typically present with relatively acute onset bilateral exudative detachments, disc oedema and peripheral choroiditis, which can then progress to chronic refractory anterior uveitis. However, children are more likely to have sight-threatening disease than adults.93
Multiple sclerosis
Between 0.1% and 1% of adults with intermediate uveitis (IU), particularly those with peripheral retinal vascular sheathing, go on to develop demyelinating disorders.97 A similar pattern has not been seen in intermediate uveitis of childhood onset, although there is a paucity of published research of sufficiently long follow-up to confirm the true later life prevalence in affected children.98 Much of intermediate uveitis in childhood is idiopathic pars planitis, a defined subtype of IU characterized by diffuse vitreous cells, peripheral retinal vasculitis, inferior vitreous inflammatory condensates (‘snowballs’), pars plana exudation (‘snowbanks’) and macular oedema. Pars planitis is not known to be associated with any systemic inflammatory disorders.
Multiple sclerosis has traditionally been known as an autoimmune disease, but there is emerging evidence of the role of inflammasomes (i.e. autoinflammatory mechanisms) in the pathogenesis of certain MS endophenotypes, particularly those of early onset and/or associated with genetic markers.18
Cryopyrin-associated periodic syndromes
The growing number of very rare monogenic autoinflammatory disorders are typically characterized by episodes of fever and inflammation due to dysregulation of the proteins involved in innate immunity, such as IL-1β. Within this group sit the cryopyrin-associated periodic syndromes (CAPS) which comprise three diseases of worsening severity: familial cold autoinflammatory syndrome (FCAS), Muckle–Wells syndrome (MWS) and neonatal-onset multisystem inflammatory disease or chronic infantile neurologic cutaneous articular syndrome (NOMID/CINCA).22
Children with CAPS can develop chronic or ‘periodic’ symptoms, with fever, rash and musculoskeletal and neurological involvement such as seizures or hydrocephalus. Over two thirds of patients get ophthalmic involvement, ranging from conjunctivitis to optic atrophy to a chronic anterior uveitis or panuveitis.99,100
The wide heterogeneity of the CAPS phenotype is partly explained by the many different mutations of the inflammasome NLRP3 gene which have been described in CAPS patients.100 Another inflammasome protein, pyrin, is encoded by the MEditerranean FeVer (MEFV) gene, mutations in which cause Familial Mediterranean Fever (FMF). Familial Mediterranean Fever is the most common inherited autoinflammatory disease, but although it is more common than CAPS, ocular manifestations (such as uveitis) are seen less common.17,101 In addition to monogenic ‘periodic fever’ disorders, there are also polygenic and/or acquired syndromes.102
Uveitis in the paediatric vasculitides
Vasculitis can be a coexisting disease seen with, or as part of, an autoinflammatory disorder (e.g. in BD). Retinal vasculitis may be a common end result of a number of inflammatory events, from raised tissue levels of IL-1, IL-6 or TNFα to tissue microdamage caused by ischaemia.103 Uveitis is a recognized feature of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (e.g. granulomatosis with polyangiitis), vasculitis within connective tissue disease (e.g. systemic lupus erythematosus or scleroderma) or small or medium vessel vasculitis [e.g. IgA vasculitis/Henoch–Schönlein purpura and Kawasaki disease (KD)].93 In KD, a mild, bilateral uveitis in a child with red eyes and a nonspecific fever can be one of the earliest signs of disease, and early diagnosis of KD is of particular value as late diagnosis can result in secondary chronic coronary artery disease.104
Managing childhood uveitis
Prompt diagnosis of uveitis affords the child the best chance of disease control before the development of inflammation-related complications. As many children with the most common form of disease, anterior uveitis, do not present with sudden onset of pain and redness, active surveillance for disease is needed to ensure case detection. This is particularly important for children known to be at risk. Those diagnosed with JIA are advised to undergo an eye examination within 6 weeks of diagnosis and to continue with 3- to 4-monthly examinations to a duration in line with national protocols in which children are stratified by JIA type, age at diagnosis and ANA status.54 As many children may have isolated idiopathic uveitis, or have uveitis as a presenting manifestation of a systemic inflammatory disease, there may be no useful indicator that the child is at risk until they present with established, sight-threatening disease.
The most common causes of sight loss in childhood uveitis are glaucoma, cataract and macular oedema.2,55,105 These occur as a sequelae of either uncontrolled inflammation or injudicious use of topical steroids.9,106–108 Thus, adequate control of ocular inflammation is key to good visual outcome for the majority of children. This often requires the use of powerful immunomodulators or immunosuppressive agents, in order to limit the dependence on the topical and systemic corticosteroids needed to control disease. The use of such systemic therapies necessitates the involvement of child health specialists and nurse specialists.
Almost one in six children and young people with uveitis develop severe visual impairment in one eye before adulthood.105,109 Although poor vision in only one eye often has a minimal impact on overall developmental and socioeconomic outcomes,110 uveitis is usually bilateral and often remains active into adulthood, putting the seeing eye at risk.111 Consequently, children who lose vision in one eye can then go on to lose vision in the other eye in adulthood, with a significant negative impact on their quality of life. In addition, children and the adults they become carry the burden of being diagnosed with a rare, often idiopathic disease of a chronic nature, which may necessitate treatment with immunosuppressive therapies which themselves carry the risk of short- and long-term morbidity.75,112–115 Multidisciplinary approaches (involving ophthalmologists, child health specialists, paediatric rheumatologists and where necessary psychosocial services) enable early diagnosis of associated conditions and holistic support for the child and family, which is key to good developmental outcomes.
Summary
Childhood uveitis comprises a collection of heterogeneous ocular phenotypes which are associated with a diverse range of childhood immune and inflammatory mediated disorders which are genetic and/or acquired. The majority of disease is idiopathic. The diagnosis of most of the associated disorders is reliant on clinical features rather than serological or genetic investigations, and no one ocular phenotype fits exactly with any particular systemic disorder, necessitating detailed medical history taking and systemic examination. Adequate control of inflammation is key to good visual outcomes, and multidisciplinary care is key to broader health outcomes.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: A.L.S. received support from the National Institute for Health Research Biomedical Research Centre (NIHR BRC) based at Great Ormond Street Hospital for Children NHS Foundation Trust and UCL GOS Institute of Child Health and is funded by an NIHR Clinician Scientist award (CS-2018-18-ST2-005). H.P. received support from the National Institute for Health Research Biomedical Research Centre (NIHR BRC) based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The funding organizations had no role in the design or conduct of this research. This paper presents independent research. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
ORCID iD: Ameenat Lola Solebo  https://orcid.org/0000-0002-8933-5864
https://orcid.org/0000-0002-8933-5864
Contributor Information
Najiha Rahman, Moorfields Eye Hospital NHS Foundation Trust, London, UK; National Institute for Health Research Biomedical Research Centre, Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology, London, UK.
Harry Petrushkin, Moorfields Eye Hospital NHS Foundation Trust, London, UK; National Institute for Health Research Biomedical Research Centre, Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology, London, UK; Great Ormond Street Hospital for Children NHS Foundation Trust, London, UK.
Ameenat Lola Solebo, Population, Policy and Practice Programme, UCL Great Ormond Street Institute of Child Health, 30 Guilford Street, London WC1N 1EH, UK; National Institute for Health Research Biomedical Research Centre, Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology, London, UK; Great Ormond Street Hospital for Children NHS Foundation Trust, London, UK; National Institute for Health Research Biomedical Research Centre, UCL Great Ormond Street Institute of Child Health and Great Ormond Street Hospital, London, UK.
References
- 1. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005; 140: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Edelsten C, Reddy MA, Stanford MR, et al. Visual loss associated with pediatric uveitis in English primary and referral centers. Am J Ophthalmol 2003; 135: 676–680. [DOI] [PubMed] [Google Scholar]
- 3. LaMattina KC, Koreishi AF. What is new in paediatric uveitis? Curr Opin Ophthalmol 2018; 29: 412–418. [DOI] [PubMed] [Google Scholar]
- 4. Paivonsalo-Hietanen T, Tuominen J, Saari KM. Uveitis in children: population-based study in Finland. Acta Ophthalmol Scand 2000; 78: 84–88. [DOI] [PubMed] [Google Scholar]
- 5. Acharya NR, Tham VM, Esterberg E, et al. Incidence and prevalence of uveitis: results from the Pacific Ocular Inflammation Study. JAMA Ophthalmol 2013; 131: 1405–1412. [DOI] [PubMed] [Google Scholar]
- 6. Ferrara M, Eggenschwiler L, Stephenson A, et al. The challenge of pediatric uveitis: tertiary referral center experience in the United States. Ocul Immunol Inflamm 2019; 27: 410–417. [DOI] [PubMed] [Google Scholar]
- 7. Papadopoulos M, Cable N, Rahi J, et al. The British Infantile and Childhood Glaucoma (BIG) Eye Study. Invest Ophthalmol Vis Sci 2007; 48: 4100–4106. [DOI] [PubMed] [Google Scholar]
- 8. Carpentier SJ, Jung JL, Patnaik JL, et al. A cross-sectional online survey identifies subspecialty differences in the management of pediatric cataracts associated with uveitis. Ophthalmol Ther 2020; 9: 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tan SZ, Yau K, Steeples LR, et al. Incidence, management and outcome of raised intraocular pressure in childhood-onset uveitis at a tertiary referral centre. Br J Ophthalmol 2019; 103: 748–752. [DOI] [PubMed] [Google Scholar]
- 10. Forrester JV, Xu H. Good news-bad news: the Yin and Yang of immune privilege in the eye. Front Immunol 2012; 3: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weinstein JE, Pepple KL. Cytokines in uveitis. Curr Opin Ophthalmol 2018; 29: 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalinina Ayuso V, Makhotkina N, van Tent-Hoeve M, et al. Pathogenesis of juvenile idiopathic arthritis associated uveitis: the known and unknown. Surv Ophthalmol 2014; 59: 517–531. [DOI] [PubMed] [Google Scholar]
- 13. Dick AD, Forrester JV, Liversidge J, et al. The role of tumour necrosis factor (TNF-alpha) in experimental autoimmune uveoretinitis (EAU). Prog Retin Eye Res 2004; 23: 617–637. [DOI] [PubMed] [Google Scholar]
- 14. Parikh JG, Tawansy KA, Rao NA. Immunohistochemical study of chronic nongranulomatous anterior uveitis in juvenile idiopathic arthritis. Ophthalmology 2008; 115: 1833–1836. [DOI] [PubMed] [Google Scholar]
- 15. McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoS Med 2006; 3: e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanazawa N. Designation of autoinflammatory skin manifestations with specific genetic backgrounds. Front Immunol 2020; 11: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sota J, Vitale A, Fabiani C, et al. The eye involvement in monogenic autoinflammatory diseases: literature review and update. Clin Exp Rheumatol 2018; 36(Suppl. 110): 44–53. [PubMed] [Google Scholar]
- 18. Barclay W, Shinohara ML. Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). Brain Pathol 2017; 27: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McGonagle D, Aziz A, Dickie LJ, et al. An integrated classification of pediatric inflammatory diseases, based on the concepts of autoinflammation and the immunological disease continuum. Pediatr Res 2009; 65: 38R–45R. [DOI] [PubMed] [Google Scholar]
- 20. Arostegui JI, Arnal C, Merino R, et al. NOD2 gene-associated pediatric granulomatous arthritis: clinical diversity, novel and recurrent mutations, and evidence of clinical improvement with interleukin-1 blockade in a Spanish cohort. Arthritis Rheum 2007; 56: 3805–3813. [DOI] [PubMed] [Google Scholar]
- 21. Sarens IL, Casteels I, Anton J, et al. Blau syndrome-associated uveitis: preliminary results from an international prospective interventional case series. Am J Ophthalmol 2018; 187: 158–166. [DOI] [PubMed] [Google Scholar]
- 22. Dollfus H, Häfner R, Hofmann HM, et al. Chronic infantile neurological cutaneous and articular/neonatal onset multisystem inflammatory disease syndrome: ocular manifestations in a recently recognized chronic inflammatory disease of childhood. Arch Ophthalmol 2000; 118: 1386–1392. [DOI] [PubMed] [Google Scholar]
- 23. Ottaviano G, Salvatore S, Salvatoni A, et al. Ocular manifestations of paediatric inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis 2018; 12: 870–879. [DOI] [PubMed] [Google Scholar]
- 24. Hoffmann AL, Milman N, Byg KE. Childhood sarcoidosis in Denmark 1979-1994: incidence, clinical features and laboratory results at presentation in 48 children. Acta Paediatr 2004; 93: 30–36. [PubMed] [Google Scholar]
- 25. Shetty AK, Gedalia A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J 2008; 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lindsley CB, Petty RE. Overview and report on international registry of sarcoid arthritis in childhood. Curr Rheumatol Rep 2000; 2: 343–348. [DOI] [PubMed] [Google Scholar]
- 27. Mackensen F, Smith JR, Rosenbaum JT. Enhanced recognition, treatment, and prognosis of tubulointerstitial nephritis and uveitis syndrome. Ophthalmology 2007; 114: 995–999. [DOI] [PubMed] [Google Scholar]
- 28. Mandeville JT, Levinson RD, Holland GN. The tubulointerstitial nephritis and uveitis syndrome. Surv Ophthalmol 2001; 46: 195–208. [DOI] [PubMed] [Google Scholar]
- 29. Sungur GK, Hazirolan D, Yalvac I, et al. Clinical and demographic evaluation of Behcet disease among different paediatric age groups. Br J Ophthalmol 2009; 93: 83–87. [DOI] [PubMed] [Google Scholar]
- 30. Borlu M, Uksal U, Ferahbas A, et al. Clinical features of Behcet’s disease in children. Int J Dermatol 2006; 45: 713–716. [DOI] [PubMed] [Google Scholar]
- 31. Eldem B, Onur C, Ozen S. Clinical features of pediatric Behcet’s disease. J Pediatr Ophthalmol Strabismus 1998; 35: 159–161. [DOI] [PubMed] [Google Scholar]
- 32. Angeles-Han ST, Pelajo CF, Vogler LB, et al. Risk markers of juvenile idiopathic arthritis-associated uveitis in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. J Rheumatol 2013; 40: 2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moradi A, Amin RM, Thorne JE. The role of gender in juvenile idiopathic arthritis-associated uveitis. J Ophthalmol 2014; 2014: 461078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clarke SL, Sen ES, Ramanan AV. Juvenile idiopathic arthritis-associated uveitis. Pediatr Rheumatol Online J 2016; 14: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walscheid K, Glandorf K, Rothaus K, et al. Enthesitis-related arthritis: prevalence and complications of associated uveitis in children and adolescents from a population-based nation-wide study in Germany. J Rheumatol. Epub ahead of print 15 March 2020. DOI: 10.3899/jrheum.191085. [DOI] [PubMed] [Google Scholar]
- 36. Stoll ML, Zurakowski D, Nigrovic LE, et al. Patients with juvenile psoriatic arthritis comprise two distinct populations. Arthritis Rheum 2006; 54: 3564–3572. [DOI] [PubMed] [Google Scholar]
- 37. Zisman D, Gladman DD, Stoll ML, et al. The Juvenile Psoriatic Arthritis Cohort in the CARRA Registry: clinical characteristics, classification, and outcomes. J Rheumatol 2017; 44: 342–351. [DOI] [PubMed] [Google Scholar]
- 38. Hamade IH, Al Shamsi HN, Al Dhibi H, et al. Uveitis survey in children. Br J Ophthalmol 2009; 93: 569–572. [DOI] [PubMed] [Google Scholar]
- 39. Tugal-Tutkun I. Pediatric uveitis. J Ophthalmic Vis Res 2011; 6: 259–269. [PMC free article] [PubMed] [Google Scholar]
- 40. Banwell B, Ghezzi A, Bar-Or A, et al. Multiple sclerosis in children: clinical diagnosis, therapeutic strategies, and future directions. Lancet Neurol 2007; 6: 887–902. [DOI] [PubMed] [Google Scholar]
- 41. Lee-Kirsch MA, Wolf C, Gunther C. Aicardi-Goutieres syndrome: a model disease for systemic autoimmunity. Clin Exp Immunol 2014; 175: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith JA, Mackensen F, Sen HN, et al. Epidemiology and course of disease in childhood uveitis. Ophthalmology 2009; 116: 1544–1551, 1551.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heiligenhaus A, Niewerth M, Ganser G, et al. Prevalence and complications of uveitis in juvenile idiopathic arthritis in a population-based nation-wide study in Germany: suggested modification of the current screening guidelines. Rheumatology 2007; 46: 1015–1019. [DOI] [PubMed] [Google Scholar]
- 44. Thierry S, Fautrel B, Lemelle I, et al. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine 2014; 81: 112–117. [DOI] [PubMed] [Google Scholar]
- 45. Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004; 31: 390–392. [PubMed] [Google Scholar]
- 46. Prahalad S, Glass DN. A comprehensive review of the genetics of juvenile idiopathic arthritis. Pediatr Rheumatol Online J 2008; 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hyrich KL, Baildam E, Pickford H, et al. Influence of past breast feeding on pattern and severity of presentation of juvenile idiopathic arthritis. Arch Dis Child 2016; 101: 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sabri K, Saurenmann RK, Silverman ED, et al. Course, complications, and outcome of juvenile arthritis-related uveitis. J AAPOS 2008; 12: 539–545. [DOI] [PubMed] [Google Scholar]
- 49. Heiligenhaus A, Heinz C, Edelsten C, et al. Review for disease of the year: epidemiology of juvenile idiopathic arthritis and its associated uveitis: the probable risk factors. Ocul Immunol Inflamm 2013; 21: 180–191. [DOI] [PubMed] [Google Scholar]
- 50. Heiligenhaus A, Niewerth M, Mingels A, et al. Epidemiology of uveitis in juvenile idiopathic arthritis from a national paediatric rheumatologic and ophthalmologic database. Klin Monbl Augenheilkd 2005; 222: 993–1001. [DOI] [PubMed] [Google Scholar]
- 51. Rosenberg AM. Uveitis associated with childhood rheumatic diseases. Curr Opin Rheumatol 2002; 14: 542–547. [DOI] [PubMed] [Google Scholar]
- 52. Carvounis PE, Herman DC, Cha S, et al. Incidence and outcomes of uveitis in juvenile rheumatoid arthritis, a synthesis of the literature. Graefes Arch Clin Exp Ophthalmol 2006; 244: 281–290. [DOI] [PubMed] [Google Scholar]
- 53. Haasnoot AJW, Kuiper JJW, de Boer JH. Predicting uveitis in juvenile idiopathic arthritis: from biomarkers to clinical practice. Expert Rev Clin Immunol 2019; 15: 657–666. [DOI] [PubMed] [Google Scholar]
- 54. British Society for Paediatric and Adolescent Rheumatology. Guidelines for screening for uveitis in juvenile idiopathic arthritis (JIA). London: BSPAR, 2006. [Google Scholar]
- 55. Angeles-Han ST, McCracken C, Yeh S, et al. Characteristics of a cohort of children with juvenile idiopathic arthritis and JIA-associated uveitis. Pediatr Rheumatol Online J 2015; 13: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Solebo AL, Rahi JS, Dick AD, et al. Areas of agreement in the management of childhood non-infectious chronic anterior uveitis in the UK. Br J Ophthalmol 2020; 104: 11–16. [DOI] [PubMed] [Google Scholar]
- 57. Oray M, Khachatryan N, Ebrahimiadib N, et al. Ocular morbidities of juvenile idiopathic arthritis-associated uveitis in adulthood: results from a tertiary center study. Graefes Arch Clin Exp Ophthalmol 2016; 254: 1841–1849. [DOI] [PubMed] [Google Scholar]
- 58. Tappeiner C, Klotsche J, Schenck S, et al. Temporal change in prevalence and complications of uveitis associated with juvenile idiopathic arthritis: data from a cross-sectional analysis of a prospective nationwide study. Clin Exp Rheumatol 2015; 33: 936–944. [PubMed] [Google Scholar]
- 59. Rathinam SR, Gonzales JA, Thundikandy R, et al. Effect of corticosteroid-sparing treatment with mycophenolate mofetil vs methotrexate on inflammation in patients with uveitis: a randomized clinical trial. JAMA 2019; 322: 936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ramanan AV, Dick AD, Jones AP, et al. Adalimumab plus methotrexate for uveitis in juvenile idiopathic arthritis. N Engl J Med 2017; 376: 1637–1646. [DOI] [PubMed] [Google Scholar]
- 61. Munoz-Gallego A, Barral E, Enriquez E, et al. Adalimumab for the treatment of refractory noninfectious paediatric uveitis. Int Ophthalmol 2017; 37: 719–725. [DOI] [PubMed] [Google Scholar]
- 62. Vazquez-Cobian LB, Flynn T, Lehman TJ. Adalimumab therapy for childhood uveitis. J Pediatr 2006; 149: 572–575. [DOI] [PubMed] [Google Scholar]
- 63. Castiblanco C, Meese H, Foster CS. Treatment of pediatric uveitis with adalimumab: the MERSI experience. J AAPOS 2016; 20: 145–147. [DOI] [PubMed] [Google Scholar]
- 64. Simonini G, Katie D, Cimaz R, et al. Does switching anti-TNFalpha biologic agents represent an effective option in childhood chronic uveitis: the evidence from a systematic review and meta-analysis approach. Semin Arthritis Rheum 2014; 44: 39–46. [DOI] [PubMed] [Google Scholar]
- 65. Saeed MU, Raza SH, Goyal S, et al. Etanercept in methotrexate-resistant JIA-related uveitis. Semin Ophthalmol 2014; 29: 1–3. [DOI] [PubMed] [Google Scholar]
- 66. Saurenmann RK, Levin AV, Feldman BM, et al. Risk of new-onset uveitis in patients with juvenile idiopathic arthritis treated with anti-TNFalpha agents. J Pediatr 2006; 149: 833–836. [DOI] [PubMed] [Google Scholar]
- 67. Foeldvari I, Becker I, Horneff G. Uveitis events during adalimumab, etanercept, and methotrexate therapy in juvenile idiopathic arthritis: data from the Biologics in Pediatric Rheumatology Registry. Arthritis Care Res 2015; 67: 1529–1535. [DOI] [PubMed] [Google Scholar]
- 68. Tappeiner C, Mesquida M, Adan A, et al. Evidence for tocilizumab as a treatment option in refractory uveitis associated with juvenile idiopathic arthritis. J Rheumatol 2016; 43: 2183–2188. [DOI] [PubMed] [Google Scholar]
- 69. Calvo-Rio V, Santos-Gomez M, Calvo I, et al. Anti-interleukin-6 receptor tocilizumab for severe juvenile idiopathic arthritis-associated uveitis refractory to anti-tumor necrosis factor therapy: a multicenter study of twenty-five patients. Arthritis Rheumatol 2017; 69: 668–675. [DOI] [PubMed] [Google Scholar]
- 70. Vegas-Revenga N, Calvo-Río V, Mesquida M, et al. Anti-IL6-receptor tocilizumab in refractory and noninfectious uveitic cystoid macular edema: multicenter study of 25 patients. Am J Ophthalmol 2019; 200: 85–94. [DOI] [PubMed] [Google Scholar]
- 71. Paley MA, Karacal H, Rao PK, et al. Tofacitinib for refractory uveitis and scleritis. Am J Ophthalmol Case Rep 2019; 13: 53–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. D’Ambrosio EM, La Cava M, Tortorella P, et al. Clinical features and complications of the HLA-B27-associated acute anterior uveitis: a metanalysis. Semin Ophthalmol 2017; 32: 689–701. [DOI] [PubMed] [Google Scholar]
- 73. Rosenbaum JT, Asquith M. The microbiome and HLA-B27-associated acute anterior uveitis. Nat Rev Rheumatol 2018; 14: 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Haasnoot AJ, Vernie LA, Rothova A, et al. Impact of juvenile idiopathic arthritis associated uveitis in early adulthood. PLoS ONE 2016; 11: e0164312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Haasnoot AJW, Sint Jago NFM, Tekstra J, et al. Impact of uveitis on quality of life in adult patients with juvenile idiopathic arthritis. Arthritis Care Res 2017; 69: 1895–1902. [DOI] [PubMed] [Google Scholar]
- 76. Nathan N, Sileo C, Calender A, et al. Paediatric sarcoidosis. Paediatr Respir Rev 2019; 29: 53–59. [DOI] [PubMed] [Google Scholar]
- 77. Scadding JG. Mycobacterium tuberculosis in the aetiology of sarcoidosis. Br Med J 1960; 2: 1617–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol 2012; 12: 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mochizuki M, Smith JR, Takase H, et al. Revised criteria of International Workshop on Ocular Sarcoidosis (IWOS) for the diagnosis of ocular sarcoidosis. Br J Ophthalmol 2019; 103: 1418–1422. [DOI] [PubMed] [Google Scholar]
- 80. Sardar E, Dusser P, Rousseau A, et al. Retrospective study evaluating treatment decisions and outcomes of childhood uveitis not associated with juvenile idiopathic arthritis. J Pediatr 2017; 186: 131–137.e1. [DOI] [PubMed] [Google Scholar]
- 81. Gedalia A, Khan TA, Shetty AK, et al. Childhood sarcoidosis: Louisiana experience. Clin Rheumatol 2016; 35: 1879–1884. [DOI] [PubMed] [Google Scholar]
- 82. Blau EB. Familial granulomatous arthritis, iritis, and rash. J Pediatr 1985; 107: 689–693. [DOI] [PubMed] [Google Scholar]
- 83. The International Criteria for Behçet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol 2014; 28: 338–347. [DOI] [PubMed] [Google Scholar]
- 84. Criteria for diagnosis of Behçet’s disease. International Study Group for Behçet’s disease. Lancet 1990; 335: 1078–1080. [PubMed] [Google Scholar]
- 85. Batu ED. Diagnostic/classification criteria in pediatric Behçet’s disease. Rheumatol Int 2019; 39: 37–46. [DOI] [PubMed] [Google Scholar]
- 86. Koné-Paut I, Shahram F, Darce-Bello M, et al. Consensus classification criteria for paediatric Behçet’s disease from a prospective observational cohort: PEDBD. Ann Rheum Dis 2016; 75: 958–964. [DOI] [PubMed] [Google Scholar]
- 87. Krause I, Uziel Y, Guedj D, et al. Childhood Behçet’s disease: clinical features and comparison with adult-onset disease. Rheumatology 1999; 38: 457–462. [DOI] [PubMed] [Google Scholar]
- 88. Koné-Paut I, Darce-Bello M, Shahram F, et al. Registries in rheumatological and musculoskeletal conditions. Paediatric Behçet’s disease: an international cohort study of 110 patients. One-year follow-up data. Rheumatology 2011; 50: 184–188. [DOI] [PubMed] [Google Scholar]
- 89. Kadowaki T, Ohnishi H, Kawamoto N, et al. Haploinsufficiency of A20 causes autoinflammatory and autoimmune disorders. J Allergy Clin Immunol 2018; 141: 1485–1488.e11. [DOI] [PubMed] [Google Scholar]
- 90. Nanthapisal S, Klein NJ, Ambrose N, et al. Paediatric Behcet’s disease: a UK tertiary centre experience. Clin Rheumatol 2016; 35: 2509–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Caso F, Costa L, Rigante D, et al. Biological treatments in Behçet’s disease: beyond anti-TNF therapy. Mediators Inflamm 2014; 2014: 107421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Saarela V, Nuutinen M, Ala-Houhala M, et al. Tubulointerstitial nephritis and uveitis syndrome in children: a prospective multicenter study. Ophthalmology 2013; 120: 1476–1481. [DOI] [PubMed] [Google Scholar]
- 93. Edelsten C. Uveitis. In: Hoyt CTD. (ed.) Pediatric ophthalmology and strabismus. 4th ed. Edinburgh: Elsevier Saunders, 2012, pp. 377–392. [Google Scholar]
- 94. Joyce E, Glasner P, Ranganathan S, et al. Tubulointerstitial nephritis: diagnosis, treatment, and monitoring. Pediatr Nephrol 2017; 32: 577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tugal-Tutkun I, Ozyazgan Y, Akova YA, et al. The spectrum of Vogt-Koyanagi-Harada disease in Turkey: VKH in Turkey. Int Ophthalmol 2007; 27: 117–123. [DOI] [PubMed] [Google Scholar]
- 96. Yang P, Zhong Y, Du L, et al. Development and evaluation of diagnostic criteria for Vogt-Koyanagi-Harada disease. JAMA Ophthalmol 2018; 136: 1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Prieto JF, Dios E, Gutierrez JM, et al. Pars planitis: epidemiology, treatment, and association with multiple sclerosis. Ocul Immunol Inflamm 2001; 9: 93–102. [DOI] [PubMed] [Google Scholar]
- 98. de Boer J, Berendschot TT, van der Does P, et al. Long-term follow-up of intermediate uveitis in children. Am J Ophthalmol 2006; 141: 616–621. [DOI] [PubMed] [Google Scholar]
- 99. Lachmann HJ. Periodic fever syndromes. Best Pract Res Clin Rheumatol 2017; 31: 596–609. [DOI] [PubMed] [Google Scholar]
- 100. Levy R, Gerard L, Kuemmerle-Deschner J, et al. Phenotypic and genotypic characteristics of cryopyrin-associated periodic syndrome: a series of 136 patients from the Eurofever Registry. Ann Rheum Dis 2015; 74: 2043–2049. [DOI] [PubMed] [Google Scholar]
- 101. Petrushkin H, Stanford M, Fortune F, et al. Clinical review: familial Mediterranean fever-an overview of pathogenesis, symptoms, ocular manifestations, and treatment. Ocul Immunol Inflamm 2016; 24: 422–430. [DOI] [PubMed] [Google Scholar]
- 102. Alejandre N, Ruiz-Palacios A, García-Aparicio AM, et al. Description of a new family with cryopyrin-associated periodic syndrome: risk of visual loss in patients bearing the R260W mutation. Rheumatology 2014; 53: 1095–1099. [DOI] [PubMed] [Google Scholar]
- 103. Rosenbaum JT, Sibley CH, Lin P. Retinal vasculitis. Curr Opin Rheumatol 2016; 28: 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Choi HS, Lee SB, Kwon JH, et al. Uveitis as an important ocular sign to help early diagnosis in Kawasaki disease. Korean J Pediatr 2015; 58: 374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. de Boer J, Wulffraat N, Rothova A. Visual loss in uveitis of childhood. Br J Ophthalmol 2003; 87: 879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Blum-Hareuveni T, Seguin-Greenstein S, Kramer M, et al. Risk factors for the development of cataract in children with uveitis. Am J Ophthalmol 2017; 177: 139–143. [DOI] [PubMed] [Google Scholar]
- 107. Thorne JE, Woreta FA, Dunn JP, et al. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology 2010; 117: 1436–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Stroh IG, Moradi A, Burkholder BM, et al. Occurrence of and risk factors for ocular hypertension and secondary glaucoma in juvenile idiopathic arthritis-associated uveitis. Ocul Immunol Inflamm 2017; 25: 503–512. [DOI] [PubMed] [Google Scholar]
- 109. Gregory AC, 2nd, Kempen JH, Daniel E, et al. Risk factors for loss of visual acuity among patients with uveitis associated with juvenile idiopathic arthritis: the Systemic Immunosuppressive Therapy for Eye Diseases Study. Ophthalmology 2013; 120: 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Solebo AL, Cumberland PM, Rahi JS. Whole-population vision screening in children aged 4-5 years to detect amblyopia. Lancet 2015; 385: 2308–2319. [DOI] [PubMed] [Google Scholar]
- 111. Kolomeyer AM, Crane ES, Tu Y, et al. Adult patients with uveitis associated with juvenile idiopathic arthritis: a retrospective review. Can J Ophthalmol 2017; 52: 458–462. [DOI] [PubMed] [Google Scholar]
- 112. Sen ES, Morgan MJ, MacLeod R, et al. Cross sectional, qualitative thematic analysis of patient perspectives of disease impact in juvenile idiopathic arthritis-associated uveitis. Pediatr Rheumatol Online J 2017; 15: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Angeles-Han ST, Yeh S, McCracken C, et al. Using the effects of youngsters’ eyesight on quality of life questionnaire to measure visual outcomes in children with uveitis. Arthritis Care Res 2015; 67: 1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mulligan K, Wedderburn LR, Newman S. The experience of taking methotrexate for juvenile idiopathic arthritis: results of a cross-sectional survey with children and young people. Pediatr Rheumatol Online J 2015; 13: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Seid M, Huang B, Niehaus S, et al. Determinants of health-related quality of life in children newly diagnosed with juvenile idiopathic arthritis. Arthritis Care Res 2014; 66: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]