Abstract
Introduction
Nipple discharge is a common symptom of breast disease. We aimed to perform a descriptive statistical analysis of the cases we evaluated and establish a model to predict intraductal tumors.
Materials and Methods
We conducted a retrospective study of patients from 2007 to 2019. In total, 1333 patients who completed the fiberoptic ductoscopy (FDS) were evaluated. The variables were analyzed by χ2 test. Logistic regression was used to analyze the relationship between the patient’s clinical characteristics and intraductal tumors and establish a predictive model. Receiver operating characteristic (ROC) curve analysis was used to assess the sensitivity and specificity of the predictive ability of the model. Calibration curves and decision curve analysis (DCA) were used to evaluate the model.
Results
Patients with spontaneous, single-duct, bloody discharge and a smooth ductal wall were more likely to be diagnosed with tumors by ductoscopy. A model was established based on five variables: age, side of discharge, spontaneous discharge status, duration of discharge, and color of discharge. The model was subsequently validated in 183 patients with complete data on the variables in the validation cohort. The area under the ROC curve (AUC) was calculated to be 0.716, indicating good predictive ability.
Conclusion
Patients with the clinical characteristics of unilateral, bloody, single-duct, spontaneous discharge and a smooth ductal wall were more likely to have intraductal tumors by ductoscopy. Our nomogram can effectively predict intraductal tumors in patients with nipple discharge.
Keywords: nipple discharge, ductoscopy, nomogram, breast tumor
Introduction
Nipple discharge (ND) is an early manifestation of most intraductal lesions.1 ND can be caused by various breast diseases, including benign lesions represented by intraductal papilloma, ductal dilatation, and papillomatosis, as well as malignant lesions represented by ductal carcinoma in situ (DCIS) and invasive ductal carcinoma (IDC).2,3 Some benign breast diseases also have the risk of malignancy.4 Therefore, identification of the nature of breast diseases causing ND is of great significance to guide the decision-making of clinical diagnosis and treatment programs.
Approximately 85% of malignant and premalignant breast diseases originate in the ductal epithelium.5 Indirect methods such as cytological examination of discharge smears, ultrasonography and molybdenum target mammography have low sensitivity to diagnose neoplasm and cannot objectively reflect the situation of intraductal lesions.6,7 The fiberoptic ductoscope was developed by Okazaki in 1991 and has been widely applied in the clinic.8 It can directly observe the condition of the duct, accurately locate the lesion, identify ductal obstruction and further clarify the indication of operation.9
Although ductoscopy has proven to be very effective in detecting intraductal masses, no general guidelines exist because of the prevalence, cost, and lack of specialists. At the same time, not all patients with ND need a fiberoptic ductoscopy examination. Clinically, for patients who have a porous discharge or clear-colored and nonspontaneous colored discharge, the detection rate of the intraductal tumor is very low.10,11 According to current Chinese guidelines, there is no accurate statement concerning the indication of fiberoptic ductoscopy. In Japan, the procedure is also a routine clinical examination for ND.12 To avoid medical and legal disputes, any ND with negative imaging findings is subjected to fiberoptic ductoscopy in China, causing an unnecessarily large amount of resource waste. The procedure also adds pain and economic burden to patients. How to clinically distinguish patients who are truly required for fiberoptic ductoscopy is an urgent topic.
We have performed fiberoptic ductoscopy to diagnose the cause of ND for 13 years. This study, for the first time, aimed to assess which symptoms accompanying ND correlate with neoplasms in lactiferous ducts. Moreover, specific features of intraductal neoplasms were identified and used to evaluate potential indications before ductoscopy.
Materials and Methods
Patients
In total, 1333 patients with ND who were admitted to our hospital from October 2007 to June 2019 and had successfully completed fiberoptic ductoscopy were evaluated. The inclusion criteria were as follows: 1) the patient presented with nipple discharge as the main complaint; 2) the patient was willing to undergo fiberoptic ductoscopy; 3) the serum prolactin content was normal. The exclusion criteria were as follows: 1) women who were breastfeeding or pregnant; 2) patients had been diagnosed with breast cancer or other breast-related diseases; 3) patients had serious organic disease, mental illness or other diseases that affected patients’ cognition; 4) patients had poor compliance and were difficult to follow-up for such long time. The flowchart of the patient selection is shown in Supplemental Figure 1. This study was retrospective and did not involve any experimental interventions. According to the rules of the ethics committee, it did not require special ethics approval.
Fiberoptic Ductoscopy System (FDS)
The FDS (solid fiber scope MS-611; 100–0201; FiberTech Co., Ltd., Tokyo, Japan) comprises a silicafiberscope, a light source, an image monitor, and an image recorder. The outer diameter of the silicafiberscope was 0.70 mm, its inner flow path diameter was 0.3 mm, and its maximum exploratory length was 6.5 cm.
FDS Procedure
The whole breasts with ND were sterilized with ethanol 3 times. Each discharging lactiferous duct was locally anesthetized with 2% lidocaine. Lacrimal dilators were inserted into the nipple orifice to dilate the ostium of the lactiferous duct. Next, the FDS was inserted into the dilated orifices. The major lactiferous ducts and segmental branches were investigated one by one until the duct was too thin for the scope or the scope was not sufficiently long. Any visible lesion was recorded by taking pictures, and the distances from the nipple orifice to the lesions were measured.
Statistical Analysis
All the data were expressed as frequencies, percentages and effective percentages. The relationship between the diagnosis of tumor or no tumor was identified by ductoscopy, and the clinical factors were categorical variables that were analyzed by χ2 test. Fisher’s exact test was applied when the theoretical frequency was <5 or the total observation frequency was <20. Logistic regression was used to analyze the relationship between the clinical features of patients with ND and fiberoptic ductoscopy. Receiver operating characteristic (ROC) and calibration curves were used to assess the sensitivity and specificity of fiberoptic ductoscopy and the predictive ability of the model. Decision curve analysis (DCA) was used to explore how the predictive models benefit ND patients. The sample() function in R software was used to divide the data into training and verification cohorts at a ratio of 7:3 by random sampling. All the statistical tests were two sided. A p-value<0.05 indicated a statistically significant difference. All analyses were performed using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) and R software (version 3.6.1; Institute for Statistics and Mathematics, Vienna, Austria).
Results
Patient Clinical Factors
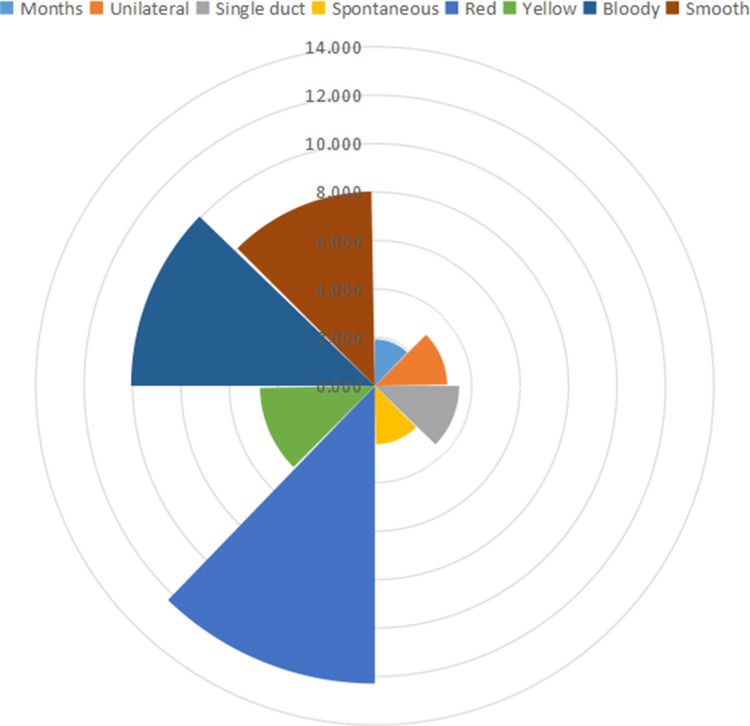
In this study, we evaluated 1333 patients with ND who had successfully completed fiberoptic ductoscopy at our hospital from October 2007 to June 2019. The age of the patients ranged from 13 to 82 years. We summarized the number of different clinical features in patients with ND and their proportions (Table 1). Nearly half (40.24%) of the patients were aged between 41 and 50 years, and most (87.70%) showed unilateral discharge. Moreover, the patients with red and yellow discharge accounted for 30.46% and 42.01%, respectively. More than half (58.06%) of the patients had nonspontaneous ND.
Table 1.
Baseline Data of Patients with Nipple Discharge
| Factors | N | % | Valid%a |
|---|---|---|---|
| Age (year) | |||
| ≤20 | 5 | 0.38 | 0.38 |
| 21–30 | 148 | 11.10 | 11.33 |
| 31–40 | 385 | 28.88 | 29.48 |
| 41–50 | 536 | 40.21 | 41.04 |
| 51–60 | 153 | 11.48 | 11.72 |
| 61–70 | 60 | 4.50 | 4.59 |
| >70 | 19 | 1.43 | 1.45 |
| Blank | 27 | 2.03 | |
| Duration of discharge | |||
| Days | 72 | 5.40 | 11.48 |
| Weeks | 115 | 8.63 | 18.34 |
| Months | 231 | 17.33 | 36.84 |
| Years | 209 | 15.68 | 33.33 |
| Blank | 706 | 52.96 | |
| Side of discharge | |||
| Unilateral | 1169 | 87.70 | 87.70 |
| Bilateral | 164 | 12.30 | 12.30 |
| Blank | 0 | 0 | |
| Location of discharge | |||
| Single duct | 1093 | 82.00 | 82.30 |
| Multiple ducts | 235 | 17.63 | 17.70 |
| Blank | 5 | 0.38 | |
| Spontaneous or not | |||
| Nonspontaneous | 418 | 31.36 | 62.02 |
| Spontaneous | 256 | 19.20 | 37.98 |
| Blank | 659 | 49.44 | |
| Color of discharge | |||
| Red | 406 | 30.46 | 30.71 |
| Yellow | 560 | 42.01 | 42.36 |
| White or colorless | 356 | 26.71 | 26.93 |
| Blank | 11 | 0.83 | |
| Characteristics of discharge | |||
| Watery | 97 | 7.28 | 7.31 |
| Bloody | 332 | 24.91 | 25.02 |
| Serosity | 774 | 58.06 | 58.33 |
| Milky | 117 | 8.78 | 8.82 |
| Solid | 7 | 0.53 | 0.53 |
| Blank | 6 | 0.45 | |
| Ductal wall | |||
| Smooth | 801 | 60.09 | 62.82 |
| Rough | 474 | 35.56 | 37.18 |
| Blank | 58 | 4.35 |
Note: aPercentages excluding “Blank”.
Distribution of the Clinical Factors of Patients Based on Ductoscopy
The association between clinical factors and intraductal tumors is shown in Table 2. According to the diagnosis of fiberoptic ductoscopy, we divided the patients into the tumor group and no tumor group. Various factors were analyzed, including age, duration of discharge, side of discharge, location of discharge, spontaneous discharge status, color of discharge, characteristics of discharge and condition of the ductal wall. Age (p<0.001), side of discharge (p<0.001), location of discharge (p<0.001), spontaneous discharge status (p<0.001), color of discharge (p<0.001), characteristic of discharge (p<0.001) and condition of the ductal wall (p<0.001) were significantly different between the two groups. However, no statistically significant difference was found between the groups in the duration of discharge (p=0.142). Compared with patients with bilateral discharge, nonspontaneous discharge and a rough ductal wall, patients with unilateral discharge, spontaneous discharge and a smooth ductal wall were more likely to be diagnosed with tumors by ductoscopy. Among the remaining variables, we performed Bonferroni correction on the p-value and performed statistical analysis on each set of data (Supplemental Table 1). Additionally, patients aged 61 to 70 years and older than 70 years with ND were more likely to be diagnosed with intraductal tumors by ductoscopy (p<0.002). Furthermore, patients with single-duct discharge (p<0.003), red-colored discharge (p<0.008) and bloody discharge (p<0.005) were more likely to be diagnosed with tumors.
Table 2.
Relationship Between the Diagnosis of Tumor or No Tumor Identified by Ductoscopy and the Clinical Factors
| Factors | N | χ2 | p-value | |||
|---|---|---|---|---|---|---|
| Tumor | % | No Tumor | % | |||
| Age(year) | 24.896 | <0.001 | ||||
| ≤20 | 2 | 0.39 | 3 | 0.38 | ||
| 21–30 | 44 | 8.54 | 104 | 13.15 | ||
| 31–40 | 134 | 26.02 | 251 | 31.73 | ||
| 41–50 | 218 | 42.33 | 318 | 40.20 | ||
| 51–60 | 72 | 13.98 | 81 | 10.24 | ||
| 61–70 | 32 | 6.21 | 28 | 3.54 | ||
| >70 | 13 | 2.52 | 6 | 0.76 | ||
| Blank | 8 | 19 | ||||
| Duration of discharge | 5.445 | 0.142 | ||||
| Days | 21 | 8.33 | 51 | 13.60 | ||
| Weeks | 48 | 19.05 | 67 | 17.87 | ||
| Months | 102 | 40.48 | 129 | 34.40 | ||
| Years | 81 | 32.14 | 128 | 34.13 | ||
| Blank | 271 | 435 | ||||
| Side of discharge | 30.511 | <0.001 | ||||
| Unilateral | 491 | 93.88 | 678 | 83.70 | ||
| Bilateral | 32 | 6.12 | 132 | 16.30 | ||
| Blank | 0 | 0 | ||||
| Location of discharge | 52.828 | <0.001 | ||||
| Single duct | 479 | 91.80 | 614 | 76.20 | ||
| Multiple ducts | 43 | 8.20 | 192 | 23.80 | ||
| Blank | 1 | 4 | ||||
| Spontaneous or not | 29.698 | <0.001 | ||||
| Nonspontaneous | 135 | 49.63 | 283 | 70.40 | ||
| Spontaneous | 137 | 50.37 | 119 | 29.60 | ||
| Blank | 251 | 408 | ||||
| Color of discharge | 204.717 | <0.001 | ||||
| Red | 255 | 49.13 | 151 | 18.80 | ||
| Yellow | 221 | 42.58 | 339 | 42.22 | ||
| White or colorless | 43 | 8.29 | 313 | 38.98 | ||
| Blank | 4 | 7 | ||||
| Characteristics of discharge | 145.852 | <0.001 | ||||
| Watery | 20 | 3.84 | 77 | 9.55 | ||
| Bloody | 208 | 39.92 | 124 | 15.38 | ||
| Serosity | 284 | 54.51 | 490 | 60.79 | ||
| Milky | 8 | 1.54 | 109 | 13.52 | ||
| Solid | 1 | 0.19 | 6 | 0.74 | ||
| Blank | 2 | 4 | ||||
| Ductal wall | 204.491 | <0.001 | ||||
| Smooth | 415 | 88.11 | 386 | 48.01 | ||
| Rough | 56 | 11.89 | 418 | 51.99 | ||
| Blank | 52 | 6 | ||||
The odds ratio (OR) was also calculated to analyze the risk of each factor. Univariate binary logistic regression analysis is presented in Supplemental Table 2. Discharge duration (OR, 1.92; 95% CI: 1.09–3.40; p=0.025), unilateral discharge (OR, 2.99; 95% CI: 2.00–4.47; p<0.001), single-duct discharge (OR, 3.48; 95% CI: 2.45–4.95; p<0.001), spontaneous discharge (OR, 2.41; 95% CI: 1.75–3.32; p<0.001), red-colored discharge (OR, 12.29; 95% CI: 8.43–17.92; p<0.001), yellow-colored discharge (OR, 4.75; 95% CI: 3.31–6.81; p<0.001), bloody discharge (OR, 10.07; 95% CI: 1.20–84.58; p=0.034) and a smooth ductal wall (OR, 8.03; 95% CI: 5.88–10.96; p<0.001) were risk factors for intraductal tumors in patients with ND. The OR values of the above variables are shown in Figure 1.
Figure 1.
The OR values of the risk factors for intraductal tumors in patients with nipple discharge. The OR values were determined by univariate binary logistic regression analysis of clinical factors with the diagnosis of tumor or no tumor identified by ductoscopy. Months (OR, 1.920; p=0.025). Unilateral (OR, 2.987; p<0.001). Spontaneous discharge (OR, 2.413; p<0.001). Red discharge (OR, 12.292; p<0.001). Yellow discharge (OR, 4.745; p<0.001). Bloody discharge (OR, 10.065; p=0.034). Smooth ductal wall (OR, 8.025; p<0.001). The position of the arc of the fan in the graph represents its OR value.
Efficiency of Breast Ductoscopy to Detect Intraductal Tumors Compared with Ultrasound and Pathology
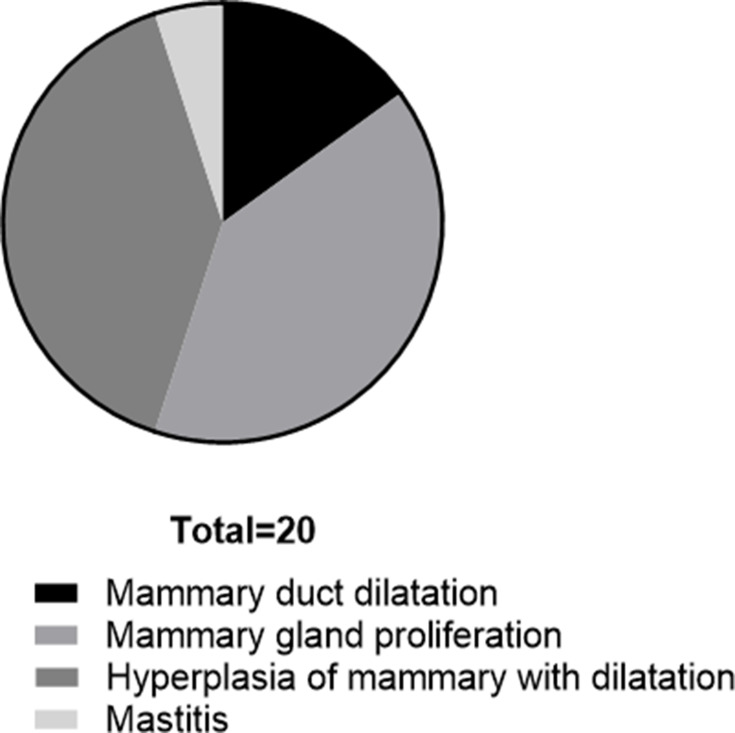
To further evaluate the effect of breast ductoscopy on intraductal tumors, we compared the results of breast ductoscopy and pathology (Supplemental Table 3), as well as breast ductoscopy and ultrasound, on tumors (Supplemental Table 4). Breast ductoscopy was sensitive to the diagnosis of intraductal tumors, while the specificity was poor. In 20 cases, the tumors were detected by ductoscopy but were not reported by pathology (Figure 2). Among them, there were 3 cases of mammary duct dilatation, 8 cases of mammary gland proliferation, 8 cases of hyperplasia of mammary with dilatation of ducts, and 1 case of mastitis.
Figure 2.
Masses were found under the mammary duct, but the pathological diagnosis was non-masses. There were 3 cases of mammary duct dilatation, 8 cases of mammary gland proliferation, 8 cases of hyperplasia of mammary with dilatation of ducts, and 1 case of mastitis.
Pathological Diagnosis of Tumor Features in Fiberoptic Ductoscopy
We collected the data of patients who had undergone breast surgery and found that 267 patients were diagnosed with benign tumors and 35 were diagnosed with malignant or borderline tumors. The following variables were analyzed: single or multiple tumors, location of tumor, distance from nipple, and color and shape of the tumor (Supplemental Table 5). However, no factor was found to have statistically significant associations with the pathological diagnosis by univariate analysis.
Baseline Data on the Clinical Factors of Patients with Malignant Tumors
After surgery, 35 patients who had undergone ductoscopy were diagnosed as malignant or precancerous and their clinical factors are shown in Supplemental Table 6. Regarding age, 14 (40%) patients were aged between 41 and 50 years. Additionally, all the patients showed unilateral discharge. The number of patients with multiple-duct discharge accounted for only 5.71% of the total patients. More than half (51.43%) of the patients had bloody discharge. Regarding the features of the tumor, patients with a single tumor number twice as many as those with multiple tumors. Yellow and spherical tumors accounted for a relatively high proportion of patients. Concerning breast cancer-specific molecular markers, the number of estrogen receptor (ER)- and progesterone receptor (PR)-positive patients was far higher than that of ER- and PR-negative patients, in contrast to the trend for human epidermal growth factor receptor-2 (HER2). In our study, the numbers of patients with HER2− and HER2+ were 17 and 6, respectively. However, the patients with HER2++ (one with FISH+, the other with FISH-) and HER2+++ numbered 2 and 6, respectively. The number of patients with Ki67<15% was 20. These data suggested that these patients were more likely to have luminal-type breast cancer, which has a good prognosis, than HER2-positive or triple-negative breast cancer with a poor prognosis. Among the 35 patients, 9 were diagnosed with atypical hyperplasia, and 15 were diagnosed with carcinoma in situ (CIS). Six and 9 patients were diagnosed with invasive and special-type carcinomas, respectively. The others were diagnosed with mixed carcinoma. Furthermore, 26 patients had undergone mastectomy and 9 had undergone breast-conserving surgery.
Nomogram to Predict the Probability of Breast Duct Tumors in Patients with ND
To predict the probability of lactiferous duct tumors in patients with ND, we reprocessed the original data. We focused on the following variables: age (year), side of discharge, spontaneous discharge status, duration of discharge, color of discharge, characteristics of discharge, and location of discharge, which can be obtained before ductoscopy is performed in ND patients. The six variables, except duration of discharge, were classified into two categories. Of the 1333 patients, 616 were included in the cohort after excluding 717 patients with unknown variables. After using R software, 433 patients were included in the training cohort and 183 were included in the validation cohort in a 7:3 ratio by random sampling. The factors of the patients between the two groups did not differ significantly (p > 0.05), a finding that was consistent with randomization (Supplemental Table 7).
Univariate and multivariate logistic regression analyses were performed to explore factors and establish a prediction model (Table 3). Univariate logistic regression analysis revealed that all the variables were correlated with tumors in the ducts. Therefore, we included all the variables in multivariate logistic regression analysis. Surprisingly, the only variables with p-values less than 0.05 were age (year) and spontaneous discharge status, a finding that contrasts our clinical experience. Therefore, we performed collinearity analysis of all the variables and found that the variance inflation factor (VIF) of the variables Side_of_discharge, Color_of_discharge, Characteristics_of_discharge, and Location_of_discharge were all greater than 3, suggesting that they have collinearity (Supplemental Table 8). Therefore, we excluded Location_of_discharge and Characteristics_of_discharge and then performed multivariate logistic regression analysis (Table 4), suggesting that all the variables were statistically significant.
Table 3.
Univariate and Multivariate Logistic Regression Analysis for Factors Associated with Tumor Under Ductoscopy in Training Cohort
| Variables | Univariate Analyses | Multivariate Analyses | ||||
|---|---|---|---|---|---|---|
| Coefficient | OR (95% CI) | p | Coefficient | OR (95% CI) | p | |
| Age(year) | ||||||
| ≤44 | – | 1 | – | 1 | – | |
| >44 | 0.601 | 1.82(1.23–2.71) | 0.003 | 0.574 | 1.77(1.16–2.73) | 0.009 |
| Side of discharge | ||||||
| Unilateral | – | 1 | – | 1 | – | |
| Bilateral | −1.311 | 0.27(0.18–0.57) | 0.001 | −1.287 | 0.28(0.06–1.30) | 0.090 |
| Spontaneous or not | ||||||
| Nonspontaneous | – | 1 | – | 1 | – | |
| Spontaneous | 0.938 | 2.56(1.70–3.84) | 0.000 | 0.902 | 2.46(1.59–3.84) | 0.000 |
| Duration of discharge | ||||||
| Days | – | 1 | – | 1 | – | |
| Weeks | 0.560 | 1.75(0.82–3.76) | 0.151 | 0.523 | 1.69(0.75–3.93) | 0.216 |
| Months | 0.730 | 2.08(1.04–4.15) | 0.039 | 0.833 | 2.30(1.11–4.99) | 0.029 |
| Years | 0.333 | 1.40(0.69–2.82) | 0.355 | 0.696 | 2.01(0.93–4.49) | 0.081 |
| Color of discharge | ||||||
| Non-red | - | 1 | - | 1 | - | |
| Red | 1.083 | 2.95(1.94–4.49) | 0.000 | 0.713 | 2.04(0.92–4.44) | 0.074 |
| Characteristics of discharge | ||||||
| Non-bloody | - | 1 | - | 1 | - | |
| Bloody | 1.196 | 3.31(2.14–5.11) | 0.000 | 0.533 | 1.70(0.76–3.88) | 0.194 |
| Location of discharge | ||||||
| Single duct | - | 1 | - | 1 | - | |
| Multiple ducts | −1.092 | 0.34(0.18–0.64) | 0.001 | 0.180 | 1.20(0.30–4.25) | 0.786 |
Table 4.
Predict the Probability of Breast Duct Tumors in Patients with Nipple Discharge
| Intercept and Variable | Coefficient | SE | OR | 95% CI | p |
|---|---|---|---|---|---|
| Age | 0.583 | 0.218 | 1.790 | 1.17–2.74 | 0.007 |
| Side of discharge | −1.162 | 0.406 | 0.310 | 0.14–0.69 | 0.004 |
| Spontaneous or not | 0.928 | 0.221 | 2.530 | 1.64–3.90 | 0.000 |
| Duration of discharge | |||||
| Weeks vs.Days | 0.504 | 0.420 | 1.660 | 0.73–3.77 | 0.230 |
| Months vs.Days | 0.801 | 0.380 | 2.230 | 1.06–4.69 | 0.035 |
| Years vs.Days | 0.657 | 0.396 | 1.930 | 0.89–4.19 | 0.097 |
| Color of discharge | 1.127 | 0.231 | 3.090 | 1.96–4.85 | 0.000 |
| Intercept | −1.918 | 0.377 | 0.147 | – | 0.000 |
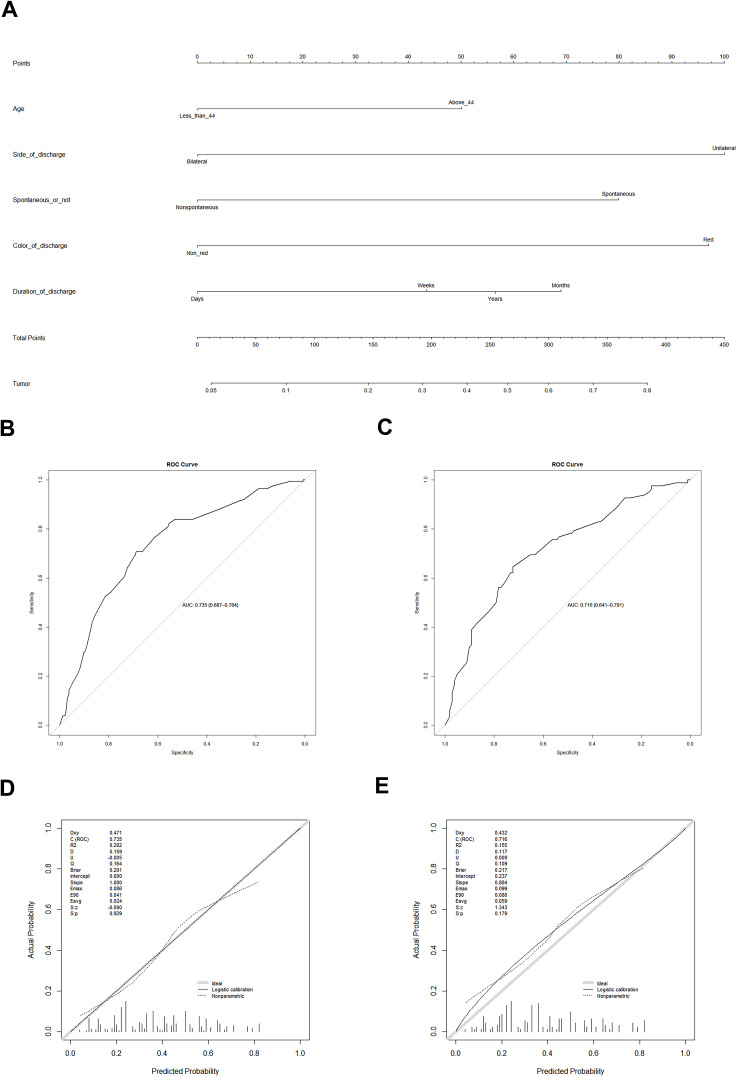
The tumor risk of patients was expressed by the following equation: ln(p/1−p) = 0.583 × a −1.162c × b + 0.928 × c + 0.504 × d1 + 0.801 × d2 + 0.657 × d3 + 1.127 × e - 1.918, where “p” represents the risk of tumor, “a” represents age, “b” represents side of discharge, “c” represents spontaneous discharge status, “d” represents duration of discharge (c1.weeks vs days; c2.months vs days; c3.years vs days), and “e” represents color of discharge. The weights of each variable in the model corresponded to different points (Figure 3A). The area under the ROC curve of the verification model using bootstrapping and external verification was 0.735 (95% CI: 0.687–0.784) and 0.716 (95% CI: 0.641–0.791) (Figure 3B and C). The calibration curve based on bootstrap resampling and verification set is shown in Figure 3D and E. When further evaluating the clinical value of the model, we found that, when the cutoff value was 0.390 in the training cohort, the specificity was 0.687, the sensitivity was 0.708, and the Youden index reached the maximum. Similarly, when we applied the model to the validation cohort, we found that when the cutoff value was 0.375, the specificity was 0.723 and the sensitivity was 0.646.
Figure 3.
A nomogram to predict the probability of breast duct tumors in patients with ND. (A) Age - age at diagnosis in years; Side_of_discharge – unilateral nipple or bilateral nipple discharge; Spontaneous_or_not – spontaneous or non-spontaneous discharge; Duration_of_discharge – duration from the time the discharge occurred to the time of the visit; Color_of_discharge – red discharge or non-red discharge. There are 8 rows in the nomogram. The variables are presented in rows 2 to 6, and points for each variable are correspond the scale in row 1. The row 8 is the total points, and the last row represents the possibility of a patient with ND suffering from a tumor in the duct. (B) ROC curve of the predictive model for the training cohort (n = 433) (ROC curve with an AUC value of 0.735 (95% CI: 0.687–0.784)). ROC, receiver-operating characteristic ROC; AUC, area under the ROC curve. (C) ROC curve of the predictive model for the validation cohort (n = 183) (ROC curve with an AUC value of 0.716 (95% CI: 0.641–0.791)). ROC, receiver-operating characteristic; AUC, area under the ROC curve. (D) Calibration curves of the predictive nomogram prediction in the training cohort. The x-axis represents the predicted risk of tumors in the ducts. The y-axis represents the actually diagnosed intraductal tumor. The diagonal gray thick line represents the ideal prediction of the ideal model. The solid line indicates the performance of the nomogram, and the dotted line closer to the diagonal indicates better prediction. (E) Calibration curves of the predictive nomogram prediction in the validation cohort. The x-axis represents the predicted risk of tumors in the ducts. The y-axis represents the actually diagnosed intraductal tumor. The diagonal gray thick line represents the ideal prediction of the ideal model. The solid line indicates the performance of the nomogram, and the dotted line closer to the diagonal indicates better prediction.
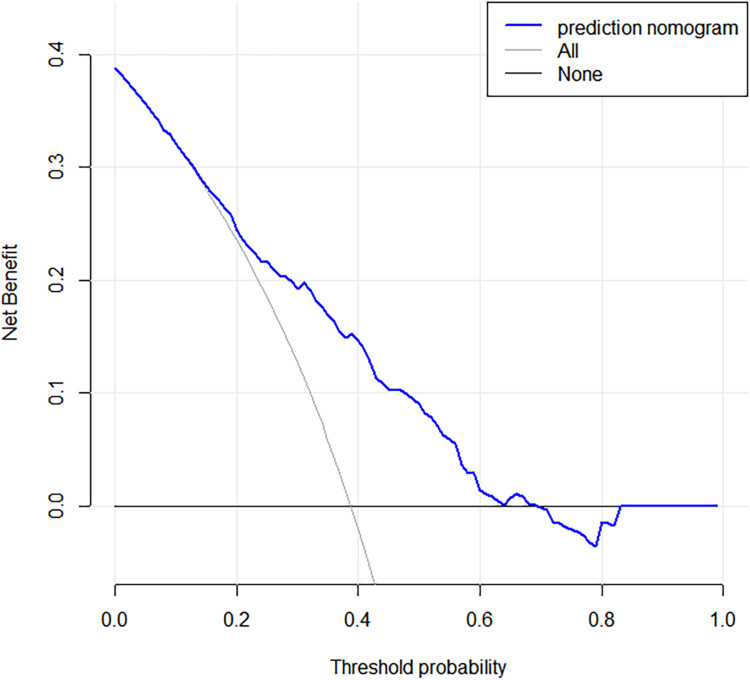
The decision curve analysis of the nomogram is shown in Figure 4. The decision curve showed that if the patient’s threshold probability was between 5% and 69%, using this model to predict intraductal tumors would give the patient more net benefits.
Figure 4.
Decision curve analysis for the predictive nomogram. The y-axis measures the net benefit. The blue line represents the intraductal tumor risk nomogram. The thin solid line represents the hypothesis that all patients have intraductal tumors. The thin solid line represents the hypothesis that no patient has an intraductal tumor.
Discussion
In the past, routine evaluation of ND mainly depended on radiographic examination (ultrasound, mammography, magnetic resonance imaging and catheterization).6,13,14 Because intraductal tumors are often small, the rate of misdiagnosis is high. With the innovation of fiberoptic ductoscopy technology, the diagnosis of ND has made a qualitative leap. It not only observes the lactiferous duct and lactiferous duct wall directly but can also wash the emulsion of the lactiferous inflammation out of the duct, achieving the integration of diagnosis and treatment. In lobectomy of the breast, the methylene blue dye can be injected into the target lactiferous duct as a marker through fiberoptic ductoscopy to facilitate the surgical search for the target lactiferous duct.15
In the clinic, it remains controversial which patients with ND need lactiferous ductoscopy. In patients with ND, over half were found to have no intraductal tumors.2 In most studies, intraductal lesions accounted for up to 35–48%.16 In our study, 523 of the 1333 patients with ND showed intraductal papillary tumors. Among patients with intraductal masses, 346 had undergone surgery at our hospital. The pathology results showed that 327 (94.5%) patients had benign intraductal papilloma, and 19 (5.5%) patients had malignant tumors (ductal carcinoma in situ or invasive ductal carcinoma).
Nomograms are considered an effective tool to quantitatively assess risk factors to maximize prediction accuracy. It can directly reflect the contribution of predictors to outcomes.17 In this study, we successfully established a nomogram model for intraductal tumor prediction. Unilateral discharge plays a key role in the diagnosis of intraductal tumors, followed by spontaneous discharge, a long period, bloody discharge, a smooth lactiferous wall, and age older than 44 years. This model was applied to the training cohort and validation cohort patients in this study. The nomograms of the two groups performed similarly (AUC = 0.7324 vs 0.7278), and the nomogram showed good predictive value in both groups. These results confirm that our nomogram is useful in different populations.
Gutman et al reported that an isolated papilloma is not always benign. Ten percent of patients are associated with breast cancer, and the risk of breast cancer is associated with all forms of papilloma.18 Yang et al recommended that patients with pathologic or benign ND both should undergo fiberoptic ductoscopy as a preoperative screening tool to avoid any cancer omission.2 Through our research, we believe that we can conduct a risk assessment of patients with ND so that some low-risk patients can be exempt from lactiferous ductoscopy. According to clinical features, ND can be classified as benign or pathological. Khan et al reported that three risk factors contribute to ND: spontaneous discharge, a single duct and bloody discharge. Fiberoptic ductoscopy can be used for these patients to provide an accurate diagnosis and appropriate surgical options.19 If we use our model to test their conclusion, we found that patients with the three symptoms above would have a nearly 40% probability of acquiring intraductal tumors, and these patients would require ductoscopy. Our studies have attempted to distinguish between different manifestations of benign and malignant tumors under lactiferous ductoscopy and failed; this result was consistent with Waaijer’s result.20 We believe that patients with any intraductal mass should undergo surgery.
Additionally, this study compared the diagnostic efficacy of ultrasound and lactiferous ductoscopy for intraductal tumors. Our analysis did not support that fiberoptic ductoscopy can replace ultrasound examination. Three reasons may explain this finding. (1) The lactiferous duct with a tumor is not the duct with ND. The intraductal tumors may be sufficiently large to block the lumen completely or the lactiferous duct is blocked somewhere congenitally. Both reasons may result in no ND. (2) The length and thickness of the fiberoptic ductoscopy may prevent the investigation of smaller lactiferous ducts. (3) There are too many branches of the lactiferous duct, and doctors miss some of them. Therefore, we still recommend that patients with ND should receive both fiberoptic ductoscopy and ultrasound.
Conclusions
This study, for the first time, established a model to predict whether a patient had intraductal tumors based on clinical manifestations worldwide. This provides evidence-based medical information for clinical patients who are evaluating ND for breast ductoscopy. At the same time, this study also confirmed that although catheteroscopy has the unique advantage of diagnosing intraductal lesions, B-ultrasound remains an irreplaceable examination to diagnose intraductal tumors.
Funding Statement
This study was supported by the National Natural Science Foundation of China (NSFC 81702633 to B. Wang).
Abbreviations
FDS, fiberoptic ductoscopy; ROC, receiver operating characteristics; ND, nipple discharge; DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; OR, odds ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor-2; AUC, area under the ROC curve.
Data Sharing Statement
All relevant data is contained within the manuscript.
Ethics Approval and Informed Consent
This study was a retrospective study and did not involve any experimental interventions, according to the rules of the ethics committee of the First Affiliated Hospital of Xi’an Jiaotong University, it did not require special ethics approval. And consent was obtained from the study participants prior to study commencement.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. These authors contributed equally to the paper: Bin Wang and Siyuan Jiang.
Disclosure
The authors declare that there is no conflict of interest.
References
- 1.Paterok EM, Rosenthal H, Säbel M. Nipple discharge and abnormal galactogram. Results of a long-term study (1964–1990). Eur J Obstet Gynecol Reprod Biol. 1993;50:227–234. doi: 10.1016/0028-2243(93)90205-Q [DOI] [PubMed] [Google Scholar]
- 2.Yang X, Li H, Gou J, et al. The role of breast ductoscopy in evaluation of nipple discharge: a chinese experience of 419 patients. Breast J. 2014;20:388–393. doi: 10.1111/tbj.12275 [DOI] [PubMed] [Google Scholar]
- 3.Liu M, Guo G, Xie F, Wang S, Yang H, Wang S. Mammary ductoscopy and follow-up avoid unnecessary duct excision in patients with pathologic nipple discharge. J Surg Oncol. 2015;112:139–143. doi: 10.1002/jso.23972 [DOI] [PubMed] [Google Scholar]
- 4.Al Sarakbi W, Worku D, Escobar PF, Mokbel K. Breast papillomas: current management with a focus on a new diagnostic and therapeutic modality. Int Semin Surg Oncol. 2006;3:1. doi: 10.1186/1477-7800-3-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grunwald S, Heyer H, Paepke S, et al. Diagnostic value of ductoscopy in the diagnosis of nipple discharge and intraductal proliferations in comparison to standard methods. Oncol Res Treat. 2007;30:243–248. doi: 10.1159/000100848 [DOI] [PubMed] [Google Scholar]
- 6.Yılmaz R, Bender Ö, Yabul FÇ, Dursun M, Tunacı M, Acunas G. Diagnosis of nipple discharge: value of magnetic resonance imaging and ultrasonography in comparison with ductoscopy. Balkan Med J. 2017;34:119–126. doi: 10.4274/balkanmedj.2016.0184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabioglu N, Hunt KK, Eva Singletary S, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg. 2003;196:354–364. doi: 10.1016/S1072-7515(02)01606-X [DOI] [PubMed] [Google Scholar]
- 8.Makita M, Sakamoto G, Akiyama F, et al. Duct endoscopy and endoscopic biopsy in the evaluation of nipple discharge. Breast Cancer Res Treat. 1991;18:179–187. doi: 10.1007/BF01990034 [DOI] [PubMed] [Google Scholar]
- 9.Valdes EK, Boolbol SK, Cohen JM, Balassanian R, Feldman SM. Clinical experience with mammary ductoscopy. Ann Surg Oncol. 2016;23:9015–9019. doi: 10.1245/ASO.2006.08.025 [DOI] [PubMed] [Google Scholar]
- 10.Winchester DJ. 1–38 outcomes of clinical and surgical assessment of women with pathological nipple discharge. Breast Dis. 2007;18:71–72. [PubMed] [Google Scholar]
- 11.Dillon MF, Mohd NS, Nasir S, et al. The role of major duct excision and microdochectomy in the detection of breast carcinoma. BMC Cancer. 2006;6:164. doi: 10.1186/1471-2407-6-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makita M, Akiyama F, Gomi N, et al. Endoscopic classification of intraductal lesions and histological diagnosis. Breast Cancer. 2002;9:220–225. doi: 10.1007/BF02967593 [DOI] [PubMed] [Google Scholar]
- 13.Yoon JH, Yoon H, Kim E, Moon HJ, Park YV, Kim MJ. Ultrasonographic evaluation of women with pathologic nipple discharge. Ultrasonography (Seoul, Korea). 2017;36:310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bahl M, Baker JA, Greenup RA, Ghate SV. Evaluation of pathologic nipple discharge: what is the added diagnostic value of MRI? Ann Surg Oncol. 2015;22:435–441. doi: 10.1245/s10434-015-4792-9 [DOI] [PubMed] [Google Scholar]
- 15.Escobar PF, Baynes D, Crowe JP. Ductoscopy-assisted microdochectomy. Int J Fertil Womens Med. 2004;49:222–224. [PubMed] [Google Scholar]
- 16.Gioffre FM, Manganaro T, Pollicino A, Scarfo P, Mical B. Surgical approach to nipple discharge: a ten-year experience. J Surg Oncol. 1999;71:235–238. [DOI] [PubMed] [Google Scholar]
- 17.Karakiewicz PI, Briganti A, Chun FK, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol. 2007;25:1316–1322. doi: 10.1200/JCO.2006.06.1218 [DOI] [PubMed] [Google Scholar]
- 18.Gutman H, Schachter J, Wasserberg N, Shechtman I, Greiff F. Are solitary breast papillomas entirely benign? Arch Surg. 2003;138:1330–1333. doi: 10.1001/archsurg.138.12.1330 [DOI] [PubMed] [Google Scholar]
- 19.Khan SA, Mangat A, Rivers A, Revesz E, Susnik B, Hansen N. Office ductoscopy for surgical selection in women with pathologic nipple discharge. Ann Surg Oncol. 2011;18(13):3785–3790. doi: 10.1245/s10434-011-1791-3 [DOI] [PubMed] [Google Scholar]
- 20.Waaijer L, van Diest PJ, Verkooijen HM, et al. Interventional ductoscopy in patients with pathological nipple discharge. Br J Surg. 2015;102:1639–1648. doi: 10.1002/bjs.9950 [DOI] [PubMed] [Google Scholar]