Abstract
Background
The tumor microenvironment plays a major tumor-supportive role in glioma. In particular, tumor-associated macrophages (TAMs), which can make up to one-third of the tumor mass, actively support tumor growth, invasion, and angiogenesis. Predominantly alternatively activated (M2-polarized) TAMs are found in late-stage glioma in both human and mouse tumors, as well as in relapse samples from patients. However, whether tumor-educated M2 TAMs can actively contribute to the emergence and growth of relapse is currently debated.
Methods
To investigate whether tumor-educated stromal cells remaining in the brain after surgical removal of the primary tumor can be long-lived and retain their tumor-supporting function, we developed a transplantation mouse model and performed lineage-tracing.
Results
We discovered that macrophages can survive transplantation and stay present in the tumor much longer than previously suggested, while sustaining an M2-polarized protumorigenic phenotype. Transplanted tumors showed a more aggressive growth and faster polarization of the TAMs toward an M2 phenotype compared with primary tumors, a process dependent on the presence of few cotransplanted macrophages.
Conclusions
Overall, we propose a new way for tumor-educated TAMs to contribute to glioma aggressiveness by long survival and stable protumorigenic features. These properties could have a relapse-supporting effect.
Keywords: glioma, long-lived macrophages, tumor-associated macrophages, tumor microenvironment, tumor transplantation
Key Points.
A model for glioma transplantation was established to perform lineage-tracing in stromal cells.
Tumor-associated macrophages can be long-lived cells in the tumor microenvironment.
Transplanted tumors show more aggressive growth than primary tumors.
Importance of the Study.
This study demonstrates for the first time that tumor-associated macrophages can be long-lived cells in the context of glioma and contribute to tumor aggressiveness independently of their microenvironment. This survival benefit is linked to a stable tumor-promoting phenotype, suggesting that macrophages have the capacity to contribute to glioma recurrence. These findings may be of clinical relevance for improving current therapeutic approaches for relapse prevention.
Gliomas are the most common type of brain tumors with an incidence of 5 in 100 000 people1 and have been identified as an area of unmet clinical need. Glioblastoma represent the most abundant and aggressive type of glioma, characterized by a diffuse infiltration into the brain parenchyma, resistance to apoptosis, genomic instability, necrosis, hypoxia, and neovascularization.2 Despite maximal surgical resection followed by a combination of radiotherapy plus concomitant and adjuvant temozolomide chemotherapy, tumor recurrence is usually observed within 7 months and the median survival time is 14 months after diagnosis.3 Current therapeutic approaches target the tumor cells mainly with limited success due to a variety of reasons, such as the highly infiltrative growth pattern of the tumor, which makes complete resection challenging, radio- and chemoresistance of the tumor cells and intratumoral genetic heterogeneity.4,5
Besides the tumor cells, cells of the tumor microenvironment (TME) also strongly influence glioma growth. In particular, immune cells have recently emerged as a novel promising target for therapy.6 Yolk sac-derived tissue-resident brain macrophages (microglia), as well as bone-marrow-derived macrophages, infiltrating from the peripheral blood, are the most abundant immune cell type in glioma tissue. These tumor-associated microglia/macrophages (TAMs) are recruited to the tumor vicinity via soluble factors, released from the tumor cells. TAMs comprise up to 30% of the tumor mass and their abundance correlates with malignancy and tumor grade.7,8 Recent studies exploring TAMs as therapeutic targets presented contradictory findings. While some demonstrated decrease in tumor size under reduction of TAM numbers,9–12 others showed increase in tumor size.13,14
Interestingly, TAMs can exhibit both tumor-suppressive and tumor-supportive functions depending on their activation state. Classically activated cytotoxic (also known as M1-type) TAMs mediate tumor resistance via the release of tumoricidal agents such as the proinflammatory cytokine tumor necrosis factor alpha. In contrast, alternatively activated TAMs (M2-type) support tumor growth via matrix remodeling, phagocytic activity and the release of angiogenic factors.7,15–18
M1-activated TAMs are abundant in early stages of tumor growth, which are often associated with chronic inflammation, whereas late-stage, aggressive tumors are populated with vast numbers of M2 TAMs. Latest research by us and others demonstrates that TAMs undergo a polarization switch from M1 to M2 during tumor progression and thus dynamically contribute to tumorigenesis.14,19 In surgically resected samples from patients, the number of M2 TAMs increased with glioma stage. Strikingly, samples from glioma relapse showed a similar density of M2 TAMs as high-grade glioma samples irrespective of the grade of the resected primary tumor.14
Despite best efforts and improved methods for complete macroscopic surgical resection of the tumor, the peritumoral brain zone (PBZ) where 80%–90% of the recurrences take place,20 often harbors tumor cells as well as other cellular and molecular components of the TME. Although most studies focus on residual tumor cells, it has been reported that nonresident macrophages can be found in the PBZ postsurgery as well.21 It is well established, that residual tumor cells can give rise to the recurrent tumor,22 but whether and how remaining TAMs can support this process has not yet been addressed.
To address the question whether tumor-educated TAMs can contribute to a recurrent tumor growth, we developed a new transplantation mouse model and saw that TAMs can survive much longer than previously anticipated in the tumor context. With lineage-tracing experiments, we demonstrate that long-lived TAMs can keep a sustained M2 polarization and a proangiogenic phenotype throughout tumor progression. Additionally, transplanted tumors containing TAMs showed an earlier switch of macrophage polarization and a faster growth rate. Altogether, we propose that long-lived TAMs can actively support the aggressiveness of glioma relapse.
Materials and Methods
Animal Experiments
All animal experiments were performed in accordance with the Belgian law and EU regulations. Experimental procedures were approved by the Institutional Animal Care and Research Advisory Committee of the KU Leuven (P105/2012, P098/2016, and P205/2017). Animals were kept in standard housing conditions and received food and water ad libitum.
Mouse strains used were: C57BL/6, ROSAmTmG, and Cx3cr1GFP/Wt. Mice used for tumor implantations were 8–14 weeks old. Both males and females were used for primary tumors. For tumor transplantations, only age-matched wild-type male mice were used.
Upon tumor implantation, animals were followed on a daily basis. Humane end-point was reached whenever the animal showed decreased mobility and hunched posture and/or had lost 20% of its body weight.
The target end-points were 2 and 4 weeks after tumor implantation. However, due to high variability of tumor growth between animals, many animals developed symptoms before the 4 weeks’ time point was reached, in which case they were sacrificed earlier. For simplicity, in this manuscript we refer to all animals, which reached a symptomatic end-point, as having “4 weeks” tumors.
Tumor Inoculations
Tumor inoculations were performed as described previously.14 A brief description can be found in Supplementary Material.
Tumor Piece Transplantations
For tumor transplantations, tumors were taken out and cut in small pieces with a scalpel and kept in DMEM complete until transplantation. Typically, pieces with a 250 µm diameter with round edges were transplanted.
Immunofluorescence
Immunofluorescence staining was performed on free-floating 200 µm vibratome sections.23 A detailed protocol and list of the antibodies used can be found in Supplementary Methods.
Tumor Size Measurement
Tumor volume was quantified using the Cavalieri’s principle. In brief, the clearly delineated tumor area in every second 200 µm thick vibratome slice was measured using FIJI software and was multiplied by the number of slices and the thickness of each slice to calculate the total volume of the tumor.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 7.02. Between-group comparisons were performed with Mann–Whitney U test or unpaired t test with or without Welch correction depending on the distribution of the data. For correlation analysis, Spearman’s rank correlation coefficient was computed. A 2-tailed value of P < .05 was considered statistically significant.
Results
Macrophages Acquire an M2 Phenotype During Glioma Growth in Correlation With Increasing Tumor Size
We used a well-established syngeneic mouse glioma model that has the advantage of slow tumor growth, develops progressive TME changes and vessel dysmorphia characteristics of different grades of human glioma14 and allows for longitudinal studies of tumor development. We implanted CT-2A24 glioma cell spheroids into the cortex of C57BL/6 mice as previously described14 and followed tumor growth. Mice were sacrificed at 2 weeks (early stage) or 4 weeks (late stage) of tumor growth, intracardially perfused and tumors were sectioned for immunofluorescence staining.
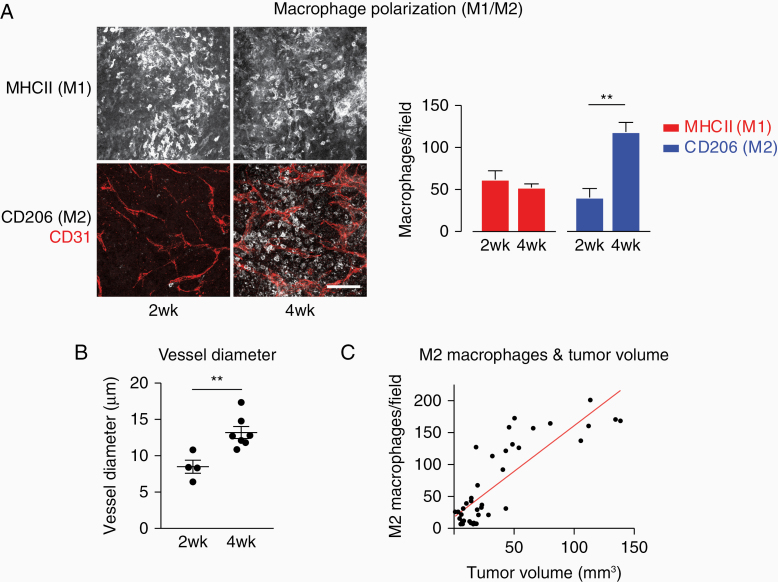
As observed previously within this mouse glioma model,14 after 2 weeks glioma growth a large number of major histocompatibility complex class II (MHCII)-positive (M1-polarized) macrophages is present in the tumor (Figure 1A), whereas the number of alternatively activated CD206-positive (MRC1 (mannose receptor); M2-polarized) macrophages is slightly lower. After 4 weeks of tumor growth, however, while the number of MHCII-positive macrophages remains unchanged, the number of CD206-positive macrophages is significantly increased. In addition, the blood vessel network as identified by CD31 (PECAM-1) staining appears more tortuous and chaotic with increased vessel diameter (Figure 1B).
Figure 1.
TAMs switch from M1 to M2 polarization during tumor growth with increasing tumor size. (A) Immunofluorescent staining of sections at 2 and 4 weeks of tumor growth. Scale bar: 100 µm; 50 µm z-stack. Quantification of M1- and M2-positive macrophages per field of view in 2 and 4 weeks tumors. N = 7 (2 weeks) and 7 tumors (4 weeks); 5 fields of view/tumor. ** P < .01. (B) Blood vessel diameters in 2 and 4 weeks tumors. **P < .01. (C) The number of M2 macrophages correlates with tumor size. Each dot represents 1 tumor, the red line indicates the linear relationship of tumor volume and M2 macrophages. Spearman’s rank correlation coefficient: 0.78; P < .0001. Data shown: mean + standard error of the mean (A and B).
Recent studies indicate that the M1/M2 dichotomy offers an oversimplified view of TAM polarization, since TAMs are a highly heterogeneous population and at any given point in a tumor, macrophages expressing a large variety of markers and receptors can be found.25 Nevertheless, when we assessed tumor size across different time points, CD206 expression (a marker for M2 polarization) strongly correlated with tumor size (Figure 1C, Spearman’s rank correlation coefficient: 0.78). The protumoral role of M2 TAMs has been demonstrated convincingly in a large body of literature.7,15–18 Thus, determining MHCII expression vs CD206 expression as a description of M1/M2 polarization bears functional significance, allowing for a simplified but efficient way to distinguish between anti- and protumorigenic TAMs.
Altogether, macrophage populations dynamically change their polarization during glioma growth and the switch from M1 to M2 polarization is a good predictor of tumor size.
Tumor Transplantation Provides a Tool to Study Macrophage Survival
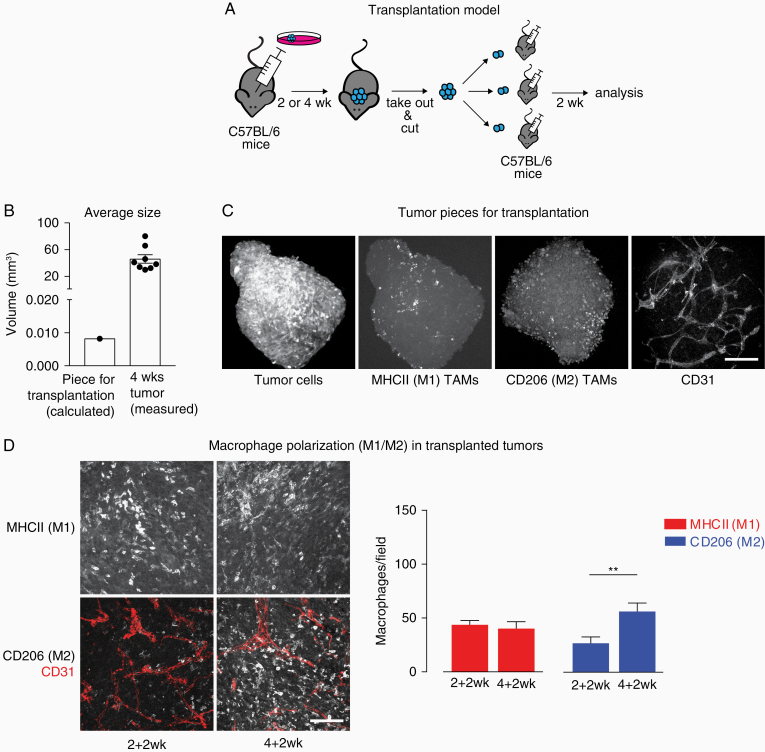
We previously reported that in human samples the number of M2 TAMs increase with increasing glioma grade and is strongly elevated in samples of tumor relapse.14 We hypothesized that tumor-educated M2 macrophages which remain in the PBZ after surgical resection of the tumor may retain their protumorigenic properties. To investigate macrophage survival in the glioma context, we sought to lineage trace macrophages from primary into secondary tumor growth. In the absence of a suitable mouse relapse model allowing for definitive lineage-tracing, we developed a transplantation model. Briefly, a tumor was grown for 2 or 4 weeks, taken out, cut in pieces and transplanted into brains of naïve mice (Figure 2A). Transplanted secondary tumors were grown for 2 weeks and then processed for immunostainings. We will refer to tumors grown after transplantation as secondary tumors to distinguish them from the primary tumors they originate from. On average, the piece of tumor used for transplantation had a diameter of 0.25 mm (V = 0.0082 mm3), whereas a fully grown primary or secondary tumor had a diameter of 4.4 mm (V = 46 mm3) after 4 weeks (Figure 2B), thus expanding its volume approximately 5600-fold.
Figure 2.
Transplantation model used in the study. The progression state of the primary tumor induces an earlier switch of TAM polarization in the secondary. (A) Schematic depicting the transplantation model. (B) Average size of the tumor piece used for transplantation in comparison with a full grown (4 weeks) tumor. (C) Immunofluorescent staining of 4 weeks tumor pieces typically used for transplantation (z-stack: 100 µm). Scale bar: 150 µm. (D) Immunofluorescent staining of sections of secondary tumor growth. Scale bar: 100 µm; 50 µm z-stack. Quantification of M1- and M2-positive macrophages in 2 + 2 and 4 + 2 weeks tumors. Data shown: mean + SEM. N = 8 (2 + 2 weeks) and 13 tumors (4 + 2 weeks); 5 fields of view/tumor. **P < .01.
In order to confirm that different cell types are still present in the small tumor piece we are transplanting, we performed immunofluorescent staining of tumor pieces which would normally be used for transplantation. Apart from CT-2A-tumor cells identified by their stable expression of blue fluorescent protein, we also confirmed that M1-, M2-polarized macrophages and some CD31-positive vessel-like structures (Figure 2C) were still present.
Primary Tumors Influence the Growth of the Secondary Tumor
In transplanted secondary tumors (Figure 2D) we found that the macrophage polarization in the secondary tumors, derived from 2 weeks primary tumors (total tumor growth 4 weeks: 2 weeks primary growth + 2 weeks secondary growth) looks similar to the primary tumors at 2 weeks—with a comparable number of M1- and M2-polarized macrophages. However, in secondary tumors originating from 4 weeks primary tumors (4 weeks primary growth + 2 weeks secondary growth), significantly more M2 macrophages were present, similar to what is found in primary tumors after 4 weeks growth. Note that in both regimes the duration of secondary tumor growth is identical, 2 weeks. Therefore, it appears that the status of the primary tumor influences the macrophage switch in the new host of the transplanted secondary tumor. This finding suggests a potential memory effect which is established in the primary tumor and transferred with transplantation.
The Faster Switch in Macrophage Polarization in the Secondary Tumors Is Mediated by the Transplanted TME
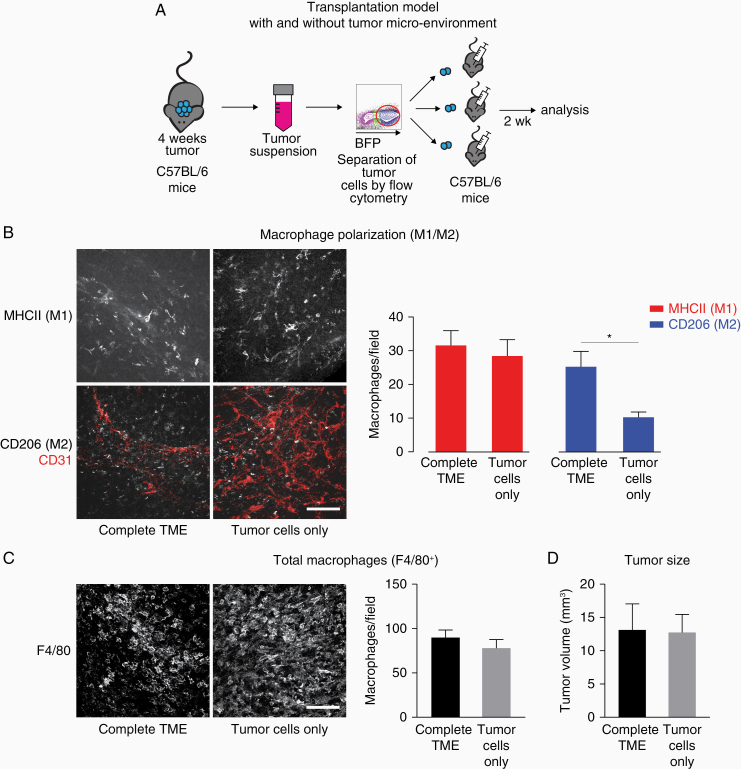
In order to understand further which cell type is responsible for the faster TAM shift to M2 polarization in 4 + 2 week tumors, we designed an experiment in which we transplanted only tumor cells. Tumor cells acquire genomic instability and accumulate mutations very rapidly,26 which could be responsible for the observed effects. To test this hypothesis, we grew primary tumors for 4 weeks, digested them enzymatically and selected the BFP+ tumor cells via flow cytometry sorting. Next, we created spheroids in hanging drops from either tumor cells only or the complete TME (which includes nonmalignant cells) as control and transplanted those spheroids into naïve mice (Figure 3A).
Figure 3.
The faster switch in macrophage polarization in the secondary tumors is mediated by the transplanted TME. (A) Schematic depicting the experimental approach. (B) Immunofluorescent staining of sections of 4 + 2 weeks tumor from complete TME and tumors from tumor cells only. Scale bar: 100 µm; 50 µm z-stack. Quantification of M1- and M2-positive macrophages. N = 10 (both groups); 5 fields of view/tumor. *P < .05. (C) Immunofluorescent staining and quantification of F4/80+ macrophages in 4 + 2 weeks tumor from complete TME and tumors from tumor cells only. Scale bar: 100 µm; 50 µm z-stack. N = 10 (complete TME) and 9 (tumor cells) tumors; 5 fields of view/tumor. (D) Tumor size of 4 + 2 weeks tumors from complete TME and tumors from tumor cells only. N = 10 (complete TME) and 8 (tumor cells) tumors. Data shown: mean + SEM (B–D).
After 2 weeks of secondary tumor growth, tumors originating from tumor cells only had a similar macrophage polarization profile as primary tumors, however in tumors grown from the complete TME there were significantly more M2 macrophages (Figure 3B). Thus, by transplanting only tumor cells we were able to reset the tumor to its initial state and could exclude a major role of the tumor cells for the faster macrophage polarization switch in the secondary tumors. However, we found no significant difference in the total number of macrophages as identified by F4/80 staining and in tumor size (Figure 3C and D).
Overall, these results suggest that potential changes in tumor cells that may occur during primary growth are not responsible for the faster macrophage switch in the secondary tumors.
TAMs Survive the Transplantation and Are M2-Polarized
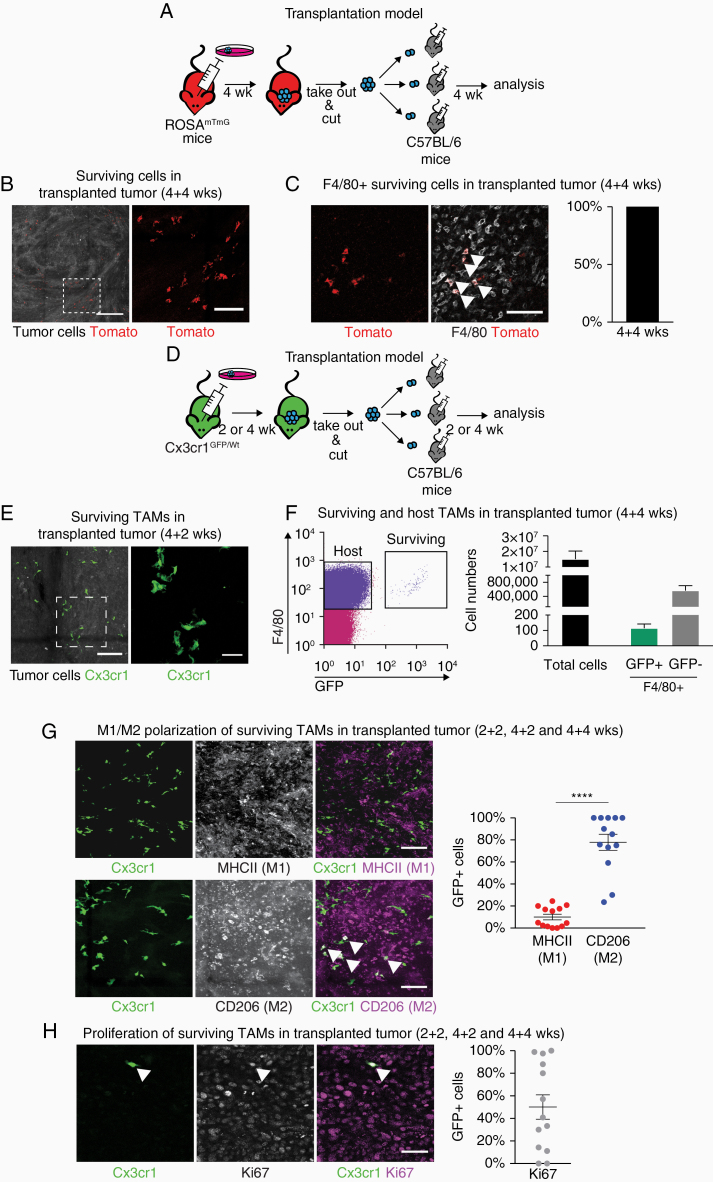
Next, we sought to understand what type of cells survives the transplantation and could potentially influence the growth of the secondary tumor. In order to lineage trace surviving cells, we grew primary tumors in ROSAmTmG mice, in which stromal cells express the Tomato fluorescent protein.27 Tumors were then transplanted in C57BL/6 mice (Figure 4A). Tomato-positive cells were present in the transplanted tumors (Figure 4B), indicating that stromal cells can survive several weeks in the secondary tumors after transplantation. After counterstaining with an F4/80 antibody, all Tomato-positive surviving cells were identified as F4/80+ macrophages (Figure 4C).
Figure 4.
TAMs survive the transplantations and are M2-polarized independently of tumor progression. (A) Schematic depicting the experimental approach for lineage-tracing of stromal cells. (B) Tomato+ (red) cells survive the transplantation and can be found in secondary tumors. Scale bar: 300 µm; 50 µm z-stack. Right: magnification of boxed area, scale bar 100 µm. (C) Tomato+ surviving cells are F4/80+. Scale bar: 100 µm. N = 4 tumors. (D) Experimental approach for macrophage-specific lineage-tracing. (E) Cx3cr1-GFP+ surviving cells in the secondary tumor. Scale bar: 150 µm; 50 µm z-stack. Right: magnification of boxed area, scale bar 50 µm. (F) Flow cytometry sort of surviving and host TAMs in a 4 + 4 weeks secondary tumor. N = 7 tumors. (G) Characterization of surviving TAMs in secondary tumors by immunofluorescent staining. Scale bar: 100 µm; 50 µm z-stack. ****P < .0001 (H) Proliferation of surviving cells in transplanted tumors. Scale bar: 50 µm. Data shown: mean + SEM (F–H). Each dot represents 1 tumor (G and H).
Since only F4/80+ macrophages survived the transplantation, we switched to a macrophage-specific reporter mouse model for the primary tumors (Figure 4D). We used Cx3cr1GFP/Wt animals,28 in which one allele of the fractalkine receptor Cx3cr1 is replaced by green fluorescent protein knock-in. In this mouse model, both brain-resident macrophages (microglia) and infiltrating macrophages originating from the bone marrow have a stable GFP expression, which does not affect cellular function.29 Indeed, after transplanting tumors originating from Cx3cr1GFP/Wt mice into C57BL/6 mice, we found GFP+ cells with a typical macrophage morphology in the secondary tumors (Figure 4E).
We quantified the number of surviving GFP+ cells per tumor via flow cytometry (Figure 4F). In large secondary tumors with an average of 15 000 000 total viable cells, we found an average of 561 824 F4/80high GFP− cells (macrophages, derived from the host mouse) and an average of only 115 F4/80high GFP+ cells (surviving macrophages, derived from the donor mouse). Thus, the ratio of surviving to host-derived macrophages is 1:5000. For further immunofluorescent analysis, we used an Alexa488-conjugated anti-GFP antibody to enhance the signal. The majority of surviving macrophages were M2-polarized (Figure 4G) and only a small percentage were M1-polarized. Some of the GFP+ surviving macrophages expressed the proliferation marker Ki67, indicating that they were proliferating, but overall the number of GFP+Ki67+ cells varied considerably between tumors (Figure 4H).
Thus, a small number of TAMs can survive longer than previously anticipated (for weeks instead of days) in the tumor context and can sustain a M2 polarization.
Surviving Macrophages in Secondary Tumors Are More Protumorigenic Than Host Macrophages
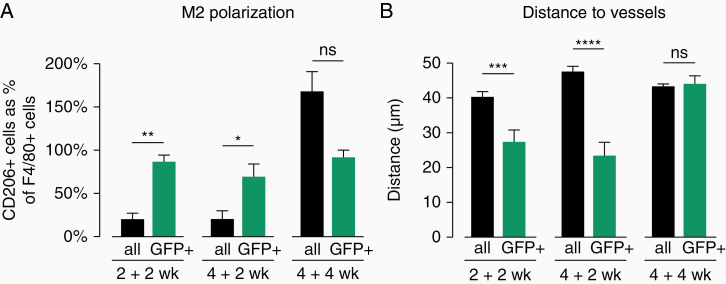
In order to better understand the properties of surviving macrophages in secondary tumors, we compared them to host macrophages in the same tumors (Figure 5A). We analyzed secondary tumors from different time points (2 + 2, 4 + 2, and 4 + 4 weeks). At 2 + 2 weeks, 20.3% of GFP− F4/80+ cells are also CD206+ (M2-polarized), whereas the percentage of GFP+ F4/80+ CD206+ cells is significantly higher (86.9%). Similarly, in 4 + 2 weeks tumors, significantly more of the GFP+ F4/80+ cells are also CD206+, compared with GFP− F4/80+ cells. However, at 4 + 4 weeks tumors, these differences are not present anymore. These results indicate that GFP+ surviving macrophages have a stable M2 polarization independently of the progression of the tumor or of the polarization of the majority of the host-derived macrophages in the TME.
Figure 5.
Characterization of host and surviving macrophages in secondary tumors. (A) M2 polarization in GFP− F4/80+ (all) and GFP+ F4/80+ (surviving) TAMs in transplanted secondary tumors. N = 3–5 tumors/group. *P < .05, **P < .01. (B) Distance to closest blood vessel of GFP− F4/80+ (all) and GFP+ F4/80+ (surviving) TAMs in transplanted secondary tumors. N = 3 tumors/group. Data shown as mean + SEM (A and B). ***P < .001, ****P < .0001.
Since M2-polarized macrophages are known to relocate in the vicinity of blood vessels and contribute to blood vessel dysmorphia, we looked into whether surviving GFP+ macrophages would associate with the blood vessels in the tumor. Tumor blood vessels were identified by CD31 staining and the distances from each macrophage to the nearest blood vessel were determined with the help of a customized Python script (for details, see Materials and methods). Consistently with the observations in Figure 5A, in 2 + 2 weeks secondary tumors GFP+ surviving cells are located significantly closer to the blood vessels in comparison to their GFP− F4/80+ counterparts. A similar difference between surviving and host-derived TAMs is also observed in the 4 + 2 weeks secondary tumors. However, in 4 + 4 weeks tumors there is no significant difference anymore between the distance to blood vessels of GFP+ surviving macrophages and GFP− host macrophages.
Overall, surviving TAMs in secondary tumors represent a unique population of TAMs in transplanted tumors, displaying a stable and TME-independent polarization status and associating with blood vessels.
Transplanted Secondary Tumors Are More Aggressive Compared With Primary Tumors
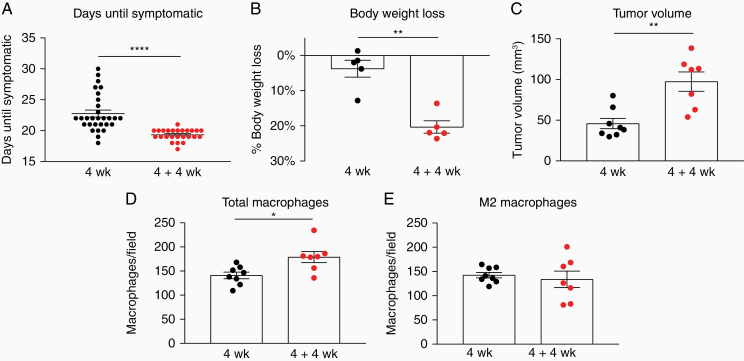
Since macrophages can survive the transplantation and have a protumorigenic phenotype, we next asked how this phenomenon would influence the overall properties of secondary tumors. We compared the growth rate of primary (4 weeks) and secondary (4 + 4 weeks) tumors. Typically, at the final stages of tumor growth, tumor-bearing animals show signs of tumor burden such as reduced mobility and weight loss. Animals bearing secondary tumors started showing tumor-related symptoms significantly earlier than animals bearing primary tumors (Figure 6A, 19.3 days vs 22.8 days on average). Furthermore, animals bearing secondary tumors had lost significantly more weight at the time point when they became symptomatic (Figure 6B, 20.3% vs 3.7%) and they were bearing larger tumors (Figure 6C). This was also reflected in an increased number of TAMs (Figure 6D), but numbers of M2-polarized TAMs were not changed. In order to better understand the possible mechanism behind these observations we performed flow cytometry analysis of the T cell subsets in primary and transplanted tumors. We observed no difference in the percentage of total T cells as identified by CD45 and CD3 marker expression, however in transplanted tumors the percentage of cytotoxic CD8+ T cells was significantly decreased (Supplementary Figure S1), whereas there was a slight but significant increase in the percentage of CD4+ T cells. In both groups, approximately 30% of these lymphocytes were Tregs which were slightly (but not significantly) higher in the transplanted tumors. These findings suggest that a reduced T cell-mediated antitumor immunity and a possibly increased immune suppression were present in the transplanted tumors.
Figure 6.
Secondary tumors display more aggressive growth than primary. (A) Days of primary and secondary tumor growth until symptoms of tumor burden are observed. N = 30 (4 weeks) and 25 (4 + 4 weeks) animals. ****P < .0001. (B) Percentage of body weight loss in mice bearing primary or secondary tumors as measured at humane end-point. **P < .01. (C) Tumor volume of primary and secondary tumors. **P < .01. (D) F4/80+ macrophages in primary and secondary tumors. *P < .05. (E) Number of M2 (CD206+) macrophages in primary and secondary tumors. Each dot represents 1 mouse/tumor (A–E). Data shown as mean ± SEM (A–E).
Altogether, secondary tumors display more aggressive growth than primary tumors.
Discussion
In a physiological context, tissue-resident macrophages can have different origins, such as embryonic yolk sac or liver, populate the target organ early in development30 and create a relatively stable population, maintained through self-renewal or longevity.31,32 In contrast to homeostatic conditions, in the context of inflammation and cancer, rapidly recruited bone-marrow-derived monocytes give rise to infiltrating macrophages33 which are generally considered to be short-lived cells with a high turnover. During circulation in the blood, monocytes are particularly short-lived with a half-life of approximately 2 days.31 After entering the tissue in an inflammatory context such as experimental autoimmune encephalitis or peritonitis, inflammatory macrophages have a strong but transient contribution to the inflammatory process and are removed upon resolution of inflammation,34,35 mainly by local apoptosis.36 However, in the context of cancer the fate of infiltrating macrophages is particularly multifaceted and dynamic. A lot of research has looked into macrophage recruitment into malignant sites, as well as TAM distribution and properties at different time points during tumor growth but little is known about what happens to TAMs in the long term within the tumor context or later on.
Here, we demonstrate that TAMs can survive for more than 6 weeks in the TME. In the used transplantation model, we found no evidence for other nonmalignant surviving cells, although CD31-positive endothelial cells were originally still present in the transplanted pieces. This is, to our knowledge, the first study to show that macrophages can be long-lived cells in a pathological context, and in a brain tumor model in particular. Moreover, TAMs are able to sustain their protumorigenic phenotype as M2-polarized cells, thus could potentially contribute to the emergence and aggressiveness of a relapse.
The role of TAMs in tumor recurrence has been a matter of intense research efforts. Following chemotherapy, M2-polarized TAMs accumulate in perivascular areas and promote relapse.37 Bone-marrow-derived monocytes are actively recruited to the hypoxic tumor site after tumor irradiation, contributing to the fast tumor recurrence38 and the postirradiation TME particularly favors M2 polarization.39 A recent Phase I/II clinical study showed that limiting macrophage recruitment to the irradiated site using an inhibitor of the SDF-1/CXCR4 pathway is a promising approach for decreasing recurrence rates.40 The rate of accumulation of M2 TAMs in the primary tumor site post-treatment can be even used for early detection of relapse growth through molecular imaging.41
However, all the aforementioned studies focused exclusively on newly recruited macrophages post-therapy. Here, we demonstrate that tumor-educated macrophages can survive for long periods of time and remain M2-polarized, thus the M2 polarization possibly confers a survival benefit. This observation is of high therapeutic relevance for several reasons. TAMs can be found in the PBZ in glioma patients postsurgery21 and are in part responsible for the resistance to treatment.42 In particular, M2-polarized macrophages are more resistant to irradiation than M1 macrophages.43 Tumors coinjected with TAMs isolated from irradiated tumors show faster growth.16
M2 polarization of macrophages is induced by a dedicated set of cytokines such as IL4 and IL13, released by immune cells upon tissue damage or helminth infection,44 and is crucial for limiting the potentially devastating effects of classical inflammation in multiple pathologies.45 During tumor progression, macrophages transition from predominantly M1-polarized to M2-polarized.46 However, the mechanisms driving this transition are currently not well understood. Previously, we showed by sequential labeling of TAMs being recruited to the tumor, that in late-stage mouse gliomas, M2 polarization occurs shortly after infiltration from the blood in the local TME.14 This switch is probably driven by locally available cytokines, such as IL4. We are currently developing models which would enable us to observe the switch dynamically in an in vivo setting.
Strikingly, in early phases of transplanted secondary tumors’ growth, surviving donor-derived macrophages display an M2 polarization, even though they are surrounded by M1-polarized host-derived macrophages, indicating that M2 donor-derived macrophages become insensitive to molecular cues in the local TME in contrast to host-derived, newly recruited macrophages. Hence in those surviving donor-derived macrophages the M2 polarization is stabilized, probably through epigenetic mechanisms.47,48 How exactly the M2 polarization contributes to the longevity of these cells is an extremely interesting question, which remains to be addressed.
The ability of cancer cells to accumulate mutations during tumor progression has been postulated as a crucial enabling characteristic for tumor growth.49 Here, we demonstrate that transplanted secondary tumors grow faster and are more aggressive than primary tumors, and that this effect is not due to intrinsic properties of the tumor cells, possibly due to the relative short time frame of our experiments which is insufficient for the tumor cells to acquire a selective growth advantage. Since we observe that the only nonmalignant donor-derived cell type surviving in the secondary tumor were TAMs, the faster switch of M1 to M2 and the increased aggressiveness of secondary tumors is likely due to macrophage-driven mechanisms. This is particularly striking considering that the ratio of surviving (donor-derived) to newly recruited (host-derived) TAMs is only 1:5000. However, this ratio is observed at the final time point, whereas during transplantation the donor-derived TAMs prevail in numbers. In the early phase of tumor growth after transplantation M2-polarized tumor-educated macrophages have the potential to reeducate newly recruited host-derived macrophages through release of molecular factors inducing M2 polarization. This becomes particularly evident when we compare the M2 polarization status and relocation close to the blood vessels in transplanted tumors after 2 and after 4 weeks growth. After 2 weeks growth, the surviving TAMs have stronger protumorigenic properties than the host TAMs, but later on (at 4 weeks growth) these differences are lost. As the tumor grows, surviving TAMs reeducate newly recruited host TAMs in an autocrine fashion, leading to an overall faster switch of TAMs to M2 and more aggressive tumors. Our analysis indicates that a reduced T cell-mediated antitumor immunity was present in the transplanted tumors. Mechanistically, this less favorable immune microenvironment could have contributed to an increased tumor aggressiveness in the transplanted tumors.
Future work will need to address the exact mechanism, by which long-lived macrophages can support tumor growth. For instance, what molecular factors are important for reeducating newly recruited macrophages? Also, testing our observations in a relapse model in the mouse and specifically targeting long-lived macrophages post-therapy to evaluate their contribution to a relapse occurrence would be of immense benefit for improving current therapeutic strategies. Stable labeling of TAMs in the PBZ of human patients during surgery and observing their longevity and phenotype over time would be extremely interesting for developing new therapeutic strategies targeting glioma relapse.
Supplementary Material
Acknowledgments
We are grateful to Dr Pavel Nedvetsky for providing critical comments on the manuscript, to Lisse Decraecker for support with mouse work, to Dr Fabio Stanchi for the stably labeled CT-2A-BFP cells and tumor inoculation expertise, to Magdalena Parys for technical support with flow cytometry experiments, and to Dr Joanna Kalucka and Dr Florian Rambow for invaluable advice. Flow cytometry was performed by the VIB-KU Leuven FACS Core Facility. CT-2A cells were kindly provided by Dr Thomas N. Seyfried (Biology Department, Boston College, USA). Cx3cr1GFP/GFP breeders were kindly provided by Dr Guy Boeckxtaens (KU Leuven).
Funding
This work was supported by Stichting Tegen Kanker [grant 2012-181]; European Research Council [311719]; Fondation Leducq [17CVD03]; European Molecular Biology Organization to T.M. and Fondation Lefoulon Delalande grant to T.M.
Conflict of interest statement. None declared.
Authorship statement: Study concept and design: P.B.G., T.M., and H.G. Data acquisition: P.B.G., T.M., M.R., and M.B. Data analysis: P.B.G., T.M., M.R., S.A., and W.G. Original draft: P.B.G. Writing, review, and editing: all authors. Supervision: H.G.
References
- 1. Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2(9):494–503. [DOI] [PubMed] [Google Scholar]
- 2. Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. [DOI] [PubMed] [Google Scholar]
- 3. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. [DOI] [PubMed] [Google Scholar]
- 4. Sottoriva A, Spiteri I, Piccirillo SG, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A. 2013;110(10):4009–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel A, Tirosh I, Trombetta J, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science (80-). 2014;345(6194):332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17(12):887–904. [DOI] [PubMed] [Google Scholar]
- 7. Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;220(2):114–125. [DOI] [PubMed] [Google Scholar]
- 8. Lu-Emerson C, Snuderl M, Kirkpatrick ND, et al. Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro Oncol. 2013;15(8):1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ries CH, Cannarile MA, Hoves S, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–859. [DOI] [PubMed] [Google Scholar]
- 11. Gabrusiewicz K, Hossain MB, Cortes-Santiago N, et al. Macrophage ablation reduces M2-like populations and jeopardizes tumor growth in a MAFIA-based glioma model. Neoplasia. 2015;17(4):374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vinnakota K, Hu F, Ku MC, et al. Toll-like receptor 2 mediates microglia/brain macrophage MT1-MMP expression and glioma expansion. Neuro Oncol. 2013;15(11):1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galarneau H, Villeneuve J, Gowing G, Julien JP, Vallières L. Increased glioma growth in mice depleted of macrophages. Cancer Res. 2007;67(18):8874–8881. [DOI] [PubMed] [Google Scholar]
- 14. Mathivet T, Bouleti C, Van Woensel M, et al. Dynamic stroma reorganization drives blood vessel dysmorphia during glioma growth. EMBO Mol Med. 2017;9(12):1629–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. [DOI] [PubMed] [Google Scholar]
- 16. Tsai CS, Chen FH, Wang CC, et al. Macrophages from irradiated tumors express higher levels of iNOS, arginase-I and COX-2, and promote tumor growth. Int J Radiat Oncol Biol Phys. 2007;68(2):499–507. [DOI] [PubMed] [Google Scholar]
- 17. Zhou W, Ke SQ, Huang Z, et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol. 2015;17(2):170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rolny C, Mazzone M, Tugues S, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19(1):31–44. [DOI] [PubMed] [Google Scholar]
- 19. Zhou J, Qu Z, Sun F, et al. Myeloid STAT3 promotes lung tumorigenesis by transforming tumor immunosurveillance into tumor-promoting inflammation. Cancer Immunol Res. 2017;5(3):257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petrecca K, Guiot MC, Panet-Raymond V, Souhami L. Failure pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with glioblastoma. J Neurooncol. 2013;111(1):19–23. [DOI] [PubMed] [Google Scholar]
- 21. Parney IF, Waldron JS, Parsa AT. Flow cytometry and in vitro analysis of human glioma-associated macrophages. Laboratory investigation. J Neurosurg. 2009;110(3):572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glas M, Rath BH, Simon M, et al. Residual tumor cells are unique cellular targets in glioblastoma. Ann Neurol. 2010;68(2):264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iulianella A. Cutting thick sections using a vibratome. Cold Spring Harb Protoc. 2017(6):pdb.prot094011. [DOI] [PubMed] [Google Scholar]
- 24. Seyfried TN, el-Abbadi M, Roy ML. Ganglioside distribution in murine neural tumors. Mol Chem Neuropathol. 1992;17(2):147–167. [DOI] [PubMed] [Google Scholar]
- 25. Szulzewsky F, Pelz A, Feng X, et al. Glioma-associated microglia/macrophages display an expression profile different from M1 and M2 polarization and highly express Gpnmb and Spp1. PLoS One. 2015;10(2):e0116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. [DOI] [PubMed] [Google Scholar]
- 27. Muzumdar M, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(6):418–426. [DOI] [PubMed] [Google Scholar]
- 28. Jung S, Aliberti J, Graemmel P, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20(11):4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feng X, Szulzewsky F, Yerevanian A, et al. Loss of CX3CR1 increases accumulation of inflammatory monocytes and promotes gliomagenesis. Oncotarget. 2015;6(17):15077–15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoeffel G, Chen J, Lavin Y, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42(4):665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14(9):1142–1149. [DOI] [PubMed] [Google Scholar]
- 35. Bellingan GJ, Xu P, Cooksley H, et al. Adhesion molecule-dependent mechanisms regulate the rate of macrophage clearance during the resolution of peritoneal inflammation. J Exp Med. 2002;196(11):1515–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gautier EL, Ivanov S, Lesnik P, Randolph GJ. Local apoptosis mediates clearance of macrophages from resolving inflammation in mice. Blood. 2013;122(15):2714–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hughes R, Qian B-Z, Rowan C, et al. Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res. 2015;75(17):3479–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu SC, Alomran R, Chernikova SB, et al. Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats. Neuro Oncol. 2014;16(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chiang CS, Fu SY, Wang SC, et al. Irradiation promotes an m2 macrophage phenotype in tumor hypoxia. Front Oncol. 2012;2:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thomas RP, Nagpal S, Iv M, et al. Macrophage Exclusion after Radiation Therapy (MERT): a first in human Phase I/II trial using a CXCR4 inhibitor in glioblastoma. Clin Cancer Res. 2019:clincanres.1421. 2019;25(23): 6948–6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang C, Yu X, Gao L, et al. Noninvasive imaging of CD206-positive M2 macrophages as an early biomarker for post-chemotherapy tumor relapse and lymph node metastasis. Theranostics. 2017;7(17):4276–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23(3):277–286. [DOI] [PubMed] [Google Scholar]
- 43. Leblond MM, Pérès EA, Helaine C, et al. M2 macrophages are more resistant than M1 macrophages following radiation therapy in the context of glioblastoma. Oncotarget. 2017;8(42):72597–72612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. [DOI] [PubMed] [Google Scholar]
- 45. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang B, Li Q, Qin L, Zhao S, Wang J, Chen X. Transition of tumor-associated macrophages from MHC class II(hi) to MHC class II(low) mediates tumor progression in mice. BMC Immunol. 2011;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ivashkiv LB. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013;34(5):216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ishii M, Wen H, Corsa CAS, et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 2015;114(15):3244–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.