Abstract
The nervous system consists of hundreds of billions of neurons interconnected into the functional neural networks that underlie behaviors. The capacity of a neuron to innervate and function within a network is mediated via specialized cell junctions known as synapses. Synapses are macromolecular structures that regulate intercellular communication in the nervous system, and are the main gatekeepers of information flow within neural networks. Where and when synapses form determines the connectivity and functionality of neural networks. Therefore, our knowledge of how synapse formation is regulated is critical to our understanding of the nervous system and how it goes awry in neurological disorders.
Synapse formation involves pairing of the pre- and postsynaptic partners at a specific neurospatial coordinate. The specificity of synapse formation requires the precise execution of multiple developmental events, including cell fate specification, cell migration, axon guidance, dendritic growth, synaptic target selection, and synaptogenesis (Juttner and Rathjen in Cell. Mol. Life Sci. 62:2811, 2005; Salie et al., in Neuron 45:189, 2005; Waites et al., in Annu. Rev. Neurosci. 28:251, 2005). Remarkably, during the development of the vertebrate nervous system, these developmental processes occur almost simultaneously in billions of neurons, resulting in the formation of trillions of synapses. How this remarkable specificity is orchestrated during development is one of the outstanding questions in the field of neurobiology, and the focus of discussion of this chapter.
We center the discussion of this chapter on the early developmental events that orchestrate the process of synaptogenesis prior to activity-dependent mechanisms. We have therefore limited the discussion of important activity-dependent synaptogenic events, which are discussed in other chapters of this book. Moreover, our discussion is biased toward lessons we have learned from invertebrate systems, in particular from C. elegans and Drosophila. We did so to complement the discussions from other chapters in this book, which focus on the important findings that have recently emerged from the vertebrate literature.
The chapter begins with a brief history of the field of synaptic biology. This serves as a backdrop to introduce some of the historically outstanding questions of synaptic development that have eluded us during the past century, and which are the focus of this review. We then discuss some general features of synaptic structure as it relates to its function. In particular, we will highlight evolutionarily conserved traits shared by all synaptic structures, and how these features have helped optimize these ancient cellular junctions for interneural communication.
We then discuss the regulatory signals that orchestrate the precise assembly of these conserved macromolecular structures. This discussion will be framed in the context of the neurodevelopmental process. Specifically, much of our discussion will focus on how the seemingly disparate developmental processes are intimately linked at a molecular level, and how this relationship might be crucial in the developmental orchestration of circuit assembly. We hope that the discussion of the multifunctional cues that direct circuit development provides a conceptual framework into understanding how, with a limited set of signaling molecules, precise neural wiring can be coordinated between synaptic partners.
1. Introduction
1.1. A historical perspective
The history of synapse biology starts at the end of the eighteenth century, with the studies of Luigi Galvani and his descriptions of “animal electricity.” In these classical studies, Galvani observed that he could induce the contraction of limb muscles when he inserted a metal hook into the medulla of the frog and attached the other end to an iron railing. These observations marked the first experimental demonstrations of synaptic transmission (Cowan and Kandel, 2001).
Most of the subsequent synaptic studies in the nineteenth century and earlier half of the twentieth century also focused around the functionality of synapses, or synaptic transmission. It is therefore befitting that the actual term “synapse” was not coined by a neuroanatomist, but by a physiologist named Charles Sherrington. Sherrington coined the term “synapse” to refer to the special connections from one nerve cell to another that facilitated the transmission of nervous impulses (Cowan and Kandel, 2001).
While physiologists and neuropharmacologists were functionally defining the concept of synapses, neuroanatomists tangled in a bitter debate on their existence. The main reason for this debate was that during the nineteenth century and earlier part of the twentieth century, nobody could visualize cell membranes and establish conclusively the existence of synapses. However, in spite of these technological limitations, some insightful neurobiologists garnered enough experimental evidence to propose the anatomical existence of synapses.
Most of these early observations came from a specialized synapse: the neuromuscular junction (NMJ). Because of its size, morphology and functional readouts, NMJs informed then, as they do now, most of our knowledge on synaptic biology. Taking advantage of this system, physiologist Willy Kühne and anatomist Wilhelm Krause independently hypothesized the existence of synapses at the site of contact between nerve cells and muscles (Cowan and Kandel, 2001).
The question of the existence of interneuronal synapses was much harder to settle. Synapses in the central nervous system are much smaller than NMJs, in closed apposition to one another and packed at very high densities. This made their visualization with the methods used during nineteenth century downright impossible and triggered the postulation of the “reticular theory”: the idea that the nervous system lacked functional separation of nerve cells and was syncytial, rather than synaptic, in nature (Cowan and Kandel, 2001; Westfall, 1996).
The theory turned out, of course, to be wrong. Although this was not conclusively shown until the advent of electron microscopy in the 1950s, the first evidence that neurons were discrete units came from developmental, pathological, and anatomical observations in the nineteenth century. Most notable among these early studies are Santiago Ramón y Cajal’s. By using a method derived by Golgi, which stains only 1% of the cells, Cajal was able to visualize the morphology of individual cells in the context of the nervous system. His detailed characterization of neurons not only provided critical evidence for the neuron doctrine, but also stated the “Principios de la Especificidad de la Conexión”: the idea that nerve cells connect to each other in a specific fashion to form precise networks (Cowan and Kandel, 2001).
Although it would take another half a century for cell biologists to visualize synapses, Cajal’s observations and insights at the turn of the nineteenth century provided the conceptual basis that has driven most of the neurodevelopmental questions since then. Over a century after Cajal’s initial descriptions, we are still untangling the complex morass that is the central nervous system and tackling the questions staged by his landmark observations: How are the numerous cell types in the nervous system specified? What directs neurites to connect to each other? What arc the cellular and molecular factors that underlie the “Principles of connection specificity”?
1.2. Synaptic structure and function
During the last century however, and thanks in great part to technical advances in cell biology, the field has made great progress in its understanding of the synaptic structure as it relates to synaptic function. Most notably, electron microscopy allowed the visualization of synapses for the first time in the 1950s. This work, spearheaded by George Palade and Keith Porter, provided unequivocal evidence for the neuron doctrine and the existence of synapses, and identified the different types of synapses and their structural components (Cowan and Kandel, 2001; De Camilli et al., 2001).
There are two general categories of synapses: electrical synapses and chemical synapses. Physiologists and neuropharmacologists functionally defined these two categories of synapses well before they were visualized by cell biologists (Cowan and Kandel, 2001). But the cell biological work that proceeded from the physiological studies demonstrated that these two functional categories corresponded to completely different structures. Electrical synapses arc gap junctions that allow bidirectional propagation of signals, including electrical stimuli. They allow the fastest mode of electrical propagation across cells, and are now known to be important in synchronizing neural activity across networks (De Camilli et al., 2001). These gap junctions will not be further discussed in this chapter.
Chemical synapses allow communication between discontinuous neurons via the highly regulated secretion of chemical intermediate signals. Unlike electrical synapses, chemical synapses are polarized junctions that allow the flow of information in just a single direction. Because of their highly regulated and directional transfer of information, chemical synapses have been the focus of most of the synaptic biology studies, and as such will remain the focus of our chapter.
Although there is great morphological and molecular variability among chemical synapses, all chemical synapses share common structural and functional features (De Camilli et al., 2001). They consist of two asymmetrically juxtaposed components linking two separate cells: a presynaptic specialization and a postsynaptic region. The presynaptic specializations are specialized regions in the presynaptic cell with an abundance of neurotransmitter-filled synaptic vesicles. Presynaptic specializations also contain the active zone structures that facilitate vesicle fusion and the release of neurotransmitter content to the intersynaptic space, called the synaptic cleft. The postsynaptic region is an area of the postsynaptic cell with a high concentration of neurotransmitter receptors, channels, and downstream signaling molecules. The neurotransmitters released by the presynaptic specializations are sensed by the receptors at the postsynaptic site, activating downstream signaling molecules, opening channels, and propagating the nervous impulse to the postsynaptic partner. These general features of the presynaptic and postsynaptic specializations are shared by all classes of synaptic structures.
The synaptic structure as described above is also very well conserved across evolution. Sea anemones and hydra (Phylum Cnidaria) have the most primitive nervous system, which consists of a diffuse network of neurons. These nerve nets, however, are connected via chemical and electrical synapses that are fully capable of transmitting and regulating information flow (Anderson and Spencer, 1989; Peteya, 1973; Westfall, 1996). Close inspection of these synaptic structures reveal that Cnidarian synapses have similar structural components as those of higher organisms, with defined presynaptic and postsynaptic specializations in close juxtaposition (Anderson and Spencer, 1989; Peteya, 1973; Westfall, 1996).
The presence of a conserved synaptic structure in these primitive nervous systems reveals that synapses are as ancient as the nervous system itself. This evolutionary conservation of the synaptic structure also underscores the importance of these specialized cell junctions in interneuronal communication and the functioning of the neural networks (Anderson and Spencer, 1989; Peteya, 1973; Westfall, 1996).
Interestingly, a recent study suggests that the evolution of the synaptic molecular machine might even precede the evolution of the nervous system (Sakarya et al, 2007). Although sponges (Phylum Porifera) are the only metazoans without a nervous system, it was found that sponges express a nearly complete set of postsynaptic protein homologues that are hypothesized to assemble into synaptic-like scaffolds. Although sponges do not have neurons, these postsynaptic-like structures are hypothesized to act as chemosensory structures capable of responding to environmental cues (Sakarya etal, 2007).
Other molecular components of the presynaptic machine, such as the synaptic vesicle cycle regulators, also predate the existence of the nervous system and are very well conserved across evolution (Sudhof, 2004). It is provocative that these macromolecular machines, presumable “building blocks” of the synapse, might be found even in the absence of a nervous system itself, an observation that underscores the importance and conservation of these signaling complexes throughout evolution (Sakarya et al, 2007).
Molecular and genetic studies in model invertebrate and vertebrate animals have also supported the notion that the ultrastructural conservation of synapses corresponds to a conservation at the molecular level. For instance, in the simple nervous system of the nematode Caenorhabditis elegans, which consists of only 302 neurons, the number of neurotransmitters and receptors required for the proper functioning of its ~5000 synapses approaches in complexity those used by the hundreds of trillions of synapses in the vertebrate nervous system (Rand and Nonet, 1997).
This suggests that throughout evolution, the increased capacity of information processing and storage observed in higher organisms is not the result of marked changes in the complexity of the synaptic structure. We speculate that this complexity results from an increasingly sophisticated neural framework in way of the abundance and organization of neural networks. Where, when, and how synapses form during development play critical roles on the wiring and function of neural networks. Although the neural network organization varies vastly across animals, the biological basis of synapses is shared from the simplest networks of Cnidaria to the complex neuropils of the human brain.
2. Synaptogenesis During Development
The organization of where, when, and how synapses are formed plays an instrumental role in directing the connectivity of circuits and organizing the neuroarchitecture that enables information processing, storage, and ultimately behaviors. As such, the developmental questions postulated by Cajal in his “Principles of connection specificity” are of great importance to our understanding of the assembly and function of the nervous system. What arc the molecular and cellular factors that direct the precise innervation of hundreds of trillions of synapses during development?
Neural circuit formation requires the intricate orchestration of multiple developmental events including cell fate specification, cell migration, axon guidance, dendritic growth, synaptic target selection, and synaptogenesis (Juttncr and Rathjen, 2005; Salie et al., 2005; Waites et al., 2005). The correct innervation of a given circuit requires the successful completion of all of these developmental steps in both synaptic partners. As such, synaptogenesis marks the final step of a complicated developmental dance where, after successful completion of the aforementioned steps, both synaptic partners converge at a specific location to form a specialized junction.
Although the field has now identified a number of molecules required for each of these developmental steps, we know much less about how these different developmental steps act in concert to direct the development of circuits. In the next sections, we will discuss the process of synaptic formation in the context of the complex developmental dance that brings neurons together.
2.1. Neuronal cell fate and synaptogenesis
Circuit formation begins with cell fate specification. During cell fate specification, the seemingly homogeneous neuroepithelium of the developing embryo differentiates into a hugely diverse number of neurons, each tailored morphologically and structurally for its particular functional role. Each of these neuron types has a distinct morphology, axonal and dendritic trajectory, and, of particular interest to this chapter, synaptic property in the way of synaptic specificity and neurotransmitter content. How each neuron adopts a particular identity and how this identity directs its connectivity remain outstanding problems in neurobiology.
Embryological studies have shown that morphogens and transcription factors play crucial roles in the specification of cell fate during the development of neural circuits. The expression of morphogens by discrete tissues establishes gradients along multiple developmental axes. These morphogenic gradients create a unique grid that conveys positional information in the developing embryo. Neural precursor cells respond to this positional information by expressing a specific set of transcription factors. The combinatorial expression of these transcription factors confers the neural precursor cells with an identity that can then be inherited by its descendants (O’Leary et al., 2007). Thus, the extrinsic positional information delineated by a grid of morphogenic gradients is translated into an intrinsic and inheritable cellular identity via the expression of a combinatorial code of transcription factors.
A growing body of literature supports the notion that this combinatorial code of transcription factors can confer important connectivity information to certain neurons (Polleux et al., 2007). For instance, transcription factors have now been shown to be important for proper projections of retino-ganglion cells from the retina to the thalamus, for projections of axons from thalamic nuclei to cortical areas and for the patterning of cortical efferent projections (Polleux et al., 2007). But are transcription factors directly required for synaptic specificity? Could the combinatorial transcriptional code also direct circuit innervation at the level of synapse formation?
Several lines of evidence suggest that transcription factors can direct where and how synapses are formed even after the process of axonal and dendritic guidance has concluded. The strongest evidence for the importance of transcription factors directly controlling synaptic specification comes from studies in the motor neurons of the ventral nerve cord of C. elegans (Von Stetina et al., 2006). Synapses in C. elegans are formed en passant, or along the length of the axon. This biological trait allows for an easier developmental dissection of the axon guidance versus the synaptogenesis steps as, unlike end-button synapses, en passant synapses are formed on the side of axons, which contact many potential synaptic targets (White et al., 1986). Early genetic studies in the motor neurons of the ventral nerve cord showed that mutant animals lacking the gene unc-4, which encodes a Prd-like homeodomain transcription factor, display a strong motor movement defect in backward locomotion. Ultrastructural studies on the innervation and morphology of nerve cord neurons showed that absence of the transcription factor did not alter the organization of the nerve cord, and all neurons looked normal in terms of morphology, position in the nerve cord fascicle and guidance. Interestingly, unc-4 mutant animals displayed abnormal synaptic specificity, with motor neurons innervating their incorrect partners. This suggested that the Prd-like homeodomain transcription factor UNC-4 directly controls synaptic choice without affecting other neural traits such as outgrowth and fasciculation (Miller et al., 1992; White et al., 1992).
Further studies in other neurons determined that the UNC-4 transcription factor also controls the expression of molecules involved in synaptic strength (Lickteig et al., 2001; Von Stetina et al., 2007). This capacity to regulate the expression of molecules involved in synaptic strength is independent from its capacity to regulate synaptic specificity (Lickteig et al., 2001). Although the identity of the targets of UNC-4 remains unknown, these studies show that transcription factors can directly regulate different aspects of synaptic biology, from formation of the synapse during development to the strength of synaptic connections (Von Stetina et al., 2006).
Another example of how transcription factors can regulate synapse formation is found in the cockroach cercal system. The cerci are an appendages in the rear of the cockroach where filiform hairs are innervated by a single sensory neuron. In newly hatched (first-instar) roaches there are just two sensory neurons that respond to stimuli: the lateral sensory neuron, which responds to stimuli form the front of the animal, and the medial sensory neuron, which responds to stimuli from the rear of the animal (Fig. 2.1). Although the arborization of these two sensory neurons overlaps, the two sensory neurons connect specifically and exclusively to different subsets of giant interneurons. This synaptic specificity directs directional sensitivity to stimuli, allowing the animal to discern if the stimuli come from the front or the rear, and eliciting the corresponding escape response (Blagburn and Bacon, 2004).
Figure 2.1.
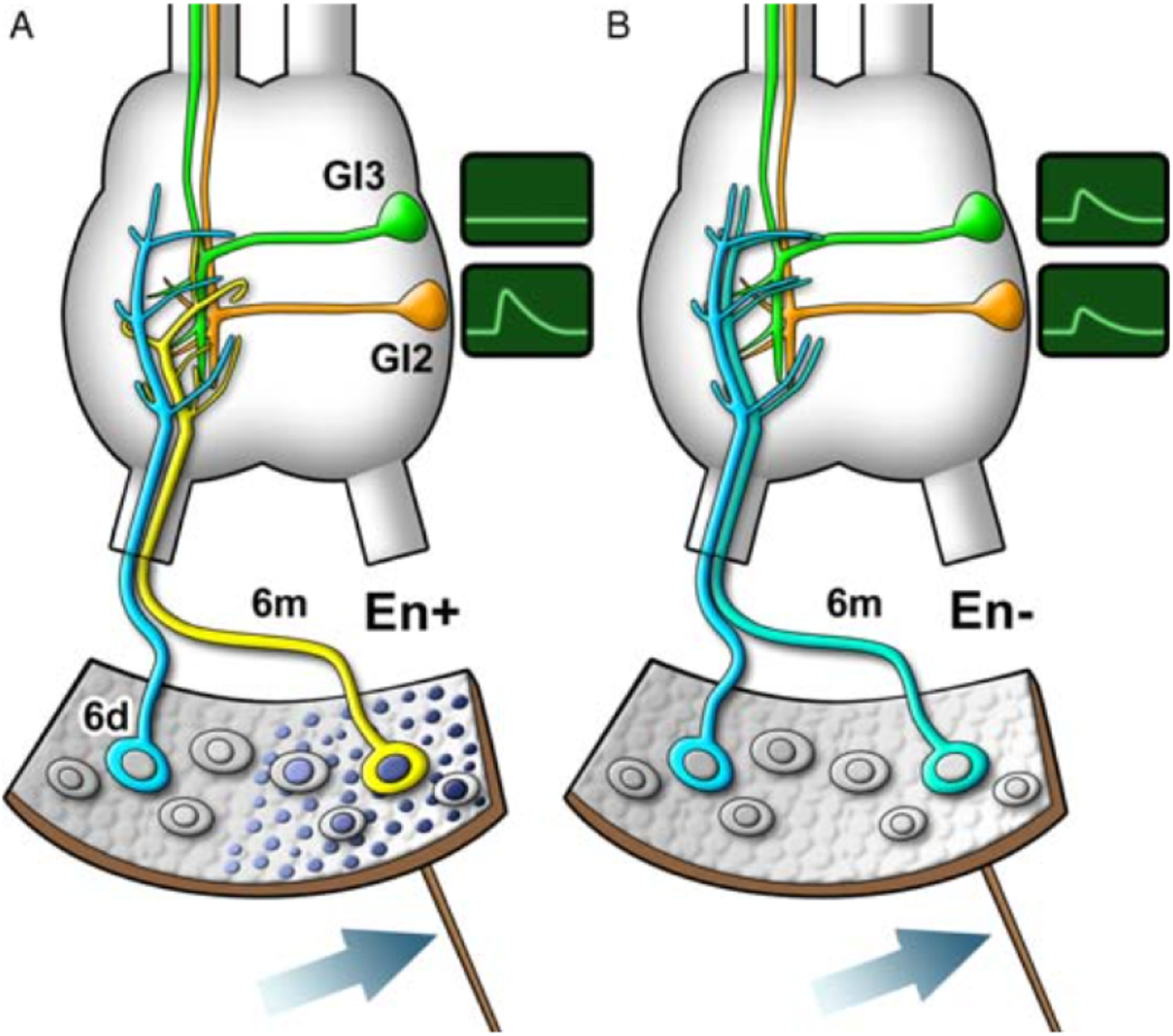
Transcription factor Engrailed directs synaptic specificity in the cockroach cercal system. Wiring diagram of the cockroach cercal system in the second instar cockroach. (A) In wild-type animals, medial sensory neuron (neuron 6m, in yellow) expresses Engrailed (represented by dark nuclei), while lateral sensory neuron (6d, in blue) does not (represented by clear nuclei). Although the axonal arbors of the medial and lateral sensory neurons overlap, they display specificity by connecting with specific target interneurons: medial sensory neuron connects to interneuron G12 (in orange), while lateral sensory neuron connects to interneuron G13 (in green). This specificity can be physiologically recorded, so that, for instance, stimuli in the filiform hair linked to the medial sensory neuron (dark arrow) results in depolarization of interneuron G12 and not G13 (represented by physiological recording to the right of the schematic). (B) Loss of Engrailed by dsRNA disrupts the medial sensory neuron identity and connectivity. In the absence of Engrailed, the medial sensory neuron (6m) adopts an identity similar to the lateral sensory neuron in terms of the branching structure (compare branching schematic of 6m in A (yellow) with 6m in B (blue)). Loss of Engrailed also disrupts medial sensory neuron synaptic specificity (represented by physiological recording to the right of the schematic).
Since these two filiform hair afferents have overlapping arborizations, the axonal projections cannot be the primary determinant of synaptic specificity. Instead, synaptic specificity must be directed by additional cues that allow these overlapping arbors to innervate specific targets. Loss-of-function studies in this system showed that the homeodomain transcription factor Engrailed is critical in directing this specification of synaptic connections (Marie et al., 2000).
Engrailed is expressed by the medial half of the central epidermis of developing animals, including the medial sensory neuron which responds to stimuli from the rear of the animal. Engrailed is not expressed by the lateral sensory neuron that responds to stimuli from the front of the animal. In the absence of Engrailed, the medial sensory neuron adopts a pattern of synaptic connections similar to that of the Engrailed-negative, lateral sensory neuron (Fig. 2.1). These results indicate that transcription factor Engrailed is required for correct specification of synaptic connections (Marie et al., 2000).
Interestingly, persistent expression of Engrailed was also shown to be required for the specification of other developmental traits of the medial sensory neuron. By manipulating Engrailed levels at different developmental stages, the authors went on to show that Engrailed is required in postmitotic neurons to control axon arborization and synaptic specification. They showed that these two events are developmentally separable, but are both dependent on the same transcription factor. These findings demonstrate that Engrailed can direct discrete connectivity decisions at different developmental stages (Marie and Blagburn, 2003; Marie et al., 2002). Furthermore, it highlights the role of transcription factors in coordinating and integrating the different developmental decisions that need to be made to direct neural connectivity.
Therefore, transcription factors can act as conveying points, receiving inputs, and directing different developmental steps that range from cell identity and neurite guidance to synapse assembly. When integrated (as in the case of Engrailed), these different activities could orchestrate the interdependent development and innervation of circuits. Although we understand the importance of transcription factors in directing discrete developmental steps, our knowledge on how their combinatorial and interdependent activity leads to correct innervation is still limited. For instance, in most of the experimental systems in which transcriptional regulation has been shown to affect circuit formation we do not yet know the identity of the guidance or synaptic specificity cues, how these cues are controlled and how their activity directs connectivity.
2.2. Axon guidance and synaptogenesis
Once neural cell fate is specified and neuron precursors have migrated to the appropriate regions, they extend polarized projections that become their axons and dendrites. The axonal processes can extend long distances, navigating complex cellular environments before reaching their postsynaptic partner. This guidance is mediated through the growth cone, a specialized sensing device at the tip of the outgrowing axon. Growth cones express a series of guidance receptors that are capable of sensing a variety of long-range (diffusible) and short-range (surface-bound) guidance cues. These guidance cues, which can be attractive or repulsive, are secreted by guidepost cells and intermediate targets. The spatial and temporal presence of the guidance cues, combined with the expression of the receptors in the growth cone, enables the axon to navigate through the labyrinth that is the developing nervous system to reach its target (Plachez and Richards, 2005; Tessier-Lavigne and Goodman, 1996). Upon reaching and contacting its target, the axon transforms into a presynaptic specialization capable of transducing synaptic signals to the postsynaptic target.
One of the outstanding questions in the field of synaptogenesis is how this transformation is mediated. How does the axon identify its correct postsynaptic target? During guidance, how does the growth cone differentiate between intermediate guidepost targets and its final target? Upon reaching the target region, how does it discriminate between potential partners to innervate its correct postsynaptic partner? And what are the cell biological changes that occur in the axon to transform it into a specialized presynaptic junction?
Clues on how this transformation occurs have come from studies in one of the better-understood systems of growth cone guidance and synaptic targeting: the RP3 motoneuron in the Drosophila embryos. The RP3 motoneuron can be visualized during development of the Drosophila embryonic CNS with single-cell resolution and in the context of the intact nervous system. This is done by using immunocytochemistry techniques that allow detailed characterization of the developmental decisions made by this motoneuron. These studies demonstrated that the RP3 axon undergoes a stereotypical sequence of guidance events before reaching its final targets, two muscles known as muscles 6 and 7 (Fig. 2.2). Remarkably, upon reaching the target region RP3 comes within filopodial reach of over a dozen different muscles, yet specifically innervates only its correct targets. In wild-type embryos this stereotyped sequence of developmental events, and the innervation of the correct targets, happens with 100% accuracy (Chiba and Rose, 1998).
Figure 2.2.

Axon guidance molecule Netrin is required for synaptic targeting events in the Drosophila embryo. Schematic diagram of RP3 neuron (blue) and the body wall muscles in the Drosophila embryo (represented here are muscles 6, 7, 13, 12, 5, and 8). (A) In wild-type animals, muscles 6 and 7 express Netrin (in pink). Muscles 12, 13, 5, and 8 express repulsive cues such as semaphorins, and do not express Netrin (lack of Netrin expression represented in white). Expression of Netrin by muscles 6 and 7 induces short-range targeting and specific innervation of these muscles by the RP3 neuron. (B) In Netrin loss-of-function mutants, the RP3 neuron reaches the neighborhood of muscles 6 and 7 in a timely fashion, coming within filopodial reach of its targets, but fails to innervate these muscles correctly. These studies suggest that Netrin is not required for long-range guidance of the RP3 neuron, but is instead required for its short-range synaptic targeting.
Genetic studies in the RP3 system showed that the long-range guidance decisions and the short-range synaptic targeting choices are directed by different molecular cues. The RP3 targets, muscles 6 and 7, express the axon guidance cue Netrin. However, deletion of the Netrin gene does not affect long-range guidance decisions of the RP3 axon: in the absence of Netrin or its receptor (Frazzled), the RP3 growth cone makes its normal guidance decisions, exiting the CNS, leaving the nerve bundles and entering the appropriate ventral muscle domain in a timely fashion to reach the neighborhoods of its targets, muscles 6 and 7 (Fig. 2.2). However, although the RP3 growth cone comes within filopodial reach of its targets, in these mutants it fails to innervate them robustly (Mitchell et al., 1996). These studies suggest that Netrin is not required for the long-range guidance decisions of the RP3 motoneuron, but instead is required for its short-range synaptic targeting. Furthermore, these studies indicate that the long-range guidance decisions and the short-range, synaptic targeting decisions of the RP3 motoneuron are two different, genetically separable events that are not dependent on the same molecular factors.
Although the RP3 guidance and synaptic targeting events are genetically separable, short-range targeting in this system is still mediated by a guidance molecule. The Netrin pathway has been shown to regulate cell migration, neural polarization leading to growth cone formation, and axon guidance events (Adler et al., 2006; Kennedy and Tessier-Lavigne, 1995). The molecular nature of Netrin suggests, prima facie, that the synaptic targeting events could just be short-range guidance events that refine growth cone steering. However, the experimental data are also consistent with the possibility that Netrin plays a role in transforming the growth cone into a presynaptic structure.
Given Netrin’s requirement on directing short-range targeting, it is not possible to conclusively determine if Netrin plays any additional downstream synaptogenic roles in the RP7 motoneuron. The reason for this is that the RP7 motoneuron synapses are terminal button synapses. In terminal button synapses, the final process of synaptic targeting is intimately and seamlessly linked to the synaptic formation step. It is therefore very difficult to separate these two developmental events.
Nonetheless, another class of synapses, en passant synapses, provides a way of developmentally differentiating between short-range guidance events, such as synaptic targeting, and bona fide synapse formation. Unlike terminal button synapses, en passant synapses are formed along the length of the axon. Because it is the axonal shaft, and not the growth cone, which transforms into a presynaptic specialization, en passant synapses do not require the short-range guidance events that target the growth cones prior to synaptic assembly. Instead, during en passant synapse formation, guidance and fasciculation events bring the axonal shaft in contact with the potential postsynaptic targets. Once this neural framework is established, downstream synaptic specification events direct the formation of synaptic structures along the axonal shaft, discriminating between the fasciculating ncurites to innervate the correct synaptic partners. For en passant synapse formation, contact between fasciculating neurites is necessary, but not sufficient to direct synaptic formation, indicating the existence of downstream synaptic specificity events that direct cell–cell recognition and synapse formation (White et al., 1986). The cytoarchitecture of en passant synapses facilitates the identification, and separation, of events involved in synaptic specificity from those involved in axon targeting.
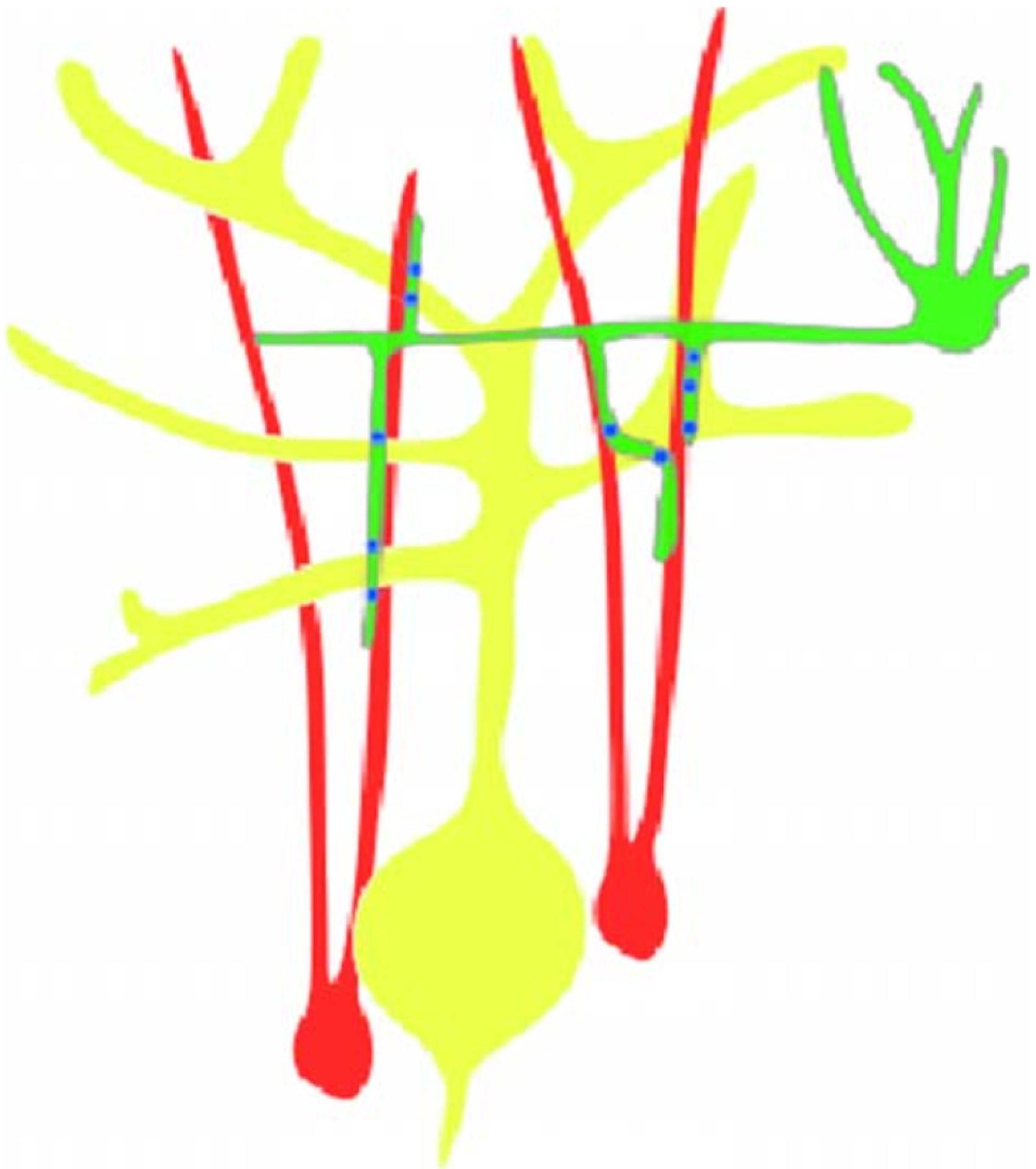
By studying the en passant synapses in the nematode C. elegans, the Netrin pathway was found to play a role in directing the formation of presynaptic specializations different from its conventional guidance role. This was done by visualizing the developmental decisions that led to the innervation of two interneurons (AIY and RIA) in the nematode nerve ring. Similar to the RP3 system, this system allowed visualization of the developmental decisions of these neurons with single-cell resolution and in the context of the intact nervous system, but also in vivo (Gray et al., 2005; Mori and Ohshima, 1995). It was observed that AIY must undergo a series of stereotyped guidance decisions to reach and contact RIA. AIY and RIA then connect to each other through en passant synapses formed at discrete regions of their respective processes (White et al., 1986). The resulting AIY: RIA circuit assembles in a stereotyped fashion in 100% of wild-type animals (Fig. 2.3).
Figure 2.3.

Guidepost cells direct synaptic specification. Schematic representing examples of guidepost cells directing synaptic specification in C. elegans. (A) Image of C. elegans with the discussed regions boxed. (B) Box-I: In the nematode head, interneuron AIY (black) contacts many neurons, but connects specifically to interneuron RIA (not shown) at a subcellular region of its neurite (boxed). This specificity is directed by ventral cephalic sheath cells (pink). Ventral cephalic sheath cells are glial cells that project a process posteriorly, where it contacts AIY and RIA, and also express Netrin. The expression of Netrin directs presynaptic assembly (in green) in the correct subcellular region of AIY (boxed region). Box-II: In the nematode egg-laying circuit, neuron HSNL (in black) innervates other neurons and muscles (not shown) in a specific and stereotyped fashion. The postsynaptic partners of HSNL are not required for the precise assembly of presynaptic specializations (in green) in a subcellular region of HSNL. Instead, guidepost epithelial cells (dark spheres) express an immunoglobulin superfamily receptor (SYG-2) that directs where synapses form in HSNL. Box-Ill: In the posterior part of the nematode, neuron DA9 (in black) elaborates a dendrite anteriorly within the ventral nerve cord and extends an axon commissurally and then longitudinally along the dorsal nerve cord. UNC-6/Netrin (pink) and LIN-44/Wnt (blue) direct synaptic specification (green) by inhibiting formation of synapses from discrete subcellular domains. Expression of lin-44/wnt (blue) by cells in the posterior part of the animal prevents ectopic synapse formation in the commissure, while expression of Netrin (pink) by ventral cells excludes presynaptic components from the ventral dendrite.
In the absence of Netrin or the Netrin receptor, the majority of AIY cells still make their guidance decisions correctly. In spite of the fact that the guidance decisions are normal in the majority of animals, AIY fails to properly form presynaptic specializations at the usual site of contact with RIA (Fig. 2.3). Cell-autonomous rescue of the Netrin receptor in the presynaptic neuron AIY is sufficient to rescue the presynaptic patterning defect, and when the subcellular localization of the Netrin receptor was visualized, it was observed that the Netrin receptor was enriched at the presynaptic sites. Together, these results indicate that the Netrin pathway plays a role in organizing synaptogenesis and that this role is independent of its function in guidance (Colon-Ramos et al., 2007).
A recent report on the identification of a novel interactor in the Netrin pathway supports this newfound role of Netrin in organizing presynaptic assembly. CLEC-38, a transmembrane protein with C-type-lectin-like domains, was recently shown to both regulate outgrowth activity of growth cones, and the organization of presynaptic terminals. In some developmental contexts CLEC-38 acts by inhibiting the Netrin receptor, thereby regulating neural outgrowth during guidance. In other developmental contexts, however, CLEC-38 does not regulate outgrowth, but is instead required for the organization of presynaptic terminals. Although it is not yet known if this presynaptic role of CLEC-38 is also mediated via the Netrin receptor, these data showed that CLEC-38, a regulator of Netrin activity and axon outgrowth, plays additional roles in organizing presynaptic specializations (Kulkarni et al., 2008).
Other families of guidance molecules have also been observed to play synaptogenic roles depending on the developmental context. For instance, in vertebrates, guidance molecules such as the Eph family of receptors and their ephrin ligands have been shown to play roles in growth cone guidance as well as the development of mature excitatory synapses (Dalva et al., 2000). The distinct cellular responses of these different developmental events are also likely generated by the developmental context and by diverse downstream targets (Murai and Pasqualc, 2004). Another family of receptors, the LAR-like phosphatase receptors, has also been shown to function at the level of axon guidance and presynaptic organization. Cell biological and genetic characterizations of this receptor showed that in nematodes, these two different activities are regulated by differentially spliced isoforms of the same receptor (Ackley et al., 2005).
The existence of these shared pathways underscores the molecular link between guidance and synaptogenesis. It also provides a conceptual framework on how growth cones, upon reaching their synaptic targets, could transition from being an outgrowth structure to a presynaptic terminal. But how can the same protein “molecularly multitask” and direct different developmental functions? How can the same receptor and ligand elicit diverse cellular responses?
In the case of the Netrin receptor, for instance, the same molecule can regulate cell migration, neuronal polarization, axon guidance, and synapse formation in different developmental contexts. Although these events have very different outcomes, there is an underlying similarity at the cell biological level: all of these events impinge on a polarization process that restructures the cytoskeleton. For example, during the maturation of the C. elegans neuron HSN, Netrin restricts the Netrin receptor localization to the neuronal side facing the source of Netrin. This leads to the polarized formation of the HSN growth cone (Adler et al., 2006). During guidance, Netrin polarizes the growth cone cytoskeleton to generate directed growth (Kennedy and Tessier-Lavigne, 1995). Similarly, in the AIY interneuron Netrin induces a localized polarization event to transform a region of the axon shaft into a specialized presynaptic area (Colon-Ramos et al., 2007).
It is not well understood how downstream factors and regulators parcel out these signals to result in different developmental outcomes. However, with our increasing molecular understanding of these developmental events, it has become clear that guidance and synaptogenesis are intimately linked at a molecular level. Future work on these signal transduction pathways will allow us to understand how the same ligand/receptor pair can elicit distinct developmental outcomes, and how these events can be orchestrated in different cells to enable circuit assembly.
3. Building a Synapse
The process of guidance and target recognition is followed by synapse formation. How synapses are assembled is a formidable developmental question in its own right. As discussed previously, synapses need to form onto the right partner, at the right density, and at a specific subcellular location with respect to the dendrites. Moreover, the assembly of presynaptic sites also needs to match the postsynaptic densities in terms of localization and identity of the neurotransmitter and postsynaptic receptor (Juttner and Rathjen, 2005).
3.1. Cell adhesion in synaptic assembly
Synaptogenesis can be subdivided into two developmentally distinct steps (1) synaptic specificity and (2) synaptic assembly. Synaptic specificity describes the process that directs where synapses form: from the selection of the right partner to the formation of synapses at the right subcellular compartment. Synaptic assembly describes how synapses are formed: from the assembly of the macromolecular presynaptic structure to the formation of the signaling-rich postsynaptic specializations.
How are these two processes integrated to result in correct synaptic development? The genesis of the synapse officially starts with the contact and communication between the pre- and the postsynaptic partners. Therefore, synaptic specificity is traditionally thought to be determined by cell surface molecules that mediate this synaptic partner interaction. The classical model states that contact between correct partners, mediated via cell adhesion molecules, would then trigger inductive events that lead to the assembly and/or differentiation of pre- and postsynaptic specializations (Waites etal., 2005).
Spurred by this classic model, a number of studies have focused on the identification of synaptogenic cell surface molecules. These studies led to the identification of cell adhesion molecules that direct a variety of events in synaptic biology. The nature and importance of these cell–cell signaling molecules in synapse biology have been discussed elsewhere (Akins and Biederer, 2006; Benson et al., 2001; Dalva et al., 2007; Juttner and Rathjen, 2005; Scheiffele, 2003; Yamagata et al., 2003), so in this section we will only provide a very brief summary of the conceptual findings stemming from these studies.
The molecules identified in these studies fall into four functional categories: they either (1) promote stability by linking synaptic partners, (2) direct target recognition, (3) regulate differentiation of pre- and postsynaptic specializations, or (4) modulate synaptic structure and function (Yamagata et al., 2003). For instance, cadherins have been shown to localize to puncta adherentia and direct synaptic morphology (Scheiffele, 2003). Immunoglobulin superfamily (IgSF) adhesion molecules Dscam, DscamL, Sidekick-1, and Sidckick-2 direct lamina-specific connectivity between specific interneurons and retinal ganglion cells in the vertebrate retina (Yamagata et al., 2002). In Drosophila, LRR transmembrane protein capricious directs target specificity between muscle 12 and the motoneurons that innervate it (Shishido et al., 1998). Ephrins, on the other hand, can act through the EphB receptor to induce the clustering of NMDA receptors and postsynaptic development (Dalva et al., 2000).
Interestingly, despite the focused efforts of identifying cell adhesion molecules directly involved in synaptogenesis, only two adhesion molecules have been shown to induce formation of presynaptic specializations: neuroligins and SynCAM1 (Akins and Biederer, 2006). The limited number of identified cell adhesion molecules capable of directly regulating synapse formation suggests that additional cues remain to be discovered. These findings also beg the question of how the connectivity of hundreds of trillions of synaptic connections are specified with a limited number of molecular cues.
3.2. Assembling the synaptic components
The classical view of synaptogenesis suggests that upon synaptic contact between partners, cell adhesion molecules induce the assembly of pre- and postsynaptic specifications. This places assembly downstream of the adhesion-mediated specification events. Nonetheless, in a number of developmental contexts in vivo, synaptic assembly occurs prior to synaptic contact. For instance, during muscle development in vertebrates, AChR clusters concentrate into high density “hotspots” well before the growing axon has arrived. This postsynaptic clustering of AChR receptors is also observed in aneural myotube cultures and in muscles of animals that have been genetically rendered aneural (Kummcr et al., 2006). These experiments indicate that postsynaptic AChR clusters can occur prior to synaptic contact and in the absence of presynaptic neural factors.
Presynaptic specializations can also form prior to cell–cell contact between synaptic partners. For instance, detailed ultrastructural studies in Xenopus laevis tadpoles revealed that presynaptic specializations develop prior to the association of the axon with the dendrites (Vaughn, 1989). These observations are also supported by ultrastructural studies in the developing cortex of vertebrates, which revealed the existence of pre- and postsynaptic specializations that formed prior to the contact between synaptic partners (Craig and Lichtman, 2001).
Tissue culture studies have also supported the notion that synaptic contact is not necessary for the establishment of pre- and postsynaptic specializations. For instance, studies in dissociated hippocampal neurons have demonstrated that, prior to cell–cell contact, functional NMDA and non-NMDA-type glutamate receptors are present on the cell surface (Craig and Lichtman, 2001). Functional studies indicate that these “free” pre- and postsynaptic structures contain the core molecular machinery necessary for their function (Krueger et al., 2003).
Together, these studies indicate some important aspects of the process of synapse formation. First, they demonstrate that synaptic partners are not necessary for the assembly of synaptic components: both the release machinery in presynaptic structures and the neurotransmitter receptor clusters in postsynaptic structures can be established independent of one another. Second, these experiments highlight the developmental and the genetic separation between synaptic assembly and synaptic specificity.
This functional separation is further underscored in tissue culture systems that can reconstitute assembly, but not specificity events. In tissue culture systems, neurons are dissociated, plated, and allowed to form synaptic connections onto neighboring neurons. The dissociation of neurons disrupts the architecture of the nervous system, thereby destroying much of the positional information which mediates synaptic specificity. Nonetheless, dissociated neurons still retain the ability to form synapses to neighboring neurons, to themselves, or even onto polylysine-coated beads (Vaughn, 1989). These data suggest that assembly and specificity events arc likely mediated through distinct signal transduction pathways.
Given the genetic separation between these events, how are they linked to enable the assembly of a precisely wired nervous system? For instance, is the observed assembly of “half-synapses” a transient and nonspecific feature of neuronal development? Can they actually influence where synapses will be ultimately formed?
A number of studies have now shown that these “half-synapses” can participate in directing where synapses form. Neurodevelopmental studies in zebrafish embryos showed that postsynaptic AChR aggregates formed in advance of growing axons. Although some aggregates dispersed before innervation, surprisingly, filopodia were stabilized when they contacted the AChR aggregates (Kummer et al., 2006). Furthermore, in dissociated hippocampal cultures, preformed postsynaptic scaffold protein complexes, containing PSD-95, GKAP, Shank, and neuroligin 1, localized to predefined postsynaptic hotspots. Upon contact with axons, these scaffolding complexes induced the recruitment of synaptophysin-containing transport vesicles and the formation of presynaptic specializations (Gerrow et al., 2006). These results suggest that assembly prior to synaptic contact can later influence where synapses will form.
3.3. Guidepost cells, morphogens, and connectivity
The studies discussed in the previous section indicate that the assembly of synapses does not require contact between synaptic partners. Moreover, given the role of these preformed specializations in directing where synapses form, these data suggest that in some developmental contexts, the specification of where synapses form is also not dependent on the contact between synaptic partners. Which molecular mechanisms direct synaptic specificity in these contexts?
Accumulating evidence suggests that both intrinsic and extrinsic mechanisms can influence where synapses are formed. For instance, studies in dissociated cortical neuronal cultures revealed that initial formation of presynaptic terminals preferentially occurs at predefined sites within the axonal shaft. In these studies, time-lapse imaging was conducted to track the movement of synaptic vesicle protein transport vesicles (STVs), an organelle containing synaptic vesicle-associated proteins which gets recruited to nascent synapses. It was observed that, even in the absence of postsynaptic partners or glia contact, STVs paused at predefined sites. Upon contact with presumptive postsynaptic partners, presynaptic terminals developed specifically at these predefined sites. Moreover, these sites promoted the formation of stable contacts with dendritic filopodia (Sabo et al., 2006). These studies indicate that intrinsic, predefined pause sites in axon shafts can influence the development of nascent synapses at particular sites along the axon in dissociated neurons.
Extrinsic signals generated by guidepost cells can also provide cues that direct where synapses are assembled. For instance, in C. elegans the egg-laying motor neuron (HSNL) specifically innervates muscles and VC neurons in the vulva region of the animal (Fig. 2.3). Surprisingly, the postsynaptic partners are not required for correct formation of presynaptic specializations in HSNL. Instead, guidepost epithelial cells provide a positional cue that directs HSNL presynaptic assembly. This is molecularly mediated through a pair of IgSF proteins, SYG-1 and SYG-2. In syg-1 or syg-2 mutants, presynaptic neuron HSNL contacts its normal synaptic partners but fails to form synaptic connections with them. Instead, ectopic synapses are formed onto abnormal postsynaptic targets. SYG-1 and SYG-2 both localize to synapses and bind to each other, acting as a receptor and a ligand. SYG-1 functions cell autonomously in the presynaptic neuron, while SYG-2 functions in the guidepost epithelial cells that are essential for the correct formation of HSNL synapses (Shen and Bargmann, 2003; Shen et al., 2004).
Glial cells can also serve as guideposts in directing the innervation of neurons. As discussed earlier, in the C. elegans nerve ring, two interneurons (RIA and AIY) reliably innervate each other at stereotyped locations (Fig. 2.3). A pair of nearby glia-like cells serve as guideposts for the innervation of these two interneurons (Colon-Ramos et al., 2007). In the vertebrate cerebellum, glia cells direct the innervation of two interneurons: stellate and Purkinje cells. Stellate cells exclusively innervate the Purkinje neuron dendrites, and this precision is critical for the proper functioning of these cerebellar GABAergic circuits (Fig. 2.4). It was observed that stellate cells associated with Bergmann glia (BG) during development, and followed the glia process by extending their axon through the curving contours of the BG fibers. By following the guidepost BG fibers, stellate cell processes are able to reach their targets: the dendrite of the Purkinje neurons (Ango et al., 2008).
Figure 2.4.
Bergmann glia direct the innervation of Stellate axons to the Purkinje dendrites. Purkinje neurons (yellow) are innervated by stellate interneurons (green) exclusively at the dendrites. This precision is directed by Bergmann glia (red), which act as guideposts by directing the stellate interneuron process to their Purkinje neuron targets (synapses in blue).
The factor required in both BG and stellate cells for the proper development of this circuit is an L1 family immunoglobulin cell adhesion molecule, CHL1. Interestingly, previous work had shown that another member of the L1 family, neurofascin l86, is required for the specification of another part of this GABAergic circuit: the innervation of the Basket cells and the Purkinje cell axon initial segment (Ango et al, 2004). This molecular characterization of the cerebellar GABAergic circuit suggests that different members of the L1CAM protein family contribute to circuit formation through their cell-specific expression in subsets of neurons and guidepost glial cells.
Some neurons in the vertebrate hippocampus and cortex can also act as guideposts and direct synaptogenesis. For instance, the transient population of Cajal-Retzius cells in the hippocampus serves as a placeholder to facilitate the meeting of the appropriate pre- and postsynaptic cells (Del Rio et al., 1997). Also, during the development of the mammalian cortex, the subplate neurons display a similar guidepost function to arrange the connectivity between the thalamic axons and the layer 4 cortical neurons (McConnell et al., 1989). The significance of these guidepost cells was demonstrated by ablating the guidepost cells and showing a synaptic connectivity defect in ablated animals (Del Rio et al., 1997; Ghosh et al., 1990).
Tissues can also provide inhibitory signals to direct the formation of synapses with subcellular precision. In the DA9 motoneuron of C. elegans, Wnt lin-44 is secreted by four hypodermal cells in the tail. This expression localizes receptor lin-17/Frizzled (Fz) to a subdomain of DA9, near the posterior part of the neurite adjacent to the hypodermal cells. This part of the neurite where lin-17/Fz localizes is normally devoid of presynaptic specializations (Fig. 2.3). When the Wnt pathway is compromised, however, synapses develop ectopically in this subdomain. Moreover, overexpression of WNT lin-44 in the posterior part of the animal expands LIN-17 localization and inhibits presynaptic assembly in these new sites of ectopic LIN-17 localization (Klassen and Shen, 2007). The Wnt pathway was also shown to act as a local repressive cue to direct target specificity in Drosophila. Studies in embryonic motor neurons showed that Wnt4 acts via Frizzled 2, Derailed-2, and Disheveled to generate target specificity by preventing synapse formation onto nontarget muscles (Inaki et al., 2007).
Wnts can also stimulate the formation of synapses in both vertebrates and invertebrates (Salinas and Zou, 2008). In Drosophila, Wnt/wingless is required for the correct development of presynaptic boutons, in terms of both their numbers and their structure, and this activity occurs in a transcription-independent manner (Miech et al., 2008). These observations, together with studies showing a role of Wnts in postsynaptic activation of Frizzled receptors, indicate that Wnt signaling can alter synaptic development by simultaneously modulating the development of presynaptic and postsynaptic structures (Miech et al, 2008).
But how can Wnts both promote and inhibit the formation of synapses? Recent studies in dissociated hippocampal cultures have demonstrated that the opposing effects of Wnts on synapse formation depend on different Wnt ligands. Interestingly, the different ligands differentially activate either the canonical or the noncanonical pathways: activation of the canonical pathway promotes synapse formation, while activation of the noncanonical one inhibits synapse formation (Davis et al., 2008).
Together, these studies demonstrate that in both vertebrates and invertebrates, morphogenic signals such as Wnts can spatially regulate the patterning of synaptic connections. This is achieved by subdividing the neurite into discrete domains and either preventing or promoting synapse formation at specific subcellular compartments.
Other extrinsic cues can also direct neuronal polarity that ultimately impinges on the site of synaptic assembly in vivo. In a study that also used the DA9 motoneuron system, it was shown that the axon guidance cue UNC-6/netrin and its receptor UNC-5 act to exclude synaptic vesicles and active zone proteins from the dendrite of DA9 (Fig. 2.3). In unc-6/netrin and unc-51oss-of-function mutants, presynaptic components mislocalize to the DA9 dendrite, where the level of endogenous UNC-6/netrin is high. In addition, ectopically expressed UNC-6/netrin, acting through UNC-5, was sufficient to exclude endogenous synapses from adjacent subcellular domains within the DA9 axon. Interestingly, this antisynaptogenic activity was interchangeable with that of LIN-44/Wnt (Poon et al, 2008). This suggests that extracellular cues such as netrin and Wnts not only guide axon navigation but also regulate the polarized accumulation of presynaptic components through local exclusion.
Together, these studies indicate that extrinsic signals generated from guidepost cells or neighboring tissues can direct the site of synaptic assembly, thereby modulating synaptic specificity. Although these examples indicate that synaptic contact is not required for the initial specification of synaptogenesis, it should be noted that contact with a postsynaptic-like substrate is required for the eventual stabilization and perseverance of many of these “free” pre- and postsynaptic sites (Vaughn, 1989).
Contact and communication between the synaptic partners is also required for the regulation of the size and shape of the synapse, a process known as synaptic homeostasis (Keshishian, 2002). During synaptic homeostasis, a crosstalk between pre- and postsynaptic specializations takes place across the synaptic cleft. Depending on the developmental context, this crosstalk can be mediated by a number of different molecular cues, which includes fibroblast growth factors (FGFs), bone morphogenetic protein (BMP), signal transduction pathways, and MEF-2 transcriptional responses (Aberle et al, 2002; Goold and Davis, 2007; Salinas, 2005; Simon et al., 2008). The trans-synaptic communication between partners elicits a coordinated regulation of synaptic size, shape, and functionality.
Contact between synaptic partners is also required for the proper modulation of synaptic activity. Although in this chapter we focused our discussion on the early synaptogenic decisions preceding synaptic activity, it should be noted that it is this synaptic activity that, in most developmental contexts, ultimately regulates the stabilization or elimination of many synapses (Flavell and Greenberg, 2008). The early developmental decisions discussed in this chapter generate a neural framework over which activity-dependent changes occur. The activity-dependent regulation of synaptic biology is required, in both the developing and the mature nervous systems, for the maturation, refinement, and plasticity of synaptic connections.
4. Perspective
Correct circuit formation requires an intricate orchestration of multiple developmental events including cell migration, axon guidance, dendritic growth, target selection, and synaptogenesis (Juttner and Rathjen, 2005; Salie et al., 2005; Waites et al, 2005). These events are integrated to enable correct synapse formation between neuronal partners. The developmental innervation of synaptic partners results in hundreds of trillions of precisely wired synaptic connections. Since the human genome has an estimated 25,000 genes, and not all genes are involved in synaptogenesis, these events need to be simultaneously orchestrated in billions of neurons using a limited set of molecular cues.
Genetic, biochemical, and cell biological studies have revealed some of the molecular cues that regulate these developmental processes. Studies of these signaling molecules have revealed classical roles for different protein families. For instance, morphogens, which are known to create gradients with important positional information, are critical for the specification of cell fate decisions. Transmembrane proteins that recognize diffusible factors have been shown to direct processes such as dendritic and axonal outgrowth and guidance. On the other hand, cell–cell adhesion proteins can control synaptogenesis. This has led to the conceptual understanding that gene families with evolutionarily conserved functions could play modular roles in patterning the nervous system (Salie et al., 2005).
Although different protein families can play distinct roles at discrete developmental steps, this modular model of nervous system development does not reflect the complexity of this process in vivo. A growing body of literature shows that molecular cues, far from playing exclusive roles at discrete steps, are instead capable of “molecular multitasking” (Salic et al., 2005). For example, morphogenic proteins such as Sonic hedgehog, Wnts, FGF, and BMP have long been known to direct neuronal cell fate by eliciting transcriptional programs. More recent studies have demonstrated that, depending on the cellular context, these canonical morphogens can also provide instructive, transcription-independent signals to control processes such as axon outgrowth, neuronal cell polarity, and synapse formation (Salie et al., 2005). Additionally, signal transduction cascades that have been traditionally thought of as long-range guidance cues have now also been observed to regulate synaptic formation events.
It is provocative to think that “molecular multitasking” could have profound implications for the development of neural circuits. For instance, through the same receptor:ligand pair, multiple signal transduction pathways could be simultaneously activated in different cells with different developmental outcomes. One could speculate that this would allow a single molecular cue to simultaneously direct several independent developmental outcomes in different cells, thereby coordinating circuit assembly by orchestrating the innervation of multiple partners.
The identification of molecular factors and signal transduction cascades involved in synapse formation, combined with an increased understanding of how these molecular factors are integrated to direct circuit formation in vivo, will provide us with an increasingly clear picture on how precise synaptogenesis is orchestrated during nervous system development.
ACKNOWLEDGMENTS
I thank M. Hammarlund, S. Margolis, M. Margeta, and G. Maro for thoughtful comments concerning this chapter. I particularly thank K. Shen for helpful discussions, generous advice, and thoughtful comments on the chapter. I also thank J. Blagbum, V. Poon, and F. Ango for contributing images. I apologize to those whose work I did not cite here due to oversight or space constrains. During the preparation of this chapter, I was supported by NIH grant 4R00NS057931-03.
REFERENCES
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, and Goodman CS (2002). Wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33, 545–558. [DOI] [PubMed] [Google Scholar]
- Ackley BD, Harrington RJ, Hudson ML, Williams L, Kenyon CJ, Chisholin AD, and Jin Y (2005). The two isoforms of the Caenorhabditis elegans leukocyte-common antigen related receptor tyrosine phosphatase PTP-3 function independently in axon guidance and synapse formation. J. Neurosci 25, 7517–7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler CE, Fetter RD, and Bargmann CI (2006). UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat. Neurosci 9, 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins MR, and Biederer T (2006). Cell-cell interactions in synaptogenesis. Curr. Opin. Neurobiol 16, 83–89. [DOI] [PubMed] [Google Scholar]
- Anderson PA, and Spencer AN (1989). The importance of cnidarian synapses for neurobiology. J. Neurobiol 20, 435–457. [DOI] [PubMed] [Google Scholar]
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, and Huang ZJ (2004). Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell 119, 257–272. [DOI] [PubMed] [Google Scholar]
- Ango F, Wu C, Van der Want JJ, Wu P, Schachner M, and Huang ZJ (2008). Bergmann glia and the recognition molecule CHL1 organize GABAergic axons and direct innervation of Purkinje cell dendrites. PLoS Biol. 6, el03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DL, Colman DR, and Huntley GW (2001). Molecules, maps and synapse specificity. Nat. Rev. Neurosci 2, 899–909. [DOI] [PubMed] [Google Scholar]
- Blagburn JM, and Bacon JP (2004). Control of central synaptic specificity in insect sensory neurons. Annu. Rev. Neurosci 27, 29–51. [DOI] [PubMed] [Google Scholar]
- Chiba A, and Rose D (1998). “Painting” the target: How local molecular cues define synaptic relationships. Bioessays 20, 941–948. [DOI] [PubMed] [Google Scholar]
- Colon-Ramos DA, Margeta MA, and Shen K (2007). Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science 318, 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan WM, and Kandel ER (2001). A brief history of synapses and synaptic transmittion In “Synapses” (Sudhof TC, Cowan WM, and Stevens CF, Eds.), pp. 1–88. The Johns Hopkins University Press, Baltimore. [Google Scholar]
- Craig AM, and Lichtman JW (2001). Synapse formation and maturation In “Synapses” (Sudhof TC, Cowan WM, and Stevens CF, Eds.), pp. 1–88. The Johns Hopkins University Press, Baltimore. [Google Scholar]
- Dalva MB, McClelland AC, and Kayser MS (2007). Cell adhesion molecules: Signalling functions at the synapse. Nat. Rev. Neurosci 8, 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, and Greenberg ME (2000). EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell 103, 945–956. [DOI] [PubMed] [Google Scholar]
- Davis EK, Zou Y, and Ghosh A (2008). Wnts acting through canonical and noncanonical signaling pathways exert opposite effects on hippocampal synapse formation. Neural Develop. 3, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P, Haucke V, Takei K, and Mugnaini E (2001). The structure of synapses In “Synapses” (Sudhof TC, Cowan WM, and Stevens CF, Eds.), pp. 1–88. The Johns Hopkins University Press, Baltimore. [Google Scholar]
- Del Rio JA, Heimrich B, Borrell V, Forster E, Drakew A, Alcantara S, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Derer P, Frotsher M, and Soriano E (1997). A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature 385, 70–74. [DOI] [PubMed] [Google Scholar]
- Flavell SW, and Greenberg ME (2008). Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu. Rev. Neurosci 31, 563–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrow K, Romorini S, Nabi SM, Colicos MA, Sala C, and El-Husseini A (2006). A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron 49, 547–562. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell SK, and Shatz CJ (1990). Requirement for subplate neurons in the formation of thalamocortical connections. Nature 347, 1792–181. [DOI] [PubMed] [Google Scholar]
- Goold CP, and Davis GW (2007). The BMP ligand Gbb gates the expression of synaptic homeostasis independent of synaptic growth control. Neuron 56, 109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Hill JJ, and Bargmann CI (2005). Inaugural article: A circuit for navigation in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 102, 3184–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaki M, Yoshikawa S, Thomas JB, Aburatani H, and Nose A (2007). Wnt4 is a local repulsive cue that determines synaptic target specificity. Curr. Biol 17, 1574–1579. [DOI] [PubMed] [Google Scholar]
- Juttner R, and Rathjen FG (2005). Molecular analysis of axonal target specificity and synapse formation. Cell. Mol. Life Sci 62, 2811–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy TE, and Tessier-Lavigne M (1995). Guidance and induction of branch formation in developing axons by target-derived diffusible factors. Curr. Opin. Neurobiol 5, 83–90. [DOI] [PubMed] [Google Scholar]
- Keshishian H (2002). Is synaptic homeostasis just wishful thinking? Neuron 33, 491–492. [DOI] [PubMed] [Google Scholar]
- Klassen MP, and Shen K (2007). Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell 130, 704–716. [DOI] [PubMed] [Google Scholar]
- Krueger SR, Kolar A, and Fitzsimonds RM (2003). The presynaptic release apparatus is functional in the absence of dendritic contact and highly mobile within isolated axons. Neuron 40, 945–957. [DOI] [PubMed] [Google Scholar]
- Kulkami G, Li H, and Wadsworth WG (2008). CLEC-38, a transmembrane protein with C-type lectin-like domains, negatively regulates UNC-40-mediated axon outgrowth and promotes presynaptic development in Caenorhabditis elegans. J. Neurosci 28, 4541–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer TT, Misgeld T, and Sanes JR (2006). Assembly of the postsynaptic membrane at the neuromuscular junction: Paradigm lost. Curr. Opin. Neurobiol 16, 74–82. [DOI] [PubMed] [Google Scholar]
- Lickteig KM, Duerr JS, Frisby DL, Hall DH, Rand JB, and Miller DM 3rd. (2001). Regulation of neurotransmitter vesicles by the homeodomain protein UNC-4 and its transcriptional corepressor UNC-37/groucho in Caenorhabditis elegans cholinergic motor neurons. J. Neurosci 21, 2001–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie B, Bacon JP, and Blagburn JM (2000). Double-stranded RNA interference shows that Engrailed controls the synaptic specificity of identified sensory neurons. Curr. Biol 10, 289–292. [DOI] [PubMed] [Google Scholar]
- Marie B, and Blagburn JM (2003). Differential roles of engrailed paralogs in determining sensory axon guidance and synaptic target recognition. J. Neurosci 23, 7854–7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie B, Cruz-Orengo L, and Blagburn JM (2002). Persistent engrailed expression is required to determine sensory axon trajectory, branching, and target choice. J. Neurosci 22, 832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, and Shatz CJ (1989). Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science 245, 978–982. [DOI] [PubMed] [Google Scholar]
- Miech C, Pauer HU, He X, and Schwarz TL (2008). Presynaptic local signaling by a canonical wingless pathway regulates development of the Drosophila neuromuscular junction. J. Neurosci 28, 10875–10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DM, Shen MM, Shamu CE, Burglin TR, Ruvkun G, Dubois ML, Ghee M, and Wilson L (1992). C. elegans unc-4 gene encodes a homeodomain protein that determines the pattern of synaptic input to specific motor neurons. Nature 355, 841–845. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Doyle JL, Serafini T, Kennedy TE, Tessier-Lavigne M, Goodman CS, and Dickson BJ (1996). Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron 17, 203–215. [DOI] [PubMed] [Google Scholar]
- Mori I, and Ohshima Y (1995). Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 376, 344–348. [DOI] [PubMed] [Google Scholar]
- Murai KK, and Pasquale EB (2004). Eph receptors, ephrins, and synaptic function. Neuroscientist 10, 304–314. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Chou SJ, and Sahara S (2007). Area patterning of the mammalian cortex. Neuron 56, 252–269. [DOI] [PubMed] [Google Scholar]
- Peteya DJ (1973). A light and electron microscope study of the nervous system of Ceriantheopsis americanus (Cnidaria, Ceriantharia). Z. Zellforsch Mikrosk Anat 141, 301–317. [DOI] [PubMed] [Google Scholar]
- Plachez C, and Richards LJ (2005). Mechanisms of axon guidance in the developing nervous system. Curr. Top. Dev. Biol 69, 267–346. [DOI] [PubMed] [Google Scholar]
- Polleux F, Ince-Dunn G, and Ghosh A (2007). Transcriptional regulation of vertebrate axon guidance and synapse formation. Nat. Rev. Neurosci 8, 331–340. [DOI] [PubMed] [Google Scholar]
- Poon VY, Klassen MP, and Shen K (2008). UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature 455, 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand JB, and Nonet ML (1997). C. elegans II In “Synaptic Transmission” (Riddle DL, Blumenthal T, Meyer BJ, and Priess JR, Eds.), vol. II, pp. 611–643. Cold Spring Harbor Laboratory Press, Cold Spring Harbor. [PubMed] [Google Scholar]
- Sabo SL, Gomes RA, and McAllister AK (2006). Formation of presynaptic terminals at predefined sites along axons. J. Neurosci 26, 10813–10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakarya O, Armstrong KA, Adamska M, Adamski M, Wang IF, Tidor B, Degnan BM, Oakley TH, and Kosik KS (2007). A post-synaptic scaffold at the origin of the animal kingdom. PLoS ONE 2, e506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salie R, Niederkofler V, and Arber S (2005). Patterning molecules; multitasking in the nervous system. Neuron 45, 189–192. [DOI] [PubMed] [Google Scholar]
- Salinas PC (2005). Signaling at the vertebrate synapse: New roles for embryonic morphogens? J. Neurobiol 64, 435–445. [DOI] [PubMed] [Google Scholar]
- Salinas PC, and Zou Y (2008). Wnt signaling in neural circuit assembly. Annu. Rev. Neurosci 31, 339–358. [DOI] [PubMed] [Google Scholar]
- Scheiffele P (2003). Cell-cell signaling during synapse formation in the CNS. Annu. Rev. Neurosci 26, 485–508. [DOI] [PubMed] [Google Scholar]
- Shen K, and Bargmann CI (2003). The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell 112, 619–630. [DOI] [PubMed] [Google Scholar]
- Shen K, Fetter RD, and Bargmann CI (2004). Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell 116, 869–881. [DOI] [PubMed] [Google Scholar]
- Shishido E, Takeichi M, and Nose A (1998). Drosophila synapse formation: Regulation by transmembrane protein with Leu-rich repeats, CAPRICIOUS. Science 280, 2118–2121. [DOI] [PubMed] [Google Scholar]
- Simon DJ, Madison JM, Conery AL, Thompson-Peer KL, Soskis M, Ruvkun GB, Kaplan JM, and Kim JK (2008). The microRNA miR-1 regulates a MEF-2-dependent retrograde signal at neuromuscular junctions. Cell 133, 903–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC (2004). The synaptic vesicle cycle. Annu. Rev. Neurosci 27, 509–547. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, and Goodman CS (1996). The molecular biology of axon guidance. Science 274,1123–1133. [DOI] [PubMed] [Google Scholar]
- Vaughn JE (1989). Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse 3, 255–285. [DOI] [PubMed] [Google Scholar]
- Von Stetina SE, Fox RM, Watkins KL, Starich TA, Shaw JE, and Miller DM 3rd. (2007). UNC-4 represses CEH-12/HB9 to specify synaptic inputs to VA motor neurons in C. elegans. Genes Dev. 21 332–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Stetina SE, Treinin M, and Miller DM 3rd. (2006). The motor circuit. Int. Rev. Neurobiol 69 125–167. [DOI] [PubMed] [Google Scholar]
- Waites CL, Craig AM, and Garner CC (2005). Mechanisms of vertebrate synaptogenesis. Annu. Rev. Neurosci 28, 251–274. [DOI] [PubMed] [Google Scholar]
- Westfall IA (1996). Ultrastructure of synapses in the first-evolved nervous systems. J. Neurocytol 25, 735–746. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, and Thomson JN (1992). Mutations in the Caenorhabditis elegans unc-4 gene alter the synaptic input to ventral cord motor neurons. Nature 355, 838–841. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, and Brenner S (1986). The structure of the nervous system of the nematode Caenorhabditis elegans. Philosophical Transactions of the Royal Society of London 314, 1–340. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR, and Weiner JA (2003). Synaptic adhesion molecules. Curr. Opin. Cell. Biol 15, 621–632. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, and Sanes JR (2002). Sidekicks: Synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell 110, 649–660. [DOI] [PubMed] [Google Scholar]


