Abstract
Versican is a large chondroitin sulfate/dermatan sulfate proteoglycan belonging to the aggrecan/lectican family. In adults, this proteoglycan serves as a structural macromolecule of the extracellular matrix in the brain and large blood vessels. In contrast, versican is transiently expressed at high levels during development and under pathological conditions when the extracellular matrix dramatically changes, including in the inflammation and repair process. There are many reports showing the upregulation of versican in cancer, which correlates with cancer aggressiveness. Versican has four classical splice variants, and all the variants contain G1 and G3 domains at N- and C-termini, respectively. There are two glycosaminoglycan attachment domains CSα and CSβ. The largest V0 variant contains both CSα and CSβ, V1 contains CSβ, V2 contains CSα, and the shortest G3 variant has neither of them. Versican degradation is initiated by cleavage at a site in the CSβ domain by ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) proteinases. The N-terminal fragment containing the G1 domain has been reported to exert various biological functions, although its mechanisms of action have not yet been elucidated. In this review, we describe the role of versican in inflammation and cancer and also address the biological function of versikine.
Keywords: extracellular matrix, glycosaminoglycan, hyaluronan, matrikine, microenvironment, proteoglycan
Introduction
The extracellular matrix (ECM) is a three-dimensional network of extracellular macromolecules, and it provides structural and biochemical support to the surrounding cells and regulates cell behavior.1,2 Formation of the ECM is essential for different cellular processes, including proliferation, migration, and differentiation. The ECM consists of fibrous and non-fibrous components. Whereas the fibrous component contains collagens, and fibrillin-1 and fibrillin-2, the non-fibrous component contains molecules such as proteoglycans (PGs),3,4 glycosaminoglycans (GAGs), and other glycoproteins. PGs of the ECM type include aggrecan (Acan)/lectican family members and small leucine-rich proteoglycans (SLRPs).5 Of the Acan/lectican family members, both Acan6 and versican (Vcan)7–10 have a large core protein with several domains and contribute to the formation of ECM. In contrast, SLRPs regulate cell behavior by modulating cell surface receptors.11,12 In this review, we will discuss the structure, function, and turnover of Vcan, focusing on inflammation and cancer invasion. In addition, we will also address the role of Vcan fragment, named versikine, generated by a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs).
Core Protein Structure and Variant Forms
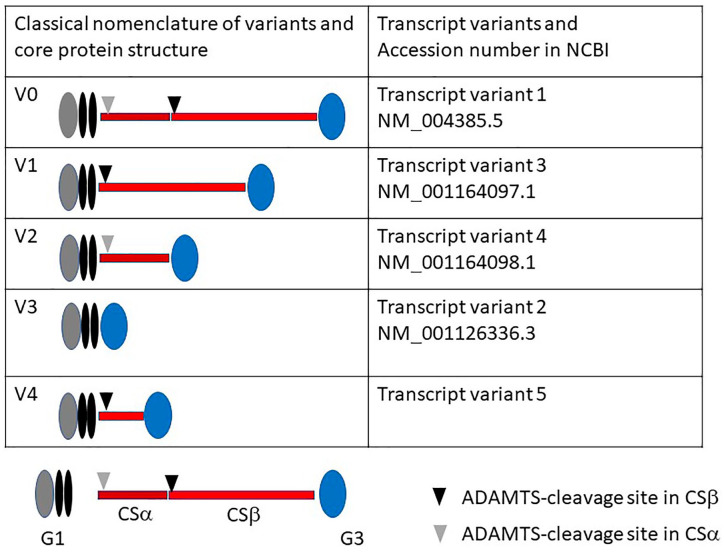
Vcan is a large chondroitin sulfate (CS)/dermatan sulfate (DS) PG, secreted and incorporated into the ECM. Vcan exists in five different isoforms generated by alternative splicing. There were four classical variant forms: V0 (G1-CSα-CSβ-G3), V1 (G1-CSβ-G3), V2 (G1-CSα-G3), and V3 (G1-G3). Later, another variant form, V4 (G1 portion of CSβ-G3), was found in human breast cancer.13 These splice variants have been registered differently in other sites (for comparison, see Fig. 1). Usually, V0 and V1 are expressed when tissue formation or remodeling occurs rapidly during development and in the tissue repair process. In contrast, V2 expression is restricted to adult central nervous systems. In the brain, both V0 and V1 are expressed during development, and after birth, V2 initiates and increases.14 Studies using Vcantm1Dzim homozygote mice, whose Vcan genome lacks exon 7 encoding the CSα domain, have revealed the relevance of the V2 variant. V2 is localized at the node of Ranvier and is necessary for the ECM structure of the node, although the clustering of nodal sodium channels and paranodal structure is normal.15 As Vcantm1Dzim homozygote mice lack both V0 and V2 variants, the contribution of V0 is evaluated in tissues where V2 is absent. Detailed analysis of their developing heart has revealed ventricular septal defect (VSD), smaller valve leaflets, decreased myocardialization, and alteration of pulmonary and aortic outflow tracts,16 indicating contributions of individual variants. The expression of the V3 variant is mainly analyzed by RT-PCR. As V3 lacks GAG chains, anion exchange chromatography for the isolation of PGs is unavailable. The presence of V3 protein will be evaluated by recognition of a band at 72 to 80 kDa by both G1- and G3-specific antibodies. The V4 variant consists of G1, an N-terminal portion of CSβ, and G3 domains. V4 is found in human breast cancer and is upregulated in breast cancer tissues (Fig. 1).
Figure 1.
Vcan splice variants. Four classical variants V0, V1, V2, and V3 and another variant V4 are depicted. These variants are named differently in the database of NCBI. The N-terminal globular domain G1 consists of A, B, and B’ subdomains. The C-terminal globular domain G3 consists of two EGF-like, a lectin-like, and complement reactive protein (CRP)-like subdomains. There are two chondroitin sulfate binding domains, CSα and CSβ. V0 contains both CSα and CSβ, V1 contains CSβ, V2 contains only CSα, and V3 has neither of the two. V4 has an N-terminal region of CSβ. Abbreviations: ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs; NCBI, National Center for Biotechnology Information.
Functional Domains of Vcan
As all the splice variants of Vcan contain both G1 and G3 domains, as well as other Acan/lectican family members, they appear to exert essential functions commonly shared among the family members. The CS domains are different from their variant forms. The largest V0 contains both CSα and CSβ domains, whereas V3 lacks CS domains. As CS domains contain proteinase cleavage sites as described below, the extent of Vcan degradation may vary depending on variant forms (Fig. 1).
N-terminal G1 Domain
The G1 domain comprises A, B, and B′ subdomains. The A subdomain forms an Ig-like structure, and each of B and B′ subdomains forms a “link module,” a module for specific binding to hyaluronic acid (HA). Whereas TSG6 and CD44 contain only one link module and exhibit HA-binding ability,17 the G1 domain of Acan or Vcan, and hyaluronan and proteoglycan link protein-1 (HAPLN-1) require tandemly aligned two link modules18,19 for HA binding. Acan or Vcan binds both HA and HAPLN1 and forms stable PG aggregates, where HAPLN1 enhances the binding of G1 and HA. With high homology among Acan G1, Vcan G1, and HAPLN1, HAPLN1 binds to Acan G1 at the A subdomain, whereas it does bind to Vcan G1 at the B-B′ stretch.19 Binding of Vcan to HA leads to a rich pericellular matrix.20,21 Haploinsufficient Vcan∆3/∆3 fibroblasts whose Vcan binds to HA with less affinity attain premature senescence through constitutive activation of EKR1/2 via CD44.22 Therefore, the action of the G1 domain appears to be mediated by HA and cell surface HA-receptors.
C-terminal G3 Domain
The G3 domain interacts with several ECM molecules, including tenascins, fibrillins, and fibulin-1 and fibulin-2.23 G3 seems to regulate the signaling of transforming growth factor beta (TGF-β) and bone morphogenic proteins through its interacting molecules, including fibrillins. The mechanisms by which Vcan regulates the signaling of these growth factors remain to be elucidated. The G3 domain contains two epidermal growth factor (EGF)-like repeats, which may exhibit biological activity similar to that of EGFs.
CS-binding Domains
Vcan has two CS-attachment domains, CSα and CSβ. Although these domains contain approximately 20 CS-attachment sites, the number of CS chains in native Vcan has not yet been determined. In several experiments performed for our Vcan conditional knockout studies, chondroitinase ABC treatment did not cause any similar abnormalities,24 and overexpression of the V3 variant rescues the phenotype,25 suggesting a minimal role of CS chains in Vcan. Although the in vivo and in vitro function of CS has been studied extensively,26 the function of CS chains in the Vcan core protein is still unclear.
Expression Patterns as Both Structural and Provisional Matrix Molecules
Vcan as a Structural Macromolecule in Adult Tissues
Vcan is expressed in both transient and stable modes, based on tissue and timing. Immunohistochemical analysis of human adult tissues revealed their distributions in epithelia, loose connective tissue, perichondrium, smooth muscle cells except tunica media of muscular arteries, and neural tissues.27 Indeed, Vcan is extracted and purified from the adult aorta and brain, indicating its abundance in these tissues.14,19,28 Whereas Vcan is present in the human ascending aorta, it is barely expressed in mice.29 In large and thick ascending aorta of humans and bovines, Vcan may contribute to water retention together with hyaluronan in the aorta.
Vcan in Development
Many reports have shown the involvement of Vcan in tissue morphogenesis and organogenesis. Analysis of cumulative Vcan expression in lung, liver, heart, and brain by embryonic days demonstrates its highest expression at E13 when that of Vcan fragment is low.30
The role of Vcan in cardiac development has been studied extensively. The hearts of Vcan-null hdf/hdf mice31 and haploinsufficient Vcan∆3/∆3 mice with C57Bl6 background32 exhibit a lack of cardiac jelly at ~E9.5, indicating its essential role in cardiac wall formation. Vcan generates a space, that is, provisional cardiac wall, together with HA, where endocardial cells migrate into space and differentiate into myocardial cells. The localization of Vcan in the developing heart moves rapidly, suggesting that Vcan plays various roles at different time points.33 The heart of Vcan∆3/∆3 mice with mixed background forms cardiac jelly and develops VSD due to impaired migration of endocardial cells and their myocardial cell differentiation.32 Milder VSD is observed in hdf heterozygotes and HAPLN1-null mice,34 suggesting the presence of PG aggregates of Vcan, HA, and HAPLN1. Stable deposition of the aggregates may contribute to ventricular septal formation.
Chondrocyte cell lines such as ATDC5 and N1511 show sequential expression patterns of Vcan and Acan.13 In the early stage of their chondrocyte differentiation, Vcan is transiently expressed and incorporated into the ECM, and it disappears while being replaced with Acan. Micromass culture of limb bud mesenchymal cells obtained from hdf/hdf embryos fails to differentiate into chondrocytes, suggesting an essential role for Vcan in chondrogenesis.35 Prx1-Cre; Vcanflox/flox (Prx1-Vcan) mice, which lack Vcan expression in the limb bud, achieve endochondral ossification, indicating that Vcan is not crucial for cartilage development.24 These mice display distorted digits due to delayed chondrocyte differentiation and impaired joint formation. Whereas the joint interzone of control mice contains adequate levels of TGF-β colocalized with Vcan, that of Prx1-Vcan mice exhibits decreased levels of TGF-β and the absence of Vcan. Vcan∆3/∆3 mice with mixed background exhibit thin dermis with scant collagen fibers and a reduced number of fibroblasts, suggesting that Vcan facilitates fibroblasts proliferation and collagen fiber formation.36 Taken together, these observations in developing heart, joints, and dermis strongly suggest that Vcan forms a space for cell migration and differentiation, and regulates local concentrations of growth factors and cytokines.
Vcan in Inflammation
In an early stage of inflammation, inflammatory cells infiltrate into the inflamed lesion and secrete various proteinases and cytokines/chemokines. These proteinases degrade the ECM, and cytokines/chemokines activate stromal fibroblasts and endothelial cells, leading to granulation and tissue repair. Vcan is expressed in activated fibroblasts, endothelial cells, and infiltrating macrophages. There are at least two major issues. One is the mechanism(s) of Vcan action. Vcan may regulate inflammation by binding to HA or by mediating signaling of cytokines/growth factors, including TGF-β. Another issue is the source of Vcan. In vivo models of inflammation generated in conditional Vcan knockout mice will clarify the role of Vcan expressed by individual cell types. Recent reports on Vcan function in inflammation are discussed below.
The role of Vcan in inflammation, especially of lung, has been extensively studied. Although Vcan is constitutively expressed at low levels in the lungs of healthy adults, it is rehashed and accumulates in many lung diseases.30,37,38 Vcan and HA increase in the lungs of mice infected with gram-negative bacteria.30,39 Analysis of Vcan expression in bone marrow–derived macrophages and conditional knockout mice whose Vcan expression is depleted in macrophages demonstrated that Vcan takes fine control in innate immunity. Toll-like receptor (TLR)/Trif/type I IFN signaling pathway upregulates Vcan, which induces the production of two critical anti-inflammatory molecules interferon-β and interleukin (IL)-10.40 In a mouse model of lipopolysaccharide-induced acute lung injury (ALI), Vcan V1 was upregulated, and its knockdown aggravated ALI via the activation of TLR2-NF-κB.41 Polyinosinic–polycytidylic acid (poly(I:C))-induced lung inflammation in a tamoxifen-inducible Vcan-deficient mouse model showed a reduced number of infiltrating leukocytes and decreased HA–Vcan matrix formation.42
As Vcan is involved in HA matrix formation, Vcan secreted by monocyte/macrophages and stromal fibroblasts may regulate inflammation via the HA matrix.43 Coculturing of human CD4+ T-cells and human lung fibroblasts led to an increase in the deposition of HA with cable-like structures within the ECM, together with increased expression and accumulation of TSG-6 but not Vcan, which promotes the adhesion of monocytes in the ECM.44 Induction of poly(I:C) in cultured human lung fibroblasts stimulates Vcan and HA-rich ECM formation with increased capacity for monocyte adhesion.45 Cockroach antigen–sensitized murine models of allergic asthma demonstrated increased subepithelial accumulation of Vcan together with HA in the airways and increased inflammatory cell infiltration. This suggests that Vcan plays an essential role in the establishment of airway inflammation in asthma.46 Vcan accumulates in vascular lesions of pulmonary arterial hypertension. In vitro analysis of human pulmonary artery smooth muscle cells demonstrates that the level of Vcan expression is regulated by mechanical stress and hypoxia.47
Vcan in Cancer
Vcan plays important roles in both malignant transformation and tumor progression. Increased Vcan expression has been observed in a wide range of malignant tumors, and it is associated with both cancer relapse and poor patient outcomes in the breast, prostate, and other cancer types. As inflammation is often associated with tumor progression, Vcan may be involved in an inflammatory tumor microenvironment. To date, the majority of studies indicate that Vcan facilitates tumor growth and metastasis. However, the results appear to be dependent on the experimental systems, such as the means of overexpression and inhibition of Vcan, cell lines used, and involvement of stromal fibroblasts and endothelial cells. As described above, Vcan has four functional domains/motifs. The domain/motif that affects tumor cell behavior may depend on the local microenvironment. Of the various cancers that originated from various tissues, some tumors have been extensively studied, partly because the cancer cells or the originating tissues express Vcan. Here, we focus on gliomas and cancers of liver, prostate, and ovaries.
Vcan in Gliomas
Several reports show the involvement of Vcan in gliomas that originated from glial cells of the brain or the spine. In primary brain tumors, HA and many other ECM such as vitronectin, osteopontin, tenascin-C, secreted protein acidic and rich in cysteine (SPARC), brevican, and Vcan are upregulated within both the tumor stroma and at the advancing edge of the tumor.48–51 An in vitro study shows that Vcan V0/V1 interacts with TGF-β2 and promotes proliferation and migration of high-grade gliomas.52 Vcan secreted from glioma promotes tumor expansion through glioma-associated microglia/macrophage TLR2 signaling.53 Yun-Yan Xiang et al.54 demonstrated that the Vcan G3 domain, abundant in human brain astrocytoma, regulates neurite growth and synaptic transmission of hippocampal neurons by activation of EGF receptors (EGFRs) via EGF-like motifs. The degree of neovascularization in high-grade glioblastoma is a histological indicator of malignancy and patient prognosis. In vivo experiments and coculturing the V2-transfected U87 cells with YPEN rat endothelial cells demonstrated that V2-expressing cells have a high affinity to endothelial cells, facilitating the formation of tube-like structures in Matrigel.55 Glioma grows in the central nervous system, where collagen fibers are almost absent. In vivo studies of the effects of Vcan on glioma will be advantageous as it could exclude the influence of such fiber components.
Vcan in Liver Cancers
Regarding liver cancers, microRNA (miRNA) that binds to the Vcan 3′-untranslated region (3′-UTR) has been shown to regulate cancer cell behavior. Ectopic expression of the Vcan 3′-UTR in transgenic mice and the liver cancer cell line HepG2 increased expression of V0 and V1, and induced the development of hepatocellular carcinoma by regulating miRNA activity.56 Vcan was identified as a novel direct miR-126a-5p target that induces telomere shortening, bone mesenchymal stem cell (BMSC) senescence, and NF-κB pathway activation that contributes to senescence-associated inflammation and hepatic metabolism. The miR-126a-5p-Vcan axis may have profound effects on hepatic aging and function, and BMSCs may have therapeutic effects against cirrhosis.57 Vcan transcriptional regulation may affect liver cancer cell behavior. Forkhead transcription factor forkhead box Q1–mediated V1 expression promotes hepatocellular carcinoma metastasis.58
Vcan in Prostate Cancers
Prostate cancer (PC) is one of the most common malignancies in the world. Over one fourth of the patients develop PC with a biochemical recurrence after radical surgery,59 which becomes castration-resistant prostate cancer (CRPC). Thalidomide in combination with docetaxel (DT therapy) improved the prognosis of patients with CRPC also in docetaxel-resistant CRPC. Generation of docetaxel-resistant PC3 (DR-PC3) cells and their in vitro analysis identified Vcan as a sophisticated biomarker for use in CRPC treatment. Vcan expression increases with the acquisition of docetaxel resistance and decreases after thalidomide treatment. Furthermore, Vcan small interfering RNA (siRNA) could substitute for thalidomide in docetaxel-resistant PC3 cells. Therefore, Vcan has a clinical role as a biomarker for PC therapy, and its siRNA expression may provide a clue to its treatment.60 Several studies have demonstrated that high levels of HA and Vcan in the peritumoral stroma are associated with the metastatic spread of clinical PC. Analysis using human PC cell lines LNCaP, PC3, and DU145 demonstrated that PC cells can recruit ECM components produced by stromal cells that formed HA- and Vcan-rich pericellular matrix, and this matrix facilitates cell motility and leads to metastasis.61 Increased accumulation of Vcan in the primary cultures of prostatic fibroblasts is induced by PC cell–derived TGF-β1. Remodeling of the ECM with Vcan may be a mechanism by which PC cells control their microenvironment to facilitate local invasion and metastasis.62 Elevated levels of Vcan expression during the progression of the early stage of PC may be a useful marker of disease progression in patients.63
Vcan in Ovarian Cancers
Under the physiological conditions of the ovary, Vcan helps the expansion of the cumulus oophorus in the preovulatory period and therefore plays an important role during folliculogenesis as a component of the granulosa layer.64 Increased levels of Vcan that correlated with increased HA expression were also observed in the ECM of the epithelial ovarian cancer (EOC), suggesting that they may assist EOC survival and spread.65,66 In vitro studies have demonstrated the production of Vcan by cancer-associated fibroblast in the tumor microenvironment, which modulates ovarian cancer (OC) invasion by TGF-β.67 cDNA microarray analysis demonstrated that downregulation of FOX2 and upregulation of Vcan gene by miR-590-3p promote OC growth and metastasis.68 Elevated levels of Vcan expression in the cancer microenvironment regulate TGF-β signaling and affect cancer initiation, development, and metastasis. LY2157299 monohydrate (LY), an inhibitor of TGF-β receptor I (TGFBRI), was examined for its ability to inhibit OC growth both in high-grade serous ovarian cancer (HGSOC) cell lines and in xenograft models. In vitro study revealed that LY alone inhibited the proliferation, migration, and invasion of HGSOC cells. In vivo study demonstrated LY-blocked fibroblast activation and delayed tumor growth and suppressed ascites formation by TGF-β1. Analysis of OVCAR8 xenograft specimens confirmed that LY downregulates the expression of stromal collagen type XI chain 1 (COL11A1) and Vcan.69
Mechanisms of Vcan Function in Cancer Cell Behavior
As described above, accumulating studies have proposed several mechanisms of Vcan function in cancer cell behavior. First, Vcan produces and expands HA-rich pericellular matrix, reduces cell–cell contact, and facilitates cell migration. Cell migration–inducing hyaluronidase (CEMIP)-1 is an inducer of cancer cell migration, highly expressed in human cancers and associated with poor prognosis. Its characterization revealed that it is present in the endoplasmic reticulum, and its binding to binding immunoglobulin protein (BiP) increases cytoplasmic calcium levels and activates protein kinase C, resulting in enhanced cell migration.70 Further studies have shown that CEMIP promotes epithelial–mesenchymal transition,71,72 and its repression inhibits colon cancer cell proliferation by attenuating Wnt signaling73 and depolymerizes HA via the cell membrane–associated clathrin-coated pit endoplasmic pathway.74 The presence of HA-degrading activity in cell migration–inducible protein sounds contradictory to the concept that HA-rich pericellular matrix promotes cell migration. Further studies on the role of HA in cancer cell behavior remain to be performed. Second, Vcan regulates TGF-β signaling. So far, this regulation is thought to be via fibrillins/latent transforming growth factor-beta binding proteins. However, the precise mechanism of regulation of TGF-β signaling by Vcan has not been elucidated yet. Whether TGF-β signaling facilitates or inhibits cell proliferation depends on cancer cell types. The effects of Vcan on cancer cells may be affected by the levels of their response to TGF-β. Third, the EGF repeat in the G3 domain may bind to the EGFR directly, aggravating cancer cell behavior.75,76 Again, the extent of the effects of Vcan appears to be dependent on the expression of EGFR on cancer cells.
The tumor microenvironment contains cancer cells, stromal fibroblasts, endothelial cells, and inflammatory cells such as macrophages and T-cells, and any cell type except T-cells may express substantial levels of Vcan. To determine the effects of Vcan synthesized by host stroma, we performed in vivo cancer cell implantation to Vcan conditional knockout mice. Vcan-deficient QRsP11 fibrosarcoma cells were subcutaneously implanted together with Cre-expressing adenovirus or PBS. QRsP11 cells grew faster and formed a larger tumor mass during the depletion of stromal Vcan expression, and overexpression of V3 in QRsP cells reversed this phenomenon. These results indicate that host stromal Vcan facilitates cancer cell proliferation. Differences in angiogenesis occurred after the alteration of the tumor mass size, and similar experiments using endothelial cell–specific Vcan conditional knockout mice showed a similar tumor mass size. These results indicate that Vcan depletion of stromal cells rather than endothelial cells has a profound effect on cancer proliferation. Interestingly, a similar effect was observed for B16F1 melanoma cells but not for Lewis lung carcinoma (LLC) cells.25 Silencing of Vcan in B16F1 effectively inhibits cell proliferation but not in LLC cells.77 As LLC cells express much higher levels of Vcan than B16F1 cells, silencing Vcan in LLC may be inadequate for inhibition of cell proliferation. In tumor implant studies, stromal Vcan is accumulated around the capillary at the tumor periphery. When implanted into Vcan hdf/+ mice, tumor size was reduced, indicating that Vcan contributes to tumor angiogenesis.78
Physiological and Pathological Relevance of Vcan Proteolysis
Biochemical studies revealed that many proteinases cleave Vcan core protein. For example, matrix metalloproteinase (MMP)-1,79 MMP-2,80 MMP-3,80 MMP-7,81 and MMP-980 have been shown to degrade Vcan in vitro. Plasmin degrades Vcan core protein in vitro.82 Of the ADAMTS family proteinases,83–85 six members, that is, ADAMTS-1, -4, -5, -9, -15, and -20, are known to cleave Vcan, and therefore they are named “versicanases.”86 Whereas Vcan core protein is susceptible to different proteinases, a series of evidence support that cleavage at a specific site by ADAMTSs is crucial for development and diseases.
Impact of the Initial Cleavage Site of Vcan Core Protein
The initial cleavage site of the Vcan core protein is located at the N-terminal region of the CSβ domain, present in V0, V1, and V4 (Glu1428-Ala1429 in V0, and Glu441-Ala442 in V1 and V4 variants in humans, respectively). Both V0 and V2 variants in brain contain another ADAMTS cleavage site at Glu405-Gln406.87 The initial report identifying glial hyaluronate-binding protein as an N-terminal fragment of Vcan showed different N-terminal fragments.79 Further analysis revealed 11 species, all of which contained the G1 domain. The major species of 64 kDa had a C-terminus at Glu405,87 presumably generated by ADAMTS-4. These results indicate that the ADAMTS cleavage site is the most susceptible to V2 core protein and that the G1 domain is resistant to proteinases.
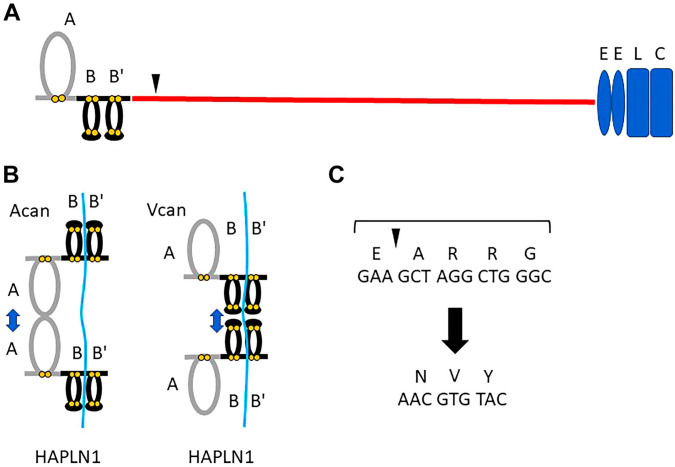
To evaluate the relevance of the initial cleavage site in Vcan turnover, we generated knockin mice, V1R, whose Vcan is mutated at the initial cleavage site in V0 and V1 (Fig. 2A and C). V1R homozygote (R/R) mice show a decreased number of littermates. When analyzed, some embryos are small, and the others exhibited organ hemorrhage. Some mice exhibit syndactyly of hindlimbs, concordant with the previous report demonstrating that versikine generated by Vcan cleavage with ADAMTSs is required for apoptosis of mesenchymal cells in the interdigit.88 These observations indicate that the initial cleavage of Vcan at this site is necessary for the normal development of blood vessels and digit formation.
Figure 2.
Schematic diagram of V1 core protein. (A) G1 consists of A subdomain, B and B’ subdomains, CSβ contains an initial cleavage site susceptible to ADAMTS-1,4,5,9,15, and 20, termed versicanases. (B) Scheme of interactions among hyaluronan, Vcan G1, and HAPLN1, in comparison with Acan G1 and HAPLN1. By biochemical analysis, the B-B’ stretch is a minimal segment for HA-binding in Acan and Vcan G1 domains, and HAPLN1. Whereas Acan G1 binds to HAPLN1 at both A subdomains, Vcan G1 binds HAPLN1 at both B-B’ stretches. Although Vcan A subdomain enhances the HA-binding affinity of Vcan G1 with HA, it is not required for the HAPLN1 interaction. C, Amino acid sequence of the initial ADAMTS-cleavage site. V1R mice were generated by CRISPR/Cas9 technology. The mutated sequence of both amino acid and DNA is shown. Abbreviation: HAPLN, hyaluronan and proteoglycan link protein.
Although most V1R R/R mice grow normally except for syndactyly, they show faster wound healing than wild-type mice. When a full-thickness defect was made on the back of mice, the wound of R/R mice closes faster than that of wild-type mice. Histologically, the wound of R/R mice reveals higher levels of intact Vcan and a negligible level of Vcan fragment as assessed by a neoepitope antibody, whereas the wound of wild-type mice exhibits both intact and cleaved Vcan. This result suggests that Vcan is cleaved by versicanases in vivo at the site in wounds of wild-type mice. By time-course analysis of Vcan deposition in the wound, Vcan in R/R accumulates more rapidly with a peak at day 7 and rapidly decreases to ~40% at day 9, whereas Vcan in wild-type continues to accumulate in the wound. These deposition patterns suggest that Vcan lacking the initial cleavage site is rapidly degraded by other proteinases during days 7 to 9, and the N-terminal fragment undetectable by neoepitope antibodies may act as versikine. Histological and immunohistochemical analysis revealed activation of TGF-β signaling and an increased number of myofibroblasts positive for α-smooth muscle actin and periostin in the wound of R/R mice. When dermal fibroblasts were isolated and analyzed in cell culture, R/R fibroblasts proliferate faster, and some of them differentiate into myofibroblasts without TGF-β treatment. Taken together, Vcan accumulation enhances TGF-β signaling, which leads to proliferation and differentiation of fibroblasts to myofibroblasts, contributing to wound healing. In the wound of R/R mice, inflammatory cell infiltration is remarkable compared with that of wild-type mice. Immunostaining and quantitative RT-PCR reveal an increase in M1 macrophages, the mechanisms of which are to be elucidated. In R/R mice, faster wound healing was already observed at day 2, when Vcan expression was upregulated together with TGF-β signaling. As versikine was detected at day 2 in the wound of wild-type mice and therefore its effect cannot be excluded, faster wound healing in R/R mice is likely due to increased Vcan expression in the wound. Studies, including versikine treatment or its local expression, would lead to an understanding of the precise mechanisms of action of Vcan and versikine.
Functional Vcan Fragment as Versikine
When proteinases degrade an ECM molecule, a released fragment may function as a biologically active molecule, thereby being termed “matrikines” and “matricryptins.”89 The examples are endostatin from type XVIII collagen, tumstatin from type IV collagen, and endorepellin from perlecan.90 There are an increasing number of reports demonstrating the biological function of the N-terminal fragment of Vcan,91 termed “versikine.” Combined knockout mice of ADAMTS-5, ADAMTS-9, and ADAMTS-20 genes exhibit syndactyly due to impaired apoptosis by an accumulation of Vcan, and local treatment with versikine rescues the phenotype, indicating that versikine leads to cellular apoptosis.88 In human multiple myeloma, Vcan serves as a potent diagnostic marker92 and regulates inflammatory milieu in the myeloma niche via TLR2/6 on the surface of myeloma-associated macrophages.93 Further studies revealed the involvement of Vcan cleavage in the disease progression.94 Vcan is abundantly expressed by myeloma-associated macrophages, and Vcan itself leads to tolerogenic polarization of antigen-presenting cells. Versikine is generated by stroma-derived ADAMTS1 and induces early expression of IL-1β and IL-6 by myeloma-associated macrophages. Therefore, versikine may be a key molecule of a damage-associated molecular pattern, facilitating immune sensing. Besides, tissue microarray analysis of colorectal cancer with chronic inflammation revealed a good correlation of CD8+ T-cell infiltration with high levels of versikine and low levels of intact Vcan. In this case, versikine facilitates the generation of conventional dendritic cells from bone marrow–derived precursor cells.95
There are two questions regarding versikine, namely, (1) the mechanisms of action and (2) the duration and in vivo localization of its activity. Versikine derived from the V1 variant contains the G1 fragment and a short stretch (~94 amino acid residue) of CSβ. As the primary function of the G1 domain is to bind to HA and increase HA-rich pericellular matrix, the activity of versikine appears to be through binding to HA. However, HA levels in the myeloma niche have not yet been determined. Like versikine, the G1 domain generated from other Acan/lectican family members and HAPLNs with homology to the G1 domain may have a similar function. Huynh et al.96 found that HAPLN1, secreted from bone marrow stromal cells, leads to constitutive NF-κB activity in myeloma cells, providing them with resistance to bortezomib, a proteasome inhibitor that inhibits multiple myeloma growth. Domain functional analysis demonstrated that either B or B′ fragment, showing a link module/proteoglycan tandem repeat, has adequate activity, and their NF-κB activation does not involve HA binding. As the B or B′ fragment was expressed in Escherichia coli in their study, the fragment may lack the correct tertiary structure. The primary structure, that is, amino acid sequence shared between B and B′, may be crucial for the action. Similarly, versikine may act with the primary sequence of B or B′, independent of HA interaction. HAPLN1 binds to the Vcan G1 domain at the B-B′ stretch,19 whereas it binds to Acan G1 at the A subdomain (Fig. 2B). Versikine may bind and sequester HAPLN1 from myeloma cells and inhibit HAPLN1 activity. So far, the expression of HAPLN1 seems to be restricted to cartilage, perineuronal nets, and endocardial cushion of developing heart. Nevertheless, versikine may exert function by interacting with HAPLN1 in tissues where these two molecules are colocalized.
As versikine is generated from Vcan, the expression level of Vcan determines the levels of versikine and proteinases that cleave Vcan core protein. In the tissue repair process, versikine will be generated until Vcan disappears when tissue repair is completed. If versikine exhibits rapid turnover like cytokines and growth factors, the duration of its action may be limited to a short period, which appears insufficient for maintaining the microenvironment. Treatment of cultured dermal fibroblasts with recombinant Vcan G1 domain has shown to alter the structure of HA into an aggregated cable-like structure, which bridges fibroblasts,97 where endogenous intact Vcan V0/V1 does not prevent the cable formation. Interestingly, G1 remains on the HA cables up to 4 weeks, presumably maintaining the HA cable structure. Vcan G1 is present not only under the tissue repair process but also in the mature dermis. In the pressure ulcers generated through chronic refractory wounds, the cleaved G1 domain colocalizes with a heavy chain (HC): HA complex (also termed as serum-derived HA-associated protein [SHAP]–HA complex). Vcan G1 directly binds to two HCs and forms a G1–HC (SHAP)–HA complex, where Vcan G1 is in the form of aggregates, facilitating the expansion of the pericellular HA matrix.98 With these observations, versikine may assemble individual HA chains to form a cable structure and alter the pericellular microenvironment. This process may involve the incorporation of HC and the formation of HC:HA complex.
Future Directions
A series of studies on the expression and function of Vcan revealed the Vcan as a key player of the ECM dynamics. There are at least two aspects that support the significance of Vcan proteolysis. First, the disappearance of intact Vcan alters the integral supramolecular structure and moiety of the ECM, changing the magnitude of cell signaling mediated by HA and growth factors. Second, the fragment generated by proteolysis may directly affect cell behavior. Detailed studies by combined alteration of expression levels of ADAMTSs and Vcan indicate that the significance of Vcan proteolysis is by the gain of biologically active fragments rather than the loss of the ECM structure.88,99,100 Yet, the precise mechanisms of how the fragments exert the function remain to be elucidated.
Acknowledgments
We thank all the researchers who kindly provided insight into our proteoglycan researches.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Both authors equally prepared the article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by JSPS KAKENHI Grant Numbers 25293096 and 18H02646 (H.W.) and Aikeikai Scholarship (S.I.).
Contributor Information
Shamima Islam, Institute for Molecular Science of Medicine, Aichi Medical University, Nagakute, Japan.
Hideto Watanabe, Institute for Molecular Science of Medicine, Aichi Medical University, Nagakute, Japan.
Literature Cited
- 1. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(Pt 24):4195–200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 3. Karamanos NK, Piperigkou Z, Theocharis AD, Watanabe H, Franchi M, Baud S, Brezillon S, Gotte M, Passi A, Vigetti D, Ricard-Blum S, Sanderson RD, Neill T, Iozzo RV. Proteoglycan chemical diversity drives multifunctional cell regulation and therapeutics. Chem Rev. 2018;118(18):9152–232. doi: 10.1021/acs.chemrev.8b00354. [DOI] [PubMed] [Google Scholar]
- 4. Iozzo RV, Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buraschi S, Neill T, Iozzo RV. Decorin is a devouring proteoglycan: remodeling of intracellular catabolism via autophagy and mitophagy. Matrix Biol. 2019;75–6:260–70. doi: 10.1016/j.matbio.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watanabe H, Yamada Y, Kimata K. Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J Biochem. 1998;124(4):687–93. doi: 10.1093/oxfordjournals.jbchem.a022166. [DOI] [PubMed] [Google Scholar]
- 7. Wight TN. Provisional matrix: a role for versican and hyaluronan. Matrix Biol. 2017;60–1:38–56. doi: 10.1016/j.matbio.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wight TN, Kang I, Evanko SP, Harten IA, Chang MY, Pearce OMT, Allen CE, Frevert CW. Versican—a critical extracellular matrix regulator of immunity and inflammation. Front Immunol. 2020;11:512. doi: 10.3389/fimmu.2020.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wight TN, Kang I, Merrilees MJ. Versican and the control of inflammation. Matrix Biol. 2014;35:152–61. doi: 10.1016/j.matbio.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wight TN, Kinsella MG, Evanko SP, Potter-Perigo S, Merrilees MJ. Versican and the regulation of cell phenotype in disease. Biochim Biophys Acta. 2014;1840(8):2441–51. doi: 10.1016/j.bbagen.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neill T, Schaefer L, Iozzo RV. Decoding the matrix: instructive roles of proteoglycan receptors. Biochemistry. 2015;54(30):4583–98. doi: 10.1021/acs.biochem.5b00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schaefer L, Tredup C, Gubbiotti MA, Iozzo RV. Proteoglycan neofunctions: regulation of inflammation and autophagy in cancer biology. FEBS J. 2017;284(1):10–26. doi: 10.1111/febs.13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kischel P, Waltregny D, Dumont B, Turtoi A, Greffe Y, Kirsch S, De Pauw E, Castronovo V. Versican overexpression in human breast cancer lesions: known and new isoforms for stromal tumor targeting. Int J Cancer. 2010;126(3):640–50. doi: 10.1002/ijc.24812. [DOI] [PubMed] [Google Scholar]
- 14. Schmalfeldt M, Dours-Zimmermann MT, Winterhalter KH, Zimmermann DR. Versican V2 is a major extracellular matrix component of the mature bovine brain. J Biol Chem. 1998;273(25):15758–64. doi: 10.1074/jbc.273.25.15758. [DOI] [PubMed] [Google Scholar]
- 15. Dours-Zimmermann MT, Maurer K, Rauch U, Stoffel W, Fassler R, Zimmermann DR. Versican V2 assembles the extracellular matrix surrounding the nodes of ranvier in the CNS. J Neurosci. 2009;29(24):7731–42. doi: 10.1523/jneurosci.4158-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burns TA, Dours-Zimmermann MT, Zimmermann DR, Krug EL, Comte-Walters S, Reyes L, Davis MA, Schey KL, Schwacke JH, Kern CB, Mjaatvedt CH. Imbalanced expression of Vcan mRNA splice form proteins alters heart morphology and cellular protein profiles. PLoS ONE. 2014;9(2):e89133. doi: 10.1371/journal.pone.0089133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Day AJ, Milner CM. TSG-6: a multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019;78–9:60–83. doi: 10.1016/j.matbio.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 18. Watanabe H, Cheung SC, Itano N, Kimata K, Yamada Y. Identification of hyaluronan-binding domains of aggrecan. J Biol Chem. 1997;272(44):28057–65. doi: 10.1074/jbc.272.44.28057. [DOI] [PubMed] [Google Scholar]
- 19. Matsumoto K, Shionyu M, Go M, Shimizu K, Shinomura T, Kimata K, Watanabe H. Distinct interaction of versican/PG-M with hyaluronan and link protein. J Biol Chem. 2003;278(42):41205–12. doi: 10.1074/jbc.M305060200. [DOI] [PubMed] [Google Scholar]
- 20. Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19(4):1004–13. [DOI] [PubMed] [Google Scholar]
- 21. Evanko SP, Potter-Perigo S, Johnson PY, Wight TN. Organization of hyaluronan and versican in the extracellular matrix of human fibroblasts treated with the viral mimetic poly I:C. J Histochem Cytochem. 2009;57(11):1041–60. doi: 10.1369/jhc.2009.953802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suwan K, Choocheep K, Hatano S, Kongtawelert P, Kimata K, Watanabe H. Versican/PG-M assembles hyaluronan into extracellular matrix and inhibits CD44-mediated signaling toward premature senescence in embryonic fibroblasts. J Biol Chem. 2009;284(13):8596–604. doi: 10.1074/jbc.M806927200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15(7):483–94. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 24. Choocheep K, Hatano S, Takagi H, Watanabe H, Kimata K, Kongtawelert P, Watanabe H. Versican facilitates chondrocyte differentiation and regulates joint morphogenesis. J Biol Chem. 2010;285(27):21114–25. doi: 10.1074/jbc.M109.096479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fanhchaksai K, Okada F, Nagai N, Pothacharoen P, Kongtawelert P, Hatano S, Makino S, Nakamura T, Watanabe H. Host stromal versican is essential for cancer-associated fibroblast function to inhibit cancer growth. Int J Cancer. 2016;138(3):630–41. doi: 10.1002/ijc.29804. [DOI] [PubMed] [Google Scholar]
- 26. Mikami T, Kitagawa H. Biosynthesis and function of chondroitin sulfate. Biochim Biophys Acta. 2013;1830(10):4719–33. doi: 10.1016/j.bbagen.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 27. Bode-Lesniewska B, Dours-Zimmermann MT, Odermatt BF, Briner J, Heitz PU, Zimmermann DR. Distribution of the large aggregating proteoglycan versican in adult human tissues. J Histochem Cytochem. 1996;44(4):303–12. doi: 10.1177/44.4.8601689. [DOI] [PubMed] [Google Scholar]
- 28. Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, Zimmermann DR, Lemire JM, Fischer JW, Wight TN, Clowes AW. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276(16):13372–8. doi: 10.1074/jbc.M009737200. [DOI] [PubMed] [Google Scholar]
- 29. Cikach FS, Koch CD, Mead TJ, Galatioto J, Willard BB, Emerton KB, Eagleton MJ, Blackstone EH, Ramirez F, Roselli EE, Apte SS. Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. JCI Insight. 2018;3(5):e97167. doi: 10.1172/jci.insight.97167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Snyder JM, Washington IM, Birkland T, Chang MY, Frevert CW. Correlation of versican expression, accumulation, and degradation during embryonic development by quantitative immunohistochemistry. J Histochem Cytochem. 2015;63(12):952–67. doi: 10.1369/0022155415610383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202(1):56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- 32. Hatano S, Kimata K, Hiraiwa N, Kusakabe M, Isogai Z, Adachi E, Shinomura T, Watanabe H. Versican/PG-M is essential for ventricular septal formation subsequent to cardiac atrioventricular cushion development. Glycobiology. 2012;22(9):1268–77. doi: 10.1093/glycob/cws095. [DOI] [PubMed] [Google Scholar]
- 33. Henderson DJ, Copp AJ. Versican expression is associated with chamber specification, septation, and valvulogenesis in the developing mouse heart. Circ Res. 1998;83(5):523–32. [DOI] [PubMed] [Google Scholar]
- 34. Wirrig EE, Snarr BS, Chintalapudi MR, O’Neal JL, Phelps AL, Barth JL, Fresco VM, Kern CB, Mjaatvedt CH, Toole BP, Hoffman S, Trusk TC, Argraves WS, Wessels A. Cartilage link protein 1 (Crtl1), an extracellular matrix component playing an important role in heart development. Dev Biol. 2007;310(2):291–303. doi: 10.1016/j.ydbio.2007.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Williams Jr, DR, Presar AR, Richmond AT, Mjaatvedt CH, Hoffman S, Capehart AA. Limb chondrogenesis is compromised in the versican deficient Hdf mouse. Biochem Biophys Res Commun. 2005;334(3):960–6. doi: 10.1016/j.bbrc.2005.06.189. [DOI] [PubMed] [Google Scholar]
- 36. Hatano S, Nagai N, Sugiura N, Tsuchimoto J, Isogai Z, Kimata K, Ota A, Karnan S, Hosokawa Y, Watanabe H. Versican A-subdomain is required for its adequate function in dermal development. Connect Tissue Res. 2018;59:178–90. doi: 10.1080/03008207.2017.1324432. [DOI] [PubMed] [Google Scholar]
- 37. Ball SL, Mann DA, Wilson JA, Fisher AJ The role of the fibroblast in inflammatory upper airway conditions. Am J Pathol. 2016;186(2):225-33. doi:10.101/j.ajpath.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nature Med. 2014;20:822-32. doi:10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chang MY, Tanino Y, Vidova V, Kinsella MG, Chan CK, Johnson PY, Wight TN, Frevert CW. A rapid increase in macrophage-derived versican and hyaluronan in infectious lung disease. Matrix Biol. 2014;34:1–12. doi: 10.1016/j.matbio.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chang MY, Kang I, Gale Jr, M, Manicone AM, Kinsella MG, Braun KR, Wigmosta T, Parks WC, Altemeier WA, Wight TN, Frevert CW. Versican is produced by trif- and type I interferon-dependent signaling in macrophages and contributes to fine control of innate immunity in lungs. Am J Physiol Lung Cell Mol Physiol. 2017;313(6):L1069–86. doi: 10.1152/ajplung.00353.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu L, Xue T, Zhang J, Qu J. Knockdown of versican V1 induces a severe inflammatory response in LPS-induced acute lung injury via the TLR2-NF-kappaB signaling pathway in C57BL/6J mice. Mol Med Rep. 2016;13(6):5005–12. doi: 10.3892/mmr.2016.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kang I, Harten IA, Chang MY, Braun KR, Sheih A, Nivison MP, Johnson PY, Workman G, Kaber G, Evanko SP, Chan CK, Merrilees MJ, Ziegler SF, Kinsella MG, Frevert CW, Wight TN. Versican deficiency significantly reduces lung inflammatory response induced by polyinosine-polycytidylic acid stimulation. J Biol Chem. 2017;292(1):51–63. doi: 10.1074/jbc.M116.753186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Front Immunol. 2014;5:101. doi: 10.3389/fimmu.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gaucherand L, Falk BA, Evanko SP, Workman G, Chan CK, Wight TN. Crosstalk between T lymphocytes and lung fibroblasts: generation of a hyaluronan-enriched extracellular matrix adhesive for monocytes. J Cell Biochem. 2017;118(8):2118–30. doi: 10.1002/jcb.25842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Potter-Perigo S, Johnson PY, Evanko SP, Chan CK, Braun KR, Wilkinson TS, Altman LC, Wight TN. Polyinosine-polycytidylic acid stimulates versican accumulation in the extracellular matrix promoting monocyte adhesion. Am J Respir Cell Mol Biol. 2010;43(1):109–20. doi: 10.1165/rcmb.2009-0081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reeves SR, Kaber G, Sheih A, Cheng G, Aronica MA, Merrilees MJ, Debley JS, Frevert CW, Ziegler SF, Wight TN. Subepithelial accumulation of versican in a cockroach antigen-induced murine model of allergic asthma. J Histochem Cytochem. 2016;64(6):364–80. doi: 10.1369/0022155416642989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang YT, Chan CK, Eriksson I, Johnson PY, Cao X, Westoo C, Norvik C, Andersson-Sjoland A, Westergren-Thorsson G, Johansson S, Hedin U, Kjellen L, Wight TN, Tran-Lundmark K. Versican accumulates in vascular lesions in pulmonary arterial hypertension. Pulm Circ. 2016;6(3):347–59. doi: 10.1086/686994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Higuchi M, Ohnishi T, Arita N, Hiraga S, Hayakawa T. Expression of tenascin in human gliomas: its relation to histological malignancy, tumor dedifferentiation and angiogenesis. Acta Neuropathol. 1993;85(5):481–7. doi: 10.1007/bf00230486. [DOI] [PubMed] [Google Scholar]
- 49. Delpech B, Maingonnat C, Girard N, Chauzy C, Maunoury R, Olivier A, Tayot J, Creissard P. Hyaluronan and hyaluronectin in the extracellular matrix of human brain tumour stroma. Eur J Cancer. 1993;29A(7):1012–7. doi: 10.1016/s0959-8049(05)80214-x. [DOI] [PubMed] [Google Scholar]
- 50. Zagzag D, Friedlander DR, Dosik J, Chikramane S, Chan W, Greco MA, Allen JC, Dorovini-Zis K, Grumet M. Tenascin-C expression by angiogenic vessels in human astrocytomas and by human brain endothelial cells in vitro. Cancer Res. 1996;56(1):182–9. [PubMed] [Google Scholar]
- 51. Varga I, Hutoczki G, Szemcsak CD, Zahuczky G, Toth J, Adamecz Z, Kenyeres A, Bognar L, Hanzely Z, Klekner A. Brevican, neurocan, tenascin-C and versican are mainly responsible for the invasiveness of low-grade astrocytoma. Pathol Oncol Res. 2012;18(2):413–20. doi: 10.1007/s12253-011-9461-0. [DOI] [PubMed] [Google Scholar]
- 52. Onken J, Moeckel S, Leukel P, Leidgens V, Baumann F, Bogdahn U, Vollmann-Zwerenz A, Hau P. Versican isoform V1 regulates proliferation and migration in high-grade gliomas. J Neurooncol. 2014;120(1):73–83. doi: 10.1007/s11060-014-1545-8. [DOI] [PubMed] [Google Scholar]
- 53. Hu F, Dzaye OD, Hahn A, Yu Y, Scavetta RJ, Dittmar G, Kaczmarek AK, Dunning KR, Ricciardelli C, Rinnenthal JL, Heppner FL, Lehnardt S, Synowitz M, Wolf SA, Kettenmann H. Glioma-derived versican promotes tumor expansion via glioma-associated microglial/macrophages toll-like receptor 2 signaling. Neuro Oncol. 2015;17(2):200–10. doi: 10.1093/neuonc/nou324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xiang YY, Dong H, Wan Y, Li J, Yee A, Yang BB, Lu WY. Versican G3 domain regulates neurite growth and synaptic transmission of hippocampal neurons by activation of epidermal growth factor receptor. J Biol Chem. 2006;281(28):19358–68. doi: 10.1074/jbc.M512980200. [DOI] [PubMed] [Google Scholar]
- 55. Yang W, Yee AJ. Versican V2 isoform enhances angiogenesis by regulating endothelial cell activities and fibronectin expression. FEBS Lett. 2013;587(2):185–92. doi: 10.1016/j.febslet.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 56. Fang L, Du WW, Yang X, Chen K, Ghanekar A, Levy G, Yang W, Yee AJ, Lu WY, Xuan JW, Gao Z, Xie F, He C, Deng Z, Yang BB. Versican 3’-untranslated region (3’-UTR) functions as a ceRNA in inducing the development of hepatocellular carcinoma by regulating miRNA activity. Faseb J. 2013;27(3):907–19. doi: 10.1096/fj.12-220905. [DOI] [PubMed] [Google Scholar]
- 57. Yan Y, Qin D, Hu B, Zhang C, Liu S, Wu D, Huang W, Huang X, Wang L, Chen X, Zhang L. Deletion of miR-126a promotes hepatic aging and inflammation in a mouse model of cholestasis. Mol Ther Nucleic Acids. 2019;16:494–504. doi: 10.1016/j.omtn.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xia L, Huang W, Tian D, Zhang L, Qi X, Chen Z, Shang X, Nie Y, Wu K. Forkhead box Q1 promotes hepatocellular carcinoma metastasis by transactivating ZEB2 and VersicanV1 expression. Hepatology. 2014;59(3):958–73. doi: 10.1002/hep.26735. [DOI] [PubMed] [Google Scholar]
- 59. Stephenson AJ, Kattan MW, Eastham JA, Dotan ZA, Bianco Jr, FJ, Lilja H, Scardino PT. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol. 2006;24(24):3973–8. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 60. Arichi N, Mitsui Y, Hiraki M, Nakamura S, Hiraoka T, Sumura M, Hirata H, Tanaka Y, Dahiya R, Yasumoto H, Shiina H. Versican is a potential therapeutic target in docetaxel-resistant prostate cancer. Oncoscience. 2015;2(2):193–204. doi: 10.18632/oncoscience.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ricciardelli C, Russell DL, Ween MP, Mayne K, Suwiwat S, Byers S, Marshall VR, Tilley WD, Horsfall DJ. Formation of hyaluronan- and versican-rich pericellular matrix by prostate cancer cells promotes cell motility. J Biol Chem. 2007;282(14):10814–25. doi: 10.1074/jbc.M606991200. [DOI] [PubMed] [Google Scholar]
- 62. Sakko AJ, Ricciardelli C, Mayne K, Tilley WD, Lebaron RG, Horsfall DJ. Versican accumulation in human prostatic fibroblast cultures is enhanced by prostate cancer cell-derived transforming growth factor beta1. Cancer Res. 2001;61(3):926–30. [PubMed] [Google Scholar]
- 63. Ricciardelli C, Mayne K, Sykes PJ, Raymond WA, McCaul K, Marshall VR, Horsfall DJ. Elevated levels of versican but not decorin predict disease progression in early-stage prostate cancer. Clin Cancer Res. 1998;4(4):963–71. [PubMed] [Google Scholar]
- 64. Russell DL, Ochsner SA, Hsieh M, Mulders S, Richards JS. Hormone-regulated expression and localization of versican in the rodent ovary. Endocrinology. 2003;144(3):1020–31. doi: 10.1210/en.2002-220434. [DOI] [PubMed] [Google Scholar]
- 65. Voutilainen K, Anttila M, Sillanpaa S, Tammi R, Tammi M, Saarikoski S, Kosma VM. Versican in epithelial ovarian cancer: relation to hyaluronan, clinicopathologic factors and prognosis. Int J Cancer. 2003;107(3):359–64. doi: 10.1002/ijc.11423. [DOI] [PubMed] [Google Scholar]
- 66. Ghosh S, Albitar L, LeBaron R, Welch WR, Samimi G, Birrer MJ, Berkowitz RS, Mok SC. Up-regulation of stromal versican expression in advanced stage serous ovarian cancer. Gynecol Oncol. 2010;119(1):114–20. doi: 10.1016/j.ygyno.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yeung TL, Leung CS, Wong KK, Samimi G, Thompson MS, Liu J, Zaid TM, Ghosh S, Birrer MJ, Mok SC. TGF-beta modulates ovarian cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res. 2013;73(16):5016–28. doi: 10.1158/0008-5472.CAN-13-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Salem M, O’Brien JA, Bernaudo S, Shawer H, Ye G, Brkic J, Amleh A, Vanderhyden BC, Refky B, Yang BB, Krylov SN, Peng C. miR-590-3p promotes ovarian cancer growth and metastasis via a Novel FOXA2-versican pathway. Cancer Res. 2018;78(15):4175–90. doi: 10.1158/0008-5472.Can-17-3014. [DOI] [PubMed] [Google Scholar]
- 69. Zhang Q, Hou X, Evans BJ, VanBlaricom JL, Weroha SJ, Cliby WA. LY2157299 monohydrate, a TGF-betaR1 inhibitor, suppresses tumor growth and ascites development in ovarian cancer. Cancers. 2018;10(8):260. doi: 10.3390/cancers10080260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Evensen NA, Kuscu C, Nguyen HL, Zarrabi K, Dufour A, Kadam P, Hu YJ, Pulkoski-Gross A, Bahou WF, Zucker S, Cao J. Unraveling the role of KIAA1199, a novel endoplasmic reticulum protein, in cancer cell migration. J Natl Cancer Inst. 2013;105(18):1402–16. doi: 10.1093/jnci/djt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sun J, Hu J, Wang G, Yang Z, Zhao C, Zhang X, Wang J. LncRNA TUG1 promoted KIAA1199 expression via miR-600 to accelerate cell metastasis and epithelial-mesenchymal transition in colorectal cancer. J Exp Clin Cancer Res. 2018;37(1):106. doi: 10.1186/s13046-018-0771-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72. Li L, Yan LH, Manoj S, Li Y, Lu L. Central role of CEMIP in tumorigenesis and its potential as therapeutic target. J Cancer. 2017;8(12):2238–46. doi: 10.7150/jca.19295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Birkenkamp-Demtroder K, Maghnouj A, Mansilla F, Thorsen K, Andersen CL, Oster B, Hahn S, Orntoft TF. Repression of KIAA1199 attenuates Wnt-signalling and decreases the proliferation of colon cancer cells. Br J Cancer. 2011;105(4):552–61. doi: 10.1038/bjc.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yoshida H, Nagaoka A, Kusaka-Kikushima A, Tobiishi M, Kawabata K, Sayo T, Sakai S, Sugiyama Y, Enomoto H, Okada Y, Inoue S. KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization. Proc Natl Acad Sci USA. 2013;110(14):5612–7. doi: 10.1073/pnas.1215432110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Du WW, Fang L, Yang W, Sheng W, Zhang Y, Seth A, Yang BB, Yee AJ. The role of versican G3 domain in regulating breast cancer cell motility including effects on osteoblast cell growth and differentiation in vitro—evaluation towards understanding breast cancer cell bone metastasis. BMC Cancer. 2012;12:341. doi: 10.1186/1471-2407-12-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Du WW, Yang BB, Shatseva TA, Yang BL, Deng Z, Shan SW, Lee DY, Seth A, Yee AJ. Versican G3 promotes mouse mammary tumor cell growth, migration, and metastasis by influencing EGF receptor signaling. PLoS ONE. 2010;5(11):e13828. doi: 10.1371/journal.pone.0013828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang Z, Li Z, Wang Y, Cao D, Wang X, Jiang M, Li M, Yan X, Li Y, Liu Y, Luo F. Versican silencing improves the antitumor efficacy of endostatin by alleviating its induced inflammatory and immunosuppressive changes in the tumor microenvironment. Oncol Rep. 2015;33(6):2981–91. doi: 10.3892/or.2015.3903. [DOI] [PubMed] [Google Scholar]
- 78. Asano K, Nelson CM, Nandadasa S, Aramaki-Hattori N, Lindner DJ, Alban T, Inagaki J, Ohtsuki T, Oohashi T, Apte SS, Hirohata S. Stromal versican regulates tumor growth by promoting angiogenesis. Sci Rep. 2017;7(1):17225. doi: 10.1038/s41598-017-17613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Perides G, Asher RA, Lark MW, Lane WS, Robinson RA, Bignami A. Glial hyaluronate-binding protein: a product of metalloproteinase digestion of versican? Biochem J. 1995;312(Pt 2):377–84. doi: 10.1042/bj3120377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Passi A, Negrini D, Albertini R, Miserocchi G, De Luca G. The sensitivity of versican from rabbit lung to gelatinase A (MMP-2) and B (MMP-9) and its involvement in the development of hydraulic lung edema. FEBS Lett. 1999;456(1):93–6. [DOI] [PubMed] [Google Scholar]
- 81. Halpert I, Sires UI, Roby JD, Potter-Perigo S, Wight TN, Shapiro SD, Welgus HG, Wickline SA, Parks WC. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci USA. 1996;93(18):9748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kenagy RD, Fischer JW, Davies MG, Berceli SA, Hawkins SM, Wight TN, Clowes AW. Increased plasmin and serine proteinase activity during flow-induced intimal atrophy in baboon PTFE grafts. Arterioscler Thromb Vasc Biol. 2002;22(3):400–4. [DOI] [PubMed] [Google Scholar]
- 83. Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284(46):31493–7. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Apte SS. ADAMTS proteins: concepts, challenges, and prospects. Methods Mol Biol. 2020;2043:1–12. doi: 10.1007/978-1-4939-9698-8_1. [DOI] [PubMed] [Google Scholar]
- 85. Mead TJ, Apte SS. ADAMTS proteins in human disorders. Matrix Biol. 2018;71–2:225–39. doi: 10.1016/j.matbio.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nandadasa S, Foulcer S, Apte SS. The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis. Matrix Biol. 2014;35:34–41. doi: 10.1016/j.matbio.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Westling J, Gottschall PE, Thompson VP, Cockburn A, Perides G, Zimmermann DR, Sandy JD. ADAMTS4 (aggrecanase-1) cleaves human brain versican V2 at Glu405-Gln406 to generate glial hyaluronate binding protein. Biochem J. 2004;377(Pt 3):787–95. doi: 10.1042/BJ20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. McCulloch DR, Nelson CM, Dixon LJ, Silver DL, Wylie JD, Lindner V, Sasaki T, Cooley MA, Argraves WS, Apte SS. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev Cell. 2009;17(5):687–98. doi: 10.1016/j.devcel.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ricard-Blum S, Salza R. Matricryptins and matrikines: biologically active fragments of the extracellular matrix. Exp Dermatol. 2014;23(7):457–63. doi: 10.1111/exd.12435. [DOI] [PubMed] [Google Scholar]
- 90. Poluzzi C, Iozzo RV, Schaefer L. Endostatin and endorepellin: a common route of action for similar angiostatic cancer avengers. Adv Drug Deliv Rev. 2016;97:156–73. doi: 10.1016/j.addr.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Timms K, Maurice SB. Context-dependent bioactivity of versican fragments. Glycobiology. 2019;30:365–73. doi: 10.1093/glycob/cwz090. [DOI] [PubMed] [Google Scholar]
- 92. Gupta N, Khan R, Kumar R, Kumar L, Sharma A. Versican and its associated molecules: potential diagnostic markers for multiple myeloma. Clin Chim Acta. 2015;442:119–24. doi: 10.1016/j.cca.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 93. Hope C, Ollar SJ, Heninger E, Hebron E, Jensen JL, Kim J, Maroulakou I, Miyamoto S, Leith C, Yang DT, Callander N, Hematti P, Chesi M, Bergsagel PL, Asimakopoulos F. TPL2 kinase regulates the inflammatory milieu of the myeloma niche. Blood. 2014;123(21):3305–15. doi: 10.1182/blood-2014-02-554071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hope C, Foulcer S, Jagodinsky J, Chen SX, Jensen JL, Patel S, Leith C, Maroulakou I, Callander N, Miyamoto S, Hematti P, Apte SS, Asimakopoulos F. Immunoregulatory roles of versican proteolysis in the myeloma microenvironment. Blood. 2016;128(5):680–5. doi: 10.1182/blood-2016-03-705780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hope C, Emmerich PB, Papadas A, Pagenkopf A, Matkowskyj KA, Van De Hey DR, Payne SN, Clipson L, Callander NS, Hematti P, Miyamoto S, Johnson MG, Deming DA, Asimakopoulos F. Versican-derived matrikines regulate Batf3-dendritic cell differentiation and promote T cell infiltration in colorectal cancer. J Immunol. 2017;199(5):1933–41. doi: 10.4049/jimmunol.1700529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Huynh M, Pak C, Markovina S, Callander NS, Chng KS, Wuerzberger-Davis SM, Bakshi DD, Kink JA, Hematti P, Hope C, Asimakopoulos F, Rui L, Miyamoto S. Hyaluronan and proteoglycan link protein 1 (HAPLN1) activates bortezomib-resistant NF-kappaB activity and increases drug resistance in multiple myeloma. J Biol Chem. 2018;293(7):2452–65. doi: 10.1074/jbc.RA117.000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Merrilees MJ, Zuo N, Evanko SP, Day AJ, Wight TN. G1 domain of versican regulates hyaluronan organization and the phenotype of cultured human dermal fibroblasts. J Histochem Cytochem. 2016;64(6):353–63. doi: 10.1369/0022155416643913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Murasawa Y, Nakamura H, Watanabe K, Kanoh H, Koyama E, Fujii S, Kimata K, Zako M, Yoneda M, Isogai Z. The versican G1 fragment and serum-derived hyaluronan-associated proteins interact and form a complex in granulation tissue of pressure ulcers. Am J Pathol. 2018;188(2):432–49. doi: 10.1016/j.ajpath.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 99. Enomoto H, Nelson CM, Somerville RP, Mielke K, Dixon LJ, Powell K, Apte SS. Cooperation of two ADAMTS metalloproteases in closure of the mouse palate identifies a requirement for versican proteolysis in regulating palatal mesenchyme proliferation. Development. 2010;137(23):4029–38. doi: 10.1242/dev.050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hong CC, Tang AT, Detter MR, Choi JP, Wang R, Yang X, Guerrero AA, Wittig CF, Hobson N, Girard R, Lightle R, Moore T, Shenkar R, Polster SP, Goddard LM, Ren AA, Leu NA, Sterling S, Yang J, Li L, Chen M, Mericko-Ishizuka P, Dow LE, Watanabe H, Schwaninger M, Min W, Marchuk DA, Zheng X, Awad IA, Kahn ML. Cerebral cavernous malformations are driven by ADAMTS5 proteolysis of versican. J Exp Med. 2020;217(10):e20200140. doi: 10.1084/jem.20200140. [DOI] [PMC free article] [PubMed] [Google Scholar]