Abstract
Aggrecan is a large proteoglycan that forms giant hydrated aggregates with hyaluronan in the extracellular matrix (ECM). The extraordinary resistance of these aggregates to compression explains their abundance in articular cartilage of joints where they ensure adequate load-bearing. In the brain, they provide mechanical buffering and contribute to formation of perineuronal nets, which regulate synaptic plasticity. Aggrecan is also present in cardiac jelly, developing heart valves, and blood vessels during cardiovascular development. Whereas aggrecan is essential for skeletal development, its function in the developing cardiovascular system remains to be fully elucidated. An excess of aggrecan was demonstrated in cardiovascular tissues in aortic aneurysms, atherosclerosis, vascular re-stenosis after injury, and varicose veins. It is a product of vascular smooth muscle and is likely to be an important component of pericellular matrix, where its levels are regulated by proteases. Aggrecan can contribute to specific biophysical and regulatory properties of cardiovascular ECM via the diverse interactions of its domains, and its accumulation is likely to have a significant role in developmental and disease pathways. Here, the established biological functions of aggrecan, its cardiovascular associations, and potential roles in cardiovascular development and disease are discussed.
Keywords: ADAMTS, aorta, aortic aneurysm, atherosclerosis, cartilage, chondroitin sulfate, extracellular matrix, glycosaminoglycan, heart valve, hyaluronan, intimal hyperplasia, proteoglycan, smooth muscle cell, vascular disease
Introduction
All tissues and organs contain extracellular matrix (ECM) having varying abundance, composition, and specific arrangements with respect to resident cells.1 Whereas considerable attention is given to cell lineage origins, cell proliferation, differentiation, and apoptosis, the regulatory impact of the ECM in these contexts has received less attention. It is well accepted that ECM provides structural integrity to tissues and organizes tissue-specific architectures. In contrast to these roles as a construction material, it is not as widely appreciated that at the cellular level, pericellular ECM in particular has a strong regulatory impact on cell polarity, adhesion, migration, proliferation, differentiation, and cell death.2–5 ECM can signal directly via specific interactions with cellular ECM receptors such as integrins,2 or indirectly by binding and regulating several classes of signaling proteins including morphogens, growth factors, and pro-inflammatory cytokines.6,7
Developmental processes are characterized by a dynamic ECM in keeping with rapidly evolving cell numbers and lineages, extensive cell movements, and dramatic shape changes in the embryo. Normal adult ECM is relatively stable, although it likely also undergoes regulated remodeling, albeit at a slower rate than embryonic ECM. However, dysregulated ECM remodeling that occurs in diseases is of profound significance for cell and tissue function. In all settings, the pericellular matrix, which is the ECM juxtaposed to the cell surface, may be especially dynamic, since it is the most accessible and thus most readily modified by cellular synthetic and proteolytic activities. It is well-positioned to influence cell behavior via its content of pro- and anti-adhesive molecules, as well as growth factors, morphogens, and cytokines. Thus, its components and their turnover are correspondingly of great interest. During early embryogenesis, the ECM is of a primordial and provisional nature, whereas adult tissues and organs have an architecturally mature ECM with a specialized and distinctive composition. The broad importance of ECM components such as fibrillar collagens and fibrillin-1, which are widely distributed, is evident from syndromic inherited disorders of connective tissues such as the Ehlers-Danlos syndromes and Marfan syndrome, which result from well-defined gene mutations.8,9 Many ECM components are specialized and have a narrower tissue distribution, reflected in rare organ-specific genetic conditions, such as inherited kidney and skin disorders resulting from ECM gene mutations.10,11 Conversely, in nearly all acquired disorders, ECM undergoes broad changes such as a general increase or decrease in its abundance relative to cell numbers or altered abundance of specific components which leads to inappropriate ECM stoichiometry and imperils cell function. For example, tendons, ligaments, and bone rely for their tensile strength on the specific rope-like arrangement and high abundance of the fibril-forming component collagen I. Yet, an inappropriate excess of collagen I in other organs is disruptive and a hallmark of a severe tissue derangement called fibrosis.12
The cardiovascular system follows similar developmental principles as other body systems, in that early stages of its development are characterized by a primordial ECM such as cardiac jelly in the ventricular wall and endocardial cushions, whereas later developmental stages are characterized by resorption of this matrix and transition to a mature collagen- or elastin-rich ECM, a process which continues into the neonatal period. Cardiac ECM is altered dramatically in the neonatal period in mice by the expansion of cardiac fibroblasts,13 which produce fibrillar collagen, resulting in an increase in ECM stiffness which may restrict the proliferative capacity of cardiomyocytes.5 Elastic lamellae of blood vessels are laid down during late gestation and the neonatal period,14 and endocardial cushions undergo compaction in late gestation to form mature valve leaflets with a stereotypical three-layered ECM structure.15 Conversely, dysregulation of adult cardiovascular ECM during disease, including fibrosis or reversion to ECM with embryonic characteristics, may accompany or cause cellular changes with significant impact on disease outcomes and are thus deserving of further research.
Proteoglycans are important, diverse and widely distributed ECM components. They are composite molecules comprising a core protein and covalently attached glycosaminoglycan (GAG) chains of various kinds. Over 40 proteoglycans are known, with several belonging to well-characterized families16 and their number is growing with the application of improved methods for their identification, such as glycoproteomics.17 The proteoglycan aggrecan is best known as a quantitatively major, essential, and defining cartilage component. In this review, we examine it in the context of development of the cardiovascular system and its emerging associations with several cardiovascular disorders.
Aggrecan—A Member of the Large Aggregating Proteoglycan Family
The structure and properties of aggrecan are similar to those of other large chondroitin sulfate (CS) proteoglycans collectively known as hyalectans, lecticans, or aggregating proteoglycans, which bind to the GAG hyaluronan (HA) to form large aggregates.18 The other hyalectans, namely versican, brevican, and neurocan, have fewer CS chains than aggrecan, with versican having the most, yet only one-tenth as many as aggrecan. Brevican and neurocan are thought to be restricted to the nervous system (which also contains aggrecan and versican), whereas versican is widely distributed.19 It is abundant in the cardiovascular system, especially during development and its accumulation is implicated in cardiovascular development and disease.20,21 Notably, mouse embryos homozygous for a mutation (Vcanhdf) that abolishes production of all versican splice variants die during early development with major cardiac developmental defects.22–24 Versican is upregulated in cardiac intimal hyperplasia following intimal damage or ablation and is associated with atheromatous lesions, where it is thought to promote vascular smooth muscle cell (VSMC) proliferation and migration as well as cytokine and lipoprotein binding.21 Some of the elucidated roles and mechanisms of versican are likely to be highly relevant to and perhaps even directly applicable to aggrecan given the many structural similarities of the two proteoglycans.
Aggrecan Domain Structure and Established Biological Roles
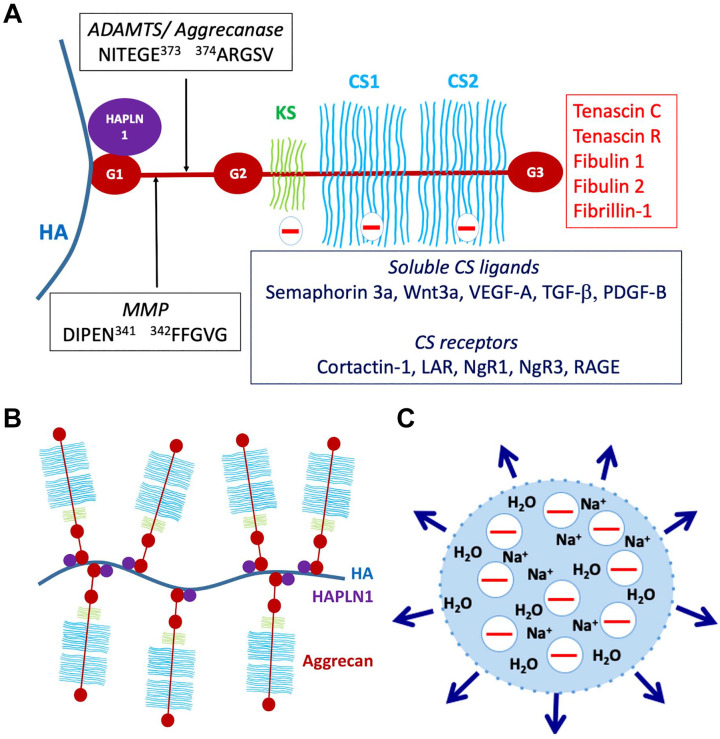
Aggrecan consists of a large core protein of ~250 kDa with covalently attached GAG chains, specifically ~100 CS chains and ~30 keratan sulfate (KS) chains (KS is present in bovine and human aggrecan, but not rat and mouse)25 (Fig. 1A). The GAG chains have variable lengths and sulfation and can increase the mass of an average aggrecan molecule to 3 MDa.25 The core protein has three globular domains, termed G1, G2, and G3, with the GAG chains attached in three main clusters to a long, extended region between the G2 and G3 domains25 (Fig. 1A). Expression of the aggrecan gene is a defining feature of chondrogenesis during development of much of the bony skeleton in vertebrates. During this process, axial and limb bud mesenchymal cells aggregate to form condensations which initiate aggrecan synthesis in response to specific transcription factors and morphogens. The condensations are subsequently identified as cartilage by the presence of aggrecan and another quantitatively major cartilage ECM component, collagen II.26 These chondrogenic rudiments form the anlagen (models) of the future bony skeleton, which are initially wholly cartilaginous. Aggrecan forms giant aggregates with HA, which are located both in the pericellular matrix of chondrocytes and interstitial ECM of cartilage27 (Fig. 1B). The HA-aggrecan complex is stabilized by a link protein structurally homologous to the aggrecan G1 domain called cartilage link protein or HAPLN1, which binds to aggrecan with 1:1 stoichiometry28 (Fig. 1A and B). Binding of the aggregates to cell-surface HA receptors, as well as CS receptors (Fig. 1A) ensures their proximity to the cell surface. During the subsequent postnatal period, cartilage is present only within well-defined growth plates near the ends of bones and the adjacent joint surfaces. Growth plate cartilage is gradually resorbed by endochondral ossification during the embryonic and juvenile periods, leaving only articular cartilage at the ends of bones upon completion of skeletal growth. In articular cartilage, aggrecan is the major compression load-bearing component, acting via two potential mechanisms: charge repulsion between adjacent aggrecan molecules and/or a Donnan equilibrium effect, in which the net negative charge of the aggrecan GAG chains draws in salt and water, resulting in a swelling pressure (Fig. 1C). Swelling of the aggregates is constrained by the collagen II network to about 10–20% of their free solution volume,25 resulting in a unique composite structure with resilience against tension, shear and compression. Aggrecan is also enriched in the inner two-thirds of fibrocartilaginous menisci in knee joints, and in regions of tendons subjected to compression loading.29,30
Figure 1.
Structure and functional attributes of aggrecan. (A) Aggrecan domains and their intermolecular interactions. G1, G2, and G3 indicate globular domains. The interglobular domain is the region between G1 and G2. HA is hyaluronan, HAPLN1, cartilage link protein. The major MMP and ADAMTS (aggrecanase) cleavage sites in the interglobular domain are shown, as well as the relative locations of the keratan sulfate (KS)—and chondroitin sulfate (CS)—bearing domains. Molecules known to bind to CS chains, including cell-surface receptors and soluble ligands and known ligands of the G3 domain are indicated. (B) The aggrecan-hyaluronan (HA) aggregate can vary enormously in size depending on the length of HA and the number of available aggrecan and HAPLN1 molecules. (C) Representation of the Donnan equilibrium concept. The net negative charge of aggrecan-HA aggregates draws in sodium ions and with them, water, contributing to exertion of a swelling pressure (indicated by the outward pointing arrows) by the aggregates. Abbreviations: MMP, matrix metalloproteases; RAGE, receptor for advanced glycation end-products.
In addition to cartilage, aggrecan has been investigated in the central nervous system, especially in the context of perineuronal nets (PNNs), a pericellular matrix present around the soma (body) of parvalbumin-positive interneurons.31 PNNs are assembled on a scaffold of cell-surface attached HA and also contain other aggregating proteoglycans.31 They appear toward the end of the critical period of neuronal plasticity and are thought to insulate the neurons from further synaptic remodeling,32 a possibility supported by the recently reported effect of conditional in vivo aggrecan deletion in these cells.33 PNNs are implicated in the loss of neuronal plasticity occurring after the end of the critical period, which happens around the age of 5 years in humans and they have been considered as the basis of long-term memory.34
Aggrecan Interactions
The aggrecan G1 domain is divided into three sub-domains, A (an immunoglobulin repeat), B, and B′ (each comprising a proteoglycan tandem repeat). Binding to HAPLN1 occurs through the N-terminal A sub-domain, whereas the B- B′ subdomains bind HA and are not known to have additional interactions.35 Whereas the G1 and G3 domains are present in all the aggregating proteoglycans, the G2 domain is unique to aggrecan, homologous to the proteoglycan tandem repeat, and lacks known interactions. The G3 domain has a variety of known ligands including tenascin-C, tenascin-R, fibulin-1 and -2, fibrillin-1, and cell-surface glycolipids and carbohydrates and connects the HA-aggrecan pericellular matrix to an extensive ECM network.36 Like G1, the G3 domain also has distinct sub-domains, namely two epidermal growth factor (EGF)–like repeats, a C-type lectin domain (CLD) and a complement regulatory protein (CRP, also known as a sushi domain) repeat.36 Unlike G1, these G3 sub-domains can undergo alternative splicing, which affects the intermolecular interactions of aggrecan.37 The KS and CS chains attached to the aggrecan core protein are believed to be of heterogenous number, length and charge density, with further modifications occurring during development and disease. CS chains have numerous known ligands including the growth factors VEGF-A, TGF-β, semaphorin-3a, Wnt3a, and platelet-derived growth factor B (PDGF-B), and bind to diverse receptors on the cell surface, such as cortactin-1, the receptor for advanced glycation end-products (RAGE), leukocyte common antigen-related phosphatase and nogo receptors NgR1 and NgR338,39 (Fig. 1A). The interglobular domain (IGD) between G1 and G2 is susceptible to cleavages that separate the GAG-bearing region from the G1 domain and HA40 (Fig. 1A). These cleavages are considered critical for aggrecan loss from articular cartilage in osteoarthritis (OA), since they release that part of aggrecan which carries the compression-load bearing element (the GAG chains), and disrupt the network linking the cell-surface to the interstitial matrix.40 Two cleavage sites identified in the IGD have attracted considerable interest. One is attributed to matrix metalloproteases (MMPs) (occurring at IDIPEN341↓F342FGVG) and the other to A disintegrin-like and metalloprotease domain with thrombospondin type 1 motifs (ADAMTS) proteases (also referred to as the aggrecanase site, occurring at NITEGE373↓A374RGSVIL) (Fig. 1A).41 They are among numerous cleavage sites in the core protein targeted by proteases (only the IGD sites are shown in Fig. 1A). Among them, sites of ADAMTS protease activity are well-characterized, since these proteases were implicated as major contributors to aggrecan proteolysis in OA.41,42 Although ADAMTSs are secreted, they have a propensity for binding cell-surfaces and/or pericellular ECM, and can be regarded as operational cell-surface proteases.24 ADAMTS cleavage of versican and aggrecan is known to release bioactive matrikines.24,43,44 The 32-mer aggrecan peptide resulting from cleavages at the major MMP and ADAMTS cleavage sites (Fig. 1A) has been shown to interact with toll-like receptors, potentially acting as a damage associate molecular pattern (DAMP) component, and also to function in stimulation of pain in the arthritic joint.45,46
Aggrecan Genetics
That aggrecan is indispensable for skeletal development and function is evident from the severe impact of mutations in several species (Table 1). Naturally occurring recessive ACAN mutants in chicks (the nanomelia [nm] mutant)47 and the bulldog ACAN mutation in Dexter cattle48 have an embryonically lethal effect. Two naturally occurring mouse null mutants, one termed cartilage matrix deficiency (Acancmd)49 resulting from a 7-bp N-terminal deletion affecting the G1 domain, and the Acancmd-Bc mutation, a near-complete Acan deletion,50 lead to a severe, lethal recessive chondrodysplasia and have been extensively characterized. Mutant mice die soon after birth with respiratory insufficiency likely resulting from soft rib and tracheal cartilage. Mice with Acan hemizygosity are apparently normal at birth, but develop mild dwarfism after 4 weeks of age and abnormal spinal curvature is noticeable in 1-year old mice.51 In another mouse mutant, Acanb2183Clo, biventricular cardiac hypertrophy was reported in addition to the expected skeletal and craniofacial defects, but few details of this phenotype are presently available.52 Complete loss of function in humans has not been reported and is likely also lethal at birth. ACAN mutations in humans were identified in spondyloepimetaphyseal dysplasia (Online Mendelian Inheritance in Man [OMIM] 612813),53 osteochondritis dissecans with short stature and early OA (OMIM 165800),54 or spondylometaphyseal dysplasia, Kimberly type (OMIM608361).55 No cardiovascular manifestations are reported in these genetic disorders. Hapln1 knockout in mice leads to a milder chondrodysplasia than aggrecan deficiency, but is nevertheless lethal at birth in the majority of Hapln1-null mice,28,56 emphasizing the important supporting role that link protein plays in forming stable HA-aggrecan and HA-versican aggregates. Hapln−/− mice have a cardiac developmental phenotype comprising atrioventricular (AV) septal and myocardial defects similar to that seen in Vcan haploinsufficient mice.57 Since versican linkage to HA is also stabilized by HAPLN1,58 and because cardiac anomalies have not been reported in Acan mouse mutants other than the Acanb2183Clo allele, it is possible that the cardiac roles of HAPLN1 are primarily related to loss of ability to form stable versican-HA complexes.
Table 1.
Naturally Occurring Mutations in the Aggrecan Gene.
| Mutant Allele or Genetic Disorder | Species | Mutation Type | Inheritance/Phenotype |
|---|---|---|---|
| Nanomelia (nm),59,60 | Chick | Nonsense mutation in exon 10, which encodes the chondroitin sulfate domain. | Recessive lethal; shortened limbs and beak, underdevelopment of the chest wall and death around 17–19 days of embryonic development. |
| ACAN, bulldog mutation48,61 | Cow, Dexter breed | Homozygosity or compound heterozygosity for one of two founder mutations: 1. A 4 bp insertion that generates a frameshift and premature stop codon in exon 11. 2. A missense mutation that leads to a new start codon upstream of the canonical one. |
Recessive lethal. Disproportionate dwarfism, a short vertebral column, marked micromelia, a large abdominal hernia, and a relatively large head with a retruded muzzle, cleft palate, and protruding tongue. Heterozygotes show a milder form of dwarfism, with noticeably shorter legs. |
|
Acancmd (MGI:1856465) 62 |
Mouse | Spontaneous; 7-bp deletion in exon 5 leading to frameshift with premature stop codon. | Recessive lethal; severe skeletal and craniofacial defects leading to respiratory and locomotor failure. Mild dwarfism and abnormal spinal curvature were described in aged hemizygous mice. |
| Acancmd-Bc (MGI:1855999)63 | Mouse | Spontaneous; near complete Acan intragenic deletion containing exons 2 to 18. | Recessive lethal; severe skeletal and craniofacial defects leading to respiratory and locomotor failure. |
| Acanb2183Clo (MGI:5297419)52 | Mouse | N-ethyl-N-nitrosourea-induced T to A substitution at coding nucleotide 1032 in exon 6 of the cDNA (c.1032T>A, NM_007424). This changes the tyrosine residue to a translation stop at position 344 of the encoded protein (p.Y344*). | Recessive lethal; In addition to above skeletal defects, the mice are reported to have hypertrophic cardiomyopathy. |
| SEMDAG; (OMIM612813) spondyloepimetaphyseal dysplasia53 | Human | Missense mutations. | Autosomal recessive; Short stature, craniofacial, limb, and vertebral anomalies |
| SSOAOD; (OMIM 165800) osteochondritis dissecans with short stature and early osteoarthritis54 | Human | Heterozygous deletion or missense mutations. | Autosomal dominant; short stature, early onset osteoarthritis, craniofacial, limb, and vertebral anomalies |
| SEDK; (OMIM 608361) spondylometaphyseal dysplasia, Kimberly type55 | Human | Heterozygous single basepair insertion in variable repeat region of exon 12. | Autosomal dominant; short stature, early onset osteoarthritis and vertebral anomalies |
Note that additional spontaneous and engineered mouse mutations are listed at Mouse Genome Informatics (www.informatics.jax.org). Additional details about human ACAN mutations are available at www.omim.org. Abbreviations: MGI, mouse genome informatics; OMIM, online mendelian inheritance in man.
Aggrecan in Cardiovascular Development
Aggrecan localization has been described previously in various regions of the cardiovascular system, and there is a new interest in its distribution and biological roles because of numerous recent associations with cardiovascular disease. Moreover, the observation of cardiac hypertrophy in embryos recessive for the Acanb2183Clo allele may have uncovered a hitherto cryptic role for aggrecan in cardiovascular development.52 The cellular impact of excess aggrecan has not been sufficiently investigated. Here, we review the literature on the cardiovascular developmental and disease associations of aggrecan to identify possible directions for future research.
Aggrecan in Zebrafish Cardiovascular Development
Zebrafish have two paralogous genes encoding aggrecan, aggrecan a and aggrecan b (symbols: acana and acanb), but only acana is expressed in cardiac development, specifically in the outflow tract (OFT), and its expression is dependent on normal hemodynamic forces.64 Knockdown of acana using morpholinos and knockout using CRISPR-Cas9 impaired OFT development and led to a larger atrium and associated edema at 3 days post-fertilization.64 acana morphants had reduced cardiac output and stroke volume, and AV regurgitation, which were attributed to failed OFT development.64 Adult zebrafish valves, unlike those of humans and mice, lack distinct stratified layers of elastin, collagen and proteoglycan, and the valve leaflets comprise two major regions along their proximal-distal axis, a base to mid-region zone containing large polygonal cells and a tip region of small cells with high cellularity. A morphologic analysis of valvular interstitial cells in zebrafish and comparison with mouse valves noted chondrocyte-like morphology of cells in the mid-region, which expressed Sox9 (a transcription factor driving aggrecan and collagen II expression) and a surrounding ECM containing aggrecan and collagen II.65
Aggrecan in Avian Cardiovascular Development
Zanin et al.66 studied aggrecan and versican in chick heart development using immunostaining, western blot, RT-PCR, and RNA in situ hybridization, concluding that aggrecan and versican were expressed in distinct regions. VCAN expression precedes that of ACAN, which is primarily present between Hamburger-Hamilton (HH) stages 16–21 in the developing chick heart, with highest expression around HH stage 20.66 At HH stage 18/19, ACAN mRNA is strongly expressed within the endocardial cushions of the OFT and the AV junction, albeit at higher levels in the inferior cushion of the AV endocardial cushion tissue than the superior side.66 ACAN mRNA was also present in the sino-atrial junction as well as in a population of migrating cells in the dorsal mesocardium of the inflow tract. ACAN mRNA expression was not detected in HH stage 45 hearts or adult hearts.66
By immunohistochemistry, aggrecan core protein was detected in multiple groups of migrating mesenchymal cells in the chick heart. HH stage 16/17 is marked by cells migrating into the thickened basement membrane to form the cardiac jelly, separating the myocardium, and endocardium and differentiating into cushion tissue. At this stage, aggrecan was present in the epicardium and in mesenchymal cells within the cardiac jelly of the OFT.66 However, no aggrecan was seen in the AV cushions, except at the sino-atrial junctions.66 By HH stage 18/19, AV cushions were moderately infiltrated with cells, locations of which correlated with aggrecan staining. By HH stage 20/21, AV cushions were fully infiltrated, showing strong aggrecan staining.66
Zanin et al.66 concluded that ACAN was expressed where mesenchymal cells contributed to the future septation of the primary heart tube, such as the ventricles, common atrium, and sinus venosus. In contrast, they showed VCAN expression to be associated with myocardium and cardiac jelly in the OFT and ventricle, and to the border between the myocardium and OFT in the atrium and AV region.66 Weaker ACAN expression was observed in the septal conal cushion, consistent with the presence of fewer mesenchymal cells.66 Chicken ACAN seems to be under the regulation of T-box transcription factor 20 (TBX20) in the endocardial cushion, since TBX20 overexpression reduced aggrecan expression, whereas siRNA-knockdown of TBX20 in primary chicken endocardial cushion cells increased ACAN expression.67 In this system, Shelton and Yutzey also found that TWIST1 acted upstream of TBX20 to repress ACAN mRNA expression.68
Another study noted the expression of ACAN during avian cardiac cushion elongation and cusp remodeling, and persistence of expression in the juvenile valve cusps (8 weeks).69 During cushion elongation, ACAN is expressed throughout the valve cusp, with increased expression in the fibrosa along with type III collagen and tenascin.69 During cusp remodeling, ACAN is strongly expressed in the annulus, while at the juvenile stage, aggrecan is absent in the annulus and is only present in the fibrosa and spongiosa.69 In the pre-fused endocardial cushion, BMP2 and FGF4 regulate the diverging differentiation of valve precursor cells into valves or the valve-supporting structures, such as chordae tendineae.70 ACAN is co-expressed with transcription factors SOX8 and SOX9, the latter being strongly associated with chondrogenesis, in the cushion tissue and differentiating valve leaflets.71 Increased BMP2 or decreased FGF4 signaling induces ACAN and SOX9, mediated by the phosphorylation of SMAD1/5/8. Conversely, decreased BMP2 or increased FGF4 signaling induces tenascin and scleraxis through phosphorylated MAPK.70 Consistent with this, at HH stage 25, ACAN was not expressed in the unfused AV endocardial cushions but was expressed after cushion fusion at HH stage 29, where ACAN and SOX9 mRNA were co-expressed in the primitive mural and septal tricuspid valve, septal mitral valve primordia in the AV canal, and in the atrial sub-septal area of the central cushion.70 At HH stage 36, ACAN and SOX9 are only expressed in the septal and parietal mitral valve leaflets extending to the tips and in the trans-septal fibrous continuity across the ventricular septum, but not in the chordae tendineae.70 Treatment of cultured valve precursor cells from the pre-fused endocardial cushions with BMP2 or FGF inhibitor SU5402 increased the percentage of cells expressing aggrecan by 20–30% and led to a 62-fold increase in the ACAN transcriptional level compared to untreated controls.70 These findings suggested the involvement of aggrecan in the cell lineage determination of bipotent valve precursor cells.
In the developing chick aorta, aggrecan is mostly localized in the outer region of the aortic wall. In embryos, little or no aggrecan was present on day 7 (HH stage 31), but weak staining was detected by day 14 (HH stage 40) around the cells organized into lamellae, which continued to day 21 (HH stage 46).72 By immunoblotting, aggrecan was detected at low levels at day 14 (HH stage 40). These observations contrasted to versican, which was present throughout the aortic wall and at higher levels.72
Aggrecan in Mammalian Cardiovascular Development
The expression and localization of aggrecan in the mammalian cardiovascular system has not been investigated in the same detail as in chick embryos. Although aggrecan staining was reported in mouse cardiac jelly during cardiomyogenesis,73 there are no reports of aggrecan expression during mouse cardiac valve development. In gestational day 18.5 (E18.5) embryonic mouse aorta, Acan mRNA is significantly higher than in 12-week-old adult aorta (delta Ct values 3.88 ± 0.58 versus 0.28 ± 0.10, p=0.001).74 At postnatal day 16 (P16), aggrecan is immunolocalized to the inner third of the aortic tunica media and limited to half of the aortic circumference, but disappears by P30 and is absent at P45, P60, and P90.75 On the other hand, ADAMTS-cleaved aggrecan increases from P16 to P60, but is absent at P45 and slightly reduced at P90.75 Acan is also upregulated by 3.67-fold in the ductus arteriosus (DA) of Brown-Norway rats, which are used as an in vivo animal model for patent DA and display dysregulated DA elastogenesis.76 No cardiovascular anomalies have been described in mouse Acan mutants, other than in Acanb2183Clo which was identified in a chemical mutagenesis screen that specifically sought cardiac developmental anomalies, probably because the severe phenotypes readily evident in the skeleton have attracted greater attention. The observed cardiomyopathy could plausibly result from a primary defect in myocardial development, since aggrecan is present in cardiac jelly.73 Alternatively, it could be secondary to an obstructive defect in the aorta and/or heart valves.
Adamts5−/− mice, which lack the major ADAMTS protease involved in aggrecan cleavage in OA, have aortic thickening with aggrecan accumulation.77 Aggrecan immunostaining in the ascending aorta of mutants was more intense around E14.5, that is, at the beginning of elastogenesis and following septation of the cardiac OFT, and was seen up to 1 month postnatally, preferentially on the aortic arch lesser curvature. Aggrecan co-localized with smooth muscle actin positive cells, suggesting that differentiated VSMCs accumulated aggrecan in their pericellular matrix in the absence of ADAMTS5.77 This possibility is consistent with RNA in situ hybridization and immunostaining which demonstrated Acan expression by mouse and human aortic VSMC.75 Aggrecan staining in Adamts5−/− aorta was polarized, with stronger staining on the adventitial aspect of VSMCs, accompanying widened interlamellar distance and elastic fiber breakage. The findings suggest that ADAMTS5 participates in physiological aggrecan turnover to maintain optimal levels during mouse aortic development, but reduced cleavage of other ADAMTS5 substrates could potentially also occur.
It was observed that inhibition of the MEK pathway by the chemical U0126 in the wild-type E13.5 heart in vitro led to 2.9 ± 0.4-fold induction of Acan and 56 ± 4% increase in Sox9 expression.78 MEK/ERK are upstream regulators of Ets-1, which is an effector transcription factor for FGF signaling.78 Ets1−/− mice exhibit perinatal lethality and develop an abnormal focus of intra-cardiac cartilage as well as a membranous ventricular septal defect due to anomalous neural crest cell migration.78
Knowledge of ACAN expression in human embryonic cardiovascular tissues is limited. Rambeau et al.64 determined relative ACAN mRNA abundance in developing and adult human aortic valves by RT-qPCR and detected low expression at 9 weeks and 10 weeks of gestation, robust expression at 13 weeks of gestation, and higher mRNA levels in adult human valves. They compared relative ACAN mRNA abundance between tricuspid aortic valves and bicuspid type 0 valves and found reduced ACAN mRNA (16-fold difference) in the latter, although the number of valves analyzed was far too small (4 tricuspid valves, 2 bicuspid) to be conclusive.64 Low levels of ACAN mRNA were identified in VSMCs of adult non-diseased human ascending aorta using RNA in situ hybridization.75
Aggrecan in Cardiovascular Disease
Although ACAN mRNA is expressed constitutively in normal arteries75,79 aggrecan accumulation has been identified in several pathologic conditions affecting the vasculature (see Table 2).
Table 2.
Major Cardiovascular Disease Associations of Aggrecan.
| Disease | Summary of Main Findings |
|---|---|
| Atherosclerosis | ACAN mRNA is expressed in the neointima associated with pre-atherosclerotic lesions in human carotid arteries but was reduced in calcified lesions, indicating that aggrecan plays a role in the progression of atherosclerosis.80 Aggrecan has been shown by immunostaining to be present in both stable and unstable advanced plaques, including calcified lesions, in both human and mice.81,82 VSMCs or derivatives from the VSMC lineage arising by phenotypic modulation are the primary source of aggrecan in atherosclerosis. Aggrecan accumulation promotes VSMC apoptosis in atherosclerotic plaques.83 |
| Aortic aneurysms | Aggrecan antibodies strongly stain proteoglycan-rich areas (aortic medial degeneration) as well as other regions of the tunica media of human TAAs. Moreover, aggrecan accumulation was strongly associated with aortic dissection and rupture in a mouse model of severe Marfan syndrome.75 In contrast to TAA, aggrecan was shown by mass spectrometry to be reduced in human AAA tissue.84 While VCAN polymorphisms showed an association with intra-cranial (brain) aneurysms,85–87 aggrecan has not been similarly implicated. |
| Vascular injury and restenosis | Significant ECM remodeling, including neointima formation, was observed after bare metal stent or drug eluting stent placement in a porcine arterial restenosis model.88 Proteomic evaluation of the neointima by mass spectrometry revealed a delayed (>7 days post-stent placement) accumulation of large proteoglycans including aggrecan and versican. Proteoglycan accumulation was accompanied by an increase in ADAMTS-cleaved aggrecan and versican neoepitopes, indicating their dynamic role in restenosis-associated ECM remodeling. These findings were confirmed by immunohistochemistry in stented human coronary arteries, with aggrecan concentrated in the neo-intimal layer and at the points of stent strut contact.88 |
| Venous hypertension | Proteomic comparison of human saphenous vein to aorta suggested that aggrecan was more abundant in arteries than veins.88 When veins were grafted into the carotid arteries of mice, aggrecan accumulation in arterialized veins was shown by immunohistochemistry, suggesting aggrecan production was induced by mechanical stress associated with increased hemodynamic pressure.88 Conversely, aggrecan was significantly reduced in varicose versus normal saphenous veins by shotgun and targeted proteomic analyses.89 |
| Cardiac valve anomalies | Cartilage proteins, including aggrecan, were identified in human myxomatous valves by immunohistochemistry90 and RNA expression analysis.91 ACAN expression is reduced in bicuspid aortic valve type 0.64 Aggrecan expression in calcific bicuspid aortic valves, however, is similar to that in tricuspid aortic valves.92 |
Abbreviations: AAA, abdominal aortic aneurysm; ADAMTS, A disintegrin-like and metalloproteinase domain with thrombospondin type 1 motif; ECM, extracellular matrix; TAA, thoracic aortic aneurysm; VSMC, vascular smooth muscle cells.
Atherosclerosis
The presence of aggrecan throughout the temporal development of atherosclerotic lesions has been described in mice, rats, and humans. Because versican, biglycan, decorin, and perlecan have long been known to be the predominant proteoglycans of the arterial wall, more attention was given to their interactions with low density lipoproteins (LDL) in mechanisms of atherosclerosis.93–96 Despite containing up to 10-fold more constitutive CS attachments sites than versican,97 aggrecan’s role in GAG-mediated LDL retention has not been investigated. In Apo−/− mice, loss of ADAMTS5 was associated with proteoglycan accumulation and ADAMTS5 was shown to reduce the proteoglycan content of atherosclerotic lesions by release of cleaved core protein fragments, primarily arising from versican, but also biglycan and aggrecan, from the arterial wall; ADAMTS5 activity also contributed to release of proteoglycan bound lipoproteins.93 ACAN mRNA is expressed in the neointima associated with pre-atherosclerotic lesions in human carotid arteries but was reduced in calcified lesions, suggesting that aggrecan plays a role in the progression of atherosclerosis.80 Aggrecan has been shown by immunostaining to be present in both stable and unstable advanced plaques, including calcified lesions, in both human and mice.81,82
Several studies have attempted to identify the cells responsible for aggrecan production in atherosclerotic lesions as well as the underlying mechanisms driving its expression. VSMCs, specifically pericytes, can display chondrocyte-like biosynthesis in response to BMP-2 and Wnts and produce aggrecan as part of an intermediate phenotype.98–100 A growing body of evidence suggests that VSMCs or derivatives from the VSMC lineage arising by phenotypic modulation are the primary source of aggrecan in atherosclerosis. Indeed, a number of studies have described VSMC trans-differentiation to a chondrocyte-like phenotype in vascular pathologies. In a uremic rat model for chronic kidney disease, chondrogenic VSMCs led to calcification in the tunica media of the aorta.101 In mouse and human atherosclerotic lesions, smooth muscle cells with transitional phenotypes, described as myochondrocytes, were identified in which chondrocyte markers such as SOX9 were increased while SMC markers were present but reduced. While the exact mechanism of chondrocyte-like phenotype transition has not been determined, several studies have provided potential insights. In response to vascular injury, typical SMC markers such as SM α-actin (SMA) and myosin heavy chain (MYH11) are downregulated concomitantly with the upregulation of chondrogenic markers including collagen II, bone morphogenetic protein-2 (BMP-2), and aggrecan.102 In mouse models of atherosclerosis, VSMC transition to a chondrocytic phenotype can be driven by BMP-2.103 Disruption of matrix GLA protein, which inhibits BMP-2 activity, is known to result in arterial calcification.104 Inhibition of BMP-2 in Ldlr−/− mice reduced both atheroma development and arterial calcification, although aggrecan was not evaluated.105 While these studies suggest that BMP-2 is a potential driver of a chondrocyte-like phenotype transition in VSMCs, its effect on aggrecan production has not yet been directly tested. It is also unclear what events trigger VSMCs transition to an aggrecan-producing phenotype. Aggrecan accumulation was enhanced in Tagln−/− mice in response to endothelial injury.102 VSMCs of the rat aorta express aggrecan and other chondrogenic markers when cultured on calcified elastin or hydroxyapatite and revert to their original phenotype when removed from calcific conditions,106 suggesting SMC phenotype modulation/ trans-differentiation can also be induced through interactions with the surrounding ECM in which they reside.
While its production by VSMCs appears to be part of an injury response mechanism, the accumulation of aggrecan promotes apoptosis of VSMCs in atherosclerotic plaques.83 Conversely, cleavage of aggrecan by the macrophage-derived proteases MMP9, ADAMTS4, and ADAMTS8 is increased in unstable plaques.107 It is unclear, however, if aggrecan cleavage itself causes plaque instability and whether it is contributory to the severity and progression of atherosclerosis. Other ADAMTS proteases implicated in versican turnover and expressed in the vascular wall, such as ADAMTS1108–110 and ADAMTS9,111–114 which can both cleave aggrecan, may also be involved in vascular aggrecan turnover.
Aneurysms
Proteoglycan accumulation is a defining feature of medial degeneration, a histopathologic hallmark of thoracic aortic aneurysms (TAAs).115 The swelling pressure thus exerted was postulated to contribute to dissection risk by creating stress risers in the aortic wall.116 Aggrecan antibodies strongly stain these proteoglycan-rich areas as well as other regions of the tunica media of human TAAs. Moreover, aggrecan and versican accumulation are strongly associated with aortic dissection and rupture.75 While TAA can arise from several genetic defects and thus unique disease pathways, the consistent presence of medial degeneration and the associated aggrecan accumulation in diseased aortic tunica media suggests a common final pathogenic sequence leading to vessel wall failure, with aggrecan accumulation making an important contribution75,117 (Fig. 2). Aggrecan accumulation is asymmetric within the ascending aortic wall and has been shown to favor the aorto-pulmonary septum.118 Its production has been proposed to be influenced by both VSMC cell lineage and vessel hemodynamics.118 Consistent with this hypothesis, aggrecan expression in the normal mouse aorta decreases as the vessel progresses from the aortic arch, an area that subjected to high shear stress, toward the abdominal aorta, an area with relatively lower shear stress.119 This heterogeneous distribution of mural proteoglycans could reflect regional vascular wall adaptations to hemodynamic stresses.120 Aortic stiffness is associated with a number of cardiovascular diseases and also occurs with aging as loss of elastic fibers reduces the ability of the aortic wall to store elastic energy.121 Reduced aggrecan has been observed in aging, and postulated to contribute to increased vessel stiffness.122 Taken together, these studies support an adaptive mechanism in which vascular proteoglycans with high load-bearing capabilities, like aggrecan, assist elastic lamellae in resisting hemodynamic forces.88 This process appears to be dynamic and controlled not only by the production of aggrecan but also by its proteolysis.123 Evidence for this comes from mouse models lacking the aggrecanase ADAMTS5 in which aggrecan accumulation and aneurysmal disease progression is exacerbated.77,119 ADAMTS5 mRNA was significantly reduced in human TAA tissue as was Adamts5 mRNA in a mouse model of Marfan syndrome, the Fbn1 mutant Fbn1mgR/mgR.75
Figure 2.
Schematic depicting the impact of aggrecan accumulation in ascending thoracic aortic aneurysm and dissection (TAAD). In a normal aortic wall (left), aggregates of hyaluronan and the proteoglycans (PG) aggrecan and versican provide compressive stiffness to the elastic lamellar units and place elastic fiber/microfibril-cell connections into tension, aiding mechano-sensing by vascular smooth muscle cells (VSMC). Pathologic accumulation of aggrecan and versican in TAAD (right), occurring together with elastic fiber fragmentation disrupts VSMC-extracellular matrix connections. This loss of adhesive and mechanical input to VSMC may result in phenotypic modulation, depicted here as loss of the typical spindle shape of VSMC and assumption of a more rounded appearance. The figure is modified from Cikach et al.75
In contrast to TAA, aggrecan accumulation is not implicated in the pathogenesis of other types of aneurysms. Abdominal aortic aneurysm (AAA) VSMCs have increased production of the proteoglycans versican, biglycan, and perlecan but an increase in aggrecan production was not observed.124 Indeed, aggrecan was shown by mass spectrometry to be reduced in human AAA tissue.84 While VCAN polymorphisms showed an association with intra-cranial (brain) aneurysms,85–87 ACAN has not been similarly implicated.
Vascular Injury and Restenosis
Neointima formation is a common complication following stenting for coronary artery disease.125 Significant ECM remodeling, including neointima formation, was observed after bare metal stent (BMS) or drug eluting stent (DES) placement in a porcine arterial restenosis model.88 Proteomic evaluation of the neo-intima by mass spectrometry revealed a delayed (>7 days post-stent placement) accumulation of large proteoglycans including aggrecan and versican. Proteoglycan accumulation was accompanied by an increase in ADAMTS-mediated aggrecan and versican neoepitopes, indicating a dynamic role in restenosis-associated ECM remodeling. These findings were confirmed by immunohistochemistry in stented human coronary arteries, with aggrecan concentrated in the neo-intimal layer and at the points of stent strut contact.88
Venous Hypertension
Proteomic comparison of human saphenous vein to aorta suggested that aggrecan was more abundant in arteries than veins.88 When veins were grafted into the carotid arteries of mice, aggrecan accumulation in arterialized veins was shown by immunohistochemistry, suggesting aggrecan production was induced by mechanical stress associated with increased hemodynamic pressure.88 Conversely, aggrecan was significantly reduced in varicose versus normal saphenous veins by shotgun and targeted proteomic analyses.89 Although varicose veins arise from venous hypertension, venous pressures are lower than arterial, and other biological differences between arteries and veins may be contributory to the distinct responses. Taken together, the findings suggest that vascular aggrecan remodeling is a major adaptation to altered hemodynamic pressure, and that aggrecan levels could additionally be altered by a structural failure of vessels.
Cardiac Valve Disorders
Cartilage proteins, including aggrecan, were identified in human myxomatous valves by immunohistochemistry90 and RNA expression analysis.91 Mice deficient in Axin2, an inhibitor of Wnt/β-catenin signaling, develop myxomatous valve disease (MVD)126 and the same signaling pathway is activated in human MVD.127 Adult aortic valves of Axin2 KO mice had both increased thickness and aggrecan immunofluorescence.128 The role of Wnt-β-catenin signaling remains unclear as it has also been shown to reduce nuclear localization of Sox9, preventing aggrecan expression.126 Increased aggrecan expression in valves has also been shown by PCR and in situ hybridization in mice lacking periostin, a matricellular protein thought to play a major role in valve development and homeostasis.129 Conversely, ACAN expression is reduced in bicuspid aortic valve type 0.64 Aggrecan expression in calcific bicuspid aortic valves, however, is similar to that in tricuspid aortic valves.92
Myocardial Overload
Proteoglycans have an important role in cardiac remodeling and fibrosis.130 In a study designed to evaluate the contribution of ADAMTS proteases to myocardial ECM remodeling, Vcan and Acan expression were found elevated in a rat model of cardiac overloading induced by aortic banding.131 Acan mRNA expression was increased 4- to 6-fold over sham-operated rats. Concomitant increases of both mRNA and protein levels of the aggrecanases ADAMTS 1, 4, and 8 were observed. Other major aggrecanases, such as ADAMTS5 were not evaluated. Pharmacological inhibition of ADAMTS4 reduced versican cleavage at the ADAMTS cleavage-site and mitigated the decline in cardiac contractility, suggesting an association between versican cleavage and deterioration of systolic function.131 The role of aggrecan and its proteolytic turnover on cardiac biomechanics requires further exploration.
Future Research Needs
This literature review leads us to conclude that aggrecan is likely to participate in cardiovascular development, with definitive data from different vertebrates (zebrafish, chick, mouse, and human) each showing aggrecan gene expression and localization. However, its roles are likely to be more subtle than those of versican. Aggrecan accumulation around mouse aortic VSMC in the absence of ADAMTS5,77 suggests that the aggrecan produced by these cells is normally rapidly turned over by the action of this protease (Fig. 3), and possibly, related proteases such as ADAMTS9113 and ADAMTS1.132 Further work is needed to understand the factors that regulate aggrecan production by VSMCs and to better define the dynamics of aggrecan turnover by these proteases. Eliciting the potential developmental cardiovascular impact of aggrecan requires conditional Acan deletion, for example, in mouse VSMCs, which are major aggrecan-producing cells in blood vessels. Aggrecan, like versican, also has a potential role in myocardiogenesis, but it is a quantitatively minor component of cardiac jelly compared to versican. Precisely which cells in the heart express aggrecan at different developmental stages or during cardiac failure and repair needs to be carefully determined before conditional deletion strategies can be effectively employed.
Figure 3.
Cellular impact of aggrecan accumulation in vascular smooth muscle cells (VSMC). The cartoon shows that normal VSMCs (left) have a low level of pericellular aggrecan, because of continuous trimming away by ADAMTS5 and potentially other proteases. This may optimize adhesion receptor access to pro-adhesive ECM, promoting formation of a robust cytoskeleton (red hatching) and maintaining the contractile phenotype. Altered hemodynamics, injury, or inflammation may lead to increased aggrecan production or reduced proteolytic turnover as an adaptive response, resulting in net pericellular aggrecan accumulation, which may impair focal adhesion formation and lead to VSMC phenotype modulation (light red broken hatching) toward a poorly characterized phenotype designated here as a “myochondrocyte.” Abbreviations: ADAMTS, A disintegrin-like and metalloproteinase domain with thrombospondin type 1 motif; ECM, extracellular matrix; LDL, low density lipoproteins; MMP, matrix metalloproteases.
We have summarized considerable evidence for aggrecan accumulation in diverse vascular and valve anomalies, as well as in the cardiac response to pressure overload. Because aggrecan is likely to be highly disruptive in excess, its roles in diseases may be more dramatic than in development. Mechanisms of aggrecan accumulation in disease are not yet fully understood, although it is clear that reduced turnover by proteases such as ADAMTS5 may be important in specific disease settings such as TAAD and atherosclerosis (Fig. 3). Among the approaches that could be considered in future are conditional Acan deletion in VSMCs applied to mouse models of aortic aneurysms and atherosclerosis. In particular, the potential mechanistic role of aggrecan in atherosclerosis, a relatively common vascular disorder, deserves further analysis. Rapid aggrecan turnover by ADAMTS proteases may be an important physiological requirement if excess pericellular aggrecan is deleterious to VSMCs and/or vascular wall biomechanics. This possibility could be tested by generating transgenic mice with targeted VSMC over-expression of aggrecan which would also be useful for further investigation of its roles in aneurysms and atherosclerosis in vivo. For example, it was previously shown that excess aggrecan, link protein and HA impaired fibroblast adhesion to the substratum in vitro,133 and similar approaches could be applied to cultured VSMCs. In this regard, versican accumulation in pericellular ECM of uterine smooth muscle cells impaired the formation of focal adhesions and led to their dedifferentiation.4 Recent work from our group shows that aggrecan accumulation in the umbilical artery, which does not occur in the umbilical vein, is developmentally and evolutionarily ensured by robust arterial ACAN transcription and limited aggrecan degradation.134 Computational analysis suggested that abundant aggrecan in the inner umbilical artery tunica media could promote rapid closure of this vessel at birth.134 Furthermore, both Acancmd-bc/cmd-bc embryos and mice lacking ADAMTS1, the most highly expressed proteoglycan-degrading enzyme in the umbilical vein, have a severely altered VSMC phenotype.134 There is thus a definite possibility that aggrecan accumulation around VSMCs may lead to phenotype modulation in disease (Fig. 3) and perhaps even compromise their survival in the setting of TAAD, such as via induction of anoikis. For these reasons, exploring the cardiovascular roles of aggrecan at the organ and cellular level is a pressing research need in the cardiovascular disease arena.
Acknowledgments
We thank David Shumick for the artwork that is the basis for Figure 2.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the NIH-NHLBI awards HL107147 and HL141130 (to S.S.A.), and by the Allen Distinguished Investigator Program, through support made by The Paul G. Allen Frontiers Group and the American Heart Association (to S.S.A).
Contributor Information
Christopher D. Koch, Department of Laboratory Medicine, Yale University, New Haven, Connecticut Department of Biomedical Engineering, Cleveland Clinic Lerner Research Institute, Cleveland, Ohio; Department of Chemistry, Cleveland State University, Cleveland, Ohio.
Chan Mi Lee, Department of Biomedical Engineering, Cleveland Clinic Lerner Research Institute, Cleveland, Ohio; Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, Ohio.
Suneel S. Apte, Department of Biomedical Engineering, Cleveland Clinic Lerner Research Institute, Cleveland, Ohio.
Literature Cited
- 1. Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol. 2014;15(12):771–85. doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix—cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2(11):793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 3. Hattori N, Carrino DA, Lauer ME, Vasanji A, Wylie JD, Nelson CM, Apte SS. Pericellular versican regulates the fibroblast-myofibroblast transition: a role for ADAMTS5 protease-mediated proteolysis. J Biol Chem. 2011;286(39):34298–310. doi: 10.1074/jbc.M111.254938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mead TJ, Du Y, Nelson CM, Gueye NA, Drazba J, Dancevic CM, Vankemmelbeke M, Buttle DJ, Apte SS. ADAMTS9-regulated pericellular matrix dynamics governs focal adhesion-dependent smooth muscle differentiation. Cell Rep. 2018;23(2):485–98. doi: 10.1016/j.celrep.2018.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yahalom-Ronen Y, Rajchman D, Sarig R, Geiger B, Tzahor E. Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. eLife. 2015;4:e07455. doi: 10.7554/eLife.07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaudhry SS, Cain SA, Morgan A, Dallas SL, Shuttleworth CA, Kielty CM. Fibrillin-1 regulates the bioavailability of TGFbeta1. J Cell Biol. 2007;176(3):355–67. doi: 10.1083/jcb.200608167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGFbeta and BMP signaling. Curr Opin Cell Biol. 2009;21(5):616–22. doi: 10.1016/j.ceb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malfait F. Vascular aspects of the Ehlers-Danlos syndromes. Matrix Biol. 2018;71–2:380–95. doi: 10.1016/j.matbio.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 9. Robinson PN, Arteaga-Solis E, Baldock C, Collod-Beroud G, Booms P, De Paepe A, Dietz HC, Guo G, Handford PA, Judge DP, Kielty CM, Loeys B, Milewicz DM, Ney A, Ramirez F, Reinhardt DP, Tiedemann K, Whiteman P, Godfrey M. The molecular genetics of Marfan syndrome and related disorders. J Med Genet. 2006;43(10):769–87. doi: 10.1136/jmg.2005.039669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chew C, Lennon R. Basement membrane defects in genetic kidney diseases. Front Pediatr. 2018;6:11. doi: 10.3389/fped.2018.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nystrom A, Bruckner-Tuderman L. Matrix molecules and skin biology. Semin Cell Dev Biol. 2019;89:136–46. doi: 10.1016/j.semcdb.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 12. Weiskirchen R, Weiskirchen S, Tacke F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol Aspects Med. 2019;65:2–15. doi: 10.1016/j.mam.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 13. Ivey MJ, Kuwabara JT, Pai JT, Moore RE, Sun Z, Tallquist MD. Resident fibroblast expansion during cardiac growth and remodeling. J Mol Cell Cardiol. 2018;114:161–74. doi: 10.1016/j.yjmcc.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89(3):957–89. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lincoln J, Lange AW, Yutzey KE. Hearts and bones: shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev Biol. 2006;294(2):292–302. doi: 10.1016/j.ydbio.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 16. Iozzo RV, Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noborn F, Gomez Toledo A, Sihlbom C, Lengqvist J, Fries E, Kjellen L, Nilsson J, Larson G. Identification of chondroitin sulfate linkage region glycopeptides reveals prohormones as a novel class of proteoglycans. Mol Cell Proteomics. 2015;14(1):41–9. doi: 10.1074/mcp.M114.043703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol. 2008;130(4):635–53. doi: 10.1007/s00418-008-0485-9. [DOI] [PubMed] [Google Scholar]
- 19. Bode-Lesniewska B, Dours-Zimmermann MT, Odermatt BF, Briner J, Heitz PU, Zimmermann DR. Distribution of the large aggregating proteoglycan versican in adult human tissues. J Histochem Cytochem. 1996;44(4):303–12. [DOI] [PubMed] [Google Scholar]
- 20. Henderson DJ, Copp AJ. Versican expression is associated with chamber specification, septation, and valvulogenesis in the developing mouse heart. Circ Res. 1998;83(5):523–32. doi: 10.1161/01.res.83.5.523. [DOI] [PubMed] [Google Scholar]
- 21. Wight TN, Merrilees MJ. Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ Res. 2004;94(9):1158–67. doi: 10.1161/01.RES.0000126921.29919.51. [DOI] [PubMed] [Google Scholar]
- 22. Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202(1):56–66. [DOI] [PubMed] [Google Scholar]
- 23. Yamamura H, Zhang M, Markwald RR, Mjaatvedt CH. A heart segmental defect in the anterior-posterior axis of a transgenic mutant mouse. Dev Biol. 1997;186(1):58–72. [DOI] [PubMed] [Google Scholar]
- 24. Nandadasa S, Foulcer S, Apte SS. The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis. Matrix Biol. 2014;35:34–41. doi: 10.1016/j.matbio.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vertel BM. The ins and outs of aggrecan. Trends Cell Biol. 1995;5(12):458–64. doi: 10.1016/s0962-8924(00)89115-1. [DOI] [PubMed] [Google Scholar]
- 26. Watanabe H, Yamada Y, Kimata K. Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J Biochem. 1998;124(4):687–93. doi: 10.1093/oxfordjournals.jbchem.a022166. [DOI] [PubMed] [Google Scholar]
- 27. Hascall VC, Heinegard D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 1974;249(13):4232–41. [PubMed] [Google Scholar]
- 28. Watanabe H, Yamada Y. Mice lacking link protein develop dwarfism and craniofacial abnormalities. Nat Genet. 1999;21(2):225–9. doi: 10.1038/6016. [DOI] [PubMed] [Google Scholar]
- 29. Robbins JR, Evanko SP, Vogel KG. Mechanical loading and TGF-beta regulate proteoglycan synthesis in tendon. Arch Biochem Biophys. 1997;342(2):203–11. doi: 10.1006/abbi.1997.0102. [DOI] [PubMed] [Google Scholar]
- 30. Robbins JR, Vogel KG. Regional expression of mRNA for proteoglycans and collagen in tendon. Eur J Cell Biol. 1994;64(2):264–70. [PubMed] [Google Scholar]
- 31. Fawcett JW, Oohashi T, Pizzorusso T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci. 2019;20(8):451–65. doi: 10.1038/s41583-019. [DOI] [PubMed] [Google Scholar]
- 32. Lensjo KK, Lepperod ME, Dick G, Hafting T, Fyhn M. Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. J Neurosci. 2017;37(5):1269–83. doi: 10.1523/JNEUROSCI.2504-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rowlands D, Lensjo KK, Dinh T, Yang S, Andrews MR, Hafting T, Fyhn M, Fawcett JW, Dick G. Aggrecan directs extracellular matrix-mediated neuronal plasticity. J Neurosci. 2018;38(47):10102–13. doi: 10.1523/JNEUROSCI.1122-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsien RY. Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc Natl Acad Sci USA. 2013;110(30):12456–61. doi: 10.1073/pnas.1310158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Watanabe H, Cheung SC, Itano N, Kimata K, Yamada Y. Identification of hyaluronan-binding domains of aggrecan. J Biol Chem. 1997;272(44):28057–65. doi: 10.1074/jbc.272.44.28057. [DOI] [PubMed] [Google Scholar]
- 36. Aspberg A. The different roles of aggrecan interaction domains. J Histochem Cytochem. 2012;60(12):987–96. doi: 10.1369/0022155412464376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Day JM, Olin AI, Murdoch AD, Canfield A, Sasaki T, Timpl R, Hardingham TE, Aspberg A. Alternative splicing in the aggrecan G3 domain influences binding interactions with tenascin-C and other extracellular matrix proteins. J Biol Chem. 2004;279(13):12511–8. doi: 10.1074/jbc.M400242200. [DOI] [PubMed] [Google Scholar]
- 38. Le Jan S, Hayashi M, Kasza Z, Eriksson I, Bishop JR, Weibrecht I, Heldin J, Holmborn K, Jakobsson L, Soderberg O, Spillmann D, Esko JD, Claesson-Welsh L, Kjellen L, Kreuger J. Functional overlap between chondroitin and heparan sulfate proteoglycans during VEGF-induced sprouting angiogenesis. Arterioscler Thromb Vasc Biol. 2012;32(5):1255–63. doi: 10.1161/ATVBAHA.111.240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mizumoto S, Yamada S, Sugahara K. Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr Opin Struct Biol. 2015;34:35–42. doi: 10.1016/j.sbi.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 40. Lark MW, Bayne EK, Flanagan J, Harper CF, Hoerrner LA, Hutchinson NI, Singer II, Donatelli SA, Weidner JR, Williams HR, Mumford RA, Lohmander LS. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J Clin Invest. 1997;100(1):93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lark MW, Gordy JT, Weidner JR, Ayala J, Kimura JH, Williams HR, Mumford RA, Flannery CR, Carlson SS, Iwata M, Sandy JD. Cell-mediated catabolism of aggrecan. Evidence that cleavage at the “aggrecanase” site (Glu373-Ala374) is a primary event in proteolysis of the interglobular domain. J Biol Chem. 1995;270(6):2550–6. [DOI] [PubMed] [Google Scholar]
- 42. Tortorella MD, Pratta M, Liu RQ, Austin J, Ross OH, Abbaszade I, Burn T, Arner E. Sites of aggrecan cleavage by recombinant human aggrecanase-1 (ADAMTS-4). J Biol Chem. 2000;275(24):18566–73. [DOI] [PubMed] [Google Scholar]
- 43. Hope C, Foulcer S, Jagodinsky J, Chen SX, Jensen JL, Patel S, Leith C, Maroulakou I, Callander N, Miyamoto S, Hematti P, Apte SS, Asimakopoulos F. Immunoregulatory roles of versican proteolysis in the myeloma microenvironment. Blood. 2016;128(5):680–5. doi: 10.1182/blood-2016-03-705780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCulloch DR, Nelson CM, Dixon LJ, Silver DL, Wylie JD, Lindner V, Sasaki T, Cooley MA, Argraves WS, Apte SS. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev Cell. 2009;17(5):687–98. doi: 10.1016/j.devcel.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lees S, Golub SB, Last K, Zeng W, Jackson DC, Sutton P, Fosang AJ. Bioactivity in an aggrecan 32-mer fragment is mediated via toll-like receptor 2. Arthritis Rheumatol. 2015;67(5):1240–9. doi: 10.1002/art.39063. [DOI] [PubMed] [Google Scholar]
- 46. Miller RE, Ishihara S, Tran PB, Golub SB, Last K, Miller RJ, Fosang AJ, Malfait AM. An aggrecan fragment drives osteoarthritis pain through Toll-like receptor 2. JCI Insight. 2018;3(6):e95704. doi: 10.1172/jci.insight.95704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li H, Schwartz NB, Vertel BM. cDNA cloning of chick cartilage chondroitin sulfate (aggrecan) core protein and identification of a stop codon in the aggrecan gene associated with the chondrodystrophy, nanomelia. J Biol Chem. 1993;268(31):23504–11. [PubMed] [Google Scholar]
- 48. Cavanagh JA, Tammen I, Windsor PA, Bateman JF, Savarirayan R, Nicholas FW, Raadsma HW. Bulldog dwarfism in Dexter cattle is caused by mutations in ACAN. Mamm Genome. 2007;18(11):808–14. doi: 10.1007/s00335-007-9066-9. [DOI] [PubMed] [Google Scholar]
- 49. Watanabe H, Kimata K, Line S, Strong D, Gao LY, Kozak CA, Yamada Y. Mouse cartilage matrix deficiency (cmd) caused by a 7 bp deletion in the aggrecan gene. Nat Genet. 1994;7(2):154–7. doi: 10.1038/ng0694-154. [DOI] [PubMed] [Google Scholar]
- 50. Krueger RC, Jr, Kurima K, Schwartz NB. Completion of the mouse aggrecan gene structure and identification of the defect in the cmd-Bc mouse as a near complete deletion of the murine aggrecan gene. Mamm Genome. 1999;10(12):1119–25. doi: 10.1007/s003359901176. [DOI] [PubMed] [Google Scholar]
- 51. Watanabe H, Nakata K, Kimata K, Nakanishi I, Yamada Y. Dwarfism and age-associated spinal degeneration of heterozygote cmd mice defective in aggrecan. Proc Natl Acad Sci USA. 1997;94(13):6943–7. doi: 10.1073/pnas.94.13.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li Y, Klena NT, Gabriel GC, Liu X, Kim AJ, Lemke K, Chen Y, Chatterjee B, Devine W, Damerla RR, Chang C, Yagi H, San Agustin JT, Thahir M, Anderton S, Lawhead C, Vescovi A, Pratt H, Morgan J, Haynes L, Smith CL, Eppig JT, Reinholdt L, Francis R, Leatherbury L, Ganapathiraju MK, Tobita K, Pazour GJ, Lo CW. Global genetic analysis in mice unveils central role for cilia in congenital heart disease. Nature. 2015;521(7553):520–4. doi: 10.1038/nature14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tompson SW, Merriman B, Funari VA, Fresquet M, Lachman RS, Rimoin DL, Nelson SF, Briggs MD, Cohn DH, Krakow D. A recessive skeletal dysplasia, SEMD aggrecan type, results from a missense mutation affecting the C-type lectin domain of aggrecan. Am J Hum Genet. 2009;84(1):72–9. doi: 10.1016/j.ajhg.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dateki S, Nakatomi A, Watanabe S, Shimizu H, Inoue Y, Baba H, Yoshiura KI, Moriuchi H. Identification of a novel heterozygous mutation of the Aggrecan gene in a family with idiopathic short stature and multiple intervertebral disc herniation. J Hum Genet. 2017;62(7):717–21. doi: 10.1038/jhg.2017.33. [DOI] [PubMed] [Google Scholar]
- 55. Gleghorn L, Ramesar R, Beighton P, Wallis G. A mutation in the variable repeat region of the aggrecan gene (AGC1) causes a form of spondyloepiphyseal dysplasia associated with severe, premature osteoarthritis. Am J Hum Genet. 2005;77(3):484–90. doi: 10.1086/444401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Czipri M, Otto JM, Cs-Szabo G, Kamath RV, Vermes C, Firneisz G, Kolman KJ, Watanabe H, Li Y, Roughley PJ, Yamada Y, Olsen BR, Glant TT. Genetic rescue of chondrodysplasia and the perinatal lethal effect of cartilage link protein deficiency. J Biol Chem. 2003;278(40):39214–23. doi: 10.1074/jbc.M303329200. [DOI] [PubMed] [Google Scholar]
- 57. Wirrig EE, Snarr BS, Chintalapudi MR, O’Neal JL, Phelps AL, Barth JL, Fresco VM, Kern CB, Mjaatvedt CH, Toole BP, Hoffman S, Trusk TC, Argraves WS, Wessels A. Cartilage link protein 1 (Crtl1), an extracellular matrix component playing an important role in heart development. Dev Biol. 2007;310(2):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Matsumoto K, Shionyu M, Go M, Shimizu K, Shinomura T, Kimata K, Watanabe H. Distinct interaction of versican/PG-M with hyaluronan and link protein. J Biol Chem. 2003;278(42):41205–12. [DOI] [PubMed] [Google Scholar]
- 59. Landauer W. Nanomelia, a lethal nutation of the fowl. J Hered. 1965;56:131–8. doi: 10.1093/oxfordjournals.jhered.a107392. [DOI] [PubMed] [Google Scholar]
- 60. Primorac D, Stover ML, Clark SH, Rowe DW. Molecular basis of nanomelia, a heritable chondrodystrophy of chicken. Matrix Biol. 1994;14(4):297–305. doi: 10.1016/0945-053x(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 61. Harper PA, Latter MR, Nicholas FW, Cook RW, Gill PA. Chondrodysplasia in Australian Dexter cattle. Aust Vet J. 1998;76(3):199–202. doi: 10.1111/j.1751-0813.1998.tb10129.x. [DOI] [PubMed] [Google Scholar]
- 62. Rittenhouse E, Dunn LC, Cookingham J, Calo C, Spiegelman M, Dooher GB, Bennett D. Cartilage matrix deficiency (cmd): a new autosomal recessive lethal mutation in the mouse. J Embryol Exp Morphol. 1978;43:71–84. [PubMed] [Google Scholar]
- 63. Bell L, Juriloff M, Harris MJ. A new mutation at the cmd locus in the mouse. J Hered. 1986;77(3):205–6. doi: 10.1093/oxfordjournals.jhered.a110215. [DOI] [PubMed] [Google Scholar]
- 64. Rambeau P, Faure E, Theron A, Avierinos JF, Jopling C, Zaffran S, Faucherre A. Reduced aggrecan expression affects cardiac outflow tract development in zebrafish and is associated with bicuspid aortic valve disease in humans. Int J Cardiol. 2017;249:340–3. doi: 10.1016/j.ijcard.2017.09.174. [DOI] [PubMed] [Google Scholar]
- 65. Schulz A, Brendler J, Blaschuk O, Landgraf K, Krueger M, Ricken AM. Non-pathological chondrogenic features of valve interstitial cells in normal adult zebrafish. J Histochem Cytochem. 2019;67(5):361–73. doi: 10.1369/0022155418824083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zanin MK, Bundy J, Ernst H, Wessels A, Conway SJ, Hoffman S. Distinct spatial and temporal distributions of aggrecan and versican in the embryonic chick heart. Anat Rec. 1999;256(4):366–80. [DOI] [PubMed] [Google Scholar]
- 67. Shelton EL, Yutzey KE. Tbx20 regulation of endocardial cushion cell proliferation and extracellular matrix gene expression. Dev Biol. 2007;302(2):376–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shelton EL, Yutzey KE. Twist1 function in endocardial cushion cell proliferation, migration, and differentiation during heart valve development. Dev Biol. 2008;317(1):282–95. doi: 10.1016/j.ydbio.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hinton RB, Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98(11):1431–8. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- 70. Lincoln J, Alfieri CM, Yutzey KE. BMP and FGF regulatory pathways control cell lineage diversification of heart valve precursor cells. Dev Biol. 2006;292(2):292–302. [DOI] [PubMed] [Google Scholar]
- 71. Montero JA, Giron B, Arrechedera H, Cheng YC, Scotting P, Chimal-Monroy J, Garcia-Porrero JA, Hurle JM. Expression of Sox8, Sox9 and Sox10 in the developing valves and autonomic nerves of the embryonic heart. Mech Dev. 2002;118(1–2):199–202. doi: 10.1016/s0925-4773(02)00249. [DOI] [PubMed] [Google Scholar]
- 72. Arciniegas E, Neves CY, Candelle D, Parada D. Differential versican isoforms and aggrecan expression in the chicken embryo aorta. Anat Rec A Discov Mol Cell Evol Biol. 2004;279(1):592–600. [DOI] [PubMed] [Google Scholar]
- 73. Del Monte-Nieto G, Ramialison M, Adam AAS, Wu B, Aharonov A, D’Uva G, Bourke LM, Pitulescu ME, Chen H, de la Pompa JL, Shou W, Adams RH, Harten SK, Tzahor E, Zhou B, Harvey RP. Control of cardiac jelly dynamics by NOTCH1 and NRG1 defines the building plan for trabeculation. Nature. 2018;557(7705):439–45. doi: 10.1038/s41586-018-0110-6. [DOI] [PubMed] [Google Scholar]
- 74. Adhikari N, Carlson M, Lerman B, Hall JL. Changes in expression of proteoglycan core proteins and heparan sulfate enzymes in the developing and adult murine aorta. J Cardiovasc Transl Res. 2011;4(3):313–20. doi: 10.1007/s12265-011-9261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cikach FS, Koch CD, Mead TJ, Galatioto J, Willard BB, Emerton KB, Eagleton MJ, Blackstone EH, Ramirez F, Roselli EE, Apte SS. Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. JCI Insight. 2018;3(5):e97167. doi: 10.1172/jci.insight.97167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hsieh Y-T, Liu NM, Ohmori E, Yokota T, Kajimura I, Akaike T, Ohshima T, Goda N, Minamisawa S. Transcription profiles of the ductus arteriosus in Brown-Norway rats with irregular elastic fiber formation. Circulation J. 2014;78(5):1224–33. [DOI] [PubMed] [Google Scholar]
- 77. Dupuis LE, Nelson EL, Hozik B, Porto Sarah C, Rogers-DeCotes A, Fosang A, Kern CB. Adamts5−/− mice exhibit altered aggrecan proteolytic profiles that correlate with ascending aortic anomalies. Arterioscler Thromb Vasc Biol. 2019;39(10):2067–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gao Z, Kim GH, Mackinnon AC, Flagg AE, Bassett B, Earley JU, Svensson EC. Ets1 is required for proper migration and differentiation of the cardiac neural crest. Development. 2010;137(9):1543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23(3):489–94. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 80. Talusan P, Bedri S, Yang S, Kattapuram T, Silva N, Roughley PJ, Stone JR. Analysis of intimal proteoglycans in atherosclerosis-prone and atherosclerosis-resistant human arteries by mass spectrometry. Mol Cell Proteomics. 2005;4(9):1350–7. doi: 10.1074/mcp.M500088-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Novikova OA, Laktionov PP, Karpenko AA. Mechanisms underlying atheroma induction: the roles of mechanotransduction, vascular wall cells, and blood cells. Ann Vasc Surg. 2018;53:224–33. doi: 10.1016/j.avsg.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 82. Strom A, Ahlqvist E, Franzen A, Heinegard D, Hultgardh-Nilsson A. Extracellular matrix components in atherosclerotic arteries of Apo E/LDL receptor deficient mice: an immunohistochemical study. Histol Histopathol. 2004;19(2):337–47. doi: 10.14670/HH-19.337. [DOI] [PubMed] [Google Scholar]
- 83. Kim SM, Huh JW, Kim EY, Shin MK, Park JE, Kim SW, Lee W, Choi B, Chang EJ. Endothelial dysfunction induces atherosclerosis: increased aggrecan expression promotes apoptosis in vascular smooth muscle cells. BMB Rep. 2019;52(2):145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Didangelos A, Yin X, Mandal K, Saje A, Smith A, Xu Q, Jahangiri M, Mayr M. Extracellular matrix composition and remodeling in human abdominal aortic aneurysms: a proteomics approach. Mol Cell Proteomics. 2011;10(8):M111.008128. doi: 10.1074/mcp.M111.008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ruigrok YM, Rinkel GJ, Wijmenga C. The versican gene and the risk of intracranial aneurysms. Stroke. 2006;37(9):2372–4. doi: 10.1161/01.STR.0000236499.55301.09. [DOI] [PubMed] [Google Scholar]
- 86. Sathyan S, Koshy LV, Balan S, Easwer HV, Premkumar S, Nair S, Bhattacharya RN, Alapatt JP, Banerjee M. Association of Versican (VCAN) gene polymorphisms rs251124 and rs2287926 (G428D), with intracranial aneurysm. Meta Gene. 2014;2:651–60. doi: 10.1016/j.mgene.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zholdybayeva EV, Medetov YZ, Aitkulova AM, Makhambetov YT, Akshulakov SK, Kaliyev AB, Talzhanov YA, Kulmambetova GN, Iskakova AN, Ramankulov YM. Genetic risk factors for intracranial aneurysm in the Kazakh population. J Mol Neurosci. 2018;66(1):135–45. doi: 10.1007/s12031-018-1134-y. [DOI] [PubMed] [Google Scholar]
- 88. Suna G, Wojakowski W, Lynch M, Barallobre-Barreiro J, Yin X, Mayr U, Baig F, Lu R, Fava M, Hayward R, Molenaar C, White SJ, Roleder T, Milewski KP, Gasior P, Buszman PP, Buszman P, Jahangiri M, Shanahan CM, Hill J, Mayr M. Extracellular matrix proteomics reveals interplay of aggrecan and aggrecanases in vascular remodeling of stented coronary arteries. Circulation. 2018;137(2):166–83. doi: 10.1161/CIRCULATIONAHA.116.023381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Barallobre-Barreiro J, Oklu R, Lynch M, Fava M, Baig F, Yin X, Barwari T, Potier DN, Albadawi H, Jahangiri M, Porter KE, Watkins MT, Misra S, Stoughton J, Mayr M. Extracellular matrix remodelling in response to venous hypertension: proteomics of human varicose veins. Cardiovasc Res. 2016;110(3):419–30. doi: 10.1093/cvr/cvw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lacerdaa CMR, MacLeaa HB, Duncana CG, Kisidaya JD, Ortona EC. Human myxomatous mitral valves exhibit focal expression of cartilage-related proteins. J Hypertens Cardiol. 2013;1(1):21–30. [Google Scholar]
- 91. Chen YT, Wang J, Wee AS, Yong QW, Tay EL, Woo CC, Sorokin V, Richards AM, Ling LH. Differential microRNA expression profile in myxomatous mitral valve prolapse and fibroelastic deficiency valves. Int J Mol Sci. 2016;17(5):753. doi: 10.3390/ijms17050753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Padang R, Bagnall RD, Tsoutsman T, Bannon PG, Semsarian C. Comparative transcriptome profiling in human bicuspid aortic valve disease using RNA sequencing. Physiol Genomics. 2015;47(3):75–87. doi: 10.1152/physiolgenomics.00115.2014. [DOI] [PubMed] [Google Scholar]
- 93. Didangelos A, Mayr U, Monaco C, Mayr M. Novel role of ADAMTS-5 protein in proteoglycan turnover and lipoprotein retention in atherosclerosis. J Biol Chem. 2012;287(23):19341–5. doi: 10.1074/jbc.C112.350785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kunjathoor VV, Chiu DS, O’Brien KD, LeBoeuf RC. Accumulation of biglycan and perlecan, but not versican, in lesions of murine models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22(3):462–8. doi: 10.1161/hq0302.105378. [DOI] [PubMed] [Google Scholar]
- 95. Marzoll A, Melchior-Becker A, Cipollone F, Fischer JW. Small leucine-rich proteoglycans in atherosclerotic lesions: novel targets of chronic statin treatment? J Cell Mol Med. 2011;15(2):232–43. doi: 10.1111/j.1582-4934.2009.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Olin KL, Potter-Perigo S, Barrett PH, Wight TN, Chait A. Lipoprotein lipase enhances the binding of native and oxidized low density lipoproteins to versican and biglycan synthesized by cultured arterial smooth muscle cells. J Biol Chem. 1999;274(49):34629–36. [DOI] [PubMed] [Google Scholar]
- 97. Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell Res. 2002;12(1):19–32. doi: 10.1038/sj.cr.7290106. [DOI] [PubMed] [Google Scholar]
- 98. Diefenderfer DL, Brighton CT. Microvascular pericytes express aggrecan message which is regulated by BMP-2. Biochem Biophys Res Commun. 2000;269(1):172–8. doi: 10.1006/bbrc.2000.2148. [DOI] [PubMed] [Google Scholar]
- 99. Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110(15):2226–32. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 100. Kirton JP, Crofts NJ, George SJ, Brennan K, Canfield AE. Wnt/beta-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: potential relevance to vascular disease? Circ Res. 2007;101(6):581–9. doi: 10.1161/CIRCRESAHA.107.156372. [DOI] [PubMed] [Google Scholar]
- 101. Neven E, Persy V, Dauwe S, De Schuster T, De Brue ME, D’Haese PC. Chondrocyte rather than osteoblast conversion of vascular cells underlies medial calcification in uremic rats. Arterioscler Thromb Vasc Biol. 2010;30(9):1741–50. doi: 10.1161/ATVBAHA.110.204834. [DOI] [PubMed] [Google Scholar]
- 102. Shen J, Yang M, Jiang H, Ju D, Zheng JP, Xu Z, Liao TD, Li L. Arterial injury promotes medial chondrogenesis in Sm22 knockout mice. Cardiovasc Res. 2011;90(1):28–37. doi: 10.1093/cvr/cvq378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fakhry M, Roszkowska M, Briolay A, Bougault C, Guignandon A, Diaz-Hernandez JI, Diaz-Hernandez M, Pikula S, Buchet R, Hamade E, Badran B, Bessueille L, Magne D. TNAP stimulates vascular smooth muscle cell trans-differentiation into chondrocytes through calcium deposition and BMP-2 activation: possible implication in atherosclerotic plaque stability. Biochim Biophys Acta Mol Basis Dis. 2017;1863(3):643–53. doi: 10.1016/j.bbadis.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 104. El-Maadawy S, Kaartinen MT, Schinke T, Murshed M, Karsenty G, McKee MD. Cartilage formation and calcification in arteries of mice lacking matrix Gla protein. Connect Tissue Res. 2003;44(Suppl 1):272–8. [PubMed] [Google Scholar]
- 105. Derwall M, Malhotra R, Lai CS, Beppu Y, Aikawa E, Seehra JS, Zapol WM, Bloch KD, Yu PB. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(3):613–22. doi: 10.1161/ATVBAHA.111.242594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lei Y, Sinha A, Nosoudi N, Grover A, Vyavahare N. Hydroxyapatite and calcified elastin induce osteoblast-like differentiation in rat aortic smooth muscle cells. Exp Cell Res. 2014;323(1):198–208. doi: 10.1016/j.yexcr.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Doddapattar P, Jain M, Dhanesha N, Lentz SR, Chauhan AK. Fibronectin containing extra domain A induces plaque destabilization in the innominate artery of aged apolipoprotein E–deficient mice. Arterioscler Thromb Vasc Biol. 2018;38(3):500–8. doi: 10.1161/ATVBAHA.117.310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jonsson-Rylander AC, Nilsson T, Fritsche-Danielson R, Hammarstrom A, Behrendt M, Andersson JO, Lindgren K, Andersson AK, Wallbrandt P, Rosengren B, Brodin P, Thelin A, Westin A, Hurt-Camejo E, Lee-Sogaard CH. Role of ADAMTS-1 in atherosclerosis: remodeling of carotid artery, immunohistochemistry, and proteolysis of versican. Arterioscler Thromb Vasc Biol. 2005;25(1):180–5. [DOI] [PubMed] [Google Scholar]
- 109. Kuno K, Okada Y, Kawashima H, Nakamura H, Miyasaka M, Ohno H, Matsushima K. ADAMTS-1 cleaves a cartilage proteoglycan, aggrecan. FEBS Lett. 2000;478(3):241–5. [DOI] [PubMed] [Google Scholar]
- 110. Ren P, Zhang L, Xu G, Palmero LC, Albini PT, Coselli JS, Shen YH, LeMaire SA. ADAMTS-1 and ADAMTS-4 levels are elevated in thoracic aortic aneurysms and dissections. Ann Thorac Surg. 2013;95(2):570–7. doi: 10.1016/j.athoracsur.2012.10.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kern CB, Wessels A, McGarity J, Dixon LJ, Alston E, Argraves WS, Geeting D, Nelson CM, Menick DR, Apte SS. Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies. Matrix Biol. 2010;29(4):304–16. doi: 10.1016/j.matbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Koo BH, Coe DM, Dixon LJ, Somerville RP, Nelson CM, Wang LW, Young ME, Lindner DJ, Apte SS. ADAMTS9 is a cell-autonomously acting, anti-angiogenic metalloprotease expressed by microvascular endothelial cells. Am J Pathol. 2010;176(3):1494–504. doi: 10.2353/ajpath.2010.090655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nandadasa S, Nelson CM, Apte SS. ADAMTS9-mediated extracellular matrix dynamics regulates umbilical cord vascular smooth muscle differentiation and rotation. Cell Rep. 2015;11(10):1519–28. doi: 10.1016/j.celrep.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S, Wight TN, Leduc R, Apte SS. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem. 2003;278(11):9503–13. [DOI] [PubMed] [Google Scholar]
- 115. Halushka MK, Angelini A, Bartoloni G, Basso C, Batoroeva L, Bruneval P, Buja LM, Butany J, d Amati ’G, Fallon JT, Gallagher PJ, Gittenberger-de Groot AC, Gouveia RH, Kholova I, Kelly KL, Leone O, Litovsky SH, Maleszewski JJ, Miller DV, Mitchell RN, Preston SD, Pucci A, Radio SJ, Rodriguez ER, Sheppard MN, Stone JR, Suvarna SK, Tan CD, Thiene G, Veinot JP, van der Wal AC. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association For European Cardiovascular Pathology: II. Noninflammatory degenerative diseases—nomenclature and diagnostic criteria. Cardiovasc Pathol. 2016;25(3):247–57. doi: 10.1016/j.carpath.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 116. Roccabianca S, Ateshian GA, Humphrey JD. Biomechanical roles of medial pooling of glycosaminoglycans in thoracic aortic dissection. Biomech Model Mechanobiol. 2014;13(1):13–25. doi: 10.1007/s10237-013-0482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yin X, Wanga S, Fellows AL, Barallobre-Barreiro J, Lu R, Davaapil H, Franken R, Fava M, Baig F, Skroblin P, Xing Q, Koolbergen DR, Groenink M, Zwinderman AH, Balm R, de Vries CJM, Mulder BJM, Viner R, Jahangiri M, Reinhardt DP, Sinha S, de Waard V, Mayr M. Glycoproteomic analysis of the aortic extracellular matrix in marfan patients. Arterioscler Thromb Vasc Biol. 2019;39(9):1859–73. doi: 10.1161/ATVBAHA.118.312175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Krishnamurthy VK, Evans AN, Wansapura JP, Osinska H, Maddy KE, Biechler SV, Narmoneva DA, Goodwin RL, Hinton RB. Asymmetric cell-matrix and biomechanical abnormalities in elastin insufficiency induced aortopathy. Ann Biomed Eng. 2014;42(10):2014–28. doi: 10.1007/s10439-014. [DOI] [PubMed] [Google Scholar]
- 119. Fava M, Barallobre-Barreiro J, Mayr U, Lu R, Didangelos A, Baig F, Lynch M, Catibog N, Joshi A, Barwari T, Yin X, Jahangiri M, Mayr M. Role of ADAMTS-5 in aortic dilatation and extracellular matrix remodeling. Arterioscler Thromb Vasc Biol. 2018;38(7):1537–48. doi: 10.1161/ATVBAHA.117.310562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Azeloglu EU, Albro MB, Thimmappa VA, Ateshian GA, Costa KD. Heterogeneous transmural proteoglycan distribution provides a mechanism for regulating residual stresses in the aorta. Am J Physiol Heart Circ Physiol. 2008;294(3):H1197–205. doi: 10.1152/ajpheart.01027.2007. [DOI] [PubMed] [Google Scholar]
- 121. Humphrey JD, Tellides G. Central artery stiffness and thoracic aortopathy. Am J Physiol Heart Circ Physiol. 2019;316(1):H169–82. doi: 10.1152/ajpheart.00205.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yasmin Maskari RA, McEniery CM, Cleary SE, Li Y, Siew K, Figg NL, Khir AW, Cockcroft JR, Wilkinson IB, O’Shaughnessy KM. The matrix proteins aggrecan and fibulin-1 play a key role in determining aortic stiffness. Sci Rep. 2018;8(1):8550. doi: 10.1038/s41598-018-25851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rienks M, Barallobre-Barreiro J, Mayr M. The emerging role of the ADAMTS family in vascular diseases. Circ Res. 2018;123(12):1279–81. doi: 10.1161/CIRCRESAHA.118.313737. [DOI] [PubMed] [Google Scholar]
- 124. Melrose J, Whitelock J, Xu Q, Ghosh P. Pathogenesis of abdominal aortic aneurysms: possible role of differential production of proteoglycans by smooth muscle cells. J Vasc Surg. 1998;28(4):676–86. doi: 10.1016/s0741-5214(98)70094-1. [DOI] [PubMed] [Google Scholar]
- 125. Buccheri D, Piraino D, Andolina G, Cortese B. Understanding and managing in-stent restenosis: a review of clinical data, from pathogenesis to treatment. J Thorac Dis. 2016;8(10):E1150–62. doi: 10.21037/jtd.2016.10.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Fang M, Alfieri CM, Hulin A, Conway SJ, Yutzey KE. Loss of β-catenin promotes chondrogenic differentiation of aortic valve interstitial cells. Arterioscler Thromb Vasc Biol. 2014;34(12):2601–8. doi: 10.1161/ATVBAHA.114.304579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Thalji NM, Hagler MA, Zhang H, Casaclang-Verzosa G, Nair AA, Suri RM, Miller JD. Nonbiased molecular screening identifies novel molecular regulators of fibrogenic and proliferative signaling in myxomatous mitral valve disease. Circ Cardiovasc Genet. 2015;8(3):516–28. doi: 10.1161/CIRCGENETICS.114.000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hulin A, Moore V, James JM, Yutzey KE. Loss of Axin2 results in impaired heart valve maturation and subsequent myxomatous valve disease. Cardiovasc Res. 2017;113(1):40–51. doi: 10.1093/cvr/cvw229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, Firulli AB, Molkentin JD, Markwald R, Conway SJ. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008;102(7):752–60. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Christensen G, Herum KM, Lunde IG. Sweet, yet underappreciated: proteoglycans and extracellular matrix remodeling in heart disease. Matrix Biol. 2019;75–6:286–99. doi: 10.1016/j.matbio.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 131. Vistnes M, Aronsen JM, Lunde IG, Sjaastad I, Carlson CR, Christensen G. Pentosan polysulfate decreases myocardial expression of the extracellular matrix enzyme ADAMTS4 and improves cardiac function in vivo in rats subjected to pressure overload by aortic banding. PLoS ONE. 2014;9(3):e89621. doi: 10.1371/journal.pone.0089621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Oller J, Mendez-Barbero N, Ruiz EJ, Villahoz S, Renard M, Canelas LI, Briones AM, Alberca R, Lozano-Vidal N, Hurle MA, Milewicz D, Evangelista A, Salaices M, Nistal JF, Jimenez-Borreguero LJ, De Backer J, Campanero MR, Redondo JM. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat Med. 2017;23:200–12. doi: 10.1038/nm.4266. [DOI] [PubMed] [Google Scholar]
- 133. Yang BB, Zhang Y, Cao L, Yang BL. Aggrecan and link protein affect cell adhesion to culture plates and to type II collagen. Matrix Biol. 1998;16(9):541–61. [DOI] [PubMed] [Google Scholar]
- 134. Nandadasa S, Szafron JM, Pathak V, Murtada S-I, Kraft CMO ’, Donnell A, Norvik C, Hughes C, Caterson B, Domowicz MS, Schwartz NB, Tran-Lundmark K, Veigl M, Sedwick D, Philipson EH, Humphrey JD, Apte SS. Vascular dimorphism ensured by regulated proteoglycan dynamics favors rapid umbilical artery closure at birth. bioRxiv. Published electronically July 3, 2020. doi: 10.1101/2020.07.02.184978. [DOI] [PMC free article] [PubMed] [Google Scholar]