Abstract
Globally, alcohol consumption contributes to more than 3 million deaths each year. While much of its ramifications is preventable, a coherent public health discourse on how to limit alcohol-related harm has been overdue. By synthesizing information from national and global databases, we show in this analysis that alcohol consumption level and alcohol-attributable burden of diseases, particularly alcoholic liver disease (ALD), are intimately linked to national income distribution, cultural norms, religion, sex, age, and health status. Prevalence and burden of ALD are positively associated with economic standing in most countries, which necessitate active governmental control via cost-effective policies, such as the “best buys” proposed by the World Health Organization. To date, a number of critical questions remain unanswered over the molecular mechanisms underlying ALD pathophysiology; the insights gained thereof should provide new opportunities for the advancement of novel diagnostic and management strategies. In comparison with other prevailing liver diseases (e.g., viral hepatitis and nonalcoholic fatty liver disease), governmental support to ALD investigation has been sluggish in most Western countries and China, resulting in a dearth of breakthroughs on both the basic and clinical research fronts in the past decades. Emerging foci of clinical trials for ALD therapy include empirical use of probiotics, antioxidants, growth factors, monoclonal antibodies against key inflammatory mediators, and technology-enhanced behavioral interventions. In this article, we seek to provide a comprehensive analysis on the progress and challenges in tackling ALD as a global health problem, with particular emphasis on global disease burden, socioeconomic influences, research trends, government roles, and future therapies.
Key words: Alcoholic liver disease (ALD), Public health policy, Global disease burden, Disease mechanisms, Alcohol abuse, Intervention
INTRODUCTION
Alcoholic liver disease (ALD) is a prevailing disorder tied to inordinate alcohol consumption that threatens the health of millions worldwide each year1. Undisciplined alcohol drinking figures as a prominent risk factor of preventable disability or death, with definite implications for over 60 acute and chronic diseases2,3. Classically, ALD refers to a clinicohistologic spectrum that includes alcoholic fatty liver, alcoholic steatohepatitis (ASH), and cirrhosis along with its complications. To date, the global clinical burden of ALD remains unclear, in part due to a lack of definitive standards for parameterizing ALD in diagnostic practice. By a rough estimate, the prevalence of ALD in Western countries is about 6%4,5. Although previous studies had postulated tempting health benefits from low or moderate consumption, such as reduced risks for myocardial infarction6, ischemic stroke7, and type 2 diabetes8, or mitigated pathology in nonalcoholic fatty liver disease (NAFLD)9, a recent global study painted a grimier picture that current thresholds for safer alcohol use requiring lowering and that there may even be no “safe alcohol dose” per se10. In terms of underlying processes, despite the fact that much of the disease progression patterns and mediators of ALD have become known, the detailed molecular mechanisms responsible for hepatic injury (e.g., lipid dysregulation, insulin resistance, inflammation, cell death, and fibrosis) and interorgan communication are yet to be clarified11. Naturally, a dearth of theoretically informed treatment targets disincentivizes the development of novel ALD therapies. As a basic intervention strategy, alcohol abstinence can halt liver injury in most cases and resolve existing steatosis or inflammation. Nutritional support and specific pharmacological options may additionally improve ALD symptoms and prognosis. However, the efficacy of steroids, anti-tumor necrosis factor (TNF) agents, growth factors, or antioxidants in treating ALD is controversial and heavily depends on disease stage and host conditions12. For patients with advanced alcoholic cirrhosis unresponsive to chemotherapies, liver transplantation may be a last resort. From a comparative perspective of other major infectious liver diseases (e.g., hepatitis B and hepatitis C) and noninfectious liver diseases [e.g., NAFLD and drug-induced liver injury (DILI)], the current global inputs in ALD research almost certainly cannot meet the urgent medical needs of a development pipeline for more efficacious and safer therapies. In this article, we seek to provide a comprehensive update on the global disease burden, mechanistic focus, research trends, government policies, and future therapies of ALD.
GLOBAL EPIDEMIOLOGY
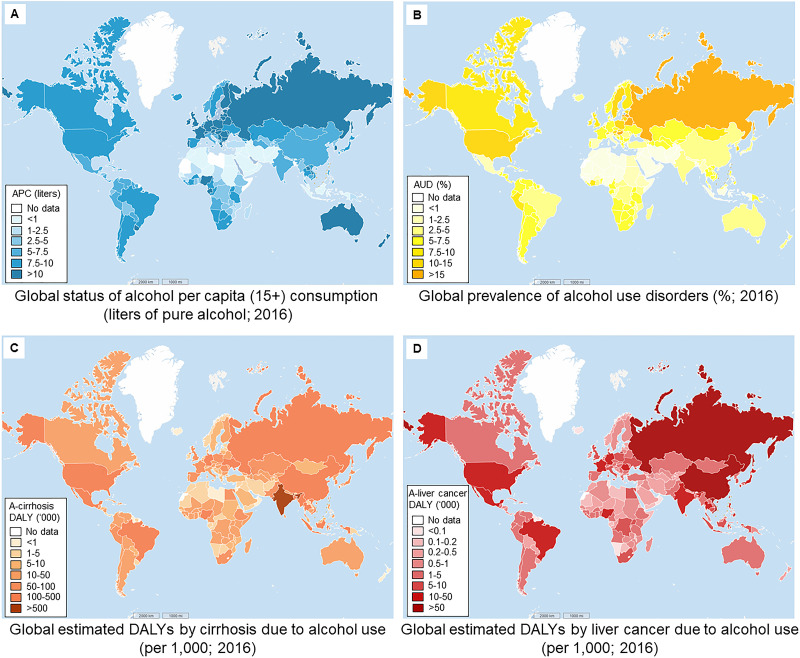
Consistent with its profound impact on human cultures since antiquity, alcohol continues to be consumed in most countries across the world. Multiple factors, including national income distribution, cultural norms, religion, sex, age, and health status, can significantly influence an individual’s alcohol use. In terms of alcohol per capita (APC) consumption (15+ of both recorded and unrecorded data in 2016), high-income countries (World Bank income group; e.g., most Western countries, Japan, and South Korea) have higher APC levels according to the Global Status Report on Alcohol and Health 2018 from the World Health Organization (WHO)13. Several countries in Africa (e.g., Nigeria and Equatorial Guinea), Asia (e.g., Lao), and Latin America (e.g., Uruguay) also have relatively higher APC consumption. Lower APC rates can be found in the Middle Eastern region and some Muslim-dominant countries (e.g., Niger, Indonesia, and Azerbaijan) (Fig. 1A). In medical diagnosis, alcohol use disorder (AUD) refers to alcohol drinking that elicits distress or harm, whose manifestation can range from mild to severe (alcoholism). In 2016, it was estimated that 283 million people (15+; population of those 15 years and older) had some form of AUD worldwide. Most prevalent AUDs are typically observed in European countries (e.g., Hungary, 21.2%; Russia, 20.9%; and Belarus, 18.8%) and in several Asian, American, and African countries (e.g., the US, 13.9%; South Korea, 13.9%; and Côte d’Ivoire, 10%). Similar to the case of APC levels, the lowest AUD prevalence occurs in countries in the Middle Eastern region (Fig. 1B). Unhealthy alcohol use causes a substantial burden of disease and injury, including communicable diseases, noncommunicable diseases, and organ dysfunction, which translated into 132.6 million disability-adjusted life years (DALYs) in 2016 (5.1% of all DALYs). Quite disconcertingly, alcohol has been the most frequent causal agent of liver cirrhosis in the Western world. In epidemiological terms, DALYs (per 1,000 people) of liver cirrhosis due to alcohol use are highest in India (2356.4), followed by the US (467.9), China (466.3), Nigeria (424.5), and Indonesia (365.1). Lowest DALYs are seen in Brunei Darussalam (0.1), Iceland (0.1), Kuwait (0.2), Qatar (0.3), and Oman (0.4%) (Fig. 1C)14. For liver cancer, China has the highest alcohol use-induced DALYS (501.4), followed by Vietnam (62.4), Russia (53.0), Thailand (40.5), and India (38.5). Brunei Darussalam, Sao Tome and Principe, Djibouti, Qatar, and Bhutan have the lowest DALYs of liver cancer linked to alcohol use (all below 0.25) (Fig. 1D)14. Geographical distributions of such DALYs are not strictly consistent with APC and AUD prevalence distribution, which may possibly be accounted for by coinfluences from other health and socioeconomic factors.
Figure 1.
Global status of alcohol consumption and prevalence of alcohol-attributable disorders and liver complications. (A) Global status of alcohol per capita (APC; 15+) consumption (in liters of pure alcohol; 2016) (data source: World Health Organization13). (B) Global prevalence of alcohol use disorders (AUDs) (%) in 2016 (data source: World Health Organization13). (C, D) Global estimates on disability-adjusted life years (DALYs) by cirrhosis and liver cancer due to alcohol use in 2016 (per 1,000 persons) (data source: World Health Organization14).
SOCIOECONOMIC FACTORS OF ALD
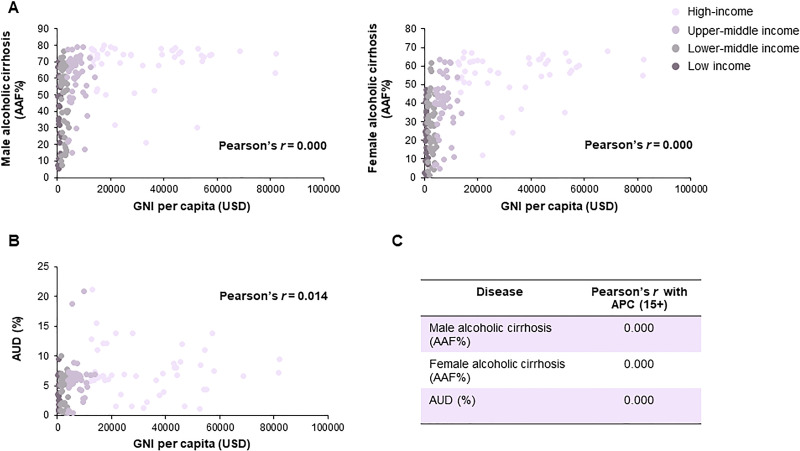
In general, alcohol consumption is positively associated with a country’s economic standing or national income distribution. In terms of ALD, our analysis further identified a robust linkage (Pearson’s correction = 0.000) between gross national income (GNI) per capita and alcohol-attributable fractions (AAFs) of liver cirrhosis in males and females in the majority of WHO member states (excluding Muslim-dominant countries; data of 2016) (Fig. 2A). National income distribution was also intimately linked to AUD prevalence (Fig. 2B). According to income group parameters defined by the World Bank, high-income and upper-middle income countries have higher cirrhotic AAFs and AUD prevalence than lower-middle income and low-income countries. This epidemiological gap can partially be explained by differences in APC in those countries (Fig. 2C). However, other than ALD, both alcohol-related and alcohol-attributable deaths occur more often among disprivileged socioeconomic groups compared with populations from more affluent areas15. This apparent “alcohol harm paradox” may be attributed to several mechanisms, namely, (1) economically disprivileged populations have higher chances of exposure to other socioenvironmental challenges (e.g., smoking and poorer nutrition status), which degrade health; (2) even when controlling for total alcohol consumption levels in considerations, people from less developed countries tend to practice more binge drinking, have different beverage formulations (e.g., greater spirits content), have risk-seeking behavioral preferences (e.g., drinking without meals), or encounter beverages of poorer quality (e.g., higher levels of toxic substances like formaldehyde and methanol); (3) economically disprivileged individuals may have a history of drinking patterns (e.g., having started drinking at a younger age) different from those of affluent individuals, although their recent consumption quantities become similar; and (4) individuals in less wealthy communities may actually consume more alcohol, as the proportion of unrecorded APC relative to total APC consumption is highest in low-income and lower-middle-income countries16,17. A recent linked cohort study confirmed that a low socioeconomic status is consistently associated with starkly increased alcohol-attributable harms, even after accounting for variables like drinking patterns, obesity, and smoking status18. Therefore, to counter the problems of AUD and ALD, stakeholders including basic researchers, clinical management, and policy decision makers need to work urgently to eliminate this inequality.
Figure 2.
Correlation analysis on national income distribution, alcohol consumption, and alcoholic liver cirrhosis. (A) Correlation analysis on gross national income (GNI) per capita (Atlas method, current US$) and male/female alcohol-attributable fractions of liver cirrhosis (AAF%) in 2016. (B) Correlation analysis on GNI per capita and AUD prevalence (%) in 2016. (C) Correlation analysis on global status of APC (15+) consumption and male/female liver cirrhosis AAFs or AUD prevalence in 2016 (data source: World Bank national accounts data, OECD national accounts data, and World Health Organization13).
GOVERNMENT CONTROL AGAINST ALCOHOL ABUSE
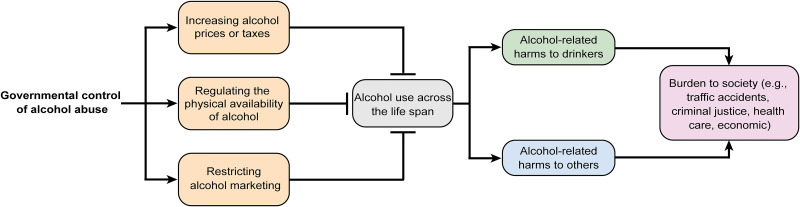
As is well known, governmental initiatives to control alcohol abuse at the national or subnational level are critical and have been well proven for the effective prevention and reduction in alcohol-related hazards19. Enforcement of government measures may take the form of legislation, ordinances, and regulations at multiple levels to put disincentives into commodity availability, prices, marketing, and drink driving. Thus far, three most cost-effective interventions (the “best buys”) have been recommended by the WHO to its member states, namely, taxation and price regulation, regulating physical availability, and restricting alcohol marketing (Fig. 3)20.
Figure 3.
Conceptual framework on government policies on the control of alcohol abuse and alcohol-related public health problems. To effectively reduce alcohol use across the life span of individuals, proper governmental control policies, such as increasing alcohol price/tax, regulating the physical availability of alcohol, and restricting alcohol marketing, are recommended by the World Health Organization. Those “best buy” interventions can decrease disease and social burdens (e.g., traffic accidents, criminal justice, and health care) caused by alcohol consumption.
Lifting alcohol prices or taxes has been proven a powerful leverage for reducing negative consequences of unhealthy alcohol consumption, such as alcohol-related mortality, end stage liver disease, lost productivity, domestic violence, teenage pregnancy, and sexually transmitted diseases21,22. In addition, greater social and welfare surcharges on alcohol beverages may contribute to an augmented financial base for educational programs23. According to the WHO Global Status Report on Alcohol and Health 2018, tax increases were the most commonly implemented “best buy” policy since 2010. About 155 countries levy duties on beer, which cover 6.8 billion people across the world. Fewer than 40% countries reported alcohol tax adjustments with inflation and income levels. There are also cases where governments adopted other price regulation strategies with success, including imposing minimum price unit, and banning predatory (i.e., below cost) selling or volume discounts, which provide further possibilities of bolstering alcohol price/tax policies13.
Restricting physical access to alcohol is a feasible and cost-effective mode of policy implementation to keep alcohol abuse at bay, particularly in low- and middle-income countries. Restriction to drinking in public places; regulation of business hours, days, and density of alcohol outlets; as well as raising the national legal age for purchase/consumption of alcohol can drastically bring down the tolls of alcohol-related harms24. Certain countries (e.g., Singapore) have restrictions on public drinking of alcoholic beverages in such places as educational premises and healthcare establishments. In addition, restricting on-premises and off-premises sales of alcohol is also an effective regulatory measure at the population level. More than half of the WHO member states reported such national regulations, mainly for beers and spirits. In contrast, few countries have regulations on alcohol outlet density (i.e., the physical density of outlets in a geographical location). Increasing the national minimum drinking age has been another effective way for preventing alcohol-related harm among young people. According to WHO statistics, in 2016, most countries (152 in total) maintain a national or subnational minimal drinking age for on-premises alcohol sales, ranging from 13 to 25 years, with the most common age of 18 years. Eleven countries (e.g., Benin and Guinea), mostly in the low-income and lower-middle income settings, do not practice such limitations14.
Consistently, legislative approaches to control the marketing, sponsorship, or promotions of alcohol beverages have been proven to promote public health and safety of the general population, particularly juniors. A positive association exists between the level of marketing exposure and alcohol consumption among youth, as well as the first use of alcohol. Such exposure also predisposes young people to risky behaviors such as binge or hazardous drinking25. Stringency of alcohol marketing policies varies substantially across regions and countries. In 2016, for example, 123 countries reported alcohol marketing restrictions in traditional media, whereas in some countries, restrictions in the Internet and social media are not available. Restrictions on alcohol product placement (e.g., on television and sports events) and sales promotion provide further brakes on alcohol consumption in many countries26.
PATHOPHYSIOLOGY OF ALD
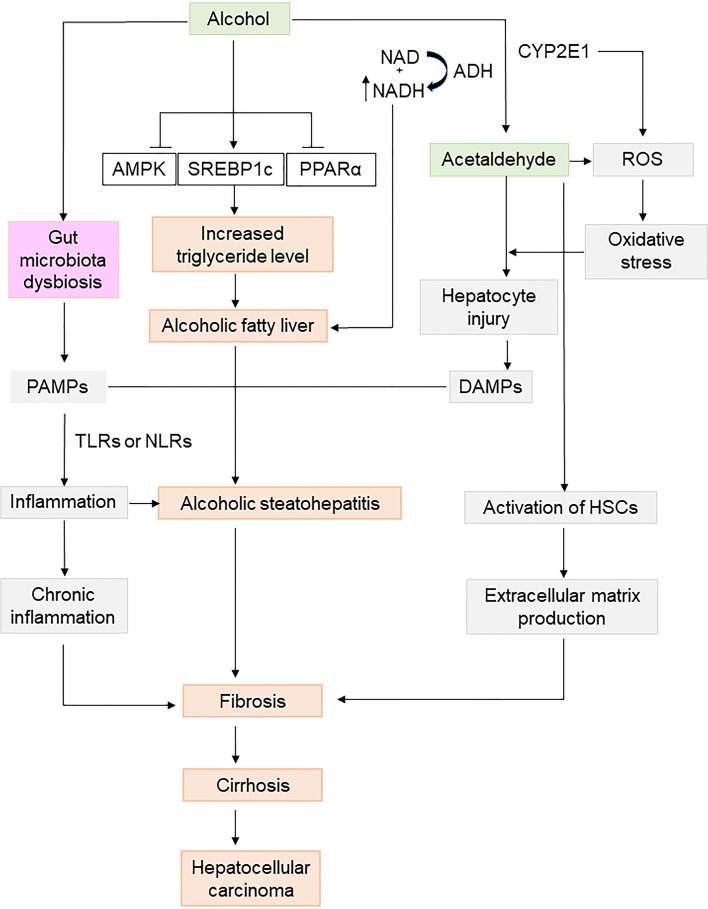
ALD is a complex disease dynamically shaped by multiple factors of the host (e.g., genetics, sex, ethnicity, malnutrition, gut microbiota, and mental conditions) and the environment (e.g., alcohol type, dose, drinking pattern, drugs, smoking, and viral infection)27. Typically, chronic alcohol consumption leads to an unwholesome accumulation of lipid droplets in the liver, primarily via dysregulated lipid metabolism (i.e., enhanced lipogenesis and suppressed lipolysis). An elevated ratio of reduced NAD/oxidized NAD (NADH/NAD+) and concomitant inactivation of nuclear hormone receptors (e.g., peroxisome proliferator-activated receptor-α) potentially pave the way for steatosis development28. During the transition from simple steatosis to ASH and other severe forms of disease manifestation, several mechanistically interconnected processes such as Kupffer cell activation, neutrophil infiltration, enteric dysbiosis, and adaptive immunity regulation are thought to be at work, while other key events could contribute to impaired immunosuppression and switching of effector cell functions with pathological implications11. Importantly, histological fibrosis staging provides a critical perspective on prognosis in both compensated and decompensated ALD. It has been postulated that profound steatosis, inflammation, and hepatocellular ballooning are responsible for driving hepatic stellate cell (HSC) activation and extracellular matrix production, which consequently set in motion pericellular/perisinusoidal fibrosis, dense fibrous septa, and, finally, cirrhosis29. Alcoholic cirrhosis has by far the worst prognosis when compared with cirrhosis of other etiologies, accounting for up to 48% of cirrhosis-associated deaths in the US30. Alcohol alone claims around one third of global incident cases of primary liver cancer. As a subclinical immunosuppressive agent, alcohol can also work cooperatively with other risk factors of secondary insults (e.g., bacterial or viral infection, and obesity) to induce hepatocellular carcinoma. Mechanistically, detrimental cellular events such as chemical modifications on proteins and DNA by excessive acetaldehyde, runaway production of reactive oxygen species (ROS), antioxidant depletion, impaired DNA repair machineries, and epigenetic remodeling (e.g., methyl group transfer caused gene expression) have been mooted as important factors in the pathophysiology of alcohol-specific carcinogenesis in some patients (Fig. 4)31.
Figure 4.
Mechanisms involved in the pathophysiology of alcoholic liver disease (ALD). In the human body, ethanol is metabolized by alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH), generating toxic aldehyde and reactive oxygen species (ROS) to elicit local oxidative stress and inflammation. Hepatic lipid metabolism is disrupted by alcohol consumption to induce steatosis, causing alcoholic fatty liver and alcoholic steatohepatitis (ASH). Excessive alcohol also provokes gut microbiota dysbiosis to produce toxins such as lipopolysaccharides (LPS) and other pathogen-associated molecular patterns. Those stimuli will activate hepatic stellate cells (HSCs), leading to extracellular matrix production, and subsequent liver fibrosis, cirrhosis, and even hepatocellular carcinoma. CYP2E1, cytochrome P450 2E1; DAMPs, damage-associated molecular patterns; NLRs, nucleotide-binding oligomerization domain (NOD)-like receptors; TLRs, toll-like receptors.
CLINICAL ASSESSMENT AND DIAGNOSIS
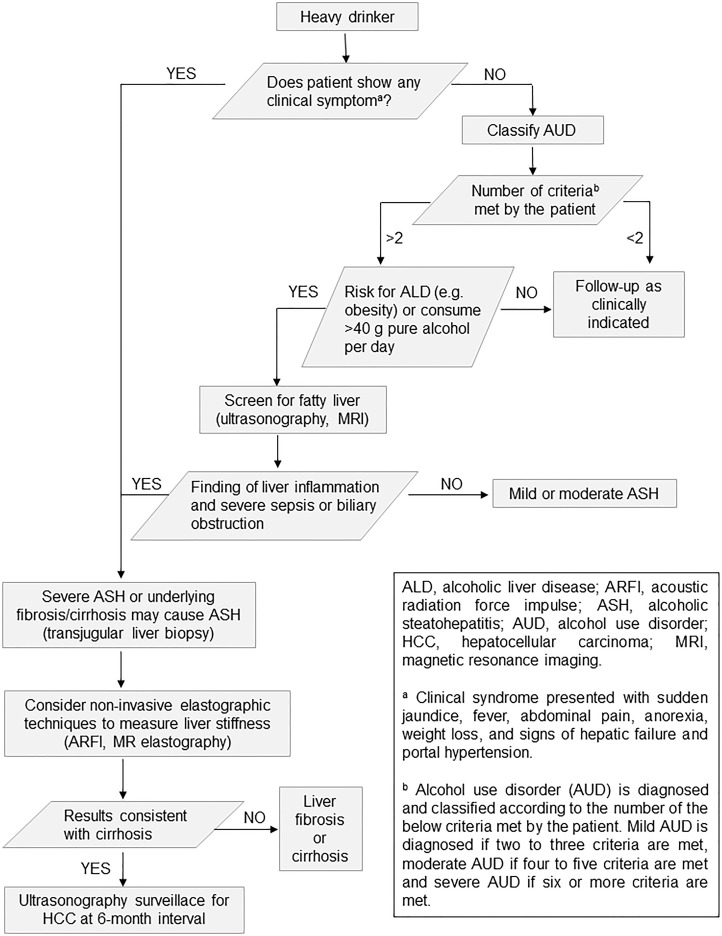
Precision in the diagnosis of ALD depends on a detailed patient history in conjunction with laboratory and imaging findings. This is an area where practical challenges may arise, as patients with elusive alcoholic steatosis are asymptomatic or present nonspecific symptoms. Thus, AUD should be classified, and risk factors of ALD (e.g., obesity) need to be taken into consideration. Screening of fatty liver by ultrasonography or magnetic resonance imaging/computed tomography and abnormal liver test facilitates the classification of nonfibrosis/mild fibrosis or advanced fibrosis/cirrhosis patients. Noninvasive elastographic techniques provides an additional analytical modality for accurate fibrosis/cirrhosis/hepatocellular carcinoma (HCC) judgment based on measurement of liver stiffness, which critically informs clinical management. On the other hand, in many cases, liver biopsy is not an obligatory item in diagnostic evaluation on fatty liver in proper management settings (Fig. 5)32.
Figure 5.
Algorithm for diagnosis and therapeutic decision of patients with ALD. Heavy drinks are under clinical diagnosis for AUD, ALD, or ASH. Noninvasive elastography or liver biopsy is needed in some cases to make precise diagnosis and therapeutic decision.
One of the major bottlenecks in improved clinical ALD assessment and diagnosis is the development of early and noninvasive prognostic markers, particularly for ASH. Emerging jaundice is a hallmark of ASH, and other signs of liver decompensation (e.g., ascites) can also appear. Recently, a three-confidence degree system for ASH classification has been proposed by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) consortia, which lays down a conceptual framework for defining and stratifying ASH, including definite alcoholic hepatitis, probable alcoholic hepatitis, and possible alcoholic hepatitis33. Essentially, prognosis of ASH and alcoholic cirrhosis tends to be poor and uncertain, even with patients with a nonsevere form of the disease. To date, only a handful of prognostic biomarkers and therapy efficacy indicators of ALD have been developed34. In addition to conventional prognostic calculators such as Maddrey discriminant function (MDF), model for end stage liver disease (MELD), and Glasgow alcoholic hepatitis score (GAHS), emerging evidence now corroborates the practical utility of circulating small noncoding RNAs (miRNAs), long noncoding RNAs (lncRNAs), and selective cytokines profiles as potential biomarkers of ALD35–37. Indeed, new methods for reliable and rapid ALD diagnosis remain in great demand in general practice, and large-scale trials on their translational potential in clinically well-characterized patient populations are much awaited38.
GLOBAL RESEARCH TRENDS
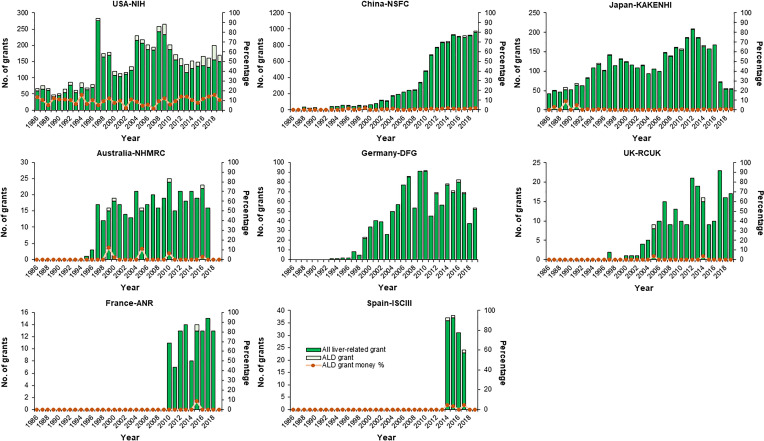
Globally, interest in ALD research is waxing, commensurate with the disorder’s deepening impact on personal health and society. To analyze the research trends in ALD during the past three decades (1986–2019), liver-related grant information from major Western countries (US, Australia, Germany, UK, France, and Spain) and East Asian countries (China and Japan) were collected and analyzed. Over the years, the National Institutes of Health (NIH) of the US has approved 4,902 grants of liver-related research, in which 470 grants concern ALD in topic (9.60%). The grant amounts of ALD accounts for 6%–20% of all liver-related grants in most financial years. In other countries that we have analyzed, the percentage of ALD-related grants among all liver-related grants is much lower than that of the NIH (e.g., China, 1.22%; Japan, 0.97%; Australia, 1.31%; Germany, 0.88%; UK, 0.91%; France, 0.93%; Spain, 2.30%), with a grant amount level typically lower than 2% in most fiscal years (Fig. 6). With respect to research topics, HCV and NAFLD are the main focus in the US and Australia, while liver cancer provides some of the most pursued topics in China, Japan, and Spain (Supplementary Fig. 1, available at https://doi.org/10.5281/zenodo.3889084).
Figure 6.
Comparison on national grant support for basic research in the fields of hepatology and ALD in major Western countries and China (1986–2019). Changes in number of funded projects and total funding amounts of all liver-related projects and corresponding percentage of ALD projects in those projects from the US [National Institutes of Health (NIH)], China [National Natural Science Foundation of China (NSFC)], Japan [Grants-in-Aid for Scientific Research (KAKENHI)], Australia [National Health and Medical Research Council (NHMRC)], Germany [German Research Foundation (DFG)], the UK [Research Councils UK (RCUK)], France [French National Research Agency (ANR)], and Spain [El Instituto de Salud Carlos III (ISCIII)]. Information on funded projects was manually collected and selected by searching project titles with the keywords “liver,” “hepatic,” “hepatitis,” “hepatoma,” “cirrhosis,” “hepatocyte,” “Kupffer,” and “Wilson” in the NSFC information system (https://isisn.nsfc.gov.cn/egrantweb/), NIH Project Reporter system (https://projectreporter.nih.gov/reporter.cfm/), KAKENHI database (https://kaken.nii.ac.jp/), NHMRC Research Funding Statistics and Database (https://www.nhmrc.gov.au/funding/data-research/research-funding-statistics-and-data), German Project Information System (https://gepris.dfg.de/gepris/OCTOPUS?language=en), RCUK Gateway to Research System (https://gtr.ukri.org/), ANR Funded Projects and Impact Database (https://anr.fr/en/funded-projects-and-impact/funded-projects/), and ISCIII Fondo de Investigación en Salud (https://portalfis.isciii.es/es/Paginas/inicio.aspx) in March, 2020. The Spanish keywords used were “hígado,” “hepático,” “hepática,” “hepatitis,” “hepatocito,” “hepatoma,” “cirrosis,” “Kupffer,” and “Wilson.”
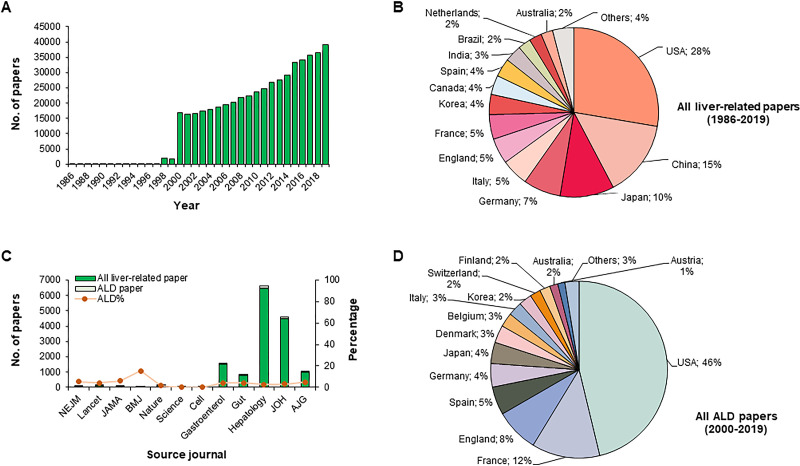
To get a panoramic view on hepatology research outputs, we extracted and analyzed publication information from the Web of Science Core Collection by using the topic keyword “liver” for works in the period of 1986–2019. The search results give a total of 510,693 research articles and review articles in this field. The annual publication volume has progressively risen since 2000 (over 15,000 articles per year) (Fig. 7A). Scientists from the US contributed 27.7% of all those liver-related publications, followed by their peers in China (14.6%), Japan (10.3%), Germany (7.2%), and Italy (5.2%) (Fig. 7B). Major publishing journals of these works include PLoS One, Transplantation Proceedings, Hepatology, World Journal of Gastroenterology, and Journal of Hepatology (Supplementary Table 1, available at https://doi.org/10.5281/zenodo.3889084). We then analyzed ALD-related articles published in leading journals in the fields of medicine, multidisciplinary sciences, cell biology, and gastroenterology from 2000 to 2019. A total of 476 ALD-related articles have been published, including 254 in medical journals (74 in New England Journal of Medicine; 116 in Lancet; 51 in Journal of the American Medical Association; and 13 in British Medical Journal), 2 in multidisciplinary sciences journal (Nature; no ALD-related articles in Science), and 460 in gastroenterology journals (62 in Gastroenterology; 32 in Gut; 181 in Hepatology; 134 in Journal of Hepatology; and 51 in American Journal of Gastroenterology), covering 4%–6% of total published liver-related articles in each journal (except ∼15% in BMJ) (Fig. 7C). Of note, 46.1% of ALD-related articles were published by authors from the US, followed by France (12.2%), England (8.3%), Spain (4.7%), and Germany (4.2%) (Fig. 7D). Reflective of the grant support strength and trends in those countries, liver cancer (including cirrhosis), HCV, NAFLD, and fibrosis/liver regeneration are the prevailing themes in liver-related articles published in Gastroenterology, Gut, Hepatology, Journal of Hepatology, and American Journal of Gastroenterology (Supplementary Fig. 2, available at https://doi.org/10.5281/zenodo.3889084).
Figure 7.
Profiles of publications of liver-related articles in the Web of Science Core Collection and in top journals of multidiscipline, medicine, and gastroenterology from 1986 to 2019. (A) Statistics for published hepatology articles and (B) author address percentage during 1986–2019. Data were collected by searching Web of Science Core Collection with the keyword “liver” during 1986–2019 at https://apps.webofknowledge.com/. (C, D) Statistics and author address percentage of all liver-related articles and ALD-related articles in top journals of medicine (New England Journal of Medicine, Lancet, Journal of the American Medical Association, British Medical Journal), multidiscipline (Nature and Science), cell biology (Cell), and gastroenterology (Gastroenterology, Gut, Hepatology, Journal of Hepatology, and American Journal of Gastroenterology) during 2000–2019. Data were collected by searching for the following items on Web of Science Core Collection (http://apps.webofknowledge.com/): “Year Published = 2000–2019” AND “Publication Name = New England Journal of Medicine” (or other 11 top journal names) in April 2020. We only counted research articles and invited reviews from those journals. All searched hits were manually selected by Dr. Jia Xiao and Dr. Fei Wang to ensure compliance with topic suitability and other vetting criteria.
NOVEL THERAPEUTICS AND MANAGEMENT OF ALD
At present, the most important and efficacious treatment modality in ALD is maintenance of abstinence, whose direct benefits include resolution of steatosis and halting of ongoing hepatitis, fibrosis, HCC, and other complications (e.g., cognitive impairment)39. Several drugs (e.g., naltrexone and acamprosate) may help a portion of the alcoholics to combat alcoholism. In addition, as obesity and cigarette smoking are known risk factors of ALD, weight control and quitting smoking are routinely encouraged in ALD lifestyle management40. It should also be noted that a poor overall nutritional status often accompanies ALD, particularly in steatohepatitis patients. The degree of malnutrition even correlates positively with the development of serious complications, including hepatic encephalopathy, ascites, and hepatorenal syndrome. Understandably, well-conceived nutrition support by oral, enteral, and parenteral routines has been seen an essential part of standard care for ASH41. Apart from high prevalence of severe protein–calorie malnutrition, micronutrient deficiencies (e.g., zinc, folate, and vitamins) have been reported in patients with heavy alcohol use and ALD42. Recent evidence also strongly implicates intestinal dysbiosis in ALD progression, which warrants efforts on the development of specific and effective therapies43.
To a great extent, successful drug treatment and management strategies for ALD rely on robust practice of disease stratification. For AUD patients to stay abstinent from alcohol, acamprosate, disulfiram, and naltrexone (oral and intramuscular) have been approved by the Food and Drug Administration (FDA). It is well known that alcohol withdrawal syndrome (AWS) can surface in heavy drinkers who stop or cut down on alcohol consumption. However, AWS is in effect a constellation of acute symptoms, which could easily include anxiety, tremors, nausea, insomnia, and in severe cases, seizures and delirium tremens. In contexts of symptom management, benzodiazepines, antipsychotic agents (e.g., haloperidol), antiepileptic agents (e.g., carbamazepine), and anesthetic agents (e.g., propofol and barbiturates) are clinically indicated for AWS patients44. For patients with severe ASH, corticosteroids is the current first-line treatment option, which brings short-term survival benefit45. Other classes of available chemotherapeutic agents include anti-TNF antibodies, pentoxifylline, antioxidants, and growth factors, although these options have produced at best mixed results in ASH patients, ranging from highly variable to weak responses12. Consistent with the need for personalized medicine, growing incentives are being put on clinical trials on novel drugs or management modalities to develop efficacious targeted therapies for patients with distinct stages of ALD. Based on their modes of action, these trials can be classified into four categories, namely, a) dietary supplements (e.g., probiotics), b) pharmacological agents/stem cells (e.g., growth factor, corticosteroids, antioxidants, antibodies against cytokine, and synthetic fatty acids), c) fecal microbiota transplantation (FMT), and d) device-related behavior change (Table 1). Evidently, there remains an urgent need to identify specific and clinically meaningful targets for ALD therapies, as current options largely depend on generic antioxidant or anti-inflammatory effects to contain symptoms. Pathologically, ALD is also known to be associated with significant changes in gut microbial composition, which involves translocation of viable bacteria, PAMPs, and eventual gut–liver inflammation. Application of probiotics/antibiotics and FMT have been explored as a strategy for modulating dysregulated microbiota43. Moreover, smartphone-based lifestyle interventions are increasingly showing promise as complementary options. Such technologies are generally cost-effective, automatable, relatively easy to implement, and highly accessible to mobile phone users46.
Table 1.
Major Ongoing Studies of Novel Drug and Management Options for Alcoholic Liver Disease (ALD)
| Type of Intervention/Treatment | Mode of Action | Disease and Inclusion | Study Design | Identifier |
|---|---|---|---|---|
| Dietary supplement | ||||
| Lactobacillus Rhamnosus GG | Probiotic | Acute ASH, MELD score < 20 | Placebo controlled RCT | NCT01922895 |
| Profermin Plus® (fermented oats, barley malt, lecithin, and Lactobacillus plantarum 299v) | Probiotic and antioxidant | Chronic ALD | RCT vs. Fresubin® (protein energy drink) | NCT03863730 |
| Omega 5 fatty acid | Antioxidant | Severe ASH | Placebo controlled RCT | NCT03732586 |
| Saturated fats | Antioxidant | ASH, Maddrey DF score > 32 | RCT vs. soybean oil | NCT04084522 |
| S-Adenosyl methionine and choline | Antioxidant | ALD | Placebo controlled RCT | NCT03938662 |
| Pharmacological agent | ||||
| G-CSF | Growth factor | ASH, Maddrey DF score ≥ 32 | RCT vs. prednisolone | NCT02442180; NCT04066179 |
| G-CSF | Growth factor | ASH, Maddrey DF score ≥ 32 | RCT | NCT03703674 |
| Pegfilgrastim | Growth factor (G-CSF analog) | ASH, Maddrey DF score ≥ 32 | RCT | NCT02776059 |
| Methylprednisolone | Corticosteroids | ASH, Maddrey DF score ≥ 32 | Placebo controlled RCT | NCT03160651 |
| N-acetylcysteine | Antioxidant | ASH, Maddrey DF score > 32 | RCT vs. prednisolone | NCT03069300 |
| Vitamin C | Antioxidant to reduce infection | ASH with sepsis | RCT | NCT03829683 |
| Bovine colostrum | Protein, growth factor, and Ig to LPS | ASH, MELD score ≥ 21, Maddrey DF score > 32 | Placebo controlled RCT | NCT02473341 |
| DS-102 (Epeleuton) | Synthetic N-3 fatty acid, improve lipid metabolism | Acute severe ASH | Placebo controlled RCT | NCT03452540 |
| Nalmefene | Opioid antagonist, controls alcoholism | AC, Child A or B | Placebo controlled RCT | NCT02824354 |
| Canakinumab | Monoclonal antibody of IL-1 | ASH, Maddrey DF score ≥ 32 and MELD score ≤ 27 | Placebo controlled RCT | NCT03775109 |
| Livitol-70 | Herbal extracts, antioxidant and hepatoprotection | ALD, Maddrey DF score < 30 | Open label | NCT03503708 |
| Biological therapy | ||||
| Cellgram™ (Bone marrow-derived MSCs) | Progenitor, improving liver regeneration | AC, Child B | Open label | NCT03838250 |
| Transplantation | ||||
| Fecal microbiota transplantation | Rectifying gut microbiota | Severe ASH | RCT vs. prednisolone | NCT03091010 |
| Fecal microbiota transplantation | Rectifying gut microbiota | ASH, Maddrey DF score > 32 | Open label | NCT03827772 |
| Device/behavior | ||||
| AlcoChange | Behavioral remodeling by Smartphone apps and breathalyzer | ALD, motivation to maintain abstinence | Open label | NCT03474328 |
| SMS/exercise | SMS-message-based lifestyle intervention | AC, run-in physical exercise programs | RCT | NCT02811887 |
| WrisTAS biosensor | Alcohol biosensor monitoring | ALD, SOCRATES score > 26 | RCT | NCT03533660 |
| A-CHESS | Smartphone apps delivering comprehensive health enhancement support | ALD | Open label | NCT03388320 |
Information was collected from the website of Clinical Trials (https://clinicaltrials.gov/) by J. Xiao and F. Wang on April 10, 2020, with key words “alcoholic,” “alcohol,” or “alcoholism” in the searching area of “Condition or disease.” This table includes most ongoing clinical trials on ALD registered in the website, except trials (1) that have current status of “withdrawn” or “unknown status,” and (2) in which ALD is not the major disease or condition studied in the trial. AC, alcoholic cirrhosis; ALD, alcoholic liver disease; ASH, alcoholic steatohepatitis; DF, discriminant function; G-CSF, granulocyte colony-stimulating factor; Ig, immunoglobin; IL, interleukin; MELD, model for end-stage liver disease; MSC, mesenchymal stem cells; LPS, lipopolysaccharide; RCT, randomized controlled trials; SMS, short-message service; SOCRATES, stages of change readiness and treatment eagerness scale.
CONCLUSIONS
Although remarkable strides have been made in understanding and tackling ALD in the past decades, it is also clear that ALD will continue to be a major cause of morbidity and mortality in global proportions. However, subtle differences in the challenges exist across countries. Despite an already high global prevalence of AUD and ALD, the world’s average APC has edged up between 2000 (5.7 L) and 2016 (6.4 L); within the same period, values of the same metric significantly declined in European countries (from 12.1 to 9.8 L). As a consequence of economic expansion and improved average incomes in developing regions, APC is surging in some countries such as China and India47. This partially explains the high incidence of alcoholic cirrhosis, liver cancer, and concurrent complications in those countries. Indeed, the link between socioeconomic factors and ALD is complex. Prevalence of AUD and alcohol-attributable liver cirrhosis is positively correlated with national income distribution in most countries. However, the “alcohol harm paradox” highlights a somewhat unforeseen situation where socioeconomically disadvantaged groups need more attention, in both clinically and sociopolitical contexts. In a similar vein, greater concerted efforts based on an international consensus must be made for the long quest of combatting ALD. These could include a judicious combination of incentives and disincentives toward reducing harmful use of alcohol, alleviating the health/societal burdens of ALD, and empowering locally relevant intervention policies as a public health priority, such as the “best buys” proposed by the WHO.
Advancing scientific knowledge on detailed pathophysiological mechanisms is a fundamental basis for attaining therapeutic innovations. Although the vital roles of several key pathways in ALD (e.g., lipid metabolism, inflammation, and immune regulation) have been partially unveiled in the past decades, still more missing pieces in the mechanistic picture over ALD pathogenesis need to be scrutinized. Some interesting questions demanding our attention include the following: (1) Why most heavy drinkers solely develop steatosis without inflammation? (2) How does inflammatory, immune response, and autophagic signaling in ALD contribute to fibrosis and carcinogenesis? (3) What are the effectors and consequences of interconnected processes like neuronal disorder, aging, and circadian clock dysfunction during ALD progression? (4) What molecular determinants drive microbiota dysregulation in ALD initiation and progression? (5) What mechanisms regulate noncoding RNAs (i.e., microRNAs, lncRNAs, and circular RNAs) in ALD pathophysiology and which of their characteristics are exploitable as novel noninvasive biomarker? (6) How can protective strategies for interorgan communication be formulated to counter systemic effects of alcohol during the development of ALD? New insights into some of these unanswered questions will certainly help guide the incubation and maturation of novel targeted therapies for ALD, which, however, also implies a necessity of intensified government roles and scientific inputs in the form of rigorously controlled clinical trials and robust grant supports. It is obvious that there is a big gap between the US and other countries in the funding and published articles on ALD. Possible explanations include the following: (1) the US has a unique national institute of alcohol-related research (NIAAA) for the prevention and treatment of alcohol abuse and alcoholism as early as 1971; (2) the US has stable annual governmental funding scheme for alcohol-related research, while similar grants in other countries primarily rely on investigators’ application; (3) in East Asian countries (e.g., China, Japan, and Korea), alcohol drinking is somehow viewed as a necessary social activity, but not a risk factor for liver diseases. Viewed as a whole, current governmental support for ALD research still lags starkly behind that for other major liver diseases (e.g., liver cancer, viral hepatitis, and NAFLD) in many developed nations and emerging economies, which is unlikely to fulfill the world’s urgent needs for better ALD care and therapy.
ACKNOWLEDGMENTS
This work has been graciously supported by grants from the National Natural Science Foundation of China (81970515, 81873573, and 81800525) and the Guangdong Natural Science Funds for Distinguished Young Scholar (2019B151502013). This article has not been motioned, financially supported, or influenced in any other ways by parties of the pharmaceutical industry. The authors declare no conflict of interest.
REFERENCES
- 1. Louvet A, Mathurin P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12(4):231–42. [DOI] [PubMed] [Google Scholar]
- 2. Rehm J, Baliunas D, Borges GL, Graham K, Irving H, Kehoe T, Parry CD, Patra J, Popova S, Poznyak V, Roerecke M, Room R, Samokhvalov AV, Taylor B. The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction 2010;105(5):817–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ventura-Cots M, Ballester-Ferre MP, Ravi S, Bataller R. Public health policies and alcohol-related liver disease. JHEP Rep. 2019;1(5):403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong T, Dang K, Ladhani S, Singal AK, Wong RJ. Prevalence of alcoholic fatty liver disease among adults in the United States, 2001–2016. Jama 2019;321(17):1723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xiao J, Wang F, Wong NK, He J, Zhang R, Sun R, Xu Y, Liu Y, Li W, Koike K, He W, You H, Miao Y, Liu X, Meng M, Gao B, Wang H, Li C. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J Hepatol. 2019;71(1):212–21. [DOI] [PubMed] [Google Scholar]
- 6. Leong DP, Smyth A, Teo KK, McKee M, Rangarajan S, Pais P, Liu L, Anand SS, Yusuf S. Patterns of alcohol consumption and myocardial infarction risk: Observations from 52 countries in the INTERHEART case–control study. Circulation 2014;130(5):390–8. [DOI] [PubMed] [Google Scholar]
- 7. Elkind MS, Sciacca R, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Moderate alcohol consumption reduces risk of ischemic stroke: The Northern Manhattan Study. Stroke 2006;37(1):13–9. [DOI] [PubMed] [Google Scholar]
- 8. Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: A meta-analysis of prospective observational studies. Diabetes Care 2005;28(3):719–25. [DOI] [PubMed] [Google Scholar]
- 9. Sookoian S, Castaño GO, Pirola CJ. Modest alcohol consumption decreases the risk of non-alcoholic fatty liver disease: A meta-analysis of 43 175 individuals. Gut 2014;63(3):530–2. [DOI] [PubMed] [Google Scholar]
- 10. Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, Paige E, Paul DS, Sweeting M, Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 2018;391(10129):1513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao B, Ahmad ,MF,Nagy LE, Tsukamoto H. Inflammatory pathways in alcoholic steatohepatitiis. J Hepatol. 2019;70(2):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singal AK, Kodali S, Vucovich LA, Darley-Usmar V, Schiano TD. Diagnosis and treatment of alcoholic hepatitis: A systematic review. Alcohol Clin Exp Res. 2016;40(7):1390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Global status report on alcohol and health 2018. Switzerland: World Health Organization; 2018. [Google Scholar]
- 14. Global health estimates 2016: Disease burden by cause, age, sex, by country and by region, 2000–2016. Switzerland, World Health Organization; 2018. [Google Scholar]
- 15. Mackenbach JP,Kulhánová I,Bopp M, Borrell C, Deboosere P, Kovács K,Looman CW, Leinsalu M, Mäkelä P, Martikainen P, Menvielle G, Rodríguez-Sanz M,Rychtaříková J,de Gelder R. Inequalities in alcohol-related mortality in 17 European countries: A retrospective analysis of mortality registers. PLoS Med. 2015;12(12):e1001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sadler S, Angus C, Gavens L, Gillespie D, Holmes J, Hamilton J, Brennan A, Meier P. Understanding the alcohol harm paradox: An analysis of sex- and condition-specific hospital admissions by socio-economic group for alcohol-associated conditions in England. Addiction 2017;112(5):808–17. [DOI] [PubMed] [Google Scholar]
- 17. Probst C, Fleischmann A, Gmel G, Poznyak V, Rekve D, Riley L, Rylett M, Shield KD, Rehm J. The global proportion and volume of unrecorded alcohol in 2015. J Glob Health 2019;9(1):010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katikireddi SV, Whitley E, Lewsey J, Gray L, Leyland AH. Socioeconomic status as an effect modifier of alcohol consumption and harm: Analysis of linked cohort data. Lancet Public Health 2017;2(6):e267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The L. Russia’s alcohol policy: A continuing success story. Lancet 2019;394(10205):1205. [DOI] [PubMed] [Google Scholar]
- 20. Rehm J, Manthey J, Lange S, Badaras R, Zurlyte I, Passmore J, Breda J, Ferreira-Borges C, Štelemėkas M. Alcohol control policy and changes in alcohol-related traffic harm. Addiction 2020;115(4):655–65. [DOI] [PubMed] [Google Scholar]
- 21. Wagenaar AC, Tobler AL, Komro KA. Effects of alcohol tax and price policies on morbidity and mortality: A systematic review. Am J Public Health 2010;100(11):2270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chaloupka FJ., Powell LM, Warner KE. The use of excise taxes to reduce tobacco, alcohol, and sugary beverage consumption. Annu Rev Public Health 2019;40:187–201. [DOI] [PubMed] [Google Scholar]
- 23. Yeh CY, Ho LM, Lee JM Hwang JY. The possible impact of an alcohol welfare surcharge on consumption of alcoholic beverages in Taiwan. BMC Public Health 2013;13:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sherk A, Stockwell T, Chikritzhs T, Andréasson S, Angus C, Gripenberg J, Holder H, Holmes J, Mäkelä P,Mills M, Norström T, Ramstedt M, Woods J. Alcohol consumption and the physical availability of take-away alcohol: Systematic reviews and meta-analyses of the days and hours of sale and outlet density. J Stud Alcohol Drugs 2018;79(1):58–67. [PubMed] [Google Scholar]
- 25. Jernigan D, Noel J, Landon J, Thornton N, Lobstein T. Alcohol marketing and youth alcohol consumption: A systematic review of longitudinal studies published since 2008. Addiction 2017;112(Suppl 1):7–20. [DOI] [PubMed] [Google Scholar]
- 26. Esser MB, Jernigan DH. Policy approaches for regulating alcohol marketing in a global context: A public health perspective. Annu Rev Public Health 2018;39:385–401. [DOI] [PubMed] [Google Scholar]
- 27. Addolorato G, Mirijello A, Barrio P, Gual A. Treatment of alcohol use disorders in patients with alcoholic liver disease. J Hepatol. 2016;65(3):618–30. [DOI] [PubMed] [Google Scholar]
- 28. Parker R, Kim SJ, Gao B. Alcohol, adipose tissue and liver disease: Mechanistic links and clinical considerations. Nat Rev Gastroenterol Hepatol. 2018;15(1):50–9. [DOI] [PubMed] [Google Scholar]
- 29. Lackner C, Tiniakos D. Fibrosis and alcohol-related liver disease. J Hepatol. 2019;70(2):294–304. [DOI] [PubMed] [Google Scholar]
- 30. Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG clinical guideline: Alcoholic liver disease. Am J Gastroenterol 2018;113(2):175–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ganne-Carrié N,Nahon P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J Hepatol. 2019;70(2):284–93. [DOI] [PubMed] [Google Scholar]
- 32. EASL clinical practice guidelines: Management of alcohol-related liver disease. J Hepatol. 2018;69(1):154–81. [DOI] [PubMed] [Google Scholar]
- 33. Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, McClain C, McCullough A, Mitchell MC, Morgan TR, Nagy L, Radaeva S, Sanyal A, Shah V, Szabo G. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: Recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 2016;150(4):785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sanyal AJ, Gao B, Szabo G. Gaps in knowledge and research priorities for alcoholic hepatitis. Gastroenterology 2015;149(1):4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013;10(9):542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mueller S, Nahon P, Rausch V, Peccerella T, Silva I, Yagmur E, Straub BK, Lackner C, Seitz HK, Rufat P, Sutton A, Bantel H, Longerich T. Caspase-cleaved keratin-18 fragments increase during alcohol withdrawal and predict liver-related death in patients with alcoholic liver disease. Hepatology 2017;66(1):96–107. [DOI] [PubMed] [Google Scholar]
- 37. Yang Z, Ross RA, Zhao S, Tu W, Liangpunsakul S, Wang L. LncRNA AK054921 and AK128652 are potential serum biomarkers and predictors of patient survival with alcoholic cirrhosis. Hepatol Commun 2017;1(6):513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moreno C, Mueller S, Szabo G. Non-invasive diagnosis and biomarkers in alcohol-related liver disease. J Hepatol. 2019;70(2):273–83. [DOI] [PubMed] [Google Scholar]
- 39. Lackner C, Spindelboeck W, Haybaeck J, Douschan P, Rainer F, Terracciano L, Haas J, Berghold A, Bataller R, Stauber RE. Histological parameters and alcohol abstinence determine long-term prognosis in patients with alcoholic liver disease. J Hepatol. 2017;66(3):610–8. [DOI] [PubMed] [Google Scholar]
- 40. Crittenden NE, McClain C. Management of patients with moderate alcoholic liver disease. Clin Liver Dis. (Hoboken) 2013;2(2):76–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Styskel B, Natarajan Y, Kanwal F. Nutrition in alcoholic liver disease: An update. Clin Liver Dis. 2019;23(1):99–114. [DOI] [PubMed] [Google Scholar]
- 42. Kozeniecki M, Ludke R, Kerner J, Patterson B. Micronutrients in liver disease: Roles, risk factors for deficiency, and recommendations for supplementation. Nutr Clin Pract. 2020;35(1):50–62. [DOI] [PubMed] [Google Scholar]
- 43. Sarin SK, Pande A, Schnabl B. Microbiome as a therapeutic target in alcohol-related liver disease. J Hepatol. 2019;70(2):260–72. [DOI] [PubMed] [Google Scholar]
- 44. Jesse S, Bråthen G, Ferrara M, Keindl M, Ben-Menachem E, Tanasescu R, Brodtkorb E, Hillbom M, Leone MA, Ludolph AC. Alcohol withdrawal syndrome: Mechanisms, manifestations, and management. Acta Neurol Scand. 2017;135(1):4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Louvet A, Thursz MR, Kim DJ, Labreuche J, Atkinson SR, Sidhu SS, O’Grady JG, Akriviadis E, Sinakos E, Carithers RL Jr, Ramond MJ, Maddrey WC, Morgan TR, Duhamel A, Mathurin P. Corticosteroids reduce risk of death within 28 days for patients with severe alcoholic hepatitis, compared with pentoxifylline or placebo – a meta-analysis of individual data from controlled trials. Gastroenterology 2018;155(2):458–68.e458. [DOI] [PubMed] [Google Scholar]
- 46. Sim, I. Mobile devices and health. N Engl J Med. 2019;381(10):956–68. [DOI] [PubMed] [Google Scholar]
- 47. Liangpunsakul S, Haber P, McCaughan GW. Alcoholic liver disease in Asia, Europe, and North America. Gastroenterology 2016;150(8):1786–97. [DOI] [PMC free article] [PubMed] [Google Scholar]