Abstract
Background:
Assessing the prognosis of patients with early-stage non-small cell lung cancer (NSCLC) has become a major clinical issue. This study aimed to devise an effective clinical nomogram and heat map for assessing the survival of patients with stage I NSCLC receiving complete resection.
Methods:
Nomograms were established based on a retrospective study of 654 patients with stage I NSCLC who underwent radical resection at Sun Yat-Sen University Cancer Center between January 2009 and December 2014. The concordance index (C-index) and calibration curve were used to measure the accuracy and discriminative ability of the final nomogram. Heat maps were constructed with prognostic factors and survival probabilities. Survival curves were depicted using the Kaplan–Meier method, and the log-rank test was used to determine significance. Patients were classified into low- and high-risk subgroups using recursive partitioning analysis based on nomogram scores.
Results:
In univariate and multivariate analyses, the independent factors for overall survival (OS) and disease-free survival (DFS) were age, sex, tumor size, and visceral pleural invasion, which were all selected in the nomogram. The C-indices of the nomogram for predicting OS and DFS were 0.694 [95% confidence interval (CI) 0.651–0.737] and 0.653 (95% CI 0.61–0.696), respectively. The calibration curves for OS and DFS probabilities showed a good agreement between the nomogram prediction and actual observation. A heat map was generated using the above independent factors for OS and DFS. High-risk patients had shorter OS [hazard ratio (HR) = 3.535, 95% CI 2.444–5.113, p < 0.001] and DFS (HR = 2.607, 95% CI 1.922–3.537, p < 0.001) than low-risk patients.
Conclusion:
We established a prognostic nomogram and heat map that can be useful for evaluating survival in patients with stage I NSCLC after complete resection. The tools resulted in more accurate prediction and may guide clinicians in making treatment decisions.
Keywords: heat map, nomogram, non-small cell lung cancer, prognosis, survival
Background
Lung cancer is the most commonly diagnosed cancer in the world, and non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer cases.1 In recent years, the increasing use of high-resolution computed tomography (CT) and low-dose CT in lung cancer screening and diagnosis have helped identify more cases of NSCLC at an early stage, and complete surgical resection has remained the mainstay treatment.
Recently, clinical nomograms have been regarded as reliable methods for quantifying risks in cancer because they incorporate and demonstrate important factors for prognosis.2–4 Several studies have proposed that nomograms confer more accurate survival prediction than the traditional tumor-node-metastasis (TNM) staging systems for various malignancies.5–8 For stage I NSCLC, the prognosis is generally favorable with 5-year survival rate ranging from 73% to 90%.9,10 The most common problems that patients encounter are tumor recurrence (including locoregional and distant), which may lead to mortality after surgical resection. Recurrence rates have been reported to be 20–37.4%, depending on length of follow-up.11–13 Therefore, outcomes remain to be heterogeneous and difficult to estimate. Thus, building a valid clinical nomogram for patients with stage I NSCLC treated with radical resection would help clinicians in identifying those with high risk and promote more individualized planning, treatment, and follow-up among the multidisciplinary team. This study therefore aimed to establish a clinical nomogram and heat map with improved prediction of long-term survival in this patient population.
Methods
Patient selection
This study included 654 patients with stage I NSCLC who underwent radical surgery at Sun Yat-Sen University Cancer Center between January 2009 and December 2014. The radical surgery consisted of complete resection of the primary tumor (lobectomy) and mediastinal lymph node dissection with microscopically radical resection (R0). All patients underwent pretreatment evaluations, which included medical history taking, physical examination, routine hematologic and serum biochemical test, chest radiography, electrocardiogram, chest and upper abdominal CT, brain magnetic resonance imaging (MRI), and bronchoscopy. Whole-body bone scan, or positron emission tomography-computed tomography (PET-CT) was conducted when metastasis was suspected. The exclusion criteria were as follows: patients who had a previous malignant disease, those with a second primary tumor, and those who received neoadjuvant and/or adjuvant treatments. Tumor stage was reclassified according to the eighth edition of the TNM classification system.9 This study was approved by the institutional ethics committee of Sun Yat-Sen University Cancer Center (number: B2020-227). All patients provided written informed consent prior to treatment.
Follow-up and outcome
After the primary treatment was completed, the patients were followed up every 3 months for 1 year, every 6 months for 2–3 years, and annually thereafter. Tumor recurrence or metastasis was recorded at the first detection of locoregional recurrence or distant metastasis after the completion of surgery confirmed based on CT, MRI, or PET-CT with or without an increase in tumor biomarkers. Follow-up data were obtained from the most recent medical review. The survival status of the patients was verified again in October 2019. Overall survival (OS) was calculated from the date of surgery to the date of death or the last day of follow-up, while disease-free survival (DFS) was defined as the interval between the date of surgery and the date of recurrence/metastasis or the last day of follow-up.
Statistical analysis
Cox proportional-hazards regression models were used to identify prognostic factors. Factors assessed included sex, age, pathology, tumor location, tumor size, grade, and pleural invasion. All factors that achieved potential significance (p < 0.1) in the univariate analysis were included in the multivariate analysis based on the Cox regression models. Survival curves were depicted using the Kaplan–Meier method, and the log-rank test was used to determine significance. All statistical analyses were performed with SPSS software version 26.0 (SPSS Inc., Chicago, IL, USA), unless otherwise indicated. A two-sided p-value of < 0.05 was considered statistically significant.
Nomograms were formulated based on the results of the univariate and multivariable analyses. Using a backward step-down selection process with the Akaike information criterion, we selected the final prognostic nomogram model.14,15 The performance of the nomogram was measured based on the concordance index (C-index) and assessed by comparing the predicted survival with the observed survival probability. Bootstraps with 1000 resamples were used for these activities. The larger the C-index, the more accurate was the prognostic stratification.16 Heat maps were provided to visualize the correlations of independent factors with survival probabilities obtained from nomogram scores. Patients were categorized into new risk groups (low-risk and high-risk) using recursive partitioning analysis (RPA) based on nomogram scores. Nomograms and heat maps were formulated, and RPA was conducted using the rms and rpart packages of R version 3.3.2 (http://www.r-project.org).17
The authenticity of this study has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn), with the approval RDD number as RDDA2020001664.
Results
Clinical and pathological characteristics of patients
Figure 1 displays the CONSORT diagram to obtain the study population. Among the 654 patients enrolled in this study, 400 (61.2%) were men. The age of the patients ranged from 25 to 82 years, and the median age was 62 years (interquartile range 55–68.8 years). Adenocarcinoma was the most common pathological type (492 patients, 75.2%). The median tumor size was 2.4 cm (range 0.3–4.0 cm). There were 31, 154, 138, and 331 patients who were classified as having a pathological status of T1a, T1b, T1c, and T2a, respectively. The clinical and pathological characteristics of the patients are detailed in Table 1.
Figure 1.
CONSORT flow diagram.
NSCLC, non-small cell lung cancer; R1/2, microscopic/macroscopic residual tumor.
Table 1.
Baseline characteristics of patients with stage I non-small cell lung cancer.
| Variable | N = 654 (%) |
|---|---|
| Sex | |
| Male | 400 (61.2) |
| Female | 254 (38.8) |
| Age, median (IQR), years | 62 (55–68.8) |
| <60 | 264 (40.4) |
| ⩾60 | 390 (59.6) |
| Pathology | |
| Squamous | 119 (18.2) |
| Adenocarcinoma | 492 (75.2) |
| Others | 43 (6.6) |
| Tumor location | |
| RUL | 220 (33.6) |
| RML | 60 (9.2) |
| RLL | 133 (20.3) |
| LUL | 168 (25.7) |
| LLL | 73 (11.2) |
| Tumor size, median ± SE, cm | 2.4 ± 0.9 |
| pT stage | |
| T1a | 31 (4.8) |
| T1b | 154 (23.5) |
| T1c | 138 (21.1) |
| T2a | 331 (50.6) |
| Grade | |
| Good | 75 (11.5) |
| Moderate | 359 (54.9) |
| Poor | 220 (33.6) |
| Pleural invasion | |
| No | 429 (65.6) |
| Yes | 225 (34.4) |
IQR, interquartile range; LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; SE, standard error.
Prognostic analysis and independent prognostic factors
To determine the independent prognostic factors for survival, we analyzed the OS and DFS using a Cox regression model. The results of the univariate and multivariate analyses are shown in Table 2. In the univariate analysis, the significant independent factors for OS included sex, age, pathology, tumor size, grade, and pleural invasion, while in the multivariate analysis, the significant independent factors were sex [adjusted hazard ratio (HR) = 0.422, p < 0.001], age (adjusted HR = 2.321, p < 0.001), tumor size (adjusted HR = 1.498, p < 0.001), and pleural invasion (adjusted HR = 1.508, p = 0.022). For DFS, the factors that showed potential significance in the univariate analysis included sex, age, tumor size, grade, and pleural invasion. In the multivariate analysis, sex (adjusted HR = 0.557, p < 0.001), age (adjusted HR = 1.853, p < 0.001), tumor size (adjusted HR = 1.391, p < 0.001), and pleural invasion (adjusted HR = 1.466, p = 0.01) were significantly associated with prognosis.
Table 2.
Prognostic factors for overall survival and disease-free survival on univariate and multivariate analyses.
| Variable | Univariate analysis |
p trend | Multivariate analysis |
p trend | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |||
| Overall survival | ||||||||
| Sex (male versus female) | 0.413 | 0.276–0.617 | <0.001 | 0.422 | 0.279–0.637 | <0.001 | ||
| Age (<60 versus ⩾60) | 2.049 | 1.397–3.006 | <0.001 | 2.321 | 1.575–3.420 | <0.001 | ||
| Pathology | ||||||||
| SCC | 1 | 0.080 | 1 | 0.403 | ||||
| ADC | 0.638 | 0.426–0.956 | 0.029 | 1.121 | 0.723–1.738 | 0.609 | ||
| Others | 0.856 | 0.431–1.698 | 0.656 | 1.624 | 0.80–3.297 | 0.179 | ||
| Tumor size (cm) | 1.563 | 1.274–1.917 | <0.001 | 1.498 | 1.219–1.842 | <0.001 | ||
| Grade | ||||||||
| Good | 1 | 0.001 | 1 | 0.066 | ||||
| Moderate | 1.749 | 0.838–3.652 | 0.137 | 1.491 | 0.712–3.122 | 0.290 | ||
| Poor | 3.052 | 1.461–6.379 | 0.003 | 2.079 | 0.981–4.367 | 0.056 | ||
| Pleural invasion (no versus yes) | 1.383 | 0.982–1.949 | 0.064 | 1.508 | 1.066–2.135 | 0.022 | ||
| Disease-free survival | ||||||||
| Sex (male versus female) | 0.542 | 0.395–0.743 | <0.001 | 0.557 | 0.403–0.771 | <0.001 | ||
| Age (<60 versus ⩾60) | 1.701 | 1.250–2.316 | 0.001 | 1.853 | 1.357–2.529 | <0.001 | ||
| Tumor size (cm) | 1.472 | 1.242–1.744 | <0.001 | 1.391 | 1.171–1.654 | <0.001 | ||
| Grade | ||||||||
| Good | 1 | 0.001 | 1 | 0.056 | ||||
| Moderate | 1.806 | 0.990–3.292 | 0.054 | 1.562 | 0.855–2.856 | 0.147 | ||
| Poor | 2.779 | 1.515–5.098 | 0.001 | 2.009 | 1.085–3.521 | 0.036 | ||
| Pleural invasion (no versus yes) | 1.406 | 1.055–1.873 | 0.020 | 1.466 | 1.096–1.961 | 0.010 | ||
ADC, adenocarcinoma; CI, confidence interval; HR, hazard ratio; SCC, squamous cell carcinoma.
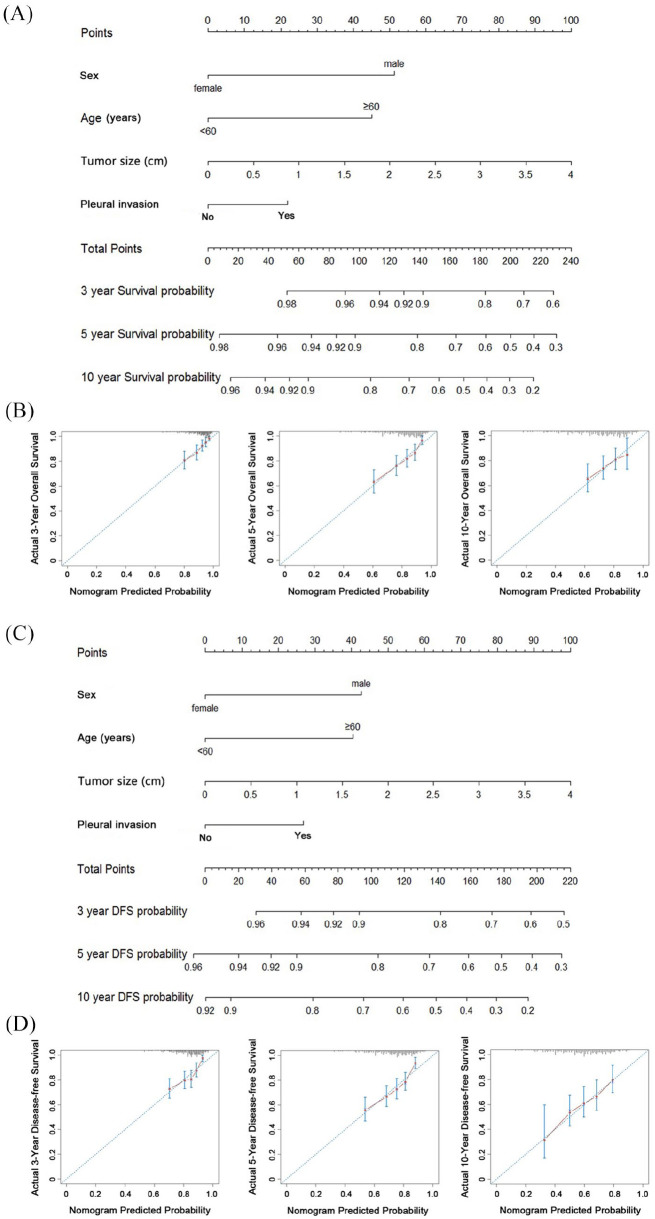
We first built a nomogram for OS that included the abovementioned independent prognostic factors [Figure 2(A)]. The nomogram showed tumor size as sharing the largest contribution to prognosis. Each patient within these variables was assigned a score on the point scale. By adding up the total score and locating it on the total point, we were easily able to draw a straight line down to determine the estimated probability of survival at each time point. Scores were totaled for each case to obtain the nomogram score of each case (median 126.4, range 7.5–218.4). The calibration curves showed a good agreement between the prediction by the nomogram and the actual observation for 3-, 5-, and 10-year OS [Figure 2(B)]. The C-index of the nomogram for predicting OS was 0.694 [95% confidence interval (CI) 0.651–0.737].
Figure 2.
Nomogram for 3-, 5-, and 10-year overall survival (OS) in patients with stage I non-small cell lung cancer (NSCLC) (A); calibration curve of nomogram for predicting OS (B); nomogram for 3-, 5-, and 10-year disease-free survival (DFS) in patients with stage I NSCLC (C); calibration curve of nomogram for predicting DFS (D). To use the nomogram, an individual patient’s value is located on each variable axis, and a line is drawn upward to determine the number of points received for each variable value. The sum of these numbers is located on the total point axis, and a line is drawn downward to the survival axes to determine the likelihood of 3-year, 5-year, and 10-year survival.
Figure 2(C) shows the prognostic nomogram for DFS incorporating all significant independent factors. This nomogram also showed tumor size as sharing the largest contribution to prognosis. The total points of each case were also calculated according to the established nomogram (median 119.8, range 7.5–210.1). The calibration curves for the probability of 3-, 5-, and 10-year DFS presented a good agreement between the nomogram prediction and actual observation [Figure 2(D)]. The C-index value of the nomogram for predicting DFS was 0.653 (95% CI 0.61–0.696).
Heat maps and survival curves based on the nomogram scores
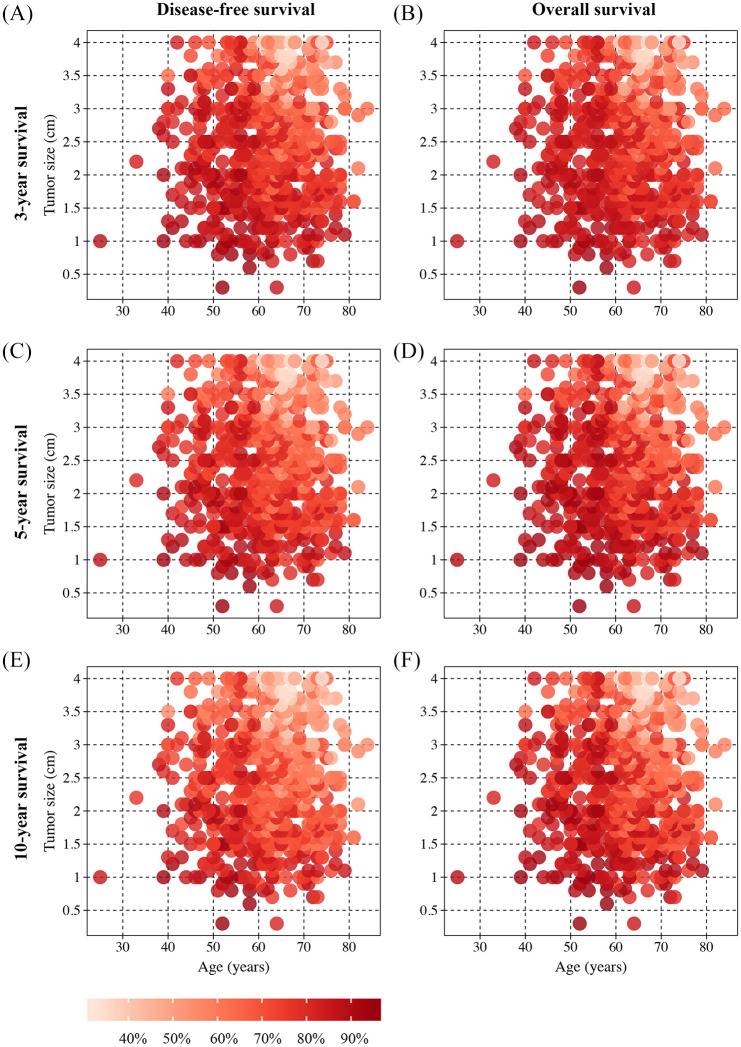
Heat maps were provided to observe the relationships of the combination of independent prognostic factors with survival probabilities extracted based on the nomogram scores. We found that the heat maps could visualize differences in variables (age and tumor size) associated with the probability of 3-, 5-, and 10-year OS and DFS (Figure 3).
Figure 3.
Heat maps for tumor size (x-axis) and age (y-axis) corresponding to 3-year disease-free survival (DFS) (A), 3-year overall survival (OS) (B), 5-year DFS (C), 5-year OS (D), 10-year DFS (E), and 10-year OS (F). Red regions indicate relatively better outcomes than pink regions.
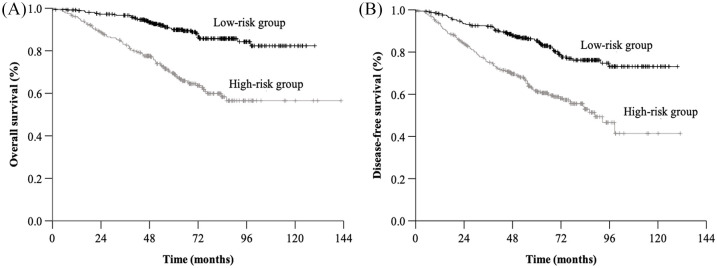
We also performed RPA for OS and DFS based on the nomogram scores. Using 134 and 120 as the cutoff points, we distinguished two risk groups in terms of OS (low-risk subgroup, <134, and high-risk subgroup, ⩾134) and DFS (low-risk subgroup, <120, and high-risk subgroup, ⩾120). The patients in the low-risk subgroup had statistically better survival than those in the high-risk subgroup (OS, HR = 3.535, 95% CI 2.444–5.113, p < 0.001; DFS, HR = 2.607, 95% CI 1.922–3.537, p < 0.001) (Figure 4).
Figure 4.
Kaplan–Meier survival curves for overall survival and disease-free survival by recursive partitioning analysis method.
Discussion
In this study, we constructed a prognostic nomogram and heat map to improve the prediction of survival in patients with stage I NSCLC who underwent radical surgery. Our findings that address the continuous nature of tumor size provided more accurate estimations for the survivals of stage I NSCLC than the methods that categorize the variables. Furthermore, our scheme could categorize patients into high-risk and low-risk subgroups with significant differences in OS and DFS.
Clinical nomograms have been recently considered as reliable methods for quantifying risk in cancer. A series of studies have revealed that nomograms present a more accurate prognostic prediction than traditional staging systems for various cancers, including colorectal cancer,3,18 gastric cancer,4 breast cancer,19,20 cholangiocarcinoma,8 and nasopharyngeal cancer.6,21 However, there has been no effective nomogram for predicting survival in patients with stage I NSCLC to date. Despite great advances in NSCLC treatment, including target therapy, chemotherapy, and immunotherapy, the primary treatment for patients with stage I NSCLC remains complete surgical resection. Moreover, although the prognosis for this cohort is generally favorable, it remains heterogeneous, with a probability of locoregional or distant recurrence.11–13 An increasing number of stage I NSCLC cases have been detected in recent years with the help of high-resolution low-dose CT for screening and diagnosis; however, the clinical nomograms for NSCLC were mostly established for patients with an advanced stage.5,22–24
Estimation of survival is important in the management of early stage NSCLC. Tumor size plays a prominent role in stage I NSCLC. In addition, T category is determined by invasion into visceral pleura. Thus, the existing scheme in predicting survival (e.g. The Eighth Edition Lung Cancer Stage Classification) is based on tumor size and visceral pleural invasion, but it is unknown whether the chosen cutoff values are optimum. More importantly, for stage I NSCLC, the available scheme categorizes tumor size, which probably results in a loss of prognostic information and causes abrupt changes in the estimated survivals when tumor size is close to a cutoff value. We think an influence of tumor size on survival of stage I NSCLC, with a larger effect per one unit of measurement at low values compared with high value. To address this issue, using tumor size as a continuous variable, we constructed user-friendly nomogram and heat maps for estimating survivals of NSCLC patients with stage I disease. This information might be useful in patient counseling. To our knowledge, this is the first study to show an innovative use of nomogram and heat map for visualizing prognosis in early stage NSCLC.
So far, there are no standard criteria for performing intentional sublobectomy (wedge resection or segmentectomy) for lung cancer.25,26 Therefore, most sublobectomy procedures were performed only for patients with compromised preoperative pulmonary function or severe underlying comorbidities. Sublobectomy is commonly considered in most studies when building nomograms for patients with early-stage NSCLC.27,28 However, most of these studies were retrospective, and the evaluations were mostly performed without distinguishing the purpose of sublobectomy (compromised or intentional resection). Therefore, one major limitation of the nomograms developed by these studies is the underlying selection bias among the different sublobectomy groups. In light of this, we strictly set the inclusion criteria in this study and included only NSCLC patients with stage I disease who were treated with standard lobectomy and mediastinal lymph node dissection.
In this study, the significant independent factors for OS in the univariate and multivariate analyses were the same for DFS, and these included sex, age, tumor size, and pleural invasion. These factors have been well established in the literature and have served as a basis for devising clinical nomograms. We also found that the calibration curves for 3-, 5-, and 10-year OS and DFS showed a good agreement between the nomogram prediction and the actual observation. The C-index of the nomogram was 0.694 (95% CI 0.651–0.737) for OS and 0.653 (95% CI 0.610–0.696) for DFS. These findings suggest that our nomogram displayed better accuracy in predicting patient clinical outcome.
Malignancies with poor cell differentiation generally indicate a more invasive biological behavior than those with good cell differentiation. Cell differentiation has been well known as an important independent prognostic factor and was therefore introduced into the TNM staging system for various human cancers. In this study, cell differentiation was significantly associated with OS and DFS. For patients with good, moderate, and poor cell differentiation, the estimated 5-year OS was 91.5%, 82.9%, and 73.8%, while the estimated 5-year DFS was 86.2%, 76.3%, and 64.4%, respectively. In the multivariate analysis, cell differentiation showed a borderline significant trend with OS (adjusted p = 0.066) and DFS (adjusted p = 0.056). There were only 75 NSCLC cases with good differentiation in this study, so we speculate that the prognostic impact of cell differentiation might achieve more statistical significance if the sample size is increased. It is plausible that the impact of cell differentiation is fragile and could be only observed in patients with early-stage NSCLC.
Administration of adjuvant therapy (chemotherapy and radiotherapy) to all patients with stage I NSCLC is unnecessary and harmful for some patients, who are cured with surgery alone. Our results indicated that nomogram and heat map accurately estimate survival and stratify risk subgroup, and their use might reduce the risk of overtreatment with adjuvant therapy in NSCLC patients with stage I disease that is likely cured by surgery. Patients in the low-risk subgroup have generally favorable survival and might not be candidates for adjuvant therapy. In contrast, adjuvant therapy might provide survival benefit to patients stratified as high risk. We believe that our findings could help clinicians in promoting individualized and appropriate treatment decisions.
This study had some limitations. First, it was retrospective in nature, and the cohort was extracted from a single-center database only. Caution should be considered when interpreting and generalizing the results. Second, this study failed to incorporate important molecular biological factors such as EGFR mutation, ALK-EML4 fusion, and T790M mutation, which may be potentially significant factors for survival in patients with early-stage NSCLC. Third, as we stated, due to the retrospective nature of the present study, it is difficult to find an external validation cohort because of diversity of standards in different hospitals (e.g. surgery and follow-up). Finally, although we validated the nomogram using bootstrap resampling, external validation was not available, and the bias could not be completely ruled out. Future prospective multicenter studies with more diverse biomarkers and external validation of results are therefore necessary.
In summary, the nomogram and heat map proposed in this study accurately predicted the prognosis of patients with stage I NSCLC treated with complete resection. The proposed nomogram could help clinicians in more accurately identifying patients with poor prognosis after surgery and further promote individualized and multidisciplinary treatment decisions.
Acknowledgments
We greatly appreciate the kind assistance of Lian-xiong Yuan (Office of Research Service, The Third Affiliation Hospital of Sun Yat-Sen University, Guangzhou, Guangdong, China) for his assistance in statistical analysis.
Footnotes
Author contributions: Conception and design: Xun Cao, Yu-zhen Zheng, Xu-dong Wang, and Xin Wang. Data collection: Xun Cao, Yu-zhen Zheng, Xiang Guo, Yong Li, Zhen Wang, Li Zhang, Xu-dong Wang, and Xin Wang. Data analysis: all authors. Data interpretation: Xun Cao, Yu-zhen Zheng, Hong-ying Liao, Yong Li, Li Zhang, and Xin Wang. Manuscript writing: Xun Cao, Yu-zhen Zheng, Hong-ying Liao, and Yong Li. Manuscript revision: Xiang Guo, Li Zhang, Xu-dong Wang, and Xin Wang. All authors approved the final manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics approval: This study was recorded and approved by the institutional ethics committee of Sun Yat-Sen University Cancer Center (number: B2020-227).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Science and Technology Project of Guangdong Province of China (grant number 2017A020215035) and the Medical Scientific Research Foundation of Guangdong Province of China (grant number A2020150).
Contributor Information
Xun Cao, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, Guangdong, China.
Yu-zhen Zheng, The Sixth Affiliated Hospital of Sun Yat-Sen University, Guangzhou, Guangdong, China.
Hong-ying Liao, The Sixth Affiliated Hospital of Sun Yat-Sen University, Guangzhou, Guangdong, China.
Xiang Guo, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, Guangdong, China.
Yong Li, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, Guangdong, China.
Zhen Wang, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, Guangdong, China.
Li Zhang, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, Guangdong, China.
Xu-dong Wang, Department of Anesthesiology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, No. 651 Dongfeng Road East, Guangzhou 510060, Guangdong, China.
Xin Wang, Department of Thoracic Surgery, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, No. 651 Dongfeng Road East, Guangzhou, Guangdong 510060, China.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. [DOI] [PubMed] [Google Scholar]
- 2. Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015; 16: e173–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valentini V, van Stiphout RG, Lammering G, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol 2011; 29: 3163–3172. [DOI] [PubMed] [Google Scholar]
- 4. Han D-S, Suh Y-S, Kong S-H, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol 2012; 30: 3834–3840. [DOI] [PubMed] [Google Scholar]
- 5. Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015; 33: 861–869. [DOI] [PubMed] [Google Scholar]
- 6. Tang XR, Li YQ, Liang SB, et al. Development and validation of a gene expression-based signature to predict distant metastasis in locoregionally advanced nasopharyngeal carcinoma: a retrospective, multicentre, cohort study. Lancet Oncol 2018; 19: 382–393. [DOI] [PubMed] [Google Scholar]
- 7. Yap WK, Shih MC, Kuo C, et al. Development and validation of a nomogram for assessing survival in patients with metastatic lung cancer referred for radiotherapy for bone metastases. JAMA Netw Open 2018; 1: e183242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013; 31: 1188–1195. [DOI] [PubMed] [Google Scholar]
- 9. Detterbeck FC, Boffa DJ, Kim AW, et al. The eighth edition lung cancer stage classification. Chest 2017; 151: 193–203. [DOI] [PubMed] [Google Scholar]
- 10. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11: 39–51. [DOI] [PubMed] [Google Scholar]
- 11. Schuchert MJ, Normolle DP, Awais O, et al. Factors influencing recurrence following anatomic lung resection for clinical stage I non-small cell lung cancer. Lung Cancer 2019; 128: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995; 109: 120–129. [DOI] [PubMed] [Google Scholar]
- 13. al-Kattan K, Sepsas E, Fountain SW, et al. Disease recurrence after resection for stage I lung cancer. Eur J Cardiothorac Surg 1997; 12: 380–384. [DOI] [PubMed] [Google Scholar]
- 14. Kee KM, Wang JH, Lee CM, et al. Validation of clinical AJCC/UICC TNM staging system for hepatocellular carcinoma: analysis of 5,613 cases from a medical center in southern Taiwan. Int J Cancer 2007; 120: 2650–2655. [DOI] [PubMed] [Google Scholar]
- 15. Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15: 361–387. [DOI] [PubMed] [Google Scholar]
- 16. Huitzil-Melendez FD, Capanu M, O’Reilly EM, et al. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol 2010; 28: 2889–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. R Core Team. R: a language and environment for statistical computing. Version 3.4.1 2017. [Google Scholar]
- 18. Collins IM, Kelleher F, Stuart C, et al. Clinical decision aids in colon cancer: a comparison of two predictive nomograms. Clin Colorectal Cancer 2012; 11: 138–142. [DOI] [PubMed] [Google Scholar]
- 19. Graesslin O, Abdulkarim BS, Coutant C, et al. Nomogram to predict subsequent brain metastasis in patients with metastatic breast cancer. J Clin Oncol 2010; 28: 2032–2037. [DOI] [PubMed] [Google Scholar]
- 20. Fujii T, Kogawa T, Wu J, et al. Nomogram to predict pathologic complete response in HER2-positive breast cancer treated with neoadjuvant systemic therapy. Br J Cancer 2017; 116: 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang LQ, Li CF, Li J, et al. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J Natl Cancer Inst 2016; 108: djv291. [DOI] [PubMed] [Google Scholar]
- 22. Xu J, Fan L, Yu H, et al. Survival value of primary tumor resection for stage IV non-small-cell lung cancer: a population based study of 6466 patients. Clin Respir J. Epub ahead of print 16 April 2020. DOI: 10.1111/crj.13194. [DOI] [PubMed] [Google Scholar]
- 23. Tang X, Li Y, Tian X, et al. Predicting severe acute radiation pneumonitis in patients with non-small cell lung cancer receiving postoperative radiotherapy: development and internal validation of a nomogram based on the clinical and dose-volume histogram parameters. Radiother Oncol 2019; 132: 197–203. [DOI] [PubMed] [Google Scholar]
- 24. Mao Q, Xia W, Dong G, et al. A nomogram to predict the survival of stage IIIA-N2 non-small cell lung cancer after surgery. J Thorac Cardiovasc Surg 2018; 155: 1784–1792.e1783. [DOI] [PubMed] [Google Scholar]
- 25. Widder J, Van De Wauwer C, Langendijk JA. Lobectomy or sublobectomy for small non-small-cell lung cancer: the question remains. J Clin Oncol 2017; 35: 572–573. [DOI] [PubMed] [Google Scholar]
- 26. Fan J, Wang L, Jiang G-N, et al. Sublobectomy versus lobectomy for stage I non-small-cell lung cancer, a meta-analysis of published studies. Ann Surg Oncol 2012; 19: 661–668. [DOI] [PubMed] [Google Scholar]
- 27. Wo Y, Yang H, Zhang Y, et al. Development and external validation of a nomogram for predicting survival in patients with stage IA non-small cell lung cancer ⩽2 cm undergoing sublobectomy. Front Oncol 2019; 9: 1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang H, Li X, Shi J, et al. A nomogram to predict prognosis in patients undergoing sublobar resection for stage IA non-small-cell lung cancer. Cancer Manag Res 2018; 10: 6611–6626. [DOI] [PMC free article] [PubMed] [Google Scholar]