Abstract
Purpose:
To evaluate the 3-year safety and effectiveness of the MDT-2113 (IN.PACT Admiral) drug-coated balloon (DCB) vs percutaneous transluminal angioplasty (PTA) in a Japanese population with femoropopliteal occlusive disease.
Materials and Methods:
The multicenter, prospective, IN.PACT SFA Japan randomized controlled trial (ClinicalTrials.gov identifier NCT01947478) was an independently adjudicated study evaluating Japanese participants randomized 2:1 to DCB (n=68) or PTA (n=32). The effectiveness endpoint was primary patency through 36 months, defined as freedom from clinically-driven target lesion revascularization (CD-TLR) and freedom from restenosis (by duplex ultrasound). The effectiveness endpoint was evaluated using the Kaplan-Meier method; estimates are presented with the 95% confidence intervals (CIs). The safety composite endpoint was freedom from device- and procedure-related death through 30 days and freedom from major target limb amputation and clinically-driven target vessel revascularization through 36 months.
Results:
Primary patency by Kaplan-Meier estimate was higher in the DCB group (68.9%, 95% CI 57.5% to 80.2%) vs the PTA group (46.9%, 95% CI 29.6% to 64.2%) at 36 months (log-rank p=0.001). The CD-TLR rates were 14.9% (10/67) for the DCB group and 20.7% (6/29) for PTA (p=0.554). The safety composite endpoint occurred in 83.6% (56/67) of DCB participants and 75.9% (22/29) of PTA participants (p=0.402). All-cause death was similar between groups at 36 months [DCB 6.0% (4/67) vs PTA 6.9% (2/29), p>0.999), with no device- or procedure-related deaths in either group.
Conclusion:
The final report of the IN.PACT SFA Japan trial showed that the IN.PACT Admiral DCB is safe and had durable outcomes through 3 years in Japanese participants with femoropopliteal occlusive disease.
Keywords: amputation, balloon angioplasty, drug-coated balloon, femoropopliteal segment, mortality, restenosis, target lesion revascularization
Introduction
Traditional endovascular approaches to the treatment of atherosclerotic disease in the femoropopliteal arteries include percutaneous transluminal angioplasty (PTA) with an uncoated balloon and implantation of a bare metal stent or stent-graft.1 Recently, drug-eluting stents (DES) and drug-coated balloons (DCBs) that transfer paclitaxel to the vessel wall during revascularization have been added to the suite of tools available to interventionists, helping to prevent restenosis after treatment.2,3
Multiple prospective studies have demonstrated the safety and effectiveness of DCBs for the treatment of patients with peripheral artery disease, including those with complex lesions that are often excluded from randomized controlled trials (RCTs).4–25 Despite the accumulation of short-term evidence supporting DCB use in a broad range of patients, there is still little evidence on DCB outcomes beyond 2 years. Three-year outcomes have been reported from the prospective, single-arm IN.PACT Global study (mean lesion length 12.1 cm),26 the ILLUMENATE US and EU trials (mean lesion lengths 8.0 and 7.2 cm, respectively),27 and the DEBATE-ISR RCT of patients with diabetes and in-stent restenosis (ISR; mean lesion length 13.2 cm).15 Five-year outcomes have been published from the THUNDER (mean lesion length 7.4 cm) and the IN.PACT SFA (mean lesion length 8.9 cm) RCTs.24,28 Five-year outcomes have been reported for AcoArt I (mean lesion length 14.7 cm).29 Beyond these studies, there is a need for more evidence on the long-term safety and efficacy of DCB use in patients with femoropopliteal occlusive disease.
Additionally, most prospective DCB studies have enrolled participants from sites in Europe or the United States, meaning that devices have not been thoroughly examined in different geographies or ethnicities or in the full range of baseline variables in a real-world population. A more global approach means that a broader variable set is tested, from vessel size to distribution of comorbidities. Furthermore, though a clinical trial setting does control for many variables, strict follow-up and medical therapy are not fully standardized and are left to the discretion of the treating physician. As such, testing medical devices in geographical regions with different standards of care is important. To date, only 5 prospective DCB studies have included populations from outside Europe and the United States; there is a distinct need for DCB studies that are focused on populations outside these geographical regions, including in Asia. Of these 5 studies, 2 are the large and single-arm studies IN.PACT Global and ILLUMENATE Global.7,18 The remaining 3 studies were conducted in Asia and include the prospective, multicenter, single-arm IN.PACT SFA China study and 2 RCTs, AcoArt I and IN.PACT (MDT-2113) SFA Japan.14,21,25,29,30 Previous reports from the IN.PACT SFA Japan trial have shown DCBs are safe and effective in an exclusively Japanese population, with a high rate of patency and low rate of clinically-driven target lesion revascularization (CD-TLR) through 2 years.25,30 This, the final IN.PACT SFA Japan report, is the first to publish 3-year DCB outcomes in an Asian population.
Materials and Methods
Study Design
IN.PACT (MDT-2113) SFA Japan was a phase III, multicenter, prospective RCT conducted in Japan. Methods have been previously reported.25 This single-blinded trial evaluated the MDT-2113 device (IN.PACT Admiral DCB; Medtronic plc, Santa Rosa, CA, USA) compared with standard uncoated PTA to report safety and effectiveness; participants were randomly assigned in a 2:1 ratio to treatment with a DCB or PTA. The trial was registered on the National Institutes of Health website (ClinicalTrials.gov identifier NCT01947478).
A Clinical Events Committee (CEC) reviewed and adjudicated all major adverse events through 36 months post-intervention. Independent core laboratories analyzed procedural and follow-up duplex ultrasonography images (VasCore, Massachusetts General Hospital, Boston, MA, USA) and angiograms (SynvaCor, Springfield, IL, USA). The CEC and independent core laboratories were blinded through the 36-month follow-up duration. The trial was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice Guidelines, and applicable laws as specified by all relevant governmental authorities. Independent oversight was provided by a data safety monitoring board.
Participant Population
Participants were enrolled in this study if they were between the ages of 20 and 85 years, with symptoms of claudication and/or ischemic rest pain (Rutherford categories 2–4) referable to a stenotic (70%–99%) lesion between 4- and 20-cm long or an occlusion ≤10 cm long involving the superficial femoral and/or proximal popliteal arteries. Lesions were required to undergo successful predilation before inclusion in the trial. Prior to enrollment, written informed consent was obtained from all participants according to the protocols approved by the institutional review boards at each investigational site.
The trial enrolled 100 participants randomized to receive treatment with DCB (n=68) or PTA (n=32). Demographic, clinical, and lesion characteristics have been reported and were well matched between groups (Table 1).25 The mean lesion length was 9.15±5.85 cm in the DCB group and 8.89±6.01 cm in the PTA group. Intravascular ultrasound was used in 39.7% (27/68) of DCB procedures and 25.0% (8/32) of PTA procedures, and the provisional stent rate was 4.4% (3/68) in the DCB group and 3.1% (1/32) in the PTA group (Table 2). Participant flow through 36 months is shown in Figure 1. Among participants still eligible for evaluation at 36 months, the rate of within and out of window follow-up was 95.1% (58/61) in the DCB group and 100% (27/27) in the PTA group.
Table 1.
Baseline Participant and Lesion Characteristics.a
| Characteristic | DCB (68 Participants, 68 Lesions) | PTA (32 Participants, 32 Lesions) | p |
|---|---|---|---|
| Age, y | 73.3±7.4 | 74.2±6.1 | 0.539 |
| Men | 73.5 (50/68) | 81.3 (26/32) | 0.461 |
| Obesity (BMI ≥30 kg/m2) | 4.4 (3/68) | 0 (0/32) | 0.549 |
| Diabetes mellitus | 58.8 (40/68) | 56.3 (18/32) | 0.831 |
| Insulin dependent | 14.7 (10/68) | 18.8 (6/32) | 0.771 |
| Current smoker | 26.5 (18/68) | 31.3 (10/32) | 0.639 |
| Carotid artery disease | 18.5 (12/65) | 16.1 (5/31) | >0.999 |
| Coronary heart disease | 50.0 (34/68) | 50.0 (16/32) | >0.999 |
| Renal insufficiency | 8.8 (6/68) | 12.5 (4/32) | 0.722 |
| Previous peripheral revascularization | 57.4 (39/68) | 59.4 (19/32) | >0.999 |
| Below-the-knee involvement | 33.8 (23/68) | 34.4 (11/32) | >0.999 |
| Previous limb amputation | 1.5 (1/68) | 0 (0/32) | >0.999 |
| ABI/TBI | 0.76±0.15 | 0.74±0.17 | 0.384 |
| Rutherford category | 0.623 | ||
| 2 | 54.4 (37/68) | 59.4 (19/32) | |
| 3 | 41.2 (28/68) | 37.5 (12/32) | |
| 4 | 4.4 (3/68) | 3.1 (1/32) | |
| Lesion typeb | 0.085 | ||
| De novo | 91.2 (62/68) | 100 (32/32) | |
| Restenotic (nonstented) | 8.8 (6/68) | 0.0 (0/32) | |
| Proximal popliteal involvement | 1.5 (1/68) | 3.1 (1/32) | 0.540 |
| Severe calcificationc,d | 7.4 (5/68) | 9.4 (3/32) | 0.708 |
| Lesion length, cmc,e | 9.15±5.85 | 8.89±6.01 | 0.838 |
| Total occlusionsc | 16.2 (11/68) | 15.6 (5/32) | >0.999 |
| TASC II classificationc | 0.852 | ||
| A | 57.4 (39/68) | 56.3 (18/32) | |
| B | 23.5 (16/68) | 21.9 (7/32) | |
| C | 19.1 (13/68) | 21.9 (7/32) | |
| Reference vessel diameter, mmc | 4.84±0.75 | 4.68±0.66 | 0.280 |
| Lesion diameter, mmc | 0.97±0.73 | 0.90±0.59 | 0.610 |
| Diameter stenosis, %c | 80.2±14.1 | 80.7±12.5 | 0.861 |
Abbreviations: ABI, ankle-brachial index; BMI, body mass index; DCB, drug-coated balloon; PTA, percutaneous transluminal angioplasty; TASC II, TransAtlantic Inter-Society Consensus II; TBI, toe-brachial index.
Continuous data are presented as the means ± standard deviation; categorical data are presented as the percent (number/sample).
Site-reported.
Assessed per lesion by core laboratory.
Severe calcification defined as calcification with circumference ≥180° (both sides of vessel at the same location) and length greater than or equal to half of the total lesion length.
Normal-to-normal by core laboratory quantitative vascular analysis.
Table 2.
Procedural Characteristics and Outcomes.a
| DCB (68 Participants, 68 Lesions) | PTA (32 Participants, 32 Lesions) | p | |
|---|---|---|---|
| Predilationb | 100 (68/68) | 100 (32/32) | >0.999 |
| Postdilationb | 23.5 (16/68) | 18.8 (6/32) | 0.796 |
| Provisional stentingb | 4.4 (3/68) | 3.1 (1/32) | 0.759 |
| IVUS use during index procedure | 39.7 (27/68) | 25.0 (8/32) | 0.181 |
| Balloons used per participantb | 1.4±0.5 | 1.1±0.2 | <0.001 |
| Dissection | 0.235 | ||
| None | 26.5 (18/68) | 28.1 (9/32) | |
| A-C | 73.5 (50/68) | 71.9 (23/32) | |
| D-F | 0 (0/68) | 0 (0/32) | |
| Hospitalization, db | 2.0±1.0 | 2.1±1.2 | 0.778 |
| Lesion length treated, cmc | 13.4±5.1 | 13.7±5.6 | 0.800 |
| Device successd | 100 (97/97) | 97.1 (33/34) | 0.260 |
| Procedure successe | 97.1 (66/68) | 100 (32/32) | >0.999 |
| Clinical successf | 97.1 (66/68) | 100 (32/32) | >0.999 |
Abbreviations: DCB, drug-coated balloon; IVUS, intravascular ultrasound; PTA, percutaneous transluminal angioplasty.
Continuous data are presented as the means ± standard deviation; categorical data are presented as the percent (number/sample).
Site-reported.
Assessed per lesion and reported by the core laboratory.
Device success was defined as successful delivery, inflation, deflation, and retrieval of the intact study balloon without burst below the rated burst pressure; device-based analysis.
Procedure success was defined as residual diameter stenosis ≤50% for nonstented participants and ≤30% for stented participants by core laboratory assessment; lesion-based analysis.
Clinical success was defined as procedure success without procedural complications (death, major target limb amputation, thrombosis of the target lesion, or target vessel revascularization) before discharge; participant-based analysis.
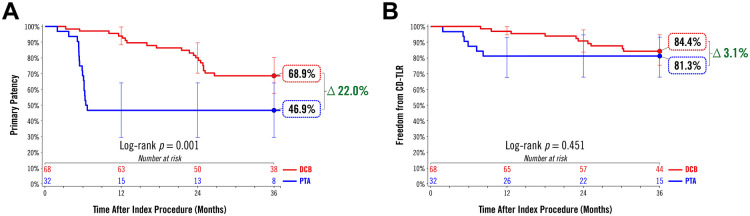
Figure 1.

Participant flow in the MDT-2113 SFA Japan trial through 36 months. Values for death, lost to follow-up, and withdrew consent are cumulative; other exits were due to progressive dementia and PTA for other disease. Compliance rates include follow-up completed in and outside of the follow-up window. DCB, drug-coated balloon; PTA, percutaneous transluminal angioplasty; SFA, superficial femoral artery.
Study Endpoints Through 36 Months
The primary effectiveness and safety endpoints were previously reported through 12 and 24 months.25,30 Through 36 months, the effectiveness endpoint was primary patency, defined as freedom from CD-TLR and freedom from restenosis as determined by a duplex ultrasound–derived peak systolic velocity ratio ≤2.4. Each component of the endpoint was independently adjudicated by the blinded CEC (for CD-TLR) or by the core laboratories (for restenosis). CD-TLR was defined as reintervention at the target lesion due to symptoms or decrease in ankle-brachial index (ABI) ≥20% or >0.15 vs the postprocedure ABI. At 36 months following the index procedure, the safety composite endpoint was freedom from (1) device- and procedure-related death through 30 days, (2) major target limb amputation through 36 months, and (3) clinically-driven target vessel revascularization (CD-TVR) through 36 months.
Other endpoints included major adverse events (a composite endpoint that included all-cause death, CD-TVR, major target limb amputation, and thrombosis at the target lesion site) at 36 months. Thrombosis was defined as a total occlusion due to rapidly evolving thrombus formation confirmed by sudden onset of symptoms and documented by duplex ultrasound and/or angiography. Additional assessments included individual components of the composite major adverse events endpoint and primary sustained clinical improvement (defined as freedom from target limb amputation, freedom from TVR, and an improvement shift of 1 Rutherford category at 36 months). Functional assessments included general appraisal through the administration of the EuroQOL (EQ-5D), a 5-dimension generic health status questionnaire, a 6-minute walk test, and evaluation of walking capacity using the Walking ImpairmentQuestionnaire (WIQ).
Statistical Analysis
The planned enrollment of 100 participants was not powered for any of the endpoints, though the trial design and endpoint assessments were the same as the IN.PACT SFA trial. The IN.PACT (MDT-2113) SFA Japan trial was intended to demonstrate consistent effectiveness and safety outcomes for the Japanese cohort compared with other studied geographies in the IN.PACT SFA trial.
All analyses were based on the intent-to-treat principle. For baseline characteristics and outcomes, continuous variables were described as mean ± standard deviation and were compared using independent t tests for between-group differences and paired t tests for changes from baseline; dichotomous and categorical variables were summarized as the counts and percentages and were compared using the Fisher exact or Cochran-Mantel-Haenszel test, respectively. The Kaplan-Meier method was used to evaluate time-to-event data for primary patency and freedom from CD-TLR over the 36-month follow-up period; estimates are given with the 95% confidence intervals (CI). Differences in the survival curves between groups were assessed using the log-rank test. There was no correction for multiple comparisons. The level of statistical significance was set at p<0.05. Statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA).
Results
Effectiveness Outcomes
As previously reported, acute procedure success was 97.1% or higher by all measures (Table 2).25 At 36 months, the Kaplan-Meier estimate of primary patency was 68.9% (95% CI 57.5% to 80.2%) in the DCB group and 46.9% (95% CI 29.6% to 64.2%) in the PTA group (log-rank p=0.001; Figure 2A). The freedom from CD-TLR estimate was 84.4% (95% CI 75.4% to 93.3%) in the DCB group and 81.3% (95% CI 67.7% to 94.8%) in the PTA group (log-rank p=0.451; Figure 2B). Time to first CD-TLR was 20.4±8.1 months in the DCB group and 5.6±2.2 months in the PTA group (p<0.001). Primary sustained clinical improvement through 36 months occurred in 70.0% (42/60) of DCB participants and 70.4% (19/27) of PTA participants (p>0.999).
Figure 2.
Kaplan-Meier analyses of (A) primary patency and (B) freedom from clinically-driven target lesion revascularization (CD-TLR) through 36 months. Bars represent the 95% confidence intervals. DCB, drug-coated balloon; PTA, percutaneous transluminal angioplasty.
Safety Outcomes
Safety outcomes are reported in Table 3. The primary safety composite endpoint (freedom from device- and procedure-related death through 30 days as well as major target limb amputation and CD-TVR through 36 months) was 83.6% (56/67) in the DCB group and 75.9% (22/29) in the PTA group (p=0.402). The rate of CD-TVR was 16.4% (11/67) in the DCB group and 24.1% (7/29) in the PTA group. There was 1 death between the 24- and 36-month follow-up periods. One participant in the PTA group died at 29.7 months of an unknown cause that was determined to be unrelated to the study device or procedure after independent adjudication by the CEC. Other deaths in the study that occurred prior to the 24-month time point included 4 deaths in the DCB group due to infection (n=1) and cancer (n=3) and 1 death in the PTA group due to infection.
Table 3.
Effectiveness and Safety Outcomes at 36 Months.a
| Outcome | DCB (68 Participants, 68 Lesions) | PTA (32 Participants, 32 Lesions) | Difference [95% CI] | p |
|---|---|---|---|---|
| CD-TLRb | 14.9 (10/67) | 20.7 (6/29) | –5.8 [–24.7 to 9.3] | 0.554 |
| Time to first CD-TLR, mo (number of participants with CD-TLR within 36 months) | 20.4±8.1 (10) | 5.6±2.2 (6) | 14.8 [8.9 to 20.8] | <0.001 |
| Primary sustained clinical improvementc | 70.0 (42/60) | 70.4 (19/27) | –0.4 [–19.0 to 21.0] | >0.999 |
| ABI/TBI | 0.91±0.14 (58) | 0.94±0.12 (27) | –0.029 [–0.092 to 0.034] | 0.363 |
| Change from baselined | 0.15±0.17 (58) | 0.20±0.18 (27) | –0.045 [–0.125 to 0.035] | 0.263 |
| Safety compositee | 83.6 (56/67) | 75.9 (22/29) | 7.7 [–8.3 to 27.0] | 0.402 |
| Major adverse eventsf | 20.9 (14/67) | 31.0 (9/29) | –10.1 [–30.0 to 7.6] | 0.306 |
| Death (all-cause) | 6.0 (4/67) | 6.9 (2/29) | –0.9 [–16.4 to 8.8] | >0.999 |
| CD-TVR | 16.4 (11/67) | 24.1 (7/29) | –7.7 [–27.0 to 8.3] | 0.402 |
| Major target limb amputation | 0 (0/67) | 0 (0/29) | NA | >0.999 |
| Thrombosis | 1.5 (1/67) | 0 (0/29) | 1.5 [–10.3 to 8.0] | >0.999 |
| Death (device- and procedure-related at 30 days) | 0 (0/68) | 0 (0/32) | NA | >0.999 |
| Any TLR | 14.9 (10/67) | 24.1 (7/29) | –9.2 [–28.4 to 6.6] | 0.382 |
| Any TVR | 16.4 (11/67) | 24.1 (7/29) | –7.7 [–27.0 to 8.3] | 0.402 |
Abbreviations: ABI, ankle-brachial index; CD-TLR, clinically-driven target lesion revascularization; CD-TVR, clinically-driven target vessel revascularization; CI, confidence interval; DCB, drug-coated balloon; PTA, percutaneous transluminal angioplasty; TBI, toe-brachial index; TLR, target lesion revascularization; TVR, target vessel revascularization.
Continuous data are presented as the means ± standard deviation and categorical data are presented as the proportion (number/sample) unless otherwise indicated. Differences are reported with 95% CI; “36 months” refers to 1080 days.
CD-TLR was defined as any reintervention within the target lesion(s) due to symptoms or drop in ABI/TBI ≥20% or >0.15 compared with postprocedure baseline ABI/TBI.
Primary sustained clinical improvement was defined as freedom from target limb amputation, freedom from TVR, and improvement of at least 1 Rutherford category at 36 months.
Changes from baseline to 36 months were significantly different in both the DCB (p<0.001) and PTA (p<0.001) groups.
Primary safety composite was defined as freedom from the following after the index procedure: (1) device- and procedure-related death through 30 days, (2) major target limb amputation through 36 months, and (3) CD-TVR through 36 months.
Major adverse events were defined as all-cause mortality, CD-TVR, major target limb amputation, and thrombosis at the target lesion site.
Functional Outcomes
There were functional improvements in both groups at 36 months. The mean ABI/TBI change from baseline was 0.15±0.17 for the DCB group (p<0.001) and 0.20±0.18 for the PTA group (p<0.001). No significant difference was observed comparing the change from baseline between groups (p=0.263). Most participants in both groups also showed improvement in the Rutherford category at 36 months (Table 4). An improvement of ≥1 Rutherford category occurred in 86.2% (50/58) of the DCB group and 88.9% (24/27) of the PTA group.
Table 4.
| Change | DCB (n=58) | PTA (n=27) |
|---|---|---|
| –4 | 3.4 (2) | 0 (0) |
| –3 | 17.2 (10) | 11.1 (3) |
| –2 | 51.7 (30) | 70.4 (19) |
| –1 | 13.8 (8) | 7.4 (2) |
| 0 | 10.3 (6) | 11.1 (3) |
| +1 | 3.4 (2) | 0 (0) |
| +2 | 0 (0) | 0 (0) |
| +3 | 0 (0) | 0 (0) |
| +4 | 0 (0) | 0 (0) |
Abbreviations: DCB, drug-coated balloon; PTA, percutaneous transluminal angioplasty.
Categorical data are presented as the percentage (count).
p=0.983 for comparison between groups from baseline to 36 months.
Discussion
The IN.PACT (MDT-2113) SFA Japan trial was the first RCT of a DCB in an Asian population with midterm outcomes. There were no safety concerns through 3 years, including all-cause mortality, major target limb amputation, or thrombosis. The rate of all-cause death was low and similar between treatment groups at 3 years. The rate of thrombosis was also low, and there were no major target limb amputations in either group.
Table 5 summarizes key IN.PACT SFA Japan outcomes between 12 months and the final follow-up at 36 months. Results are consistent with what has been reported from other DCB trials in Asian populations at 12 and 24 months. Efficacy outcomes at 12 months were consistent with IN.PACT SFA China (90.9% primary patency, 2.9% CD-TLR), which is remarkable considering that the prevalence of total occlusions was so much higher in IN.PACT SFA China (52.4%) compared with IN.PACT SFA Japan (16.2%).14,25 Safety outcomes were also similar between the 2 trials, with consistent rates of all-cause mortality (2.9% IN.PACT SFA China) and thrombosis at 12 months (2.2% IN.PACT SFA China).14,25 At 24 months, primary patency in IN.PACT SFA Japan was higher than in the AcoArt I trial of Chinese patients (64.6%), though AcoArt I may have enrolled a more challenging patient population.21,30 The DCB group in AcoArt I had longer lesions (mean length 14.7 cm) and a higher percentage of patients with total occlusions (57.0%) compared with IN.PACT SFA Japan. Other 24-month outcomes were consistent between the studies, including rates of CD-TLR (13.5% AcoArt I) and all-cause mortality (8.3% AcoArt I).21,30
Table 5.
IN.PACT SFA Japan Outcomes at 12, 24, and 36 Months.a
| Outcome | 12 Months25 |
24 Months30 |
36 Months |
||||||
|---|---|---|---|---|---|---|---|---|---|
| DCB | PTA | p | DCB | PTA | p | DCB | PTA | p | |
| Primary patency | 93.9b | 46.9b | <0.001c | 79.8b | 46.9b | <0.001c | 68.9b | 46.9b | 0.001c |
| Freedom from CD-TLR | 97.1b | 81.3b | 0.005c | 90.8b | 81.3b | 0.114c | 84.4b | 81.3b | 0.451c |
| CD-TLR | 2.9 (2/68) | 18.9 (6/32) | 0.012 | 9.1 (6/66) | 20.7 (6/29) | 0.177 | 14.9 (10/67) | 20.7 (6/29) | 0.554 |
| All-cause death | 0 (0/68) | 0 (0/32) | >0.999 | 6.1 (4/66) | 3.4 (1/29) | >0.999 | 6.0 (4/67)d | 6.9 (2/29) | >0.999 |
| Major target limb amputation | 0 (0/68) | 0 (0/32) | >0.999 | 0 (0/66) | 0 (0/29) | >0.999 | 0 (0/67) | 0 (0/29) | >0.999 |
| Thrombosis | 0 (0/68) | 0 (0/32) | >0.999 | 0 (0/66) | 0 (0/29) | >0.999 | 1.5 (1/67) | 0 (0/29) | >0.999 |
Abbreviations: CD-TLR, clinically-driven target lesion revascularization; DCB, drug-coated balloon; PTA, percutaneous transluminal angioplasty.
Data are presented as the percent (number/sample) unless otherwise indicated.
Kaplan-Meier estimate in percent.
Log-rank p.
There were 66 evaluable participants at 2-year follow-up and 67 evaluable participants at 3-year follow-up. There were no deaths in the DCB group between 2 and 3 years.
To date, the only other DCB studies that have published 36-month results are IN.PACT SFA, IN.PACT Global, REAL-PTX, ILLUMENATE EU, and ILLUMENATE US (Table 6). All-cause mortality in the DCB group from IN.PACT SFA Japan (6.0%) was within range of IN.PACT SFA (10.7%), IN.PACT Global (11.6%), REAL-PTX (10.7%), ILLUMENATE EU (9.4%), and ILLUMENATE US (10.1%).6,26,27,31 All-cause mortality from the DCB group in IN.PACT SFA Japan was also consistent with studies of other endovascular modalities that have reported outcomes at 36 months, including MAJESTIC (DES, 3.6%), RESILIENT (BMS, 10.0%), DURABILITY II (BMS, 10.1%), and STROLL (BMS, 10.1%).32–35 The rate of thrombosis after DCB angioplasty in IN.PACT SFA Japan, while low (1.5%), was consistent with what has been reported for DCBs at 36 months (IN.PACT SFA 2.0%, IN.PACT Global 5.6%, not reported in REAL-PTX, ILLUMENATE EU, or ILLUMENATE US).6,26,27,31
Table 6.
36-Month Outcomes Across Studies of Endovascular Interventions.
| Study | Study Device | N | Mean Lesion Length, cm | Primary Patency,a,b % | Freedom From CD-TLR,a % | CD-TLR, % (n/N) | All-Cause Death, % (n/N) | Major Amputation, % (n/N) | Thrombosis, % (n/N) |
|---|---|---|---|---|---|---|---|---|---|
| IN.PACT SFA Japan | |||||||||
| DCB | IN.PACT Admiral | 68 | 9.2 | 68.9 | 84.4 | 14.9 (10/67) | 6.0 (4/67) | 0 (0/67) | 1.5 (1/67) |
| PTA | Uncoated balloon | 32 | 8.9 | 46.9 | 81.3 | 20.7 (6/29) | 6.9 (2/29) | 0 (0/29) | 0.0 (0/29) |
| IN.PACT SFA RCT6 | |||||||||
| DCB | IN.PACT Admiral | 220 | 8.9 | 69.5 | 84.5 | 15.2 (30/197) | 10.7 (21/197) | 0 (0/197) | 2.0 (4/197) |
| PTA | Uncoated balloon | 111 | 8.8 | 45.1 | 70.4 | 31.1 (32/103) | 1.9 (2/103) | 0 (0/103) | 4.9 (5/103) |
| IN.PACT Global Clinical Cohort26 | |||||||||
| DCB | IN.PACT Admiral | 1406 | 12.1 | NR | 76.9 | 22.9 (289/1262) | 11.6 (147/1262) | 1.0 (12/1262) | 5.6 (71/1262) |
| REAL-PTX31 | |||||||||
| DCB | IN.PACT Admiral or Pacific, or Lutonix | 75 | 15.0 | 42.4 | 71.3 | NR | 10.7 (8/75) | NR | NR |
| DES | Zilver-PTX | 75 | 15.6 | 56.7 | 68.9 | NR | 4.0 (3/75) | NR | NR |
| ILLUMENATE EU27 | |||||||||
| DCB | Stellarex | 222 | 7.2 | 67.5 | NR | NR | 9.4 (18/192) | 0 (0/175) | NR |
| PTA | Uncoated balloon | 72 | 7.1 | 59.9 | NR | NR | 8.5 (5/59) | 0 (0/54) | NR |
| ILLUMENATE US27 | |||||||||
| DCB | Stellarex | 200 | 8.0 | 64.2 | NR | NR | 10.1 (18/178) | 0 (0/162) | NR |
| PTA | Uncoated balloon | 100 | 8.9 | 51.0 | NR | NR | 11.0 (10/91) | 0 (0/81) | NR |
| MAJESTIC34 | |||||||||
| DES | Eluvia | 57 | 7.1 | NR | 85.3c | 14.8c | 3.6 (2/56) | 0 (0/56) | NR |
| RESILIENT RCT33 | |||||||||
| BMS | LifeStent | 134 | 7.0 | NR | 75.5c | NR | 10.0d | NR | NR |
| PTA | Uncoated balloon | 72 | 6.4 | NR | 41.8c | NR | 8.3d | NR | NR |
| DURABILITY II35 | |||||||||
| BMS | EverFlex | 287 | 8.9 | 60.0 | 70.0 | 31.1 (80/257) | 10.1 (26/257) | 0.8 (2/257) | NR |
| STROLL32 | |||||||||
| BMS | SMART | 250 | 7.7 | 72.7 | 78.5 | 21.5a | 10.1a | 0.8a | NR |
Abbreviations: BMS, bare metal stent; CD-TLR, clinically-driven target lesion revascularization; DCB, drug-coated balloon; DES, drug-eluting stent; DUS, duplex ultrasonography; n/N, sample/population; NR, not reported; PSVR, peak systolic velocity ratio; PTA, percutaneous transluminal angioplasty; RCT, randomized controlled trial.
Kaplan-Meier estimate.
For IN.PACT SFA Japan, IN.PACT SFA RCT, IN.PACT Global Clinical Cohort, ILLUMENATE EU, and ILLUMENATE US, the endpoint was “primary patency,” defined as freedom from CD-TLR and freedom from restenosis (DUS PSVR ≤2.4). For REAL-PTX, patency was defined as absence of CD-TLR (reintervention performed for ≥50% diameter stenosis as confirmed by angiography after documentation of clinical symptoms following the index procedure) or binary restenosis (DUS >2.4). For MAJESTIC, patency was reported only through 2 years. For DURABILITY II, endpoint was “stent patency,” defined as PSVR <2.0. For STROLL, the endpoint was “target vessel patency,” defined as freedom from CD-TLR and freedom from binary restenosis (eg, PSVR ≥2.5).
Value refers to all TLRs.
Calculated from probability of survival at 36 months.
Efficacy outcomes from IN.PACT SFA Japan were consistent with other DCB studies at 36 months (Table 6). Primary patency was very similar between DCB groups in IN.PACT SFA Japan (68.9%), IN.PACT SFA (69.5%), ILLUMENATE EU (67.5%), and ILLUMENATE US (64.2%).6,27 Freedom from CD-TLR in the IN.PACT SFA Japan trial (84.4%) was consistent with IN.PACT SFA (84.5%) and higher than IN.PACT Global (76.9%), as well as REAL-PTX (71.3% in the DCB arm and 68.9% in the DES arm).6,26,31 The potential difference in CD-TLR between these studies may be due to underlying clinical and lesion characteristics of the patient population. The IN.PACT Global Study was a prospective, single-arm trial that was open to patients with complex lesions, including ISR and total occlusions. The inclusion of patients with challenging lesions may have contributed to a higher need for revascularization in IN.PACT Global compared with IN.PACT SFA Japan. Mean lesion lengths in the DCB groups were 9.2 cm in IN.PACT SFA Japan, 12.1 cm in IN.PACT Global, and higher in REAL-PTX (15.0 cm in the DCB arm and 15.6 cm in DES arm). The percentages of patients with total occlusions and ISR were higher in IN.PACT Global (35.5% occlusions, 18.0% ISR) and highest in REAL-PTX (occlusions were 53.3% in the DCB arm and 52.0% in DES arm; ISR not reported) compared with IN.PACT SFA Japan (16.2% occlusions, 0% ISR).25,26,31
Limitations
The trial enrolled only Japanese participants, which not only limits the generalizability of findings from this specific study but also increases the range of populations that have been included in the IN.PACT clinical program. The number of enrolled participants was lower than other IN.PACT studies, and the trial was not fully powered. While the study evaluated mid-term outcomes through 3 years, other studies (eg, IN.PACT SFA, THUNDER, AcoArt I) have followed participants up to 5 years.24,28,29
Conclusion
The IN.PACT SFA Japan trial is the first RCT of a DCB in an Asian population with published midterm outcomes. Results of this final report show that treatment with the IN.PACT Admiral DCB is safe and has durable outcomes through 3 years in a population of Japanese participants with femoropopliteal occlusive disease.
Acknowledgments
The authors would like to thank the participants for their involvement in this study and recognize the principal investigators and institutions in Japan that enrolled participants in the study. The authors also thank Zachary Harrelson, PhD, for medical writing assistance and Bridget Wall, PhD, and Eric Fernandez, MD, for technical review.
Appendix
The principal investigators and institutions in Japan participating in the IN.PACT SFA Japan study: Shigeru Saito, MD, Shonan Kamakura General Hospital, Kamakura; Masato Nakamura, MD, Toho University Medical Center, Ohashi Hospital, Tokyo; Keisuke Hirano, MD, Yokohama Tobu Hospital, Yokohama; Osamu Iida, MD, Kansai Rosai Hospital, Amagasaki; Kazushi Urasawa, MD, Tokeidai Memorial Hospital, Sapporo; Naoto Inoue, MD, Sendai Kousei Hospital, Sendai; Hiroshi Ando, MD, Kasukabe Chuo General Hospital, Kasukabe; Junko Hone, MD, Kikuna Memorial Hospital, Yokohama; and Takuo Nakagami, MD, Omihachiman Community Medical Center, Omihachiman.
Footnotes
Authors’ Note: This study was presented at the Leipzig Interventional Course (LINC) 2019; January 22–25, 2019; Leipzig, Germany.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Hong Wang and Hiroko Ookubo are full-time employees of Medtronic. Michael R. Jaff is a compensated advisor for Biotronix, Medtronic, Philips/Spectranetics, Sanofi, and Vactronix and is an equity investor of PQ Bypass.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Medtronic.
ORCID iDs: Yoshimitsu Soga  https://orcid.org/0000-0003-1931-5769
https://orcid.org/0000-0003-1931-5769
Osamu Iida  https://orcid.org/0000-0001-6829-7304
https://orcid.org/0000-0001-6829-7304
References
- 1. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69:1465–1508. [DOI] [PubMed] [Google Scholar]
- 2. Bailey SR, Beckman JA, Dao TD, et al. ACC/AHA/SCAI/SIR/SVM 2018 appropriate use criteria for peripheral artery intervention: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Heart Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, and Society for Vascular Medicine. J Am Coll Cardiol. 2019; 73:214–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feldman DN, Armstrong EJ, Aronow HD, et al. SCAI consensus guidelines for device selection in femoral-popliteal arterial interventions. Catheter Cardiovasc Interv. 2018;92:124–140. [DOI] [PubMed] [Google Scholar]
- 4. Tepe G, Laird J, Schneider P, et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laird JR, Schneider PA, Tepe G, et al. Durability of treatment effect using a drug-coated balloon for femoropopliteal lesions: 24-month results of IN.PACT SFA. J Am Coll Cardiol. 2015;66:2329–2338. [DOI] [PubMed] [Google Scholar]
- 6. Schneider PA, Laird JR, Tepe G, et al. Treatment effect of drug-coated balloons is durable to 3 years in the femoropopliteal arteries: long-term results of the IN.PACT SFA randomized trial. Circ Cardiovasc Interv. 2018;11:e005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Micari A, Brodmann M, Keirse K, et al. Drug-coated balloon treatment of femoropopliteal lesions for patients with intermittent claudication and ischemic rest pain: 2-year results from the IN.PACT Global Study. JACC Cardiovasc Interv. 2018;11:945–953. [DOI] [PubMed] [Google Scholar]
- 8. Scheinert D, Micari A, Brodmann M, et al. Drug-coated balloon treatment for femoropopliteal artery disease. Circ Cardiovasc Interv. 2018;11:e005654. [DOI] [PubMed] [Google Scholar]
- 9. Brodmann M, Keirse K, Scheinert D, et al. Drug-coated balloon treatment for femoropopliteal artery disease: the IN.PACT Global Study de novo in-stent restenosis imaging cohort. JACC Cardiovasc Interv. 2017;23:2113–2123. [DOI] [PubMed] [Google Scholar]
- 10. Ansel GM, Brodmann M, Keirse K, et al. Drug-coated balloon treatment of femoropopliteal lesions typically excluded from clinical trials: twelve-month findings from the IN.PACT Global Study. J Endovasc Ther. 2018;25:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Micari A, Vadalà G, Castriota F, et al. 1-year results of paclitaxel-coated balloons for long femoropopliteal artery disease: evidence from the SFA-Long study. JACC Cardiovasc Interv. 2016;9:950–956. [DOI] [PubMed] [Google Scholar]
- 12. Micari A, Nerla R, Vadalà G, et al. 2-year results of paclitaxel-coated balloons for long femoropopliteal artery disease: evidence from the SFA-Long study. JACC Cardiovasc Interv. 2017;10:728–734. [DOI] [PubMed] [Google Scholar]
- 13. Krankenberg H, Tübler T, Ingwersen M, et al. Drug-coated balloon versus standard balloon for superficial femoral artery in-stent restenosis: the randomized Femoral Artery In-stent Restenosis (FAIR) trial. Circulation. 2015;132:2230–2236. [DOI] [PubMed] [Google Scholar]
- 14. Chen Z, Guo W, Jiang W, et al. IN.PACT SFA clinical study using the IN.PACT Admiral drug-coated balloon in a Chinese patient population. J Endovasc Ther. 2019;26:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grotti S, Liistro F, Angioli P, et al. Paclitaxel-eluting balloon vs standard angioplasty to reduce restenosis in diabetic patients with in-stent restenosis of the superficial femoral and proximal popliteal arteries: three-year results of the DEBATE-ISR study. J Endovasc Ther. 2016;23:52–57. [DOI] [PubMed] [Google Scholar]
- 16. Rosenfield K, Jaff MR, White CJ, et al. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145–153. [DOI] [PubMed] [Google Scholar]
- 17. Krishnan P, Faries P, Niazi K, et al. Stellarex drug-coated balloon for treatment of femoropopliteal disease: twelve-month outcomes from the randomized ILLUMENATE pivotal and pharmacokinetic studies. Circulation. 2017;136: 1102–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schroë H, Holden AH, Goueffic Y, et al. Stellarex drug-coated balloon for treatment of femoropopliteal arterial disease-The ILLUMENATE Global Study: 12-month results from a prospective, multicenter, single-arm study. Catheter Cardiovasc Interv. 2018;91:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schroeder H, Werner M, Meyer DR, et al. Low-dose paclitaxel-coated versus uncoated percutaneous transluminal balloon angioplasty for femoropopliteal peripheral artery disease: one-year results of the ILLUMENATE European randomized clinical trial (randomized trial of a novel paclitaxel-coated percutaneous angioplasty balloon). Circulation. 2017;135:2227–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brodmann M, Werner M, Meyer DR, et al. Sustainable antirestenosis effect with a low-dose drug-coated balloon: the ILLUMENATE European randomized clinical trial 2-year results. JACC Cardiovasc Interv. 2018;11:2357–2364. [DOI] [PubMed] [Google Scholar]
- 21. Xu Y, Jia X, Zhang J, et al. Drug-coated balloon angioplasty compared with uncoated balloons in the treatment of 200 Chinese patients with severe femoropopliteal lesions: 24-month results of AcoArt I. JACC Cardiovasc Interv. 2018;11:2347–2353. [DOI] [PubMed] [Google Scholar]
- 22. Bausback Y, Willfort-Ehringer A, Sievert H, et al. Six-month results from the initial randomized study of the Ranger paclitaxel-coated balloon in the femoropopliteal segment. J Endovasc Ther. 2017;24:459–467. [DOI] [PubMed] [Google Scholar]
- 23. Tepe G, Gögebakan Ö, Redlich U, et al. Angiographic and clinical outcomes after treatment of femoro-popliteal lesions with a novel paclitaxel-matrix-coated balloon catheter. Cardiovasc Intervent Radiol. 2017;40:1535–1544. [DOI] [PubMed] [Google Scholar]
- 24. Tepe G, Schnorr B, Albrecht T, et al. Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER trial. JACC Cardiovasc Interv. 2015;8:102–108. [DOI] [PubMed] [Google Scholar]
- 25. Iida O, Soga Y, Urasawa K, et al. Drug-coated balloon vs standard percutaneous transluminal angioplasty for the treatment of atherosclerotic lesions in the superficial femoral and proximal popliteal arteries: one-year results of the MDT-2113 SFA Japan randomized trial. J Endovasc Ther. 2018;25:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Torsello G, Stavroulakis K, Brodmann M, et al. Three-year sustained clinical efficacy of drug-coated balloon angioplasty in a real-world femoropopliteal cohort [published online June 25, 2020]. J Endovasc Ther. doi: 10.1177/1526602820931477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mathews SJ. Stellarex in the treatment of the SFA and popliteal: late-breaking 3-year data. Paper presented at: the New Cardiovascular Horizons (NCVH) 2019 Annual Conference; May 29–31, 2019; New Orleans, LA, USA. [Google Scholar]
- 28. Laird JA, Schneider PA, Jaff MR, et al. Long-term clinical effectiveness of a drug-coated balloon for the treatment of femoropopliteal lesions. Circ Cardiovasc Interv. 2019;12: e007702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jia X, Guo W. New long-term information on PTX coated DCB 5-year results from the AcoArt I study. Abstract presented at: LINC 2020; January 28–31, 2020; Leipzig, Germany. [Google Scholar]
- 30. Iida O, Soga Y, Urasawa K, et al. Drug-coated balloon versus uncoated percutaneous transluminal angioplasty for the treatment of atherosclerotic lesions in the superficial femoral and proximal popliteal artery: 2-year results of the MDT-2113 SFA Japan randomized trial. Catheter Cardiovasc Interv. 2019;93:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bausback Y, Wittig T, Schmidt A, et al. Drug-eluting stent versus drug-coated balloon revascularization in patients with femoropopliteal arterial disease. J Am Coll Cardiol. 2019;73: 667–679. [DOI] [PubMed] [Google Scholar]
- 32. Bunte MC, Cohen DJ, Jaff MR, et al. Long-term clinical and quality of life outcomes after stenting of femoropopliteal artery stenosis: 3-year results from the STROLL study. Catheter Cardiovasc Interv. 2018;92:106–114. [DOI] [PubMed] [Google Scholar]
- 33. Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation vs. balloon angioplasty for lesions in the superficial femoral and proximal popliteal arteries of patients with claudication: three-year follow-up from the RESILIENT randomized trial. J Endovasc Ther. 2012;19:1–9. [DOI] [PubMed] [Google Scholar]
- 34. Müller-Hülsbeck S, Keirse K, Zeller T, et al. Long-term results from the MAJESTIC trial of the Eluvia paclitaxel-eluting stent for femoropopliteal treatment: 3-year follow-up. Cardiovasc Intervent Radiol. 2017;40:1832–1838. [DOI] [PubMed] [Google Scholar]
- 35. Rocha-Singh KJ, Bosiers M, Schultz G, et al. A single stent strategy in patients with lifestyle limiting claudication: 3-year results from the Durability II trial. Catheter Cardiovasc Interv. 2015;86:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]