Abstract
Background:
Targeting immune checkpoints represents an immense breakthrough in cancer therapeutics. The prognostic value of hemoglobin (Hb) has been investigated in many malignancies including non-small cell lung cancer (NSCLC). However, the prognostic impact of pretreatment Hb count for immune checkpoint inhibitors (ICIs) in advanced NSCLC patients remains unclear.
Methods:
A total of 310 late-stage NSCLC patients who received ICI therapies between January 2015 and March 2019 were prospectively enrolled. We used a propensity score-matched cohort analysis for this study. Patients’ clinicopathological characteristics and pretreatment Hb concentration were assessed against the progression-free survival (PFS) and overall survival (OS) using the Kaplan–Meier method and Cox proportional hazards regression.
Results:
A propensity score (PS)-matched cohort analysis was applied to adjust for potential bias and to create two comparable groups according to patients’ clinicopathological characteristics. The patients with normal baseline Hb levels (⩾110 g/L) had significantly longer PFS [median: 10.0 versus 4.0 months, hazard ratio (HR): 0.63, 95% confidence interval (CI): 0.46−0.86; p = 0.001] and OS [median: 17.6 versus 10.5 months, HR (95% CI): 0.56 (0.40−0.79); p < 0.001] than those with decreased Hb count (<110 g/L) in a PS-matched cohort (n = 255). For patients with normal pretreatment Hb levels, ICI combination therapy was significantly associated with better PFS [median: 11.1 versus 8.0 months, HR (95% CI): 0.74 (0.50−1.06); p = 0.09] and OS [median: 26.0 versus 12.9 months, HR (95% CI): 0.56 (0.37−0.86); p = 0.008] than monotherapy, but there was no such trend for patients with decreased baseline Hb levels.
Conclusion:
Our findings showed that normal pretreatment Hb count served as a favorable prognostic marker in advanced NSCLC patients treated with ICIs, representing an economical biomarker with readily measuring performance among all reported ones.
Keywords: efficacy, hemoglobin, immune checkpoint inhibitors, non-small cell lung cancer, predictor
Introduction
Lung cancer remains the leading cause of cancer-related deaths on a global basis.1,2 Non-small cell lung cancer (NSCLC) comprises about 85–90% of all lung cancers and is generally diagnosed at metastatic stages, indicative of its late detection and biological aggressiveness.3 Although molecular targeted therapies alone or in combination with chemotherapy and radiation have drastically improved clinical outcomes in NSCLC patients harboring well-known oncogenic drivers, such as EGFR mutations and ALK fusions, the majority of patients inevitably acquired therapeutic resistance and had disease relapse with a 5-year survival rate of less than 20%.4
Recent advances in lung cancer treatment are emerging from new immunotherapies that target T-cell inhibitory receptors, such as programmed cell death-1 (PD-1). Blockade of PD-1 and programmed cell death-ligand 1 (PD-L1) interaction has shown to result in very durable responses and prolonged survival in advanced NSCLC patients with low side-effect incidence and long-term benefits by numerous clinical trials.5–9 However, overall response rate of ICI treatment among unselected advanced NSCLC patients was about 20%.10 Hitherto, intra-tumoral PD-L1 expression and tumor mutation burden (TMB) are the most widely studied biomarkers of appropriate NSCLC patients selection for immunotherapy,9,11–16 but their predictive values are still under controversy, mainly due to the lack of standard assessment criteria.17–20 Other challenges hindering the implementation of these biomarkers in the clinic include involvement of invasive procedures, expensive cost, and complex data analysis of next-generation sequencing. These findings underline an unmet urgent need to identify novel predictors for the efficacy of immunotherapy.21
Anemia is a hematological abnormality commonly occurring in advanced cancer patients as a direct or indirect result of tumor progression or antineoplastic therapy.22 Mounting evidences demonstrated that anemia and hypoxemia may be implicated in tumor growth, motility, anti-apoptosis, and angiopoiesis, thereby influencing treatment effects.23 According to the National Comprehensive Cancer Network (NCCN) guideline, hemoglobin (Hb) level of less than 110 g/L is defined as cancer-related anemia.24 Decreased Hb levels have been shown associated with poor patient prognosis in many solid malignancies.25–27 Hence, we aim to investigate whether the pretreatment Hb level represents a valuable prognostic biomarker of benefits from immune checkpoint inhibitor (ICI) therapy in advanced NSCLC patients.
Materials and methods
Study population and data collection
A total of 410 advanced (stage IIIB to IV) NSCLC patients who received ICI treatment at the Chinese PLA General Hospital between January 2015 and March 2019 were prospectively enrolled. Among them, 310 patients met the following eligibility criteria: (1) histological confirmation of stage IIIB−IV according to the eighth edition of the TNM classification for NSCLC; (2) receiving ICI treatment for at least 6 weeks and participating in the evaluation of treatment response at least once; (3) baseline Hb levels were measured.
The clinicopathological characteristics including age, sex, stage, smoking history, histological type, Eastern Cooperative Oncology Group Performance Status (ECOG PS), prior lines of therapy, treatment type, and brain metastasis were recorded.
Radiology response assessment was performed according to the Response Evaluation Criteria in Solid Tumors version 1.1,28 including complete response, partial response, stable disease, and progressive disease. Progression-free survival (PFS) was defined as the time from the first dose of ICI to the date of disease progression or death (whichever occurred first), and overall survival (OS) was referred to as the time between the first immunotherapy and death. The last follow-up date was recorded under the situation that patients were censored. The cut-off date was 20 December 2019.
Statistical analysis
Statistical analyses were performed using the SPSS 19.0 software (IBM Corporation, Armonk, NY, USA). Baseline covariates were balanced by the method of propensity score (PS)-matching using MatchIt v3.0.2 (http://gking.harvard.edu/matchit). Patients were grouped by the suggested Hb level of 110 g/L according to the NCCN guideline.24 The Kaplan–meier method and the Log-rank test were used to analyze survival data. Univariate and multivariate analyses were performed using Cox regression model for calculating hazard ratio (HR) with its 95% confidence interval (CI).29 Categorical variables were compared using the Chi-square test. All statistical tests were bilateral and a p-value of <0.05 was considered as statistical significance.
Results
Study population and cohort
A total of 310 patients who received ICI treatment at the Chinese PLA General Hospital between January 2015 and March 2019 were included in the study. Detailed demographic and clinical data, including age, sex, histological type, stage, smoking history, ICI treatment regimen, ECOG PS, prior lines of therapy, and the presence of brain metastasis are summarized in Table 1. The median age of this cohort was 61 years (range: 33–91). There were 237 males (76.5%) and 73 females (23.5%) in the cohort. Among them, 197 cases (63.5%) were non-squamous cell carcinoma and 244 cases (78.7%) were stage IV according to the Eighth Edition TNM Staging of International Lung Cancer Research Association.30 Approximately 90% of the patients had ECOG PS of 0−1, and about 60% had smoking history.
Table 1.
Clinicopathological characteristics of patients with advanced non-small cell lung cancer.
| Characteristics | No. of patients N = 310 |
Percentage |
|---|---|---|
| Age, years, median (range) | 61 (33−91) | |
| Hb, g/L, median (range) | 119 (65−174) | |
| Sex | ||
| Male | 237 | 76.5 |
| Female | 73 | 23.5 |
| Histology | ||
| Non-squamous | 197 | 63.5 |
| Squamous | 113 | 36.5 |
| Stage | ||
| IIIB | 41 | 13.2 |
| IIIC | 25 | 8.1 |
| IV | 244 | 78.7 |
| Smoking history | ||
| Never smoked | 117 | 37.7 |
| Smoke | 193 | 62.3 |
| ICI drugs | ||
| Pembrolizumab | 139 | 44.8 |
| Nivolumab | 137 | 44.2 |
| Atezolizumab | 13 | 4.2 |
| Durvalumab | 21 | 6.8 |
| Treatment type | ||
| Monotherapy | 149 | 48.1 |
| Combination therapy | ||
| ICI plus chemotherapy | 121 | 39.0 |
| ICI plus anti-angiogenic agent | 40 | 12.9 |
| ECOG PS | ||
| 0−1 | 278 | 89.7 |
| ⩾2 | 32 | 10.3 |
| Prior lines of therapy | ||
| 1 line | 100 | 32.3 |
| 2 lines | 109 | 35.2 |
| ⩾3 lines | 101 | 32.5 |
| Brain metastasis | ||
| Yes | 53 | 17.1 |
| No | 257 | 82.9 |
ECOG PS, Eastern Cooperative Oncology Group Performance Status; Hb, hemoglobin; ICI, immune checkpoint inhibitor; PSM, propensity score matching.
Approximately half of the cohort received ICI monotherapy, while the rest were treated with ICIs in combination with either chemotherapy or anti-angiogenic agent, defined as combination therapy (Table 1). In particular, a total of 34 patients received the front-line ICI monotherapy, and about half were tested for PD-L1 expression. There was no significant association between PD-L1 levels and Hb count. Furthermore, patients with different number of prior lines of therapy were largely evenly represented (Table 1). The overall objective response rate (ORR) was 19.7%, 30% for first-line, 17.4% for second-line, and 11.9% for third-line and beyond, respectively. There was no significant correlation between Hb count and ORR (p = 0.143). A total of 208 patients were found to have normal pretreatment Hb concentration (⩾110 g/L) according to the NCCN guideline,24 while the other 102 patients had decreased Hb levels (<110 g/L). None of the patients had any severe bleeding at the initiation of ICI treatment, except for a slight hemoptysis reported for a small proportion (~6%) of patients who appeared to have normal Hb levels.
Baseline covariates after propensity score matching
We used a PS-matched cohort analysis to balance baseline covariates between normal Hb and decreased Hb subgroups of patients. The baseline characteristics in the pre-matched and post-matched cohorts were presented in Table 2; 165 patients in the normal Hb subset were matched with 90 patients in the decreased Hb subset. The matching process eliminated some significant differences that existed between the normal- and low-concentration subgroups in the pre-matched cohort, including ECOG PS and prior lines of therapy received.
Table 2.
Association between baseline Hb levels and clinicopathological characteristics in non-small cell lung cancer patients treated with immune checkpoint inhibitors.
| Characteristics | Before PSM |
After PSM |
||||
|---|---|---|---|---|---|---|
| Hb <110 g/L | Hb ⩾110 g/L | p-value | Hb <110 g/L | Hb ⩾110 g/L | p-value | |
| No. of patients | 102 | 208 | 90 | 165 | ||
| Age, median (range) | 61 (36−91) | 61 (33−85) | 0.60 | 61 (36−91) | 61 (33−85) | 0.37 |
| Sex | ||||||
| Male (%) | 78 (76.5) | 159 (76.4) | 1.00 | 69 (76.7) | 124 (75.2) | 0.63 |
| Smoking history | ||||||
| Yes (%) | 63 (61.8) | 130 (62.5) | 0.90 | 55 (61.1) | 100 (60.6) | 1.00 |
| Histology | ||||||
| Squamous (%) | 34 (33.3) | 79 (38.0) | 0.45 | 29 (32.2) | 53 (32.1) | 1.00 |
| Brain metastasis | ||||||
| Yes (%) | 21 (20.6) | 32 (15.4) | 0.26 | 19 (21.1) | 23 (13.9) | 0.19 |
| Stage | ||||||
| IV (%) | 83 (81.4) | 161 (77.4) | 0.46 | 76 (84.4) | 142 (86.1) | 0.87 |
| ECOG PS | ||||||
| ⩾ 2 (%) | 17 (16.7) | 15 (7.2) | 0.01 | 6 (6.7) | 7 (4.2) | 0.59 |
| Line (%) | 0.01 | 0.20 | ||||
| 1 line | 20 (19.6) | 80 (38.4) | 20 (22.2) | 54 (32.7) | ||
| 2 lines | 45 (44.1) | 64 (30.8) | 38 (42.2) | 58 (35.2) | ||
| ⩾ 3 lines | 37 (36.3) | 64 (30.8) | 32 (35.6) | 53 (32.1) | ||
| Treatment type | ||||||
| Combination (%) | 46 (45.1) | 115 (55.3) | 0.11 | 40 (44.4) | 90 (54.5) | 0.16 |
ECOG PS, Eastern Cooperative Oncology Group Performance Status; Hb, hemoglobin; ICI, immune checkpoint inhibitor; PSM, propensity score matching.
Univariate and multivariate analysis for survival data
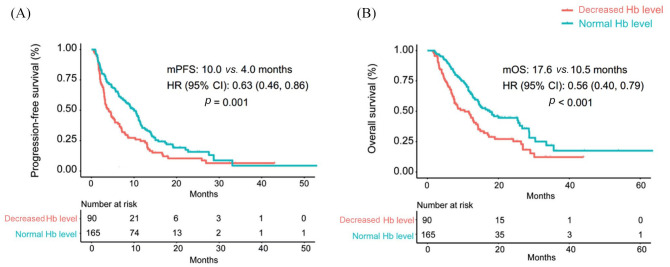
Patients who had normal pretreatment Hb levels demonstrated significantly longer PFS than those with decreased Hb levels [median: 10.0 versus 4.0 months, HR (95% CI): 0.63 (0.46−0.86); p = 0.001, Figure 1(A)]. As shown in Table 3, univariate analysis revealed that treatment type, prior lines of therapy, brain metastasis, and baseline Hb levels were all associated with PFS (p < 0.05), and multivariate analysis further demonstrated that ICI combination therapy and normal baseline Hb levels were independent favorable prognostic predictors for PFS (p < 0.05).
Figure 1.
Baseline Hb levels associated with (A) progression-free survival and (B) overall survival in patients treated with immune checkpoint inhibitors.
CI, confidence interval; Hb, hemoglobin; HR, hazard ratio; mOS, median overall survival; mPFS, median progression-free survival.
Table 3.
Univariate and multivariate logistic regression analyses for progression-free survival in non-small cell lung cancer patients treated with immune checkpoint inhibitors after propensity score matching.
| Variable | Category | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age, years | ⩾61 versus <61 | 0.78 (0.59−1.03) | 0.09 | 0.81 (0.60−1.08) | 0.16 |
| Sex | Female versus male | 1.33 (0.97−1.83) | 0.07 | 1.35 (0.87−2.07) | 0.17 |
| Smoking history | Yes versus no | 0.92 (0.69−1.22) | 0.57 | 1.24 (0.83−1.85) | 0.28 |
| Histology | Squamous versus non-squamous | 0.87 (0.64−1.17) | 0.36 | 0.80 (0.58−1.10) | 0.18 |
| Stage | IV versus III | 0.98 (0.66−1.47) | 0.96 | 0.81 (0.52−1.24) | 0.34 |
| ECOG PS | ⩾2 versus 0−1 | 1.12 (0.61−2.03) | 0.71 | 1.07 (0.56−2.05) | 0.82 |
| Treatment type | Combination therapy versus monotherapy | 0.75 (0.57−0.99) | <0.05 | 0.73 (0.54−0.99) | <0.05 |
| Prior lines of therapy | 1 line versus 2 lines versus ⩾3 lines | 1.24 (1.04−1.42) | 0.01 | 1.16 (0.9−1.42) | 0.13 |
| Brain metastasis | Yes versus no | 1.70 (1.18−2.44) | <0.01 | 1.40 (0.95−2.05) | 0.08 |
| Baseline Hb, g/L | ⩾110 versus <110 | 0.63 (0.47−0.83) | <0.01 | 0.67 (0.50−0.91) | 0.01 |
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; Hb, hemoglobin; HR, hazard ratio.
Patients who had a normal pretreatment Hb count had remarkably better OS than those in the low Hb subgroup [median: 17.6 versus 10.5 months, HR (95% CI): 0.56 (0.40−0.79); p < 0.001, Figure 1(B)). As shown in Table 4, univariate and multivariate analysis showed that ICI combination therapy, first line of therapy, absence of brain metastasis, and normal baseline Hb were significantly associated with better OS (p < 0.05).
Table 4.
Univariate and multivariate logistic regression analyses for overall survival in non-small cell lung cancer patients treated with immune checkpoint inhibitors after propensity score matching.
| Variable | Category | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age, years | ⩾61 versus <61 | 0.86 (0.62−1.18) | 0.37 | 0.82 (0.59−1.15) | 0.26 |
| Sex | Female versus male | 1.33 (0.98−1.98) | 0.06 | 1.59 (0.97−2.60) | 0.06 |
| Smoking history | Yes versus no | 0.94 (0.68−1.30) | 0.73 | 1.50 (0.95−2.37) | 0.08 |
| Histology | Squamous versus non-squamous | 0.84 (0.59−1.19) | 0.34 | 0.76 (0.52−1.12) | 0.17 |
| Stage | IV versus III | 1.17 (0.71−1.92) | 0.53 | 0.83 (0.48−1.42) | 0.51 |
| ECOG PS | ⩾2 versus 0−1 | 1.25 (0.67−2.31) | 0.48 | 1.17 (0.60−2.26) | 0.64 |
| Treatment type | Combination therapy versus monotherapy | 0.64 (0.46−0.88) | 0.01 | 0.64 (0.45−0.91) | 0.01 |
| Prior lines of therapy | 1 line versus 2 lines versus ⩾3 lines | 1.38 (1.12−1.69) | <0.01 | 1.28 (1.01−1.63) | 0.04 |
| Brain metastasis | Yes versus no | 2.00 (1.35−2.95) | <0.01 | 1.63 (1.08−2.47) | 0.02 |
| Baseline Hb, g/L | ⩾110 versus <110 | 0.56 (0.40−0.77) | <0.01 | 0.59 (0.43−0.83) | <0.01 |
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; Hb, hemoglobin; HR, hazard ratio.
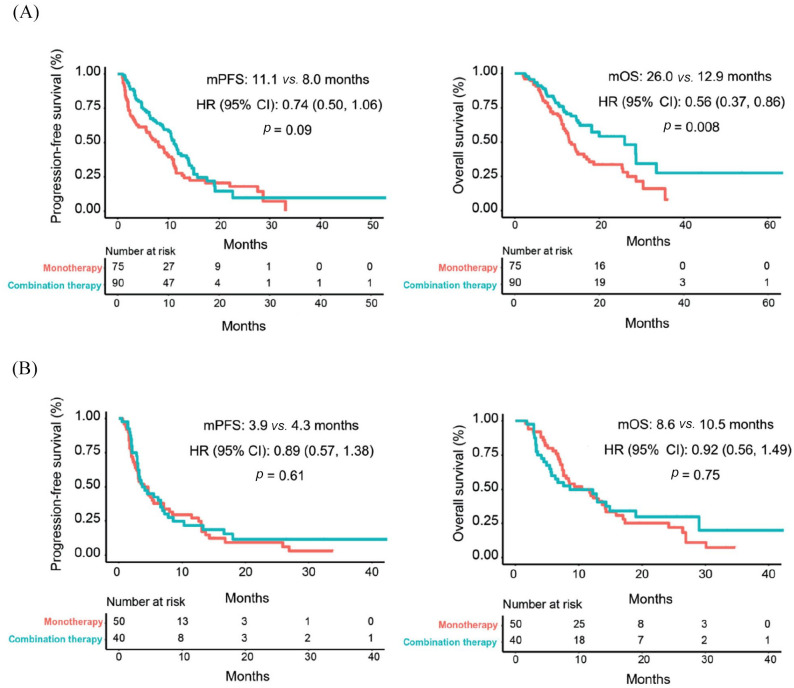
More importantly, as shown in Figure 2(A), ICI combination therapy was significantly associated with better PFS [median: 11.1 versus 8.0 months, HR (95% CI): 0.74 (0.50−1.06); p = 0.09] and OS [median: 26.0 versus 12.9 months, HR (95% CI): 0.56 (0.37−0.86); p = 0.008] than monotherapy for patients with normal pretreatment Hb levels, but there was no such trend for those with decreased baseline Hb levels [Figure 2(B)].
Figure 2.
Treatment type associated with progression-free survival and overall survival in patients with (A) normal or (B) decreased Hb levels.
CI, confidence interval; Hb, hemoglobin; HR, hazard ratio; mOS, median overall survival; mPFS, median progression-free survival.
Discussion
Most NSCLC patients receiving ICIs do not derive benefit, although recent developments in cancer immunotherapy, especially ICI immunotherapy, have exerted considerable therapeutic effects on survival outcomes in diverse solid tumor types including NSCLC. Given that the prognostic and predictive power of PD-L1 expression and TMB in tracking response to ICI therapy in advanced NSCLC is still not fully understood, additional predictive biomarkers with increased accuracy are urgently needed in the clinic to assist in identifying patients who will likely respond to ICI treatment.
Since anemia is the most frequent hematologic abnormality in cancer patients and its incidence increases with anti-cancer therapy,31 recent studies have demonstrated that reduced pretreatment Hb levels were associated with shorter OS in lung cancer patients who underwent chemotherapy, radiotherapy, or nivolumab monotherapy,32–35 but its prognostic value in advanced NSCLC patients treated with ICIs remains to be addressed. Our data showed that baseline Hb level was a prognostic marker for PFS and OS in late-stage NSCLC patients with ICI immunotherapy. Decreased pretreatment Hb level was significantly correlated with shorter PFS and OS. The effect of suboptimal Hb count on immunotherapy could be multifactorial. Zhao et al. previously reported that anemia may cause the deficiency of T cell response and induce immunosuppression in patients with late-stage tumors.36 Furthermore, hypoxia induced by Hb reduction has been shown to stimulate tumor growth and progression and decrease their sensitivity to anticancer treatments, eventually contributing to poor patient outcomes.37–39 However, it remained unclear whether oxygen levels dropped in these patients; an examination of in vivo oxygen concentration or hypoxia markers such as HIF-1α would be valuable in future research.
More importantly, we further found that immune-combination therapy (ICI plus chemotherapy or anti-angiogenic agent) was associated with better PFS and OS in patients with normal pretreatment Hb levels than ICIs alone, but this difference was not seen in patients with decreased baseline Hb levels. Kuo et al. also reported improved treatment efficacy of combined ICI plus chemotherapy over ICIs alone in metastatic NSCLC, and the additional efficacy of chemotherapy varied between histological subtypes and EGFR mutation status.40 The level of Hb could be another factor to consider in that scenario. Overall, immune-combination therapy would be more favorable for patients with normal baseline Hb levels, but for those with pretreatment decreased Hb levels, immune-monotherapy may be more suitable.
There were several potential limitations of our study. First, although we used the cutoff value of 110 g/L for pretreatment Hb count solely accordingly to the NCCN guideline, we do acknowledge that the definition of normal Hb count may vary subtly with factors including age and sex according to the World Health Organization.41,42 Second, only patients receiving more than 6 weeks of ICI treatment were included in this study, which might introduce unknown selection bias. Third, we did not explore the correlation between the change of Hb count and ICI benefits during the treatment. Fourth, though PS-matching method was applied to eliminate the bias of the listed baseline covariates between subgroups, other covariates, including PD-L1 expression and TMB, could also be potential confounders. In addition, how Hb levels affect ICI efficacy in NSCLC remains to be further explored, and the measurement of in vivo oxygen concentration may be warranted in future studies.
Conclusion
Our study demonstrated that pretreatment Hb levels served as a favorable prognostic factor for ICI treatment in advanced NSCLC patients, which represents an economical biomarker with relatively easier measuring performance compared with other available biomarkers, including TMB and PD-L1 expression. Given the limitations of the study, future investigations are needed to validate the prognostic value of baseline Hb levels in larger multi-center cohorts.
Acknowledgments
We thank the patients and their families who gave consent to participate in the study as well as all research staff involved.
Footnotes
Author contributions: Jinliang Wang and Yi Hu conceived the idea of this article; Fang Yan, Junxun Ma, Zhefeng Liu and Bo Yang completed the work of acquisition of data; Zhibo Zhang, Fan Zhang and Qiuxiang Ou shared the task of analysis, interpretation of data, and manuscript writing and revision; all authors participated in discussing and revising the manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics approval and consent to participate: This study was approved by the Institutional Ethics Committee of the Chinese PLA General Hospital of China (No: S2018-141-01) and conducted in compliance with the Declaration of Helsinki and Good Clinical Practice Guidelines defined by the International Conference on Harmonization. Informed consent was collected from each patient prior to sample collection and participation in the study.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Zhibo Zhang  https://orcid.org/0000-0001-8534-7190
https://orcid.org/0000-0001-8534-7190
Qiuxiang Ou  https://orcid.org/0000-0002-2961-2057
https://orcid.org/0000-0002-2961-2057
Contributor Information
Zhibo Zhang, Department of Oncology, The Second Medical Center of Chinese PLA General Hospital, Beijing, China; Department of Oncology, Chinese PLA General Hospital, Beijing, China; The 78th Group Army Hospital of Chinese PLA, Mudanjiang, China.
Fan Zhang, Department of Oncology, Chinese PLA General Hospital, Beijing, China.
Fang Yuan, Department of Oncology, Chinese PLA General Hospital, Beijing, China.
Ye Li, Department of Radiotherapy, Chinese PLA General Hospital, Beijing, China.
Junxun Ma, Department of Oncology, Chinese PLA General Hospital, Beijing, China.
Qiuxiang Ou, Translational Medicine Research Institute, Geneseeq Technology Inc., Toronto, Ontario, Canada.
Zhefeng Liu, Department of Oncology, Chinese PLA General Hospital, Beijing, China.
Bo Yang, Department of Oncology, Chinese PLA General Hospital, Beijing, China.
Lijie Wang, Department of Oncology, Chinese PLA General Hospital, Beijing, China.
Haitao Tao, Department of Oncology, Chinese PLA General Hospital, Beijing, China.
Sujie Zhang, Department of Oncology, Chinese PLA General Hospital, Beijing, China.
Xiaoyan Li, Department of Oncology, Chinese PLA General Hospital, Beijing, China.
Xiaoyu Zhi, Department of Oncology, The Second Medical Center of Chinese PLA General Hospital, Beijing, China; Medical School of Chinese PLA, Beijing, China.
Xiangwei Ge, Department of Oncology, The Second Medical Center of Chinese PLA General Hospital, Beijing, China; Medical School of Chinese PLA, Beijing, China.
Hua Bao, Translational Medicine Research Institute, Geneseeq Technology Inc., Toronto, Ontario, Canada.
Xue Wu, Translational Medicine Research Institute, Geneseeq Technology Inc., Toronto, Ontario, Canada.
Yi Hu, Department of Oncology, Chinese PLA General Hospital, 28 Fuxing Road, Haidian District, Beijing, China.
Jinliang Wang, Department of Oncology, The Second Medical Center of Chinese PLA General Hospital, 28 Fuxing Road, Haidian District, Beijing, China.
References
- 1. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019; 69: 363–385. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 4. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10: 1243–1260. [DOI] [PubMed] [Google Scholar]
- 5. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 7. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 2018; 19: 1468–1479. [DOI] [PubMed] [Google Scholar]
- 9. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 10. Barbee MS, Ogunniyi A, Horvat TZ, et al. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother 2015; 49: 907–937. [DOI] [PubMed] [Google Scholar]
- 11. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- 12. Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016; 34: 2980–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 2017; 376: 2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aguiar PN, Jr, Santoro IL, Tadokoro H, et al. A pooled analysis of nivolumab for the treatment of advanced non-small-cell lung cancer and the role of PD-L1 as a predictive biomarker. Immunotherapy 2016; 8: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 15. Wang Z, Duan J, Cai S, et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol 2019; 5: 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 2018; 24: 1441–1448. [DOI] [PubMed] [Google Scholar]
- 17. Garon EB. Cancer immunotherapy trials not immune from imprecise selection of patients. N Engl J Med 2017; 376: 2483–2485. [DOI] [PubMed] [Google Scholar]
- 18. Remon J, Besse B, Soria JC. Successes and failures: what did we learn from recent first-line treatment immunotherapy trials in non-small cell lung cancer? BMC Med 2017; 15: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhaijee F, Anders RA. PD-L1 Expression as a predictive biomarker: is absence of proof the same as proof of absence? JAMA Oncol 2016; 2: 54–55. [DOI] [PubMed] [Google Scholar]
- 20. Cristescu R, Mogg R, Ayers M, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018; 362: eaar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cyriac G, Gandhi L. Emerging biomarkers for immune checkpoint inhibition in lung cancer. Semin Cancer Biol 2018; 52: 269–277. [DOI] [PubMed] [Google Scholar]
- 22. Ibrahim UA, Yusuf AA, Ahmed SG. The pathophysiologic basis of anaemia in patients with malignant diseases. Gulf J Oncolog 2016; 1: 80–89. [PubMed] [Google Scholar]
- 23. Belle SJ-PV, Cocquyt V. Impact of haemoglobin levels on the outcome of cancers treated with chemotherapy. Crit Rev Oncol Hematol 2003; 47: 1–11. [DOI] [PubMed] [Google Scholar]
- 24. Rodgers GM, III, Becker PS, Blinder M, et al. Cancer- and chemotherapy-induced anemia. J Natl Compr Canc Netw 2012; 10: 628–653. [DOI] [PubMed] [Google Scholar]
- 25. Li J, Shulin C, Songguo P, et al. Prognostic nomogram for patients with nasopharyngeal carcinoma incorporating hematological biomarkers and clinical characteristics. Int J Biol Sci 2018; 14: 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watine J, Bouarioua N. Anemia as an independent prognostic factor for survival in patients with cancer. Cancer 2002; 94: 2793–2796. [DOI] [PubMed] [Google Scholar]
- 27. Koh WJ, Greer BE, Abu-Rustum NR, et al. Vulvar cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017; 15: 92–120. [DOI] [PubMed] [Google Scholar]
- 28. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 29. Fagerland MW, Hosmer DW, Bofin AM. Multinomial goodness-of-fit tests for logistic regression models. Stat Med 2008; 27: 4238–4253. [DOI] [PubMed] [Google Scholar]
- 30. Nicholson AG, Chansky K, Crowley J, et al. The international association for the study of lung cancer lung cancer staging project: proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11: 300–311. [DOI] [PubMed] [Google Scholar]
- 31. Spano JP, Namer M. Anemia and cancer. Bull Cancer 2005; 92: 428. [PubMed] [Google Scholar]
- 32. Lin Y, Yang H, Cai Q, et al. Characteristics and prognostic analysis of 69 patients with pulmonary sarcomatoid carcinoma. Am J Clin Oncol 2014; 39: 215–222. [DOI] [PubMed] [Google Scholar]
- 33. Strouse CS, Arce-Lara C, Whittle J, et al. A retrospective, population-based comparison of pemetrexed and paclitaxel for first-line treatment of stage IV non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2014; 90: S49. [Google Scholar]
- 34. Huang Y, Wei S, Jiang N, et al. The prognostic impact of decreased pretreatment haemoglobin level on the survival of patients with lung cancer: a systematic review and meta-analysis. BMC Cancer 2018; 18: 1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Svaton M, Zemanova M, Skrickova J, et al. Chronic inflammation as a potential predictive factor of nivolumab therapy in non-small cell lung cancer. Anticancer Res 2018; 38: 6771–6782. [DOI] [PubMed] [Google Scholar]
- 36. Zhao L, He R, Long H, et al. Late-stage tumors induce anemia and immunosuppressive extramedullary erythroid progenitor cells. Nat Med 2018; 24: 1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gaspar BL, Sharma P, Das R. Anemia in malignancies: pathogenetic and diagnostic considerations. Hematology 2015; 20: 18–25. [DOI] [PubMed] [Google Scholar]
- 38. Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 2001; 93: 266–276. [DOI] [PubMed] [Google Scholar]
- 39. Tas F, Eralp Y, Basaran M, et al. Anemia in oncology practice: relation to diseases and their therapies. Am J Clin Oncol 2002; 25: 371–379. [DOI] [PubMed] [Google Scholar]
- 40. Kuo CS, Wang CC, Huang YC, et al. Comparison of a combination of chemotherapy and immune checkpoint inhibitors and immune checkpoint inhibitors alone for the treatment of advanced and metastatic non-small cell lung cancer. Thorac Cancer 2019; 10: 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cappellini MD, Motta I. Anemia in clinical practice-definition and classification: does hemoglobin change with aging? Semin Hematol 2015; 52: 261–269. [DOI] [PubMed] [Google Scholar]
- 42. Lanier JB, Park JJ, Callahan RC. Anemia in older adults. Am Fam Physician 2018; 98: 437–442. [PubMed] [Google Scholar]