Abstract
WNT/β-catenin signaling promotes stemness, proliferation, and cell fate decisions in various tissue stem cell compartments, which maintain organs with a high turnover of cells (e.g., skin, stomach, and gut). Thus, the β-catenin target genes AXIN2 and LGR5 are widely considered as tissue stem cell markers. In contrast, AXIN2 and LGR5 are expressed in pericentral hepatocytes, which do not show overt proliferation during liver homeostasis. Given the low hepatocyte turnover, the liver does not require constant high rates of proliferation, whereas WNT/β-catenin signaling is critical for metabolic zonation. Yet, WNT/β-catenin pathway upregulation, including AXIN2 and LGR5 induction in hepatocytes throughout the liver, enables hepatocyte regeneration in response to various injuries. In this brief review, I discuss the role of WNT/β-catenin signaling in controlling metabolic zonation and the conundrum around pericentral hepatocytes that have been proposed as liver stem cells.
Key words: WNT signaling, Metabolic zonation, Homeostasis, Liver stem cell, Tissue stem cell, AXIN2, LGR5, Liver regeneration
WNT/β-CATENIN SIGNALING IN THE LIVER
The WNT/β-catenin pathway is a highly conserved master regulator controlling diverse critical processes, including organ patterning, cell fate decisions, and cell proliferation during development, homeostasis, and regeneration1–3. In the absence of WNT ligands, β-catenin is bound to the destruction complex, which contains the tumor suppressor adenomatous polyposis coli (APC), glycogen synthase kinase 3β (GSK3β), casein kinase 1α (CK1α), and scaffolding proteins AXIN1/2, among other components. CK1α and GSK3β phosphorylate β-catenin, targeting it for ubiquitin-dependent proteasomal degradation. When WNT ligands bind their cognate Frizzled receptor and the LRP5/6 coreceptor, phosphorylation of LRP5/6 results into decreased activity of the β-catenin destruction complex and stabilization of β-catenin. Stabilized β-catenin accumulates in the cytoplasm and can now enter the nucleus, where it binds to the TCF family of transcription factors, and enhances the transcription of β-catenin target genes1–3. R-spondin (RSPO)1–4 ligands potentiate WNT/β-catenin signaling following binding to their leucine-rich repeat-containing G protein-coupled receptors 4–6 (LGR4–6) by clearing the cell surface transmembrane E3 ubiquitin ligases, zinc and ring finger 3 (ZNRF3), and its homolog ring finger 43 (RNF43), which promote WNT receptor turnover, from the plasma membrane4–11. WNT/β-catenin signaling is also regulated via crosstalk with several other pathways, such as YAP, mTOR, HGF–cMET, Notch, SHH, USP7, and transforming growth factor-β (TGF-β) signaling12–18.
In the liver, genetic deletion of WNT/β-catenin pathway components revealed its important role in promoting hepatic cell fate specification during early development, whereas its role during hepatoblast differentiation and late-stage liver development is less clear. Moreover, the WNT/β-catenin pathway controls proliferation and metabolic zonation during early postnatal liver development. In the adult liver, WNT/β-catenin signaling maintains liver size by controlling hepatocyte proliferation while preserving metabolic zonal patterning. While WNT/β-catenin activity is restricted to the pericentral area during homeostasis, its upregulation in hepatocytes throughout the liver is important to promote hepatocyte proliferation and liver regeneration in response to injury. Deregulated hepatic WNT/β-catenin signaling is also involved in various liver diseases, including liver fibrosis and tumor formation, highlighting the importance of fine-tuning and restricting hepatic WNT/β-catenin activity19,20.
METABOLIC ZONATION
The liver is responsible for metabolizing nutrients and xenobiotics, as well as for the production and recycling of various proteins. Division of labor by hepatocytes in the three different liver zones is critical to enable liver function, since hepatocytes along the porto-central blood flow execute complementary tasks. Periportal hepatocytes perform gluconeogenesis, cholesterol biosynthesis, and urea metabolism, whereas pericentral hepatocytes perform glycolysis, bile acid biosynthesis, and glutamine synthesis. Hepatic metabolic zonation is controlled by the interplay of several pathways, often involving WNT/β-catenin signaling. A centro-portal WNT/β-catenin activity gradient is critical to establish and maintain metabolic zonation since most pericentral metabolic genes are either direct or indirect targets of β-catenin. Classical metabolic enzymes regulated by β-catenin include glutamine synthetase (GS) and CYP2E1, which are expressed only in the one to two first layers of hepatocytes around the central vein or in all pericentral and some adjacent parenchymal hepatocytes, respectively21–30. Several studies utilizing gene deletion of different WNT/β-catenin pathway components collectively suggest that inhibition of WNT/β-catenin signaling results in the loss of metabolic zonation due to lack of pericentral enzyme expression. Conversely, activation of the pathway caused the opposite effect and derailed metabolic zonation by expanding the pericentral metabolic program toward the portal vein24,25,27–32. While these studies established the importance of hepatic WNT/β-catenin signaling, they do not solve the question of how pathway activity is spatially controlled in a gradient manner. APC is expressed in a porto-central gradient, and given its inhibitory function on WNT/β-catenin activity, it may be responsible for blocking β-catenin activity in periportal hepatocytes24. In addition, local WNT and RSPO ligand secretion from central vein endothelial cells (CEVs) and liver sinusoidal endothelial cells (LSECs) seem to be responsible for activating WNT/β-catenin signaling in pericentral and adjacent parenchymal hepatocytes27,30–35. However, the exact mechanisms spatially confining expression of these ligands and thereby establishing the centro-portal WNT/β-catenin activity gradient remain elusive.
PERICENTRAL HEPATOCYTES VERSUS TISSUE STEM CELLS
The intrinsic regenerative capacity of the liver was already described in the myth of Prometheus 2,500 years ago and has been extensively studied in recent decades. While all hepatic cell types and many regenerative pathways collaboratively orchestrate liver regeneration, the WNT/β-catenin pathway stood out as a key regulator of hepatocyte proliferation19,20,36. Genetic labeling of cells with active WNT/β-catenin signaling allowed lineage tracing over time and revealed tissue stem cell compartments in several organs with high rates of homeostatic renewal (e.g., skin, stomach, and gut). WNT/β-catenin signaling controls proliferation, stemness, and cell fate decisions in several tissue stem cell compartments, and the β-catenin target genes AXIN2 and LGR5 were established as tissue stem cell markers37,38.
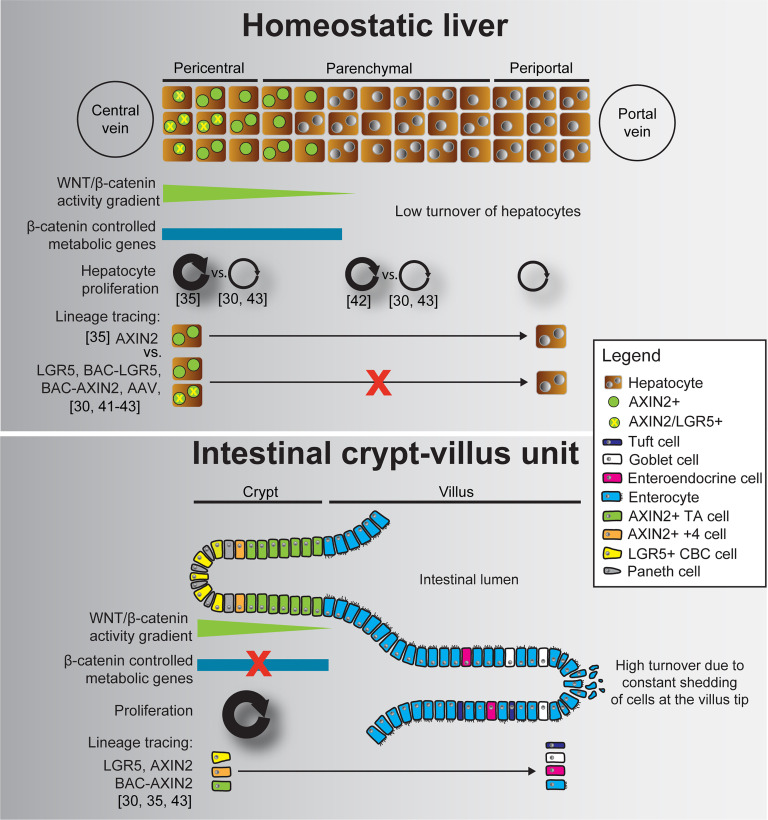
High rates of homeostatic proliferation and the short time, in which descending cells migrate and differentiate, enabled lineage tracing tools to clearly identify tissue stem cells in other organs37,38. In contrast, adult hepatocytes have an average life span ranging from 200 to 300 days, and the majority is resting in a quiescent (G0) state, while only up to 2% are actively cycling at any given time39,40. Such low turnover and the associated low proliferation rates make it more challenging to identify potential hepatocyte subpopulations with increased proliferative potential during liver homeostasis (Fig. 1). It is obvious to imagine that pericentral hepatocytes may be liver stem cells, since they express the tissue stem cell markers AXIN2 and LGR5 and harbor constant WNT/β-catenin signaling, similar to tissue stem cells in other organs. Likewise, several lineage tracing studies have asked the question whether AXIN2/LGR5+ pericentral hepatocytes may be the long sought elusive liver stem cells30,35,41–43. Using AXIN2–CreERT2 lineage tracing mice, Wang and colleagues observed that AXIN2/GS+ hepatocytes at the central vein expanded over time and repopulated large parts of the liver during liver homeostasis. Descendants of AXIN2/GS+ hepatocytes even reached the periportal zone, collectively suggesting that they are liver stem cells refueling the hepatocyte pool under homeostatic conditions35. In contrast, lineage tracing of pericentral hepatocytes using LGR5–CreERT2 mice neither confirmed increased proliferative potential of pericentral hepatocytes nor did it indicate their expansion into the periportal zone30. However, AXIN2 inhibits WNT/β-catenin signaling and is a tumor suppressor in the liver44–46, and AXIN2–CreERT2 lineage tracing mice are heterozygous mutants for Axin2 35. Likewise, LGR5–CreERT2 mice are lacking one Lgr5 allele, with unclear potential consequences in pericentral hepatocytes30.
Figure 1.
WNT/β-catenin signaling, metabolic zonation, proliferation, and tissue stem cell potential in the pericentral hepatocyte zone and the intestinal crypt–villus unit. While proliferation is low and similar in all three liver zones, the intestinal crypt shows high levels of proliferation that are required to compensate for constant loss of cells at the villus tip. Lineage tracing studies* collectively indicate that AXIN2/LGR5+ crypt base columnar (CBC) cells and AXIN2+ transit-amplifying (TA) cells repopulate the villus and give rise to more differentiated cells. In contrast, the majority of lineage tracing studies suggest that AXIN2/LGR5+ pericentral hepatocytes neither repopulate the liver during homeostasis nor do their descendants stream into the periportal zone. *We only mention lineage tracing mice in this figure that have been discussed in this review. Lineage tracing of crypt cells has been pioneered with different LGR5 lineage tracing mice51, and many more mouse lines successfully recapitulated and extended this finding.
To exclude the possibility that potential haploinsufficiency of Axin2 or Lgr5 may bias WNT/β-catenin signaling and proliferation of pericentral hepatocytes, BAC-transgenic LGR5 and AXIN2 lineage tracing mice were developed to shed additional light on the controversial role of AXIN2/LGR5+ pericentral hepatocytes41,43. Interestingly, BAC–LGR5–CreERT2 and BAC–AXIN2–CreERT2, as well as adeno-associated virus (AAV)-based lineage tracing, indicated that pericentral hepatocytes have no superior proliferative capacity over other hepatocytes, do not stream into the periportal zone, and may therefore not represent bona fide liver stem cells41–43 (Fig. 1). Importantly, the initial increase in BAC–AXIN2–CreERT2-labeled pericentral hepatocytes within the first week was due to additional CreERT2-mediated recombination by residual tamoxifen rather than proliferation, with no significant further increase in labeled hepatocytes from day 7 to 10 months of lineage tracing43. While Wang and colleagues found more EdU incorporation in AXIN2/GS+ hepatocytes when compared to AXIN2/GS− hepatocytes35, other studies observed most EdU incorporated in midzonal hepatocytes30,47. However, midzonal hepatocytes also comprise the largest hepatocyte population, and normalization to hepatocyte numbers in each zone indicated similar percentage of EdU+ hepatocytes in the three liver zones30,43. Yet, the overall low hepatocyte proliferation rates (2%–8% in hepatocytes30,35,43, compared to almost 80% proliferating AXIN2+ cells in the small intestine43), the high variability between the studies, and the short duration of EdU labeling make it difficult to judge the proliferative potential of hepatocytes in different hepatic zones30,35,43. Clonal tracing of LGR4+ hepatocytes showed similar clone size in all liver zones, suggesting no zonal dominance during homeostatic hepatocyte proliferation30. In contrast, AAV-based random lineage tracing indicated increased proliferation of midzonal hepatocytes42, which also showed higher baseline levels of the WNT/β-catenin target and cell cycle regulator CYCLIND142,48. Additional research is required to clarify the contribution of hepatocytes in different hepatic zones to maintaining a functional hepatocyte pool during liver homeostasis.
Pericentral hepatocytes did also not display increased regenerative potential in response to various liver injury models41,43. Moreover, DTA-mediated ablation of AXIN2/LGR5+ pericentral hepatocytes only transiently disrupted the pericentral zone, which was reestablished by conversion of GS− into GS+ hepatocytes and compensatory proliferation of hepatocytes in other zones43. In addition, AXIN2 and LGR5 upregulation in hepatocytes outside the pericentral niche is a hallmark of many different liver injury settings where WNT/β-catenin signaling is important to drive hepatocyte proliferation30,43,49,50. With the majority of studies arguing against a liver stem cell role for pericentral hepatocytes, it appears that hepatocytes throughout the liver can upregulate AXIN2 and LGR5 in response to injury and contribute to liver regeneration on demand.
Another conundrum in the pericentral niche is the low rate of homeostatic hepatocyte proliferation despite constant WNT/β-catenin signaling necessary to maintain metabolic zonation. While the intestinal crypt and pericentral liver zone both consist of niche cells providing WNT and RSPO ligands and epithelial cells expressing AXIN2 and LGR5, they seem to utilize WNT/β-catenin signaling differently. Comparative transcriptomic profiling suggests that intestinal stem cells do not express β-catenin-regulated metabolic enzymes but show upregulation of cell cycle genes when compared to AXIN2+ hepatocytes, and vice versa43. Although these are fundamentally different cell types, involving diverse cooperative signaling networks with other pathways, it would be interesting to identify mechanisms allowing these cells to differentially utilize WNT/β-catenin signaling. Most importantly, the mechanisms restricting proliferation in pericentral hepatocytes despite constant WNT/β-catenin necessary to maintain metabolic zonation remain elusive.
CONCLUDING REMARKS
Fine-tuning of WNT/β-catenin signaling is critical for maintaining metabolic zonation in the homeostatic liver, whereas overt proliferation, as seen in tissue stem cell compartments of other organs, must be prevented. Therefore, pericentral hepatocytes require mechanisms restricting their proliferation despite constant WNT/β-catenin activity. It remains to be studied whether epigenetic regulation may prevent induction of cell cycle genes in pericentral hepatocytes. Possibly we could learn from liver regeneration studies, where upregulation of WNT/β-catenin signaling is followed by increased hepatocyte proliferation and therefore signaling changes in hepatocytes reentering the cell cycle may inform about possible mechanistic cues. Given the high amount of liver tumors displaying hyperactive WNT/β-catenin signaling and the high susceptibility of pericentral hepatocytes to produce tumors in experimental models, learning more about mechanisms restricting proliferation in pericentral hepatocytes may further our understanding of liver tumor initiation. Additional lineage tracing studies utilizing different genetic tracing markers and monitoring hepatocyte proliferation over longer periods might further clarify the debated role of pericentral hepatocytes being liver stem cells and the zonal contribution of hepatocytes to liver homeostasis.
REFERENCES
- 1. Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006;127:469–80. [DOI] [PubMed] [Google Scholar]
- 2. Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. [DOI] [PubMed] [Google Scholar]
- 3. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell 2009;17:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hao HX, Xie Y, Zhang Y, ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 2012;485:195–200. [DOI] [PubMed] [Google Scholar]
- 5. Koo BK, Spit M, Jordens I, Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 2012;488:665–69. [DOI] [PubMed] [Google Scholar]
- 6. de Lau W, Barker N, Low TY, Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 2011;476:293–7. [DOI] [PubMed] [Google Scholar]
- 7. de Lau W, Peng WC, Gros P, Clevers H. The R-spondin/Lgr5/Rnf43 module: Regulator of Wnt signal strength. Genes Dev. 2014;28:305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Lau WB, Snel B, Clevers HC. The R-spondin protein family. Genome Biol. 2012;13:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim KA, Kakitani M, Zhao J, Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 2005;309:1256–9. [DOI] [PubMed] [Google Scholar]
- 10. Ruffner H, Sprunger J, Charlat O, R-Spondin potentiates Wnt/beta-catenin signaling through orphan receptors LGR4 and LGR5. PLoS One 2012;7:e40976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xie Y, Zamponi R, Charlat O, Interaction with both ZNRF3 and LGR4 is required for the signalling activity of R-spondin. EMBO Rep. 2013;14:1120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akhmetshina A, Palumbo K, Dees C, Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat Commun. 2012; 3:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Azzolin L, Panciera T, Soligo S, YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell 2014;158:157–70. [DOI] [PubMed] [Google Scholar]
- 14. Ji L, Lu B, Zamponi R, USP7 inhibits Wnt/beta-catenin signaling through promoting stabilization of Axin. Nat Commun. 2019;10:4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim W, Khan SK, Gvozdenovic-Jeremic J, Hippo signaling interactions with Wnt/beta-catenin and Notch signaling repress liver tumorigenesis. J Clin Invest. 2017;127:137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kolbe E, Aleithe S, Rennert C, Mutual zonated interactions of Wnt and Hh signaling are orchestrating the metabolism of the adult liver in mice and human. Cell Rep. 2019; 29:4553–67 e4557. [DOI] [PubMed] [Google Scholar]
- 17. Monga SP, Mars WM, Pediaditakis P, Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 2002;62:2064–71. [PubMed] [Google Scholar]
- 18. Zeng H, Lu B, Zamponi R, mTORC1 signaling suppresses Wnt/beta-catenin signaling through DVL-dependent regulation of Wnt receptor FZD level. Proc Natl Acad Sci USA 2018;115:E10362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perugorria MJ, Olaizola P, Labiano I, Wnt-beta-catenin signalling in liver development, health and disease. Nat Rev Gastroenterol Hepatol. 2019;16:121–36. [DOI] [PubMed] [Google Scholar]
- 20. Russell JO, Monga SP. Wnt/beta-catenin signaling in liver development, homeostasis, and pathobiology. Annu Rev Pathol. 2018;13:351–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Colnot S. PC. Liver Zonation. In: Monga SP. ed. Molecular pathology of liver diseases. New York (NY): Springer Science and Business Media; 2011. [Google Scholar]
- 22. Gebhardt R, Matz-Soja M. Liver zonation: Novel aspects of its regulation and its impact on homeostasis. World J Gastroenterol. 2014;20:8491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kietzmann T. Metabolic zonation of the liver: The oxygen gradient revisited. Redox Biol. 2017;11:622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benhamouche S, Decaens T, Godard C, Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev Cell 2006;10:759–70. [DOI] [PubMed] [Google Scholar]
- 25. Burke ZD, Reed KR, Phesse TJ, Sansom OJ, Clarke AR, Tosh D. Liver zonation occurs through a beta-catenin-dependent, c-Myc-independent mechanism. Gastroenterology 2009;136:2316–24 e2311-3. [DOI] [PubMed] [Google Scholar]
- 26. Gebhardt R, Hovhannisyan A. Organ patterning in the adult stage: The role of Wnt/beta-catenin signaling in liver zonation and beyond. Dev Dyn. 2010;239:45–5. [DOI] [PubMed] [Google Scholar]
- 27. Ma R, Martinez-Ramirez AS, Borders TL, Gao F, Sosa-Pineda B. Metabolic and non-metabolic liver zonation is established non-synchronously and requires sinusoidal Wnts. Elife 2020;9:e46206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Torre C, Perret C, Colnot S. Transcription dynamics in a physiological process: Beta-catenin signaling directs liver metabolic zonation. Int J Biochem Cell Biol. 2011;43:271–8. [DOI] [PubMed] [Google Scholar]
- 29. Yang J, Mowry LE, Nejak-Bowen KN, Beta-catenin signaling in murine liver zonation and regeneration: A Wnt-Wnt situation! Hepatology 2014;60:964–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Planas-Paz L, Orsini V, Boulter L, The RSPO-LGR4/5-ZNRF3/RNF43 module controls liver zonation and size. Nat Cell Biol. 2016;18:467–79. [DOI] [PubMed] [Google Scholar]
- 31. Preziosi M, Okabe H, Poddar M, Singh S, Monga SP. Endothelial Wnts regulate beta-catenin signaling in murine liver zonation and regeneration: A sequel to the Wnt-Wnt situation. Hepatol Commun. 2018;2:845–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rocha AS, Vidal V, Mertz M, The angiocrine factor rspondin3 is a key determinant of liver zonation. Cell Rep. 2015;13:1757–64. [DOI] [PubMed] [Google Scholar]
- 33. Halpern KB, Shenhav R, Massalha H, Paired-cell sequencing enables spatial gene expression mapping of liver endothelial cells. Nat Biotechnol. 2018;36:962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leibing T, Geraud C, Augustin I, Angiocrine Wnt signaling controls liver growth and metabolic maturation in mice. Hepatology 2018;68:707–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang B, Zhao L, Fish M, Logan CY, Nusse R. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature 2015;524:180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Michalopoulos GK, DeFrances MC. Liver regeneration. Science 1997;276:60–6. [DOI] [PubMed] [Google Scholar]
- 37. Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell 2010;7:656–70. [DOI] [PubMed] [Google Scholar]
- 38. Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014;346:1248012. [DOI] [PubMed] [Google Scholar]
- 39. Bucher NLR MR. Regeneration of liver and kidney. Boston (MA): Little, Brown; 1971. [Google Scholar]
- 40. Macdonald RA. “Lifespan” of liver cells. Autoradio-graphic study using tritiated thymidine in normal, cirrhotic, and partially hepatectomized rats. Arch Intern Med. 1961;107:335–43. [DOI] [PubMed] [Google Scholar]
- 41. Ang CH, Hsu SH, Guo F, Lgr5(+) pericentral hepatocytes are self-maintained in normal liver regeneration and susceptible to hepatocarcinogenesis. Proc Natl Acad Sci USA 2019;16:19530–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen F, Jimenez RJ, Sharma K, Broad distribution of hepatocyte proliferation in liver homeostasis and regeneration. Cell Stem Cell 2020;26:27–33 e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sun T, Pikiolek M, Orsini V, AXIN2(+) pericentral hepatocytes have limited contributions to liver homeostasis and regeneration. Cell Stem Cell 2020;26:97–107 e106. [DOI] [PubMed] [Google Scholar]
- 44. Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lustig B, Jerchow B, Sachs M, Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taniguchi K, Roberts LR, Aderca IN, Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene 2002;21:4863–71. [DOI] [PubMed] [Google Scholar]
- 47. Lin S, Nascimento EM, Gajera CR, Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature 2018;556:244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alvarado TF, Puliga E, Preziosi M, Thyroid hormone receptor beta agonist induces beta-catenin-dependent hepatocyte proliferation in mice: Implications in hepatic regeneration. Gene Expr. 2016;17:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Planas-Paz L, Sun T, Pikiolek M, YAP, but not RSPO-LGR4/5, signaling in biliary epithelial cells promotes a ductular reaction in response to liver injury. Cell Stem Cell 2019;25:39–53 e10. [DOI] [PubMed] [Google Scholar]
- 50. Zhao L, Jin Y, Donahue K, Tissue repair in the mouse liver following acute carbon tetrachloride depends on injury-induced Wnt/beta-catenin signaling. Hepatology 2019;69:2623–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barker N, van Es JH, Kuipers J, Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003–7. [DOI] [PubMed] [Google Scholar]