Abstract
Acetaminophen (APAP) overdose is the major cause of acute liver failure (ALF) in the Western world. Extensive research is ongoing to identify the mechanisms of APAP-induced ALF. APAP-induced acute liver injury is also one of the most commonly studied drug-induced liver injury models in the field of hepatotoxicity. APAP toxicity is triphasic and includes three mechanistically interlinked but temporally distinct phases of initiation, progression, and recovery/regeneration. Despite how commonly it is studied, the methods to study APAP toxicity differ significantly, often leading to confusing and contradictory data. There are number of reviews on mechanisms of APAP toxicity, but a detailed mechanism-based comprehensive method and list of assays that covers all phases of APAP hepatotoxicity are missing. The goal of this review is to provide a standard protocol and guidelines to study APAP toxicity in mice including a “test battery” that can help investigators to comprehensively analyze APAP toxicity in the specific context of their hypothesis. Further, we will identify the major roadblocks and common technical problems that can significantly affect the results. This acetaminophen test battery (ATB) will be an excellent guide for scientists studying this most common and clinically relevant drug-induced liver injury and will also be helpful as a roadmap for hypothesis development to study novel mechanisms.
Key words: Acetaminophen toxicity, Acute liver failure (ALF), Acute liver injury (ALI), Acetaminophen test battery, Liver regeneration
INTRODUCTION
Acetaminophen (APAP; also called paracetamol) is one of the most common antipyretic and analgesic agents used worldwide. Whereas it is extremely effective at therapeutic doses, overdose of APAP can cause significant acute liver injury (ALI) leading to acute liver failure (ALF). APAP overdose is the most common cause of ALF in the Western world1. The mechanisms of APAP-induced ALF have been under investigation for over four decades. Apart from investigators interested in APAP toxicity itself, many other researchers interested in studying the role of their favorite molecules also use APAP-induced ALI as a model of drug-induced liver injury (DILI)2. Investigators trying to identify therapeutic ability of herbal medicines to inhibit or prevent DILI also frequently use APAP-induced ALI mouse model3. Because of this, the literature of APAP-induced ALF is extremely vast. However, there is no consensus among researchers despite significant data on how to conduct APAP experiments, which has led to confusions about exact mechanisms of toxicity and several contradictory results. Fortunately, when developed with all necessary controls and keeping in mind its limitations and requirements, the mouse model of APAP-induced ALI faithfully mimics human disease4. APAP toxicity unfolds in three phases, including initiation, progression, and recovery/regeneration (Fig. 1). Whereas these phases are temporally distinct, they are mechanistically interlinked, and molecular changes in one phase affects the other5. Because of this, it is very important to consider investigation of all three phases while testing a new hypothesis related to APAP overdose.
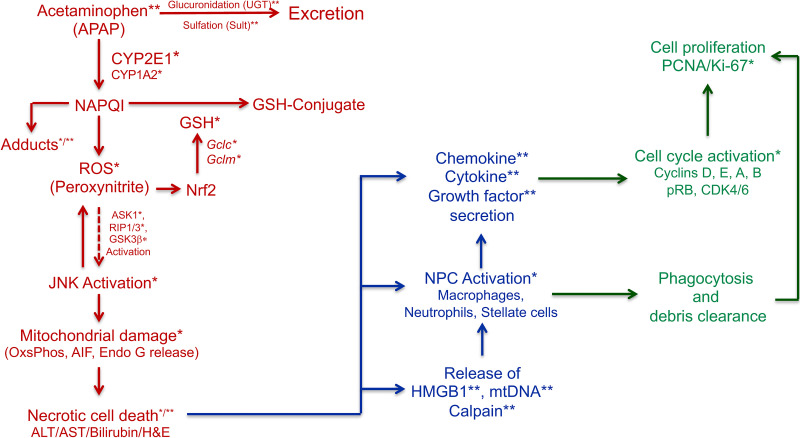
Figure 1.
Parameters of the acetaminophen test battery (ABT). This scheme outlines the most important mechanistic components of acetaminophen-induced ALI model in mice that can be easily measured. * indicates marker is measured in the liver tissue, and ** indicates marker is measured in serum. The “initiation phase” markers are in red, the “progression phase” markers are in blue, and the “recovery/regeneration phase” markers are in green.
The initiation phase is most well characterized and includes processes from APAP bioactivation to actual hepatocyte death within first 6 h after treatment of mice with toxic doses of APAP. The initiation of injury involves metabolism of APAP into its toxic metabolite N-acetylbenzoquinonimine (NAPQI), which is generally detoxified via glutathione (GSH) conjugation6. During overdose situations, GSH rapidly depletes, resulting in excessively free NAPQI, which induces many events including APAP–protein adduct formation, free radicle, and reactive oxygen species (ROS) generation especially in mitochondria7,8. This in turn triggers an intracellular cascade of kinase activation (such as JNK, RIP, and GSK3β), which significantly exacerbates mitochondrial oxidant stress, culminating in mitochondrial oxidative damage, mitochondrial permeability transition, and necrotic cell death7,9. After the initial 6 h and centrilobular cell death, the progression phase of injury begins, during which the initial injury spreads further in the liver lobule, affecting cells well beyond the centrilobular zone depending on the dose10. This phase is also associated with initial inflammatory signaling in response to cell death and infiltration of immune cells including monocytes, macrophages, and neutrophils11–13. Inflammatory signaling is triggered by release of cellular damage-associated molecular patterns (DAMPs), such as nuclear and mitochondrial DNA and molecules like HMGB111–13. The progression phase continues from 6 to 24 h after initial cell death and is characterized by expansion of injury independent of presence of APAP in the system. This is because, in most cases, APAP is eliminated from the body within the first 6 to 12 h6, but the injury continues to expand. The first signs of signaling associated with liver regeneration and recovery are seen around 24 h after APAP treatment10. The recovery phase is highly dose dependent and can last from 24 to 96 h and beyond depending on the dose of APAP and extent of initial injury10. This phase is characterized by removal of necrotic debris, presumably by the invading immune cells, and extensive autocrine, paracrine, and exocrine signaling via chemokines, cytokines, and growth factors that trigger promitogenic intracellular signaling leading to cell division5,10. The cells that are immediately next to the necrotic zone divide and replace the dead cells, reestablishing the architecture and liver function10. At very high doses, this process is either significantly slower or is completely inhibited, resulting in either delayed or total inhibition of liver regeneration and recovery, resulting in death by ALF10,14.
ASSESSING APAP-INDUCED ALI IN MICE: GENERAL CONSIDERATIONS
Dose Response
In most cases, investigators studying APAP hepatotoxicity or simply using APAP overdose as a model to test a particular hypothesis conduct the studies only at one dose. This is problematic because APAP hepatotoxicity, as for all toxic chemicals, is highly dose dependent10,15. Increasing doses of APAP result in increasing liver injury and also in increased liver regeneration up to a threshold dose, beyond which liver regeneration is inhibited10,14. This phenomenon of inhibited liver regeneration after very high liver injury induced by high doses is well documented in the case of a large number of chemicals and is not restricted to the liver15. Particularly in APAP-induced ALF, it has been demonstrated that liver pathobiology varies greatly following administration of different doses, all of which can be designated as “overdose”10,16,17. When using C57BL/6J strain of male mice and fasting before APAP administration, doses beyond 250 mg/kg produce significant liver injury that can be easily measured by biochemical assays and histopathology. However, liver injury and subsequent repair vary significantly over a dose range of 250 to 750 mg/kg. Doses up to 550 mg/kg induce a dose-dependent increase in injury followed by corresponding increase in liver regeneration leading to a complete recovery without any mortality. However, beyond the threshold dose of 550 mg/kg, liver regeneration is inhibited, recovery is delayed, and a dose-dependent mortality is observed. These outcomes are clearly driven by molecular changes that are highly dose dependent in all three phases of toxicity. The dose response of APAP is strain dependent. Different strains of mice have different sensitivity to the same dose of APAP18. Thus, it is critical to use multiple doses of APAP in the studies in order to correctly test the hypothesis under investigation. We recommend studying APAP toxicity using at least two doses: one sublethal and another partially lethal dose.
Time Course
The majority of the studies using APAP as a model generally study only one time point (24 h after APAP dose seems to be the favorite) after APAP administration and use those data to make conclusions. Like most biological processes, APAP-induced ALI unfolds over a time course of 0 to 96 h in mice10. The initiation phase of the toxicity is 0 to 6 h, the progression of injury phase is from 6 to 24 h, and the recovery and regeneration phase starts from 24 and finishes by 96 h at nonlethal doses where regeneration is robust and recovery is complete5,10. At higher doses, the recovery may be delayed and so is the progression phase, while the duration of initiation phase is generally unaffected10. It is critical to study at least two to three time points to get a clear understanding of the molecular and pathophysiological changes occurring over the three phases of APAP toxicity. For investigators who are interested in determining molecular mechanisms of both APAP-induced liver injury and compensatory liver regeneration, it is recommended to study a detailed time course including the 0, 1, 3, 6, 12, 24, 48, and 72 h time points. However, for investigators interested in using APAP overdose as a model to study the role of a particular favorite molecule (i.e., not really interested in APAP for the sake of it), an abridged time course of 0, 1, 6, 24, and 48 h (or at least 0, 6, and 24 h at the minimum) is recommended, which still covers important aspects of all three phases of APAP-induced liver injury. Of course, consideration should also be given to the design and goals of the study. For example, if the goal of a study is to understand only the mechanisms of liver injury but not regeneration, then investigating time points up to 24 h can be appropriate. Further, if a study is designed to test effect of an intervention given 2 h post-APAP, obviously investigating a time point of 1 h post-APAP will not serve any purpose.
Strain, Sex, and Nutritional Status and Other Model Considerations
Mice are the recommended species to study APAP toxicity as they most closely simulate mechanisms of APAP toxicity in humans and thus are exclusively discussed in this review2,4. Rats, in general, are resistant to APAP toxicity and do not show toxicity mechanisms (such as extensive mitochondrial dysfunction) relevant to humans, and thus are not recommended19. Extent of APAP toxicity is also greatly variable across different strains of mouse18. For instance, CD-1 and C3He/FeJ mice strains display extensive hemorrhage in the liver, but not C57BL/6J13. We recommend using C57BL/6J mice mainly because of the significantly higher mechanistic data available in this strain of mice. Even the difference in substrain of mice can significantly impact extent of APAP toxicity. Studies have shown that the C57BL/6N substrain of mice is more susceptible to APAP as compared to the C57BL/6J substrain of mice20,21. The mechanism behind this difference is associated with increased mitochondrial dysfunction in the C57BL/6N strain of mice20. These studies demonstrate that considering the background strain of the mice when using knockout mice for APAP studies is critical. Thus, we recommend that the background of all knockout mice to be used in APAP studies should be bred back to the C57BL/6J strain.
Similarly, studies have shown that female mice are resistant to APAP-induced ALI as compared to male mice22. These differences are attributed to accelerated recovery of mitochondrial GSH in the female mice after APAP overdose22. Whereas the majority of mouse studies on APAP toxicity are conducted using male mice, it is possible to conduct studies with female mice by using higher doses of APAP in the experiments considering high emphasis by NIH on determining the effect of sex as a biological variable (SAVB). However, it should be noted that the pathophysiological relevance of the female mouse model is questionable because females actually appear to be more susceptible to APAP-induced ALF compared to males in humans23.
To decrease intragroup variability in injury development due to differences in nutritional status, we also recommend overnight (12–16 h) fasting prior to APAP administration, especially when lower doses of APAP are utilized. Fasting decreases variability in basal GSH and glycogen levels, which are important for GSH conjugation and glucuronidation of APAP, respectively2. We also recommend maintaining consistency with fasting period within a study as extent of fasting makes a significant difference in magnitude of liver injury. Fresh cages should be used for fasting to avoid any variation due to chow deposits inside the cages. Fed conditions can also be utilized with higher APAP dose or higher animal numbers in specific circumstances when focus is on studying some physiological parameters that are impacted by fasting (such as autophagy).
Rodent models have a particular importance in studying mechanisms of APAP overdose because of the limited utility of the cell culture models. Commonly used hepatoma cell lines such as HepG2, Hep3B, and others (except HepaRG) are not a good model to study APAP toxicity because they lack the required enzymes to bioactivate APAP into NAPQI, and cell death mechanisms in these cell lines are not relevant to human or mice3,24. Thus, data obtained using hepatoma cells are incorrect and irrelevant for APAP toxicity. Primary rodent and human hepatocytes as well as the HepaRG cells are much better models to study APAP toxicity, especially to study initiation phase mechanisms25, but they are limited by the fact that cultured hepatocytes cannot proliferate and the regeneration and recovery phase of the APAP-induced ALF cannot be modeled in vitro. Furthermore, recent studies have indicated a significant role of nonparenchymal cells in stimulating liver regeneration after APAP overdose26, which cannot be modeled in hepatocyte-only cultures.
Markers of Liver Injury, Function, and Regeneration
The focus of APAP-induced ALI studies is to determine ALI induced by APAP and, in more recent years, to determine the subsequent liver regeneration following the injury. Few parameters of liver injury, function and liver regeneration, if that is being studied, are obligatory to all APAP overdose studies (Table 1). The most well-established markers of liver injury are serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels, which are biomarkers of hepatocyte death. ALT analysis is recommended due to its greater specificity for liver tissue. Additionally, serum total bilirubin should also be assessed in order to determine change in liver function. Whereas international normalized ratio (INR) is measured routinely in the clinical samples as marker of liver function, it is rarely investigated in mouse studies. The serum marker data should be always confirmed by performing histopathological analysis of liver hematoxylin and eosin (H&E)-stained liver section as ALT/AST levels may not always correlate with the extent of necrosis especially during the recovery phase10. Additionally, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay can be performed to determine extent of liver necrosis. TUNEL staining is an excellent way to determine both apoptotic and necrotic cell death27. In case of APAP overdose, TUNEL staining shows dark cytoplasmic staining pattern27, which is especially easy to quantify using imaging software in order to obtain percent necrosis scores, a quantitative method to assess histological damage.
Table 1.
Markers of Liver Injury and Regeneration After Acetaminophen (APAP)-Induced Acute Liver Failure (ALF)
The most common method to study liver regeneration after APAP overdose is to stain paraffin-embedded liver section for proliferating cell nuclear antigen (PCNA) and Ki-67, both markers of cell proliferation. PCNA staining is of particular use because it can detect cells in all phases of cell cycle including resting G0, G1, S, G2, and M phase10. Quantifying number of cells in each phase of cell cycle in three high-power (400×) fields per slide per mouse can provide a quantitative measure of kinetics of cell proliferation in the liver. The best time points to determine liver regeneration are 24, 48, and 72 h after APAP treatment in mice10.
ASSESSMENT OF INITIATION PHASE OF APAP-INDUCED ALI
The initiation phase of injury in a mouse model of APAP overdose runs from time of APAP administration (0 h) to approximately first 6 h. However, at higher doses, these events may be a bit prolonged. A good indicator of how long this phase can last is the half-life of APAP in mice, which is approximately 60 min28. Thus, very little APAP remains in the system at 6 h if the starting dose is 300 mg/kg, but a larger dose would remain if the starting dose is double (i.e., 600 mg/kg). The end result of a plethora of mechanistic events during the initiation phase is significant centrilobular necrosis in the liver along with rise in serum transaminases and other markers of ALI29. These mechanistic events can be broadly broken down in the following categories: APAP metabolism, GSH depletion and APAP–protein adduct formation, generation of ROS/reactive oxygen nitrogen (RNS) in mitochondria, intracellular signaling (that exacerbates mitochondrial oxidant stress), mitochondrial injury, and nuclear DNA damage29. Depending on the goal of the study, the investigators can either choose to study several events in detail or choose representative markers from each or some of these events (Table 2).
Table 2.
Assessment of Initiation of APAP-Induced Liver Injury
| Process | Marker | Sample Needed | Reference(s) |
|---|---|---|---|
| APAP metabolism | Phase II metabolism (sulfation and glucuronidation) | Plasma/serum | 31,32 |
| CYP2E1 | Liver (Western blot and activity assay) | 31,41 | |
| APAP–protein adduct formation | Liver (Western blot/HPLC) and serum (HPLC) | 24,44,45 | |
| GSH depletion | Liver (enzymatic assay) | 16,37 | |
| Intracellular signaling | JNK, GSK3β, RIP1/3, ASK, etc. | Liver (Western blot of total and active proteins) | 8,9 |
| ROS (GSSG/GSH) | Liver (enzymatic assay) | 51 | |
| RNS (nitrotyrosine–protein adducts) | Liver (Western blot), paraffin slides (IHC) | 45 | |
| Mitochondrial respiratory function | Freshly isolated mitochondria (SeaHorse) | 45 | |
| Mitochondrial injury (release of AIF, Endo G, Cyto C, SMAC into cytosol) | Cytosolic and mitochondrial fraction (both needed for Western blot) | 45,51 |
Measuring Metabolism of APAP
Metabolism of APAP has been studied in detail, and numerous methods exist to quantify APAP metabolites in serum and in the liver. APAP is primarily metabolized to glucuronide and sulfate conjugates rapidly after absorption, leading to detoxification and elimination6. Any leftover APAP is subjected to bioactivation by the CYP450 enzyme system. The primary CYP involved in metabolic activation of APAP to its reactive metabolite NAPQI is CYP2E1 in mice. Whereas other CYPs such as CYP3A4 are involved in humans, the majority of the metabolic activation in mice occurs via CYP2E16.
Metabolic activation of APAP to NAPQI is an obligatory step in APAP-induced ALI30. Because of this, it is critical to determine that CYP2E1 protein level or activity is not changed (either inhibited or induced) by any intervention or genetically modified mice under investigation. It is relatively uncommon to measure the APAP sulfate and glucuronic acid conjugates, but high-performance liquid chromatography (HPLC)-based methods exist31,32. However, it is absolutely critical to measure CYP2E1-based metabolic activation of APAP to ensure that the phenotype obtained (protection or exacerbation of injury) is not due to alteration of metabolic activation33,34. Thus, even before the actual experiments are undertaken, it is recommended that investigators determine whether the transgenic mice or other intervention being used is not modulating CYP2E1 activity. The most common ways to determine change in CYP2E1 are Western blot analysis for CYP2E1 protein (mRNA is not very informative) and, better yet, a CYP2E1 activity assay, which can be performed multiple ways including in house methods or using a kit31. Broader CYP activity assays have also been utilized in previous studies since APAP metabolism by other CYPs cannot be ruled out completely35,36. It should also be noted that a competitive inhibitor of CYP activity can only be identified by testing chemical directly in vitro, but not by measuring activity in tissue preparations after in vivo treatment37.
A major consideration in using pharmacological inhibitors or any other chemical intervention is the choice of solvents. Dimethyl sulfoxide (DMSO), the most commonly used solvent, inhibits APAP-induced liver injury by multiple mechanisms including inhibition of CYPs and quenching ROS38,39. Because DMSO can interfere with APAP metabolism at a dose as low as 0.1 ml/kg, it makes using any chemicals that are soluble only in DMSO very challenging39. A common practice is to use a high dose of APAP in experiments or use other solvents or treat the chemical 3–5 h after APAP treatment to avoid interference with APAP bioactivation40. It is critical in such cases to have a vehicle control included in the experiments. Nevertheless, posttreatment of an inhibitor is always a better approach compared to pretreatment because of its clinical relevance.
Determining APAP Bioactivation After APAP Treatment
It is not necessary to comprehensively study APAP metabolism or specific CYP activity, but it is absolutely required to assess if overall APAP bioactivation remains intact after any intervention3. There are two ways to confirm that APAP bioactivation is not interfered after APAP is administered: measuring GSH depletion and APAP–protein adduct formation. Both these assays require liver tissue and preferably at a very early time points between 30 min and 2 h after APAP administration. In our hands, 1 h post-APAP is a good time point to determine both GSH depletion and formation of APAP adduct31,41. The NAPQI produced by CYP450-mediated metabolism of APAP is rapidly quenched by cellular GSH. However, because there is a finite amount of GSH in mouse liver (approximately 8–10 μM/g liver tissue), after an overdose of APAP, it is rapidly depleted within the first 30 to 60 min42. Depletion of GSH measured within 30–60 min after APAP treatment is a good indicator of APAP metabolism to its reactive metabolite in most of the cases. Analysis of GSH depletion can also be done at 2 h or beyond, but the investigators should be aware that it has lower sensitivity to detect a delay in NAPQI formation and can also be confounded by de novo synthesis of GSH, thus it may not accurately reflect APAP metabolic activation43. Subtle delay in APAP metabolic activation by interventions under investigation has previously been reported to significantly impair injury initiation, which can be most accurately measured by analyzing rate of GSH depletion within the first 30 min after APAP administration37.
APAP toxic metabolite, NAPQI, binds to cellular macromolecules, producing APAP adducts. Measuring APAP–protein adducts is another biomarker of confirming that APAP was metabolized to NAPQI, and this obligatory step was not hampered. APAP–protein adducts can be simply analyzed by Western blot or immunohistochemical techniques (which are both less quantitative and accurate), but more reliably by HPLC coupled with electrochemical or mass spectrometric measurements24,44,45. HPLC methodology has been developed for measuring protein-derived APAP–cysteine adducts, since NAPQI is majorly bound to cysteine residues on proteins. Apart from using whole-liver tissue, APAP–protein adducts can also be directly measured in mitochondrial fractions given that mitochondria are the major target of APAP toxicity37. Clinically, APAP adducts can also be measured in serum as they are leaked from the liver and their use has been proposed for diagnosis and prognosis of APAP overdose patients46.
Depletion of GSH is an obligatory step in APAP-induced liver injury and is clearly demonstrated by the observation that N-acetylcysteine, the precursor of GSH synthesis, is currently the only therapy for APAP overdose1. N-acetylcysteine works via donating cysteine residues needed for de novo synthesis of GSH. Interestingly, within an hour after GSH depletion, hepatocytes start “replenishing” depleted GSH reserves, and studies indicate that the rate of GSH replenishment is a critical determinant of progression of APAP-induced liver injury16,31,47. At high doses, GSH replenishment is relatively slower, resulting in higher injury16. If GSH replenishment is experimentally inhibited, it results in higher injury even at lower doses48. Thus, another important aspect of the initiation phase that should be measured is the rate of GSH replenishment. This can be achieved by measuring total GSH levels over a time course of 0, 1, 3, and 6 h after APAP administration. Mechanistically, de novo synthesis of GSH is regulated by the nuclear receptor Nrf2, which controls the expression of Gclc and Gclm genes, that codes for the catalytic and modifier subunits, respectively, of the rate-limiting enzyme (i.e., glutamate cysteine ligase) in GSH production49. If a significant difference is noticed in GSH replenishment, then Gclc and Gclm mRNA expression should be measured to determine the mechanism behind the decreased GSH production.
Measuring Oxidant Stress and Mitochondrial Dysfunction
Oxidant stress initiated by APAP adduct formation specifically in the mitochondria and ensuing mitochondrial damage are considered the major cellular mechanisms of APAP hepatotoxicity in both mice and humans4. Thus, any model utilized to study APAP hepatotoxicity should show features of significant mitochondrial oxidative damage. Lipid peroxidation is not considered a relevant mechanism for cell death during APAP hepatotoxicity50. However, several studies still use MDA/TBARS assay (as a marker of lipid peroxidation) to demonstrate the protective effect of their interventions on APAP hepatotoxicity and show only minor changes in these parameters, which are not biologically relevant to cell death caused by APAP overdose3. Since oxidant stress is a popular and relevant mechanistic target to develop treatments for APAP toxicity, it should be carefully examined. Reduction in ROS by GSH is an important cellular antioxidant defense mechanism. In this process, GSH itself gets oxidized to GSSG. Thus, cellular GSSG levels or, more accurately, the ratio of GSSG/GSH (as GSH itself is depleted after APAP overdose) is a good indicator of oxidant stress, which can be measured in liver homogenates by commercially available kits or in-house methodologies51. It should be noted that use of slow-reacting GSH-trapping reagents such as 2-vinylpyridine (as utilized in many commercial assay kits) for measuring GSSG may result in artificially high GSSG values due to oxidation of samples over time. The use of fast-reacting agents such as N-ethylmaleimide is greatly preferred to obtain more accurate results52. During APAP hepatotoxicity, superoxide radicals react with nitric oxide to form the potent oxidant peroxynitrite, which is generated mainly in mitochondria53. Peroxynitrite radicals then react with tyrosine residues on proteins to form nitrotyrosine–protein adducts, which further accentuates mitochondrial damage53. Nitrotyrosine–protein adducts can be detected by immunohistochemistry in liver sections as well as by Western blot analysis using total liver lysates or mitochondrial fractions45. In our hands, using mitochondrial fractions increased the sensitivity of this assay45. Both GSSG/GSH ratio and nitrotyrosine–protein adduct formation can be investigated at 2–6 h after APAP administration in mice. We recommend analysis of these parameters at 6 h, if only one time point needs to be selected because it is difficult to detect significant changes at earlier time points. On a similar topic, it is very critical to consider preconditioning effect due to activation of antioxidant defense mechanisms when investigating the role of any molecule in APAP-induced liver injury. Any intervention (pretreatment) and gene deletion (utilized to test direct role of any protein), which causes hepatocyte stress, can result in induction of antioxidant enzymes (such as metallothioneins, HO-1, and other Nrf2 target genes) even prior to APAP administration49,54. This results in attenuation of APAP toxicity by reducing oxidant stress, which can confound interpretation of the results regarding the specific role of a molecule in other investigated mechanisms of protection. Thus, status of antioxidant defense mechanisms and preexisting stress condition should be tested early on, especially in transgene models, before performing elaborate studies.
Prolonged and unchecked oxidant stress beyond a threshold results in irreversible mitochondrial damage, which is a requisite step for APAP-induced liver injury. Extensive studies indicate that inhibition of mitochondrial damage results in halting the progression of APAP-induced liver injury7. There are several techniques and methods to assess mitochondrial injury after APAP overdose, including electron microscopy, fluorescence-based approaches to determine mitochondrial membrane potential, Western blotting of mitochondrial proteins, and measuring mitochondrial function. Out of these, the easiest are measuring release of mitochondrial proteins such as cytochrome c, second mitochondria-derived activator of caspases (SMAC), apoptosis-inducing factor (AIF), and endonuclease G into cytosol using Western blotting45,51. Of these, AIF and endonuclease G are known to be critically involved in causing nuclear DNA fragmentation leading to necrosis after APAP overdose55,56. The analysis of release of these mitochondrial proteins into cytosol requires isolation of mitochondrial and cytosolic fractions from cells of livers freshly after the APAP treatment. The most critical time points for this analysis in mice are between 2 and 6 h after APAP overdose. The freshly isolated mitochondria or hepatocytes can also be used to measure mitochondrial respiration parameters related to oxidative phosphorylation, which decreases during APAP-induced liver injury due to mitochondrial dysfunction45. The most reliable method to measure oxidative phosphorylation is utilizing SeaHorse extracellular flux analyzer. SeaHorse analysis can also be utilized to sensitively analyze early mitochondrial dysfunction (at time points such as 1.5 h post-APAP) induced by APAP before any overt oxidant stress and cell death can be detected45. Other spectrophotometric approaches can also be utilized to determine function of individual complexes of oxidative phosphorylation for detailed analysis.
Assessment of Intracellular Signaling Involved in APAP-Induced Liver Injury
The initial bioactivation of APAP to NAPQI, the subsequent GSH depletion, adduct formation and early mitochondrial dysfunction are very crucial for initiation of APAP toxicity, but not sufficient to cause hepatocyte death and necrosis7. Initial ROS generation triggers a cascade of intracellular signaling that exacerbates mitochondrial oxidant stress and ultimately results in irreversible mitochondrial injury and necrotic cell death9. This signaling process has a domino-like effect and involves several kinases including ASK1, MLK3, MKK4, GSK3β, PKC, AMPK, RIP1, RIP3, and JNK8,9,29,57. The proapoptotic protein Bax is also translocated to mitochondria and is involved in mitochondrial permeability transition but not in apoptosis58. Apoptosis is not considered a relevant mechanism of APAP toxicity8,27. The most well studied and obligatory step in this cascade of event is the activation of JNK. Pharmacological and genetic inhibition of JNK inhibits progression of APAP-induced liver injury59–61. Several studies show that ASK1, a kinase sensitive to ROS generation, is the upstream trigger for activation of JNK62,63. Whereas one can measure activation of all the different kinases involved, the most important is assessment of JNK activation, which is measured by Western blot analysis of total and phosphorylated JNK proteins using total cell lysates prepared from APAP-treated cells or liver of APAP-treated mice. Significant JNK activation can be detected as early as 1 h after APAP treatment in mice62. Phosphorylated JNK is translocated to mitochondria, which is considered important for causing mitochondrial dysfunction and damage59. Thus, total and phosphorylated JNK levels can also be measured in both cytosolic and mitochondrial fractions to study its mitochondrial translocation. Unless required to elucidate mechanisms, detailed analysis of other kinases is not required. However, it should be noted that activity of these kinases, rather than actual protein or mRNA expression, is important for the abovementioned signaling events. Analysis of only expression of some the signaling mediators in previous studies has resulted in contradictory interpretations64. It should also be emphasized that the most important time points to study any signaling events involved in initiation of APAP-induced liver injury in mice are between 2 and 6 h after APAP overdose. At later time points (such as 24 h, the most commonly studied time point), the investigated signaling mediator may also be involved in other phases of APAP-induced ALI. Further, at later time points, it is more difficult to analyze if the signaling event is merely a consequence of initial injury or actual cause.
ASSESSMENT OF THE PROGRESSION PHASE OF APAP-INDUCED ALI
By 6 h after APAP administration, extensive centrilobular necrosis is established in mice treated with an overdose of APAP45. The majority of APAP is also excreted from the body by 6 h after lower doses of APAP (up to 400 mg/kg) due to its short half-life28. However, this initial liver injury further exaggerates between 6 and 24 h after APAP administration and spreads even beyond the centrilobular area, depending upon the dose. Liver injury measured by serum transaminase levels peaks between 12 and 24 h post-APAP treatment10. This progression of injury from 6 h, mostly in the absence of APAP, is independent of bioactivation of APAP to NAPQI and may depend on delayed intracellular signaling cascade, promoting cell death or extracellular mechanisms. The mechanisms of this “progression phase” of liver injury are not completely understood, but few events that take place during the 6- to 24-h period have been consistently recorded (as described below and Table 3).
Table 3.
Assessment of Progression of APAP-Induced Liver Injury
Extensive studies have shown that the necrotic cell death after APAP overdose results in release of DAMPs such as HMGB1 and mitochondrial DNA11–13,65–67. These DAMPs are crucial in alerting the rest of the body including the immune system of the massive hepatocyte damage, recruiting inflammatory cells, and sending signals necessary for starting the regenerative process13. Serum levels of HMGB1 and mitochondrial DNA can be measured as signals emitted by damaged liver to attract the inflammatory cells.
One of the major features of APAP-induced ALI is the sterile inflammatory response that follows after initial cell death. The role of sterile inflammation in APAP-induced liver injury is highly controversial and has been extensively debated and reviewed11–13. Some studies have claimed that increased inflammatory signaling resulting in neutrophil infiltration in the liver is involved in the further progression of liver injury12. However, significant contradictory evidence using time course studies shows that inflammatory signaling, especially macrophage activation, is involved in stimulation of liver regeneration after APAP overdose by promoting phagocytosis and, also possibly, by secreting proproliferative cytokines and growth factors11–13,68,69. Methods exist to study the inflammatory response in detail using combination of serum cytokine levels, immunofluorescence staining, and flow cytometry. To quickly determine changes in major immune cell types and their chemoattractants, a combination of immunohistochemistry (or immunofluorescence) staining for Ly6G (neutrophil marker) and F4/80 (macrophage marker) combined with mRNA analysis for major pro- and anti-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interferon-γ (INF-γ), IL-10, IL-13, and IL-4 can be performed. The best time points for initial analysis are 24 and 48 h after APAP, and additional studies over a detailed time course can be conducted later depending on the goals of study13. For detailed analysis, it should be emphasized that induction of cytokines/chemokines and recruitment of inflammatory cells do not necessary mean their role in aggravating liver injury, as they can also be involved in removal of cell debris, liver regeneration, and overall recovery11–13. Thus, the causal role of any mediator under investigation in injury progression or repair should be assessed by more conclusive interventional studies.
One of the other hypotheses to explain the progression of bioactivation-independent injury is that the cellular proteases released from dying cells, termed “death proteins” (which get activated due to very high extracellular Ca2+ levels), attack the neighboring healthy cells15. An example of death protein is the cysteine protease called calpain, which is rapidly released from dying hepatocytes and can affect the neighboring cells by degrading exposed membrane proteins70,71. Studies show that blocking calpain activation using either pharmacological inhibitor or overexpression of an endogenous inhibitor results in protection from APAP-induced liver injury70,71. Measuring calpain activity either in the liver or serum can provide information about the death protein involvement in APAP-induced liver injury. Overall the notion of spreading of injury to hepatocytes that are not originally inflicted after APAP overdose is poorly understood and still controversial, which requires further future investigations.
ASSESSMENT OF RECOVERY AND REGENERATION PHASE OF APAP-INDUCED ALI
For over three decades the majority of research on APAP overdose was focused on understanding mechanisms of liver injury. However, a number of studies on APAP and other chemicals have shown that the final outcome of APAP overdose is dependent on the extent of liver regeneration in response to the injury10,15,45,72–74. Patients with rapid and proportionate liver regeneration have significantly higher transplant-free survival33,75. This has highlighted the need for understanding the mechanisms of liver regeneration after APAP overdose, which can help in developing regenerative therapies for APAP-induced ALF. Studies from our group in the last decade have revealed the kinetics of dose-dependent regenerative response after APAP overdose and several mechanisms involved in this process10,17,41,45,76–78. Whereas studying liver regeneration in detail is a highly involved process, some simple assays performed at critical time points can provide a general idea of ongoing regenerative process.
In mice, the entire regeneration and recovery phase typically starts at 12 h and can last as long as 120 h. During the recovery phase of APAP-induced ALI, cell debris in the necrotic areas is removed by infiltrating immune cells, and healthy cells surrounding the necrotic zones proliferate and replace the dead cells5. As previously described, the kinetics of the recovery phase is highly dependent on the dose of APAP. Liver regeneration increases proportionately to the dose (and injury), but with a corresponding delay in the onset of regenerative response. Up to a threshold dose, this regenerative response leads to spontaneous recovery as increased regenerative response more than compensates for moderate delay in the onset10,15. However, beyond the threshold dose, liver regeneration is significantly delayed and inhibited, leading to failed recovery and significant mortality10,15. Any investigation to study liver regeneration should include a comprehensive time course analysis to sufficiently cover the entire regeneration kinetics. If any manipulation to investigate the role of any mediator in liver regeneration causes impairment of regeneration, it is important to know if it is causing only delay or complete inhibition of liver regeneration. Further, it is highly recommended to study liver regeneration using at least two doses of APAP when testing interventions intended to improve regeneration: one where regeneration is intact leading to spontaneous recovery, and one where liver regeneration is severely impaired leading to failed recovery and progression to ALF10. Although the molecular mechanisms of liver regeneration can be studied at lower regenerating dose, from a therapeutic standpoint it is important to study the mechanisms that impair liver regeneration at higher doses in order to find strategies to improve liver regeneration in a clinically relevant setting5. Thus, if the goal of any intervention is to improve liver regeneration, the study should include a higher dose of APAP, where animals cannot regenerate spontaneously. Since liver regeneration is dependent on extent of initial injury, any direct alteration of injury by an intervention can indirectly affect amount of regenerative response34. If the goal of a study is to investigate the direct role of any mediator/intervention in liver regeneration, consideration should be given to achieve equal initial liver injury in groups under investigation. This can be achieved by performing intervention after liver injury is already established (e.g., after 12 h post-APAP)45. In cases where delayed intervention is not possible due to the nature of the experiment (for instance, transgenic mice showing different toxicity at same dose of APAP compared to control mice), different optimized dose of APAP can be utilized for different groups to achieve equal toxicity response in groups under investigation (equitoxic dose approach)33. Further, for any promising intervention targeted specifically to alter liver injury, its effect on liver regeneration should also be characterized, which is rarely investigated. Any adverse effect on liver regeneration by an intervention can hamper its potential to be a safe and effective treatment45. All the variables that affect the extent of liver injury in any experiment including the extent of fasting, metabolism, and strain can also indirectly or directly affect regenerative response, and thus should be considered while designing any study. Effect of any manipulation on both liver injury and liver regeneration should also be characterized at the basal level (without APAP treatment) in order to investigate the direct role specific to the APAP model, and this should be considered for interpretation of the results.
Assessment of Proliferative Markers and Core Cell Cycle
In order to comprehensively study liver regeneration, both immunohistochemical examination of markers of proliferations (such as PCNA and Ki-67) and molecular analysis of cell cycle machinery should be performed (Table 4). PCNA nonspecifically detects cells in all phases of cell cycle progression, but careful analysis (as described previously) of PCNA-stained liver sections can distinguish cells in different stages (including G0, G1, S, G2, and M phase)10. On the other hand, immunostaining of liver sections for Ki-67 can specifically detect cells in DNA synthesis (S phase). After treatment with the most commonly used dose (300 mg/kg) of APAP (i.e., regenerative dose) in male C57BL6J mice (12 h fasted), faint nuclear PCNA staining (indicator of G1 phase) can be observed starting at 12 h10. Some of the cells undergo DNA synthesis (S phase) by 24 h (as observed by dark nuclear PCNA staining), and by 48 h, majority of the cells are in the S phase. By 72 h, the majority of the cells are in the G2 phase (as observed by diffuse cytoplasmic PCNA staining), and regenerative response subsides by 96 h10. After regenerative (300 mg/kg) dose of APAP, significant Ki-67 staining is observed only beyond 48 h. After nonregenerative dose of APAP (600 mg/kg), a remarkably lower number of PCNA-positive cells are observed (starting at 48 h), which are mostly in the G1 phase, and do not progress further through cell cycle and do not stain positive for Ki-6710. Because of the more subjective nature of PCNA staining to analyze the specific phase of cell cycle, it is recommended to perform Ki-67 staining to confirm if cells are undergoing DNA synthesis. While both Ki-67 and PCNA staining capture the cell stage at a particular time, peak of cell proliferation can be missed if detailed time course analysis is not performed. Since BrdU permanently labels cells that have undergone DNA synthesis, it can be mixed in drinking water, and BrdU staining can be performed on liver sections to quantitate cumulatively all the cells that have undergone DNA synthesis up to a particular time point. Mitotic figures can also be observed on stained liver sections, and mitotic index can be calculated to quantitate cells in mitosis. Protein levels of PCNA can also be analyzed by immunoblotting to corroborate the immunohistochemical findings, but immunoblotting alone is not very informative. Induction of various cyclins including cyclins D, E, A, and B, which are sequentially involved in progression through various stages of cell cycle, can be analyzed at the mRNA and protein levels. Further, induction of various proteins involved in mitosis such as Polo-like kinase, Aurora B kinase, and Cdc20 can also be analyzed at the mRNA level as indicators of mitosis. Additionally, levels of cell cycle inhibitors (such as p21) can also be investigated. It is important to know if any intervention or manipulation aimed at improving liver regeneration is causing complete progression through cell cycle leading to cell division, as many strategies can only cause cells to go into the G1 phase, and cells may not progress to mitosis. Detailed analysis as mentioned above will not only provide useful information on progression through the entire cell cycle but also help dissect if any intervention is altering any specific stage of the cell cycle. Additionally, regression of liver injury is an important indicator of overall recovery and thus should be studied by quantifying the necrotic areas (described previously)10. Serum ALT/AST level is not considered a good indicator of extent of remnant necrosis during the later phase of APAP hepatotoxicity due to the limited half-life of these injury markers in the circulation. Researchers have observed discrepancy between extent of necrosis and serum ALT/AST levels especially during the recovery phase. Serum ALT declines sharply at 48 h after treatment with 300 mg/kg APAP in mice, even though significant necrotic areas are still present and notable regression of liver injury occurs only beyond 72 h10.
Table 4.
Assessment of Liver Regeneration After APAP Overdose
| Process | Marker | Sample Needed | Reference(s) |
|---|---|---|---|
| Cell proliferation | PCNA/Ki-67/mitotic index | Liver sections | 10,45 |
| Cell cycle | Cyclins D, E, A, and B; pRb and CDK4 | Liver (mRNA or protein) | 10,41 |
| Stimulus | Cytokines and their receptors (IL6/STAT3; TNF-α/NF-κB) | Liver (for mRNA/protein) and serum (for secreted protein) | 10,78 |
| Growth factors and their receptors (HGF/MET; EGFR ligands/EGFR) | Liver (for mRNA or protein) and serum (for secreted protein) | 10,45 |
Assessment of Regenerative Signaling Pathways
Further, various cell signaling pathways upstream to core cycle machinery can be investigated based on relevance to the study. Many extracellular signaling pathways involving cytokines, chemokines, and growth factors are known to orchestrate liver regeneration response after APAP overdose, which are reviewed in detail elsewhere5. Growth factors such as hepatocyte growth factor (HGF) and epidermal growth factor receptor (EGFR) ligands and their receptors MET and EGFR, respectively, are generally considered most important in liver regeneration and can be investigated79–81. Activation of MET and EGFR occurs dose dependently after APAP overdose and can be investigated by studying their phosphorylation status and phosphorylation of downstream proregenerative mitogen-activated protein kinases (MAPKs) such as extracellular signal-regulated kinase 1/2 (ERK1/2)10,45. Induction of cytokines such as TNF-α and IL-6 is also known to be important for timely liver regeneration, which can be investigated along with downstream signaling of these cytokines [i.e., nuclear localization of nuclear factor κB (NFκB) and phosphorylation of STAT3, respectively]5. Many cytokines are common to both inflammatory and regenerative pathways, and it is hard to delineate their role in the two processes merely based on their induction. It should be noted that activation of all these signaling mediators (mentioned above) may not necessarily correlate with liver regeneration as many of these signaling mediators (including MET/EGFR signaling, MAPKs, and IL-6/STAT3) remain highly activated even after severe APAP overdose, where liver regeneration as measured by actual cell division and reduction in injury (necrosis) is impaired10. This is most likely due to concomitant activation of cell cycle arrest pathways. For example, TGF-β and p53/p21 signaling pathways are known to actively inhibit liver regeneration after severe APAP overdose by causing cell cycle arrest, which can also be investigated14,17,41. Some of the cell cycle inhibitors are also transiently induced during normal liver regeneration, which should be considered for interpretation of data10,17. Upstream signaling pathways should be looked in relation to status of downstream cell cycle activation and actual proliferation. Activity of β-catenin signaling pathway (including nuclear localization of β-catenin) is one of the signaling pathways that have been highly correlated with extent of regenerative response in several studies, even in ALF patients10,33,34,76. β-Catenin signaling is activated during robust proliferative response after moderate APAP overdose and inhibited after severe APAP overdose that causes impaired liver regeneration10. It is noteworthy to mention that most of the abovementioned signaling pathways are activated very early even before observable necrosis and normalize even before peak of proliferation, which should be considered while selecting time points to study signaling underlying liver regeneration10.
WHAT IS THE MINIMUM I SHOULD DO?
It is clear that APAP-induced ALI and ALF is a complicated, multiphasic process involving hepatic and extrahepatic tissues. Studying this clinically important DILI in detail can be a highly involved and daunting task. However, for an investigator using APAP overdose simply as a model to study role of a particular biomolecule of interest, it is neither feasible nor necessary to perform a multi-time course detailed study. Here, we provide recommendations for smaller study designs, which can test the stated hypotheses without requiring extensive work.
Two study designs should be considered. In our experience, a study involving five time points including 0, 1, 6, 24, and 48 h after APAP overdose provides samples necessary to measure markers of all three phases of APAP-induced liver injury. Samples from 0 and 1 h after APAP treatment can be used to confirm the bioactivation of APAP to NAPQI. The 6-h samples can be used to determine changes in critical intracellular events such as JNK activation. Finally, the 24- and 48-h samples can be used to assess both progression and recovery regeneration phases. Additionally, samples from 0, 1, and 6 h can be used to analyze GSH depletion and replenishment. However, if it is not feasible to analyze five time points, a shorter study with 0-, 6-, and 24-h samples can be performed. Albeit definitely less informative, such a short study can still provide enough information about all three phases of APAP-induced liver injury up to a certain extent. However, caution should be taken to avoid overinterpretation of these data, and if needed, additional time points should be added later.
APAP overdose is a clinically important model of DILI and is very popular among liver pathobiologists. The apparent simple (but highly deceiving) nature of this model has resulted in its use to study various biomolecules and processes. However, because many investigators do not know the detailed mechanisms of APAP-induced ALF and use either flawed or incomplete experimental designs, significant amount of incorrect studies are published, adding to the controversies. We hope that the experimental designs and details of the model outlined in this article will help and guide investigators to not only use the APAP model more frequently but also provide excellent mechanistic information to better understand this common DILI.
REFERENCES
- 1. Stravitz RT, Lee WM. Acute liver failure. Lancet 2019;394(10201):869–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGill MR, Jaeschke H. Animal models of drug-induced liver injury. Biochim Biophys Acta Mol Basis Dis. 2019;1865(5):1031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jaeschke H, Williams CD, McGill MR, Xie Y, Ramachandran A. Models of drug-induced liver injury for evaluation of phytotherapeutics and other natural products. Food Chem Toxicol. 2013;55:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jaeschke H, Xie Y, McGill MR. Acetaminophen-induced liver injury: From animal models to humans. J Clin Transl Hepatol. 2014;2(3):153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhushan B, Apte U. Liver regeneration after acetaminophen hepatotoxicity: Mechanisms and therapeutic opportunities. Am J Pathol. 2019;189(4):719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: Recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;30(9):2174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramachandran A, Visschers RGJ, Duan L, Akakpo JY, Jaeschke H. Mitochondrial dysfunction as a mechanism of drug-induced hepatotoxicity: Current understanding and future perspectives. J Clin Transl Res. 2018;4(1):75–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jaeschke H, Ramachandran A, Chao X, Ding WX. Emerging and established modes of cell death during acetaminophen-induced liver injury. Arch Toxicol. 2019;93(12):3491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han D, Dara L, Win S, Than TA, Yuan L, Abbasi SQ, Liu ZX, Kaplowitz N. Regulation of drug-induced liver injury by signal transduction pathways: Critical role of mitochondria. Trends Pharmacol Sci. 2013;34(4):243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhushan B, Walesky C, Manley M, Gallagher T, Borude P, Edwards G, Monga SP, Apte U. Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am J Pathol. 2014;184(11):3013–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Woolbright BL, Jaeschke H. The impact of sterile inflammation in acute liver injury. J Clin Transl Res. 2017;3(Suppl 1):170–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woolbright BL, Jaeschke H. Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J Hepatol. 2017;66(4):836–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: The role of sterile inflammation and innate immunity. Liver Int 2012;32(1):8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bird TG, Muller M, Boulter L, Vincent DF, Ridgway RA, Lopez-Guadamillas E, Lu WY, Jamieson T, Govaere O, Campbell AD, TGFbeta inhibition restores a regenerative response in acute liver injury by suppressing paracrine senescence. Sci Transl Med. 2018;10(454). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehendale HM. Tissue repair: An important determinant of final outcome of toxicant-induced injury. Toxicol Pathol. 2005;33(1):41–51. [DOI] [PubMed] [Google Scholar]
- 16. McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: Dose–response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 2013;269(3):240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borude P, Bhushan B, Apte U. DNA damage response regulates initiation of liver regeneration following acetaminophen overdose. Gene Expr. 2018;18(2):115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harrill AH, Ross PK, Gatti DM, Threadgill DW, Rusyn I. Population-based discovery of toxicogenomics biomarkers for hepatotoxicity using a laboratory strain diversity panel. Toxicol Sci. 2009;110(1):235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McGill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H. Acetaminophen-induced liver injury in rats and mice: Comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol Appl Pharmacol. 2012;264(3):387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duan L, Davis JS, Woolbright BL, Du K, Cahkraborty M, Weemhoff J, Jaeschke H, Bourdi M. Differential susceptibility to acetaminophen-induced liver injury in sub-strains of C57BL/6 mice: 6N versus 6J. Food Chem Toxicol. 2016;98(Pt B):107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bourdi M, Davies JS, Pohl LR. Mispairing C57BL/6 substrains of genetically engineered mice and wild-type controls can lead to confounding results as it did in studies of JNK2 in acetaminophen and concanavalin A liver injury. Chem Res Toxicol. 2011;24(6):794–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Du K, Williams CD, McGill MR, Jaeschke H. Lower susceptibility of female mice to acetaminophen hepatotoxicity: Role of mitochondrial glutathione, oxidant stress and c-jun N-terminal kinase. Toxicol Appl Pharmacol. 2014;281(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rubin JB, Hameed B, Gottfried M, Lee WM, Sarkar M, Acute Liver Failure Study G. Acetaminophen-induced acute liver failure is more common and more severe in women. Clin Gastroenterol Hepatol. 2018;16(6):936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: A human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology 2011;53(3):974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xie Y, McGill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, Jaeschke H. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol Appl Pharmacol. 2014;279(3):266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Michalopoulos GK. Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology 2017;65(4):1384–92. [DOI] [PubMed] [Google Scholar]
- 27. Jaeschke H, Williams CD, Farhood A. No evidence for caspase-dependent apoptosis in acetaminophen hepatotoxicity. Hepatology 2011;53(2):718–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fischer LJ, Green MD, Harman AW. Levels of acetaminophen and its metabolites in mouse tissues after a toxic dose. J Pharmacol Exp Ther. 1981;219(2):281–6. [PubMed] [Google Scholar]
- 29. Ramachandran A, Jaeschke H. Acetaminophen toxicity: Novel insights into mechanisms and future perspectives. Gene Expr. 2018;18(1):19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10(4):267–78. [DOI] [PubMed] [Google Scholar]
- 31. McGreal SR, Bhushan B, Walesky C, McGill MR, Lebofsky M, Kandel SE, Winefield RD, Jaeschke H, Zachara NE, Zhang Z and others. Modulation of O-GlcNAc levels in the liver impacts acetaminophen-induced liver injury by affecting protein adduct formation and glutathione synthesis. Toxicol Sci. 2018;162(2):599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akakpo JY, Ramachandran A, Kandel SE, Ni HM, Kumer SC, Rumack BH, Jaeschke H. 4-Methylpyrazole protects against acetaminophen hepatotoxicity in mice and in primary human hepatocytes. Hum Exp Toxicol. 2018;37(12):1310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Apte U, Singh S, Zeng G, Cieply B, Virji MA, Wu T, Monga SP. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol. 2009;175(3):1056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bhushan B, Edwards G, Desai A, Michalopoulos GK, Apte U. Liver-specific deletion of integrin-linked kinase in mice attenuates hepatotoxicity and improves liver regeneration after acetaminophen overdose. Gene Expr. 2016;17(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Du K, McGill MR, Xie Y, Jaeschke H. Benzyl alcohol protects against acetaminophen hepatotoxicity by inhibiting cytochrome P450 enzymes but causes mitochondrial dysfunction and cell death at higher doses. Food Chem Toxicol. 2015;86:253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011;45(2):156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xie Y, Williams CD, McGill MR, Lebofsky M, Ramachandran A, Jaeschke H. Purinergic receptor antagonist A438079 protects against acetaminophen-induced liver injury by inhibiting p450 isoenzymes, not by inflammasome activation. Toxicol Sci. 2013;131(1):325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park Y, Smith RD, Combs AB, Kehrer JP. Prevention of acetaminophen-induced hepatotoxicity by dimethyl sulfoxide. Toxicology 1988;52(1-2):165–75. [DOI] [PubMed] [Google Scholar]
- 39. Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci 2006;78(15):1670–6. [DOI] [PubMed] [Google Scholar]
- 40. Du K, Williams CD, McGill MR, Xie Y, Farhood A, Vinken M, Jaeschke H. The gap junction inhibitor 2-aminoethoxy-diphenyl-borate protects against acetaminophen hepatotoxicity by inhibiting cytochrome P450 enzymes and c-jun N-terminal kinase activation. Toxicol Appl Pharmacol. 2013;273(3):484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Borude P, Bhushan B, Gunewardena S, Akakpo J, Jaeschke H, Apte U. Pleiotropic role of p53 in injury and liver regeneration after acetaminophen overdose. Am J Pathol. 2018;188(6):1406–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saito C, Yan HM, Artigues A, Villar MT, Farhood A, Jaeschke H. Mechanism of protection by metallothionein against acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;242(2):182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jaeschke H, McGill MR, Williams CD, Ramachandran A. Current issues with acetaminophen hepatotoxicity—A clinically relevant model to test the efficacy of natural products. Life Sci. 2011;88(17-18):737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roberts DW, Bucci TJ, Benson RW, Warbritton AR, McRae TA, Pumford NR, Hinson JA. Immunohistochemical localization and quantification of the 3-(cystein-S-yl)-acetaminophen protein adduct in acetaminophen hepatotoxicity. Am J Pathol. 1991;138(2):359–71. [PMC free article] [PubMed] [Google Scholar]
- 45. Bhushan B, Chavan H, Borude P, Xie Y, Du K, McGill MR, Lebofsky M, Jaeschke H, Krishnamurthy P, Apte U. Dual role of epidermal growth factor receptor in liver injury and regeneration after acetaminophen overdose in mice. Toxicol Sci. 2017;155(2):363–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Curry SC, Padilla-Jones A, Ruha AM, O’Connor AD, Kang AM, Wilkins DG, Jaeschke H, Wilhelms K, Gerkin RD, Acetaminophen Adduct Study G. The relationship between circulating acetaminophen-protein adduct concentrations and alanine aminotransferase activities in patients with and without acetaminophen overdose and toxicity. J Med Toxicol. 2019;15(3):143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bajt ML, Knight TR, Farhood A, Jaeschke H. Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J Pharmacol Exp Ther. 2003;307(1):67–73. [DOI] [PubMed] [Google Scholar]
- 48. van Doorn R, Leijdekkers CM, Henderson PT. Synergistic effects of phorone on the hepatotoxicity of bromobenzene and paracetamol in mice. Toxicology 1978;11(3):225–33. [DOI] [PubMed] [Google Scholar]
- 49. Ni HM, Boggess N, McGill MR, Lebofsky M, Borude P, Apte U, Jaeschke H, Ding WX. Liver-specific loss of Atg5 causes persistent activation of Nrf2 and protects against acetaminophen-induced liver injury. Toxicol Sci. 2012;127(2):438–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44(1):88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Du K, Farhood A, Jaeschke H. Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Arch Toxicol. 2017;91(2):761–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McGill MR, Jaeschke H. A direct comparison of methods used to measure oxidized glutathione in biological samples: 2-vinylpyridine and N-ethylmaleimide. Toxicol Mech Methods 2015;25(8):589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315(2):879–87. [DOI] [PubMed] [Google Scholar]
- 54. Williams CD, McGill MR, Lebofsky M, Bajt ML, Jaeschke H. Protection against acetaminophen-induced liver injury by allopurinol is dependent on aldehyde oxidase-mediated liver preconditioning. Toxicol Appl Pharmacol. 2014;274(3):417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bajt ML, Ramachandran A, Yan HM, Lebofsky M, Farhood A, Lemasters JJ, Jaeschke H. Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol Sci. 2011;122(2):598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94(1):217–25. [DOI] [PubMed] [Google Scholar]
- 57. Saberi B, Ybanez MD, Johnson HS, Gaarde WA, Han D, Kaplowitz N. Protein kinase C (PKC) participates in acetaminophen hepatotoxicity through c-jun-N-terminal kinase (JNK)-dependent and-independent signaling pathways. Hepatology 2014;59(4):1543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324(1):8–14. [DOI] [PubMed] [Google Scholar]
- 59. Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283(20):13565–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology 2006;131(1):165–78. [DOI] [PubMed] [Google Scholar]
- 61. Henderson NC, Pollock KJ, Frew J, Mackinnon AC, Flavell RA, Davis RJ, Sethi T, Simpson KJ. Critical role of c-jun (NH2) terminal kinase in paracetamol-induced acute liver failure. Gut 2007;56(7):982–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xie Y, Ramachandran A, Breckenridge DG, Liles JT, Lebofsky M, Farhood A, Jaeschke H. Inhibitor of apoptosis signal-regulating kinase 1 protects against acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 2015;286(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M and others. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology 2008;135(4):1311–21. [DOI] [PubMed] [Google Scholar]
- 64. Jaeschke H, Duan L, Akakpo JY, Farhood A, Ramachandran A. The role of apoptosis in acetaminophen hepatotoxicity. Food Chem Toxicol. 2018;118:709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122(4):1574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56(5):1070–9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67. Antoine DJ, Williams DP, Kipar A, Jenkins RE, Regan SL, Sathish JG, Kitteringham NR, Park BK. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol Sci. 2009;112(2):521–31. [DOI] [PubMed] [Google Scholar]
- 68. Antoniades CG, Quaglia A, Taams LS, Mitry RR, Hussain M, Abeles R, Possamai LA, Bruce M, McPhail M, Starling C and others. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology 2012;56(2):735–46. [DOI] [PubMed] [Google Scholar]
- 69. Williams CD, Bajt ML, Sharpe MR, McGill MR, Farhood A, Jaeschke H. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol Appl Pharmacol. 2014;275(2):122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Limaye PB, Apte UM, Shankar K, Bucci TJ, Warbritton A, Mehendale HM. Calpain released from dying hepatocytes mediates progression of acute liver injury induced by model hepatotoxicants. Toxicol Appl Pharmacol. 2003;191(3):211–26. [DOI] [PubMed] [Google Scholar]
- 71. Limaye PB, Bhave VS, Palkar PS, Apte UM, Sawant SP, Yu S, Latendresse JR, Reddy JK, Mehendale HM. Upregulation of calpastatin in regenerating and developing rat liver: Role in resistance against hepatotoxicity. Hepatology 2006;44(2):379–88. [DOI] [PubMed] [Google Scholar]
- 72. James LP, Lamps LW, McCullough S, Hinson JA. Interleukin 6 and hepatocyte regeneration in acetaminophen toxicity in the mouse. Biochem Biophys Res Commun. 2003;309(4):857–63. [DOI] [PubMed] [Google Scholar]
- 73. Hu B, Colletti LM. Stem cell factor and c-kit are involved in hepatic recovery after acetaminophen-induced liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295(1):G45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Donahower BC, McCullough SS, Hennings L, Simpson PM, Stowe CD, Saad AG, Kurten RC, Hinson JA, James LP. Human recombinant vascular endothelial growth factor reduces necrosis and enhances hepatocyte regeneration in a mouse model of acetaminophen toxicity. J Pharmacol Exp Ther. 2010;334(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schmidt LE, Dalhoff K. Alpha-fetoprotein is a predictor of outcome in acetaminophen-induced liver injury. Hepatology 2005;41(1):26–31. [DOI] [PubMed] [Google Scholar]
- 76. Bhushan B, Poudel S, Manley MW Jr., , Roy N, Apte U. Inhibition of glycogen synthase kinase 3 accelerated liver regeneration after acetaminophen-induced hepatotoxicity in mice. Am J Pathol. 2017;187(3):543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bhushan B, Borude P, Edwards G, Walesky C, Cleveland J, Li F, Ma X, Apte U. Role of bile acids in liver injury and regeneration following acetaminophen overdose. Am J Pathol. 2013;183(5):1518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bhushan B, Gunewardena S, Edwards G, Apte U. Comparison of liver regeneration after partial hepatectomy and acetaminophen-induced acute liver failure: A global picture based on transcriptome analysis. Food Chem Toxicol. 2020;139:111186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bhushan B, Michalopoulos GK. Role of epidermal growth factor receptor in liver injury and lipid metabolism: Emerging new roles for an old receptor. Chem Biol Interact. 2020;324:109090. [DOI] [PubMed] [Google Scholar]
- 80. Bhushan B, Stoops JW, Mars WM, Orr A, Bowen WC, Paranjpe S, Michalopoulos GK. TCPOBOP-induced hepatomegaly and hepatocyte proliferation are attenuated by combined disruption of MET and EGFR signaling. Hepatology 2019;69(4):1702–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Paranjpe S, Bowen WC, Mars WM, Orr A, Haynes MM, DeFrances MC, Liu S, Tseng GC, Tsagianni A, Michalopoulos GK. Combined systemic elimination of MET and epidermal growth factor receptor signaling completely abolishes liver regeneration and leads to liver decompensation. Hepatology 2016;64(5):1711–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Roth K, Strickland J, Joshi N, Deng M, Kennedy RC, Rockwell CE, Luyendyk JP, Billiar TR, Copple BL. Dichotomous role of plasmin in regulation of macrophage function after acetaminophen overdose. Am J Pathol. 2019;189(10):1986–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]