Abstract
Purpose
Staphylococcus aureus (S. aureus) is an important bacterial pathogen, which creates infective inflammation to human being and animals. Mangiferin (MG) is one of the natural flavonoids with anti-inflammatory, anti-bacterial, and anti-oxidative properties. However, the anti-apoptosis and anti-autophagy of MG are unknown. Hence, this study was aimed to research the inhibition of MG on S. aureus-induced apoptosis and autophagy in RAW264.7 cells.
Methods
The RAW264.7 cells were pretreated with MG, or pretreated with SP600125 or anisomycin synchronously, and then infected with S. aureus (MOI=100:1). The viability and proliferation status of RAW264.7 cells were detected by MTT and EdU assay. The relative expression of TNF-α, IL-6 and IL-10 protein was tested with ELISA. The levels of Bax, Bcl-2, caspase-3, c-Jun N-terminal kinase (JNK), extracellular-regulated protein kinase (ERK), p38, LC3, Beclin-1, p62, phosphorylated JNK, phosphorylated p38 and phosphorylated ERK in cells were detected by Western blotting. The apoptosis rate of RAW264.7 cells was analyzed by flow cytometric assay.
Results
The study showed that MG significantly attenuated RAW264.7 cells apoptosis and autophagy caused by S. aureus. MG alleviated S. aureus-induced apoptosis by down-regulating the protein level of active caspase-3 and Bax and up-regulating the level of Bcl-2. MG also inhibited S. aureus-induced autophagy via decreasing the protein level of LC3-II/LC3-I and Beclin-1 or increasing the protein expression of p62. This protective role was dependent on the up-regulation of JNK signal pathway, which was confirmed by using JNK agonist and inhibitor.
Conclusion
Our results demonstrated that MG might protect RAW264.7 cells from S. aureus-induced apoptosis and autophagy via inhibiting JNK/Bax-dependent signal pathway. Therefore, MG may be a potential agent against pathological cell damage induced by S. aureus infection.
Keywords: mangiferin, Staphylococcus aureus, apoptosis, autophagy
Introduction
Staphylococcus aureus (S. aureus) is a common gram-positive bacterial pathogen all over the world, which induces a series of infections ranging from skin and folliculated infection to pneumonia, meningitis, osteomyelitis, endocarditis, bacteremia, and septicemia.1 S. aureus has evolved multiple virulence factors such as alpha-hemolysin, panton-valentine leucopenia (PVL), phenol-soluble module (PSM),2,3 soluble regulatory protein and cysteine protease that are making it one of the most infectious and pathogenic bacteria, which seriously threatens the life safety of human and animal. Since antibiotics were widely used to prevent and treat S. aureus infectious diseases, it promotes the emergence of antibiotic-resistant bacteria, especially multidrug-resistant (MDR) bacteria and methicillin-resistant S. aureus (MASA).4,5 At present, research had been focused on preventing and treating S. aureus.6
Toll-like receptors (TLR) are one of the protein molecules involved in non-specific immunity (natural immunity); they are also an important link between non-specific immunity and specific immunity.7 Previous research demonstrated that TLR2 is closely related to bacterial infection. TLR2 recognises peptidoglycan and phosphatidyl acid on the cell wall of S. aureus, thus causing cellular immune response.8 The level of TLR2 and TLR4 protein was enhanced after macrophages infected with S. aureus, thus activating downstream NF-κB and MAPK signalling pathway and promoting the release of cytokines by macrophages.9–13 S. aureus activates TLR2 receptor, and MAPK signalling pathway mediates phagocytosis and autophagy in RAW264.7 macrophage cells by up-regulation of Beclin-1, LC3-II/LC3-I.14,15 Controlling the pathological changes and protecting the tissue from damage is a usual strategy. ID13 remarkably alleviated pathological status, inhibited the production of pro-inflammatory cytokines,16 and suppressed the TLR2-NF-κB signal pathway.17 Daphnetin conferred protection against S. aureus-induced pneumonia by anti-inflammation and enhanced mTOR-dependent autophagy.2 CpG-ODN promotes phagocytosis and autophagy through JNK/P38 signal pathway in S. aureus-stimulated macrophage.2
Mangiferin (MG) is a flavonoid that originally extracted from the rhizomes of traditional plant Anemarrhena.18 MG is effective in treating bronchitis19,20, and pulmonary through inhibiting TLR4/p65, TGF-beta1/Smad2/3 pathway,12,19,21 and COX-2.20 MG ameliorate fat liver through modulation of autophagy and inflammation,22 also MG boosts autophagy via inhibiting mTORC1 pathway to prevent high glucose-induced cardiomyocyte injury,23 and protect myocardial insults through inhibition of MAPK/TGF-beta pathways.24 MG prevents diabetic nephropathy by up-regulation of glyoxalase and autophagy.24 MG inhibits apoptosis and oxidative stress via BMP2/Smad-1 signalling in dexamethasone-induced MC3T3-E1 cells.26 Although a large number of research have revealed that MG has a protective role in the inflammatory response, little was known about the role of MG on S. aureus-induced apoptosis and autophagy in RAW264.7 cells model.
In this study, MG treatment protected RAW264.7 cells from S. aureus-induced apoptosis and autophagy. These effects mainly involve the inhibition of JNK/Bax-dependent pathways. It may be a potential therapy to control tissue damage caused by S. aureus.
Materials and Methods
Reagent and Antibodies
MG was purchased from Aladdin Bio-Chem Technology (Shanghai, China). JNK inhibitor SP600125 and JNK activator anisomycin were purchased from Selleck Chemicals (Houston, Texas, USA). The following primary antibodies: rabbit anti-mouse β-actin, caspase-3, Bax, Bcl-2, LC3, p62, Beclin-1, p38, P-p38, ERK, P-ERK, JNK, P-JNK were bought from Cell Signaling Technology (Boston, Mass, USA). The second antibody was Alexa Fluor 488 labelled anti-rabbit from Life Technologies Corporation (Waltham, Mass, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cells Culture
Mouse mononuclear macrophage leukaemia cells (RAW264.7 cells) (Cat No. TIB-71 ™) were purchased from American Type CultureCollection (Manassas, VA, USA). The cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM; Thermo Fisher Scientific, USA) supplemented with 10% FBS (Thermo Fisher Scientific, USA) and 1% penicillin/streptomycin in a constant temperature incubator at 37°C and 5% CO2.
Bacterial Strain
S. aureus (ATCC29213) was cultured in 10 mL of Luria-Bertani (LB; Hopebio, Qinghai, China) at 37°C and harvested at the log phase.27,28 Then, the S. aureus was diluted to achieve a multiplicity of infection (MOI = 100:1). The number of S. aureus was determined by serial dilution with the plate counting method.28
Cells Treatment
The study was composed of three parts. The first part involved the blank control group (BC) and S. aureus infection (MOI = 100:1) group (SA); on this basis, the second part was set up as three parallel groups, namely BC, SA (MOI = 100:1 S. aureus infection for 4 h) and MG (low, medium and high concentration pretreatment for 1 h) + SA; similarly, the third part was the BC, SA, MG (high concentration pretreatment for 1 h) + SA, SP (JNK inhibitor SP600125 pretreatment for 12 h), SP + SA, SP + SA + MG; finally, the fourth part was the BC, SA, MG (high concentration pretreatment for 1 h) + SA for 4 h, AM (JNK activator Anisomycin pretreatment for 30 min), AM + SA, and AM + SA + MG.
Cells Viability Assay
The effect of S. aureus and MG on RAW264.7 cells viability was assayed using MTT method (Sigma-Aldrich, America). Cells were inoculated into a 96-well plate at 2×104 CFU/well, after cells growing to monolayer, washing three times with phosphate buffer saline (PBS; Hyclone; China). Then, 200 µL of S. aureus (MOI = 100:1) was added into each well for different times (0, 0.5, 1, 2, 4, and 6 h) (three wells/each group), or 200 µL of MG of different concentrations to each well (three wells each concentration). After washing three times with PBS, the cells were incubated with color-free DMEM (80 µL/well) and 5 mg/mL MTT (20 µL/well) solution at 37°C for 4 h. After washing three times with PBS, 0.05% DMSO (100 µL/well) was added and the tray was shaken gently for 10 min. The cells were observed under a light microscope. The optical density (OD) value of the wells at a wavelength of 490 nm was measured using an enzyme-inked immunosorbent assay reader (Thermo Fisher Scientific, US).29 The percentage of viable cells was calculated according to the equation: the percentage of viable cells = (OD-value of test team/OD-value of a blank team) ×100%.30
Measurement of Cells Proliferation by Ethynyl Deoxyuridine (EdU)
RAW264.7 cells were cultured in 6-well plate with 2×104 CFU/wells under 37°C and 5% CO2 for 12 h,31 pretreated with MG (25, 50, 100 µM) for 1 h, following by infected with S. aureus (MOI=100:1) for 4 h. The supernatant was discarded. Cell Proliferation was assayed using BeyoClick™ EdU-488 Cell Proliferation Assay Kit (Beyotime, Beijing, China) following the user’s manual. These results were visualised by a fluorescence microscope (Olympus Corporation, Japan) at a magnification of 400×, and the signals were counted in five random visional fields.
Caspase-3 Activity Assay
The activity of caspase-3 in RAW264.7 cells was detected using Caspase-3 Activity Assay Kit (Beyotime Institute of Biotechnology, China) following the manufacturers’ instructions. In short, 100 μL lysate was added into the RAW264.7 cells for 15 min on dry ice and centrifuged (20,000 g, 4°C) 15 min, then 50 μL supernatant, 40 μL detection buffer, and 10 μL caspase-3 substrate acetyl-Asp-Glu-Val-Asp p-nitroanilide (2 mM)16 were added into 96-well plates for 60 min. The absorbance of p-nitroanilide was determined at 405 nm using a microtiter plate reader (Bio-Rad, USA). Caspase-3 activity was calculated as a ratio of p-nitroanilide content to total protein amount.32
Detection of IL-6, IL-10 and TNF-α by ELISA
The protein levels of IL-6, IL-10, and TNF-α in a medium of RAW264.7 cells were assayed with ELISA kits (RayBiotech, USA). In brief, RAW264.7 cells were cultured in 6-well plates with 2×104 CFU/wells under 37°C and 5% CO2 for 12 h. MG (25, 50, 100 µM) treatment 1 h before S. aureus infection (MOI=100:1) for 4 h. Then, the cell supernatant was taken for assay following the manufacturers’ instructions.
Flow Cytometry Assay
Flow cytometry assay was used to detected RAW264.7 cells apoptosis and cells cycle progression. We used the Annexin V-FITC/propidium iodide (AV/PI) dual staining commercial kits (Biosea Biotechnology, China), and tested the apoptosis rate of RAW264.7 cells following the manufacturers’ instructions. Briefly, RAW264.7 cells were cultured in 6-well plates with 2×104 CFU/wells for 12 h. After pretreatment with MG and S. aureus, the cells were digested with trypsin, collected by centrifugation, and washed with PBS, the cells were stained with Annexin V-FITC and PI, analysis by FCM (Becton Dickson).
Western Blotting
The whole protein was extracted using an extraction kit (Nanjing Key Gen Biotech, Nanjing, China). The soluble protein supernatant was quantified using Protein Assay Kit (Aid lab Biotechnologies, Beijing, China). Protein samples (20 µg/lane) were separated on polyacrylamide gel electrophoresis (Applygen Technologies, Beijing, China).33 Then, proteins were transferred onto nitrocellulose filter membranes (NC; Pierce Biotechnology, Inc. USA). NC membranes were incubated with primary antibodies overnight at 4°C and a secondary antibody at room temperature for 40 min. The images of the membranes were displayed by Odyssey dual colour infrared fluorescence imaging system (LICOR, US), and the Image J software was used to analyze these images.
Statistical Analysis
All data are expressed as the mean ± standard error of the mean (SEM) from three independent experiments performed in triplicate. Student’s t-test was used to analyse the comparison between two groups.34 One-way ANOVA followed by Dunnett’s test in GraphPad Prism version 5 (GraphPad Software, San Diego, CA, USA).
Results
MG Attenuated Cytotoxicity in RAW264.7 Cells Induced by S. aureus Infection
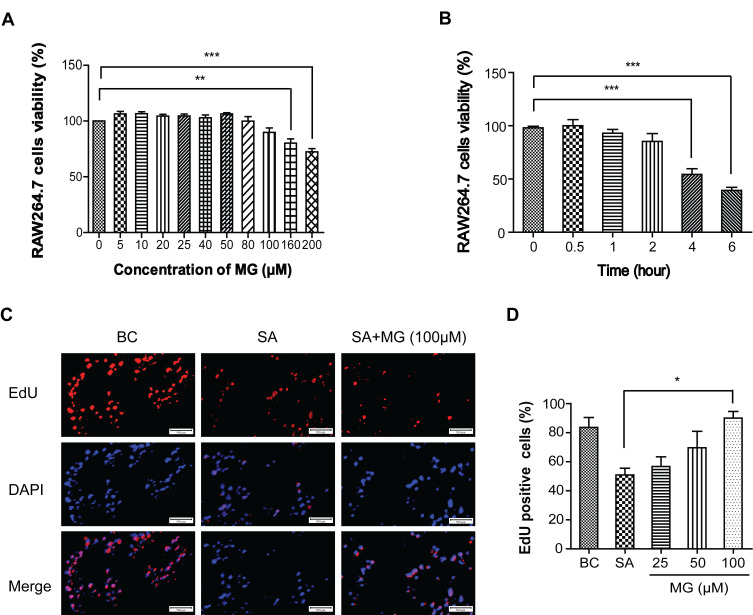
The viability of RAW264.7 cells was detected using MTT assay. Cells were pretreated with doses (0, 5, 10, 20, 25, 40, 50, 80, 100, 160 and 200 μΜ) of MG for 24 hours. As shown in Figure 1A, when the concentration was as high as 100 μM, the viability of RAW264.7 cells were 85.6 ± 0.07% (n=3) no cytotoxicity. However, 200 μM MG induced about 30% cells growth in dose-dependent (Figure 1A). It indicated MG maximum non-cytotoxicity concentration is 100 μM.
Figure 1.
MG attenuated S. aureus-induced cytotoxicity in RAW264.7 cells. (A) Cell viability of RAW264.7 cells treated with MG (0, 5, 10, 20, 25, 40, 50, 80, 100, 160 and 200 μΜ) for 24 hours was determined using MTT assay. Its maximum non-cytotoxicity was 100 μM. **P<0.01, ***P<0.001 compared with 0 μM group. (B) S. aureus infection time was determined using MTT assay. The results showed that S. aureus induced about 53.4% RAW264.7 cells growth inhibition at 4 hours. ***P<0.001 compared with 0 h group. (C) RAW264.7 cells were treated with MG (25, 50, 100 μM) for 1h and infected with S. aureus (MOI:100) for 4 h. S. aureus infection inhibited RAW264.7 cells proliferation, which was significantly reversed by 100 μM MG pretreatment. Relative fluorescence expression levels were quantified by EdU and DAPI staining. Scale bars: 500 μm. (D) The graph represents a quantitative analysis of the number of EdU positive cells. *P<0.05 compared with the SA group.
As showed in Figure 1B, S. aureus infection time-dependently inhibited RAW264.7 cells viability and induced to 53.4% cells death at 4 h. Therefore, the infection time of S. aureus (MOI=100:1) was set at 4 hours for subsequent research.
In addition, EdU fluorescence staining was used to detect whether RAW264.7 cells were in the proliferation cycle. S. aureus reduced positive RAW264.7 cells from 83.61 ± 9.66% to 50.90 ± 6.53% (n=3), 100 μM MG pretreatment increased positive RAW264.7 cells from 50.90% ± 6.53% to 90% ± 6.53% (n=3) (Figure 1C and D). These results indicated that MG within 100 μM is non-cytotoxicity to RAW264.7 cells, and 100 μM MG attenuated the cytotoxicity caused by S. aureus.
MG Suppressed Inflammation and Apoptosis in S. aureus-Induced RAW264.7 Cells
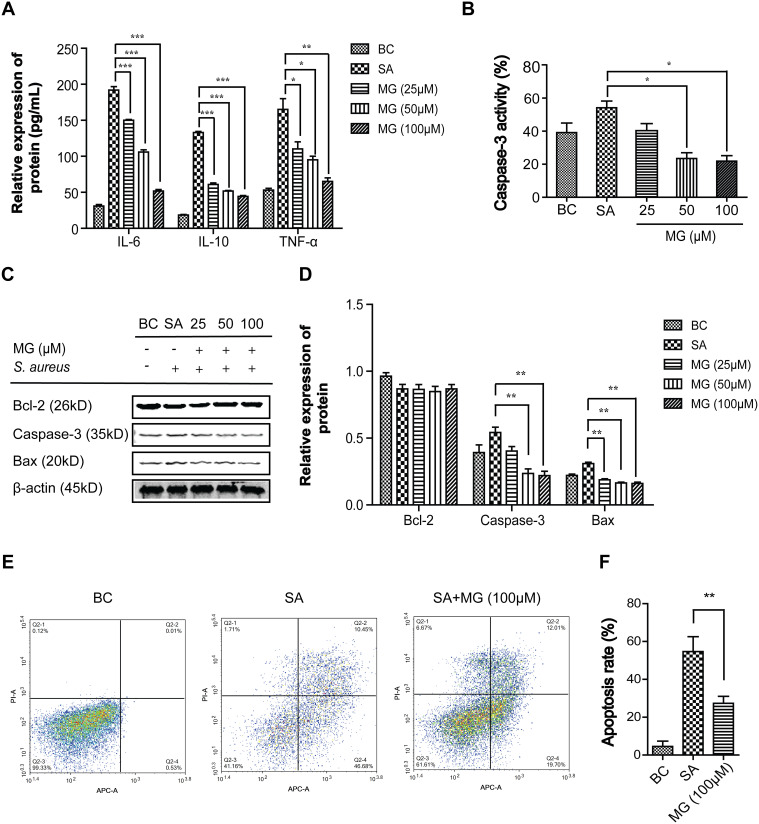
Proinflammatory cytokines in medium culture were assayed using ELISA kits. Compared with the BC group, the intracellular protein expression of IL-6, IL-10, and TNF-α in RAW264.7 cells of the SA group significantly increased (Figure 2A) (n=3). IL-6 increased from 31 ± 2.94 pg/mL to 192 ± 6.68 pg/mL; IL-10 increased from 18.37 ± 1.21 pg/mL to 133 ± 2.16 pg/mL; TNF-α increased from 52.5 ± 2.5 pg/mL to 165 ± 15 pg/mL On the contrary, compared with the SA group, the level of IL-6, IL-10, and TNF-α protein significantly decreased in the MG group (n=3). IL-6 decreased to 51.7± 3.09 pg/mL; IL-10 decreased to 44.3 ± 1.70 pg/mL; TNF-α decreased 65 ± 5 pg/mL. The result showed that MG pretreatment significantly ameliorated the inflammation reaction in a dose-dependent manner induced by S. aureus.
Figure 2.
MG attenuated inhibited apoptosis in RAW264.7 cells infected by S. aureus. RAW264.7 cells were infected with S. aureus (MOI:100) for 4 hours after pretreated with MG (25, 50, 100 μM) for 1h. (A) Detection of pro-inflammatory factor IL-6, IL-10, and TNF-α in medium culture were assayed using ELISA kit. IL-6, IL-10, and TNF-α in the SA group were significantly increased but were significantly decreased in a dose-dependent manner at MG group. *P˂0.05, **P˂0.01, ***P<0.001 compared with SA group. (B) Caspase-3 activity was determined using Caspase-3 Activity Assay Kit. Caspase-3 in the SA group were significantly increased but was significantly decreased in a dose-dependent manner at MG group. *P˂0.05 compared with SA group. (C) Expressions of Bax, Bcl-2 and active caspase-3 were analysed by Western blotting in RAW264.7 cells. (D) Bax, Bcl-2 and active caspase-3 relative expression were quantified by normalising to β-actin. **P˂0.01 compared with SA group. (E) Apoptosis was determined by a FITC-labeled Annexin V/PI staining and flow cytometry. Representative results from flow cytometry were shown. (F) The apoptosis cell rates was calculated. Apoptosis rate in the SA group were significantly increased, but was significantly decreased in the MG group. **P˂0.01 compared with SA group.
To characterise the RAW264.7 cells death process, pro-apoptotic proteins (Bax and caspase-3) and anti-apoptotic protein (Bcl-2) were assayed by Western blot. As shown in Figure 2C and D, compared with the BC group (n=3), the level of Bax and cleaved caspase-3 protein in the SA group was increased from 0.22 ± 0.01 to 0.31 ± 0.01, and from 0.39 ± 0.06 to 0.54 ± 0.04, respectively. While the level of Bcl-2 protein was decreased from 0.86 ± 0.003 to 0.87 ± 0.003. Compared with the SA group, the relative protein expression level of Bax and caspase-3 in the 100 μM MG groups was significantly decreased from 0.31 ± 0.01 to 0.16 ± 0.01 and from 0.54 ± 0.04 to 0.22 ± 0.03, respectively. Additionally, the caspase-3 activity in medium culture showed a similar trend with intracellular caspase-3 (Figure 2B). In short, MG pretreatment significantly prevented the changes induced by S. aureus in a dose-dependent manner.
The apoptotic rates of RAW264.7 cells induced by S. aureus were measured using flow cytometer. Cells assay (annexin V/PI) including early (annexin V positive and PI-negative) and late apoptotic cells (annexin V positive and PI-positive) were induced by S. aureus infection. MG significantly ameliorated the apoptotic death rate from 57.4 ± 8.6% to 27.3 ± 5.7% (n=3) induced by S. aureus infection in RAW264.7 cells (Figure 2E and F). All these results illustrated that MG attenuated S. aureus-induced inflammatory apoptosis as evidence by decreased expressions of IL-6, IL-10, TNF-α, Bax and caspase-3 and increased expression of Bcl-2 in RAW264.7 cells.
MG Repressed Autophagy Induced by S. aureus in RAW264.7 Cells
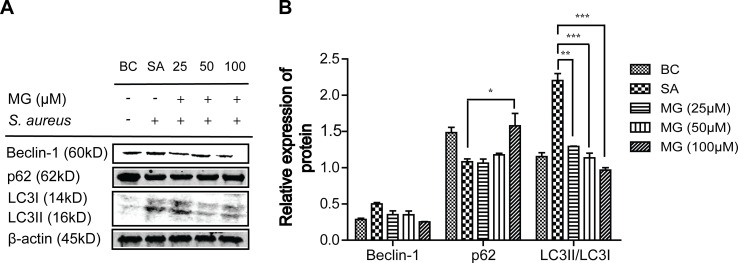
The protein levels of p62, Beclin-1 and LC3 associated with autophagy were detected using Western blot (Figure 3). Compared with BC group (n=3), the relative expression of Beclin-1 and LC3-II/LC3-I in the SA group showed a significant up-regulation, increased from 0.29 ± 0.02 to 0.50 ± 0.02, and from 1.15 ± 0.05 to 2.20 ± 0.10. However, the relative expression of p62 protein in the SA group significant down-regulated from 1.50 ± 0.13 to 1.08 ± 0.06. Compared with the SA group, the expression of Beclin-1 and LC3-II/LC3-I in MG group was significantly decreased from 0.50 ± 0.02 to 0.25 ± 0.003 and from 2.20 ± 0.10 to 0.97 ± 0.03. Besides, the expression of P62 protein significantly increased from 1.08 ± 0.06 to 1.49 ± 0.26. These results showed that MG attenuated S. aureus-induced autophagy in RAW264.7 cells.
Figure 3.
MG inhibited apoptosis in RAW264.7 cells induced by S. aureus. RAW264.7 cells were pretreated with MG (25, 50, 100 μM) for 1h, and infected with S. aureus (MOI:100) for 4 h. (A) Expressions of Beclin-1, P62 and LC3 protein were analysed by Western blotting in RAW264.7 cells. (B) The graph represents a quantitative analysis of the band intensity. *P˂0.05, **P˂0.01, ***p ˂0.001 compared with SA group.
MG Protected RAW264.7 Cells from Apoptosis and Autophagy Induced by S. aureus Infection via JNK/Bax Signal Pathway
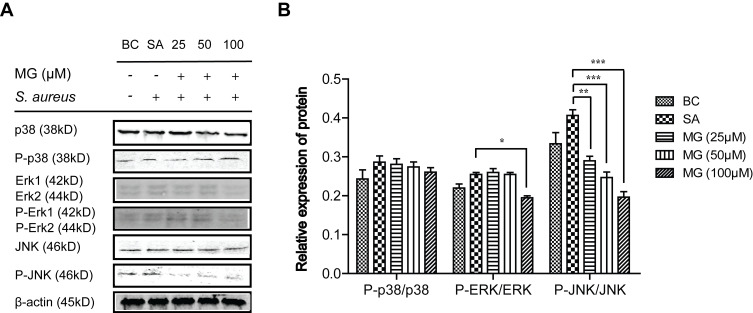
The mitogen-activated protein kinases (MAPK) signalling pathway was shared by four distinct cascades, including the extracellular signal-related kinases (ERK1/2), Jun amino-terminal kinases (JNK1/2/3), p38-MAPK and ERK.35,36 Activated MAPK transmits extracellular signals to regulate cell growth, proliferation, differentiation, migration, even control the balance of autophagy and apoptosis in response to all kinds of stress.35,36 To understand whether the protection mediated by MG was dependent on the MAPK signalling pathway, the role of these proteins were determined via used Western blot. As Figure 4 showed that S. aureus infection promoted the phosphorylation of JNK, ERK and p38 at different levels, while MG pretreatment only significantly suppressed JNK activation. In a word, S. aureus infection suppressed the cells proliferation of by activating MAPK, while MG pretreatment was down-regulated the level of proapoptotic and proinflammatory JNK protein.
Figure 4.
MG inhibited ERK signalling pathway-related proteins expression caused by S. aureus infection in RAW264.7 cells. RAW264.7 cells were treated with MG (25, 50, 100 μM) for 1h and infected with S. aureus (MOI:100) for 4 h. (A) Expressions of p38, P-p38, Erk, P-Erk, JNK and P-JNK protein were analysed by Western blotting in RAW264.7 cells. Gels were representative of three independent experiments. (B) The graph represents a quantitative analysis of the band intensity. *P˂0.05, **P˂0.01, ***p ˂0.001 compared with SA group.
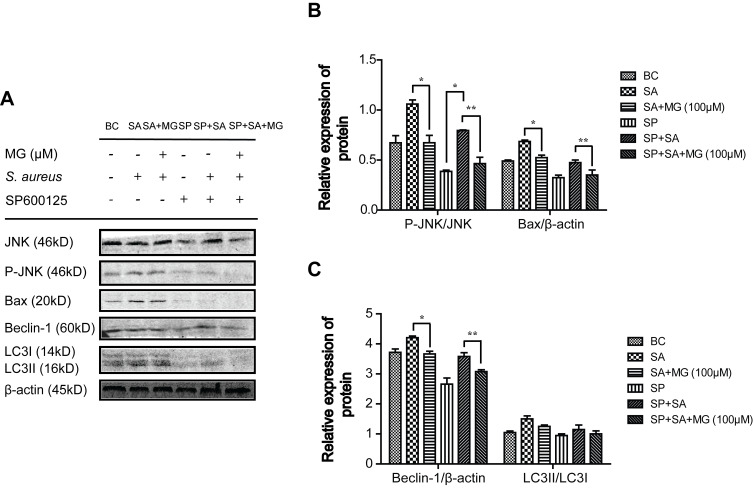
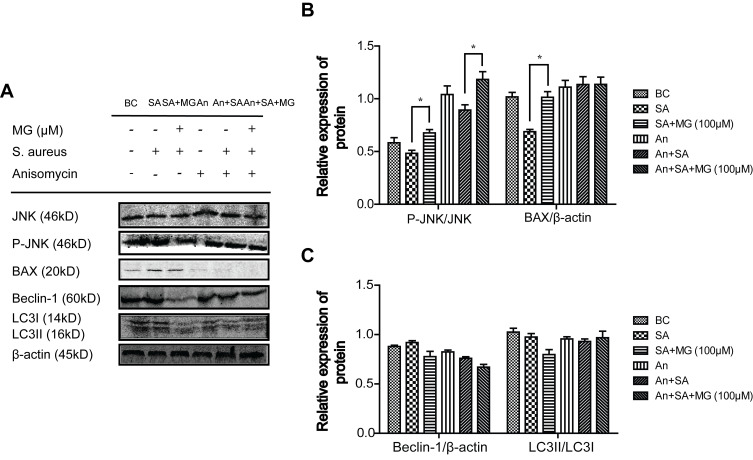
MG pretreatment inhibited JNK expression stimulated by S. aureus. To study whether the molecular mechanism of JNK phosphorylation and MG inhibition of JNK signalling pathway were related to the dissociation of Beclin1-Bcl-2 and Bax-Bcl-2 complexes, which involved in apoptosis and autophagy. RAW264.7 cells were pretreated with MG for 1 h, pretreated with JNK inhibitor SP600125 for 12 h, or pretreated with a JNK agonist anisomycin for 30 min, respectively. The expression level of JNK, P-JNK, Bax, Beclin-1, and LC3-II/LC3-I were measured with Western blot (Figures 5 and 6).
Figure 5.
JNK inhibitor SP600125 obviously affected the related protein of apoptosis and autophagy caused by S. aureus infection in RAW264.7 cells. RAW264.7 cells were treated with SP600125 for 12 hour, then treated with MG (25, 50, 100 μM) for 1h and infected with S. aureus (MOI:100) for 4 h. (A) Cell lysates were subjected to Western blot analysis: Representative JNK, P-JNK and Bax band of three independent experiments. Gels were representative of three independent experiments. (B) The graph represents quantitative analysis of the band intensity. *P˂0.05, **P˂0.01 compared with SA group. (C) The graph represents a quantitative analysis of the band intensity. *P˂0.05, **P˂0.01 compared with SA group.
Figure 6.
JNK activator Anisomycin effected the related protein of apoptosis and autophagy caused by S. aureus infection in RAW264.7 cells. RAW264.7 cells treated with a JNK agonist anisomycin for 30 min, then treated with MG (25, 50, 100 μM) for 1h and infected with S. aureus (MOI:100) for 4 h. (A) Expressions of JNK, P-JNK, BAX, Beclin-1 and LC3 protein were analysed by Western blotting in RAW264.7 cells. Gels were representative of three independent experiments. (B) The graph represents a quantitative analysis of the band intensity. *P˂0.05 compared with SA group. (C) The graph represents a quantitative analysis of the band intensity.
Pretreatment with JNK inhibitor SP600125, the phosphorylation of JNK and Bax were inhibited in S. aureus infected cells (Figure 5A and B), which showed similar trends pretreated with MG (Figure 5A and B). In addition, the JNK activator anisomycin further promoted Bax induced by S. aureus, indicating that anisomycin aggravated apoptosis induced by S. aureus, but MG attenuated the effect (Figure 6A and B).
On the other hand, the JNK inhibitor SP600125 decreased the expression of Beclin-1 and inhibited transformation of LC3-I to LC3-II significantly, indicating that SP600125 inhibited autophagy. The effect was similar to MG (Figure 5A and C). Moreover, the JNK activator anisomycin further promoted the expression of Beclin-1 and the activate of LC3 induced by S. aureus. MG pretreatment inhibited Beclin-1 and LC3, indicating that MG and anisomycin exerted an opposite effect on autophagic cell process induced by S. aureus infection (Figure 6A and C).
These results indicated that activation of JNK accelerated S. aureus-induced autophagy and apoptosis in RAW264.7 cells, and MG inhibited apoptosis and autophagy through inhibiting JNK/Bax signal pathway.
Discussion
S. aureus causes severe systemic infection with high mortality rates for sepsis.38 Studies have shown that S. aureus is recognised by host pattern recognition receptors, and polymorphonuclear cells (PMN) with potent antibacterial activity are recruited.39,40 However, S. aureus resists antimicrobial activities of PMN, requiring additional immune cells to control infection. Macrophages are widely distributed professional phagocytes and kill invading pathogens, facilitate recruitment of immune cells, coordinate adaptive immunity, promote resolution of inflammation and repair damaged tissues.41 Therefore, S. aureus infection is a challenge to the host because the control of the infection requires strong antibacterial immunity while limiting excessive inflammation.
The main mechanism of inflammation is the induction of apoptosis of innate and adaptive immune system cells, and the immunosuppressive effect of these apoptotic cells on surviving immune cells.42 Apoptosis is a type I programmed cell death, which is closely related to multiple cell systems under both physiological and pathological conditions.33 The regulation of apoptosis is important in the pathogenesis of the autoimmune disease, infection and tissue injury. Rectification of the apoptotic-inflammatory imbalance has shown obvious efficacy in animal models, which points out a new path for the clinical treatment of S. aureus infection.42 MG exerts many pharmacological properties, including its antioxidant, antiaging, antiviral, hepatoprotective, analgesic, and immunomodulatory activities.18,19,24,26,43 MG reduces renal cell apoptosis and enhances the expression of antioxidant molecules HO-1 and SOD2 through the PI3K/AKT pathway.44 Therefore, MG may be a promising agent for the protection from programmed death and tissues damage. In the study, MG attenuated S.aureus-induced inflammation and apoptosis via reducing the expressions of IL-6, IL-10, TNF-α, Bax and caspase-3 and increasing the level of Bcl-2 in RAW264.7 cells.
Autophagy is an endogenous process to remove damaged organelles, maintains essential cellular homeostasis.45 Physiologic autophagy is involved in the degradation of harmful proteins and damaged organelles to prevent the accumulation of harmful substances and limits the transmission of harmful signaling.46 Autophagy is controlled by cytokines and receptors modulating innate and adaptive immunity; but excessive autophagy will cause irreversible injury and transform cells to autophagic cellular death.47 In the study, MG attenuated S.aureus-induced autophagy, the potential mechanisms involved was further investigated, so that it may be exploited as a target for the control of excessive inflammation and tissues damage.
Bacterial recognition receptors trigger a series of cell-activating pathways, including the NF-κB, MAPK, and type I interferon (IFN I), to induce apoptosis.48 JNKs belong to the superfamily of MAPK.49 JNK signalling pathway plays a major role in antibacterial defence against bacterial infection, and inhibition of JNK impairs autophagy upon bacterial infection. S. aureus activates Toll-like receptor 2-NF-Kb/MAPK signalling and apoptosis.50,51 CpG-ODN promotes S. aureus-stimulated phagocytosis and autophagy through JNK/P38 signal pathway in macrophage.12 In our study, MG pretreatment only obviously inhibited JNK signal pathway, which protected RAW264.7 cells from apoptosis and autophagy induced by S. aureus. The anti-autophagy efficiency was mediated by inactivation of JNK-dependent Bcl-2 phosphorylation and its dissociation from the BH3 domain of Beclin. The anti-apoptosis was mediated by inactivativation of JNK-dependent Bax. These results provide important insights for controlling apoptosis and autophagy in S. aureus infection.
In a word, MG significantly attenuates S. aureus-induced apoptosis and autophagy in RAW264.7 cells. Similar effects were shown by treatment with JNK inhibitor SP600125, and opposite effect showed by the treatment with JNK agonist anisomycin. These results indicated that the protective effects of MG against S. aureus-induced apoptosis and autophagy were related to JNK inhibition. More should be done in future studies to verify the protective effect of MG on S. aureus infection, especially in vivo.
Conclusion
The present study indicated that pretreatment of MG inhibited apoptosis and autophagy in S. aureus-stimulated RAW264.7 cells, which might be mediated by inhibiting JNK/Bax signalling pathway. These findings provided novel insights into the underlying protective mechanisms for the link between JNK/BAX mediated apoptosis and autophagy upon S. aureus infection. This has provides further information regarding clinical treatment strategies using MG.
Funding Statement
The work was funded by the National Key Research and Development Program of China (2016YFD0501307).
Author Contributions
Jun Xu, Hua Yao and Shichen Wang performed the studies. Huanrong Li directed the experiment of bacterial infection. Jun Xu wrote the manuscript. Xiaolin Hou designed the experiment and revised the manuscript. All authors made substantial contributions to conception and design, study design and execution, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. First author with equal contribution: Jun Xu, Hua Yao, and Shichen Wang.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ondusko DS, Nolt D. Staphylococcus aureus. Pediatr Rev. 2018;39(6):287–298. doi: 10.1542/pir.2017-0224 [DOI] [PubMed] [Google Scholar]
- 2.Zhang W, Zhuo S, He L, et al. Daphnetin prevents methicillin-resistant Staphylococcus aureus infection by inducing autophagic response. Int Immunopharmacol. 2019;72:195–203. doi: 10.1016/j.intimp.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 3.Haslinger-Loffler B, Kahl BC, Grundmeier M, et al. Multiple virulence factors are required for Staphylococcus aureus-induced apoptosis in endothelial cells. Cell Microbiol. 2005;7:1087–1097. doi: 10.1111/j.1462-5822.2005.00533.x [DOI] [PubMed] [Google Scholar]
- 4.Mama OM, Morales L, Ruiz-Ripa L, Zarazaga M, Torres C. High prevalence of multidrug resistant S. aureus-CC398 and frequent detection of enterotoxin genes among non-CC398 S. aureus from pig-derived food in Spain. Int J Food Microbiol. 2020;320:108510. doi: 10.1016/j.ijfoodmicro.2020.108510 [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos P, Papadopoulos T, Angelidis AS, et al. Prevalence of Staphylococcus aureus and of methicillin-resistant S. aureus (MRSA) along the production chain of dairy products in north-western Greece. Food Microbiol. 2018;69:43–50. doi: 10.1016/j.fm.2017.07.016 [DOI] [PubMed] [Google Scholar]
- 6.Kim W, Hendricks GL, Tori K, Fuchs BB, Mylonakis E, Ricciardolo FLM. Strategies against methicillin-resistant Staphylococcus aureus persisters. Future Med Chem. 2018;10(7):779–794. doi: 10.4155/fmc-2017-0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balka KR, De Nardo D. Understanding early TLR signaling through the myddosome. J Leukoc Biol. 2019;105(2):339–351. doi: 10.1002/JLB.MR0318-096R [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol. 2015;109:12–14. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Hou T, Wu X, Luo F, Xie Z, Xu J. Knockdown of TNFR1 suppresses expression of TLR2 in the cellular response to Staphylococcus aureus Infection. Inflammation. 2016;39:798–806. doi: 10.1007/s10753-016-0308-4 [DOI] [PubMed] [Google Scholar]
- 10.Guo M, Cao Y, Wang T, et al. Baicalin inhibits Staphylococcus aureus-induced apoptosis by regulating TLR2 and TLR2-related apoptotic factors in the mouse mammary glands. Eur J Pharmacol. 2014;723:481–488. doi: 10.1016/j.ejphar.2013.10.032 [DOI] [PubMed] [Google Scholar]
- 11.Akhtar M, Shaukat A, Zahoor A, Chen Y, Deng G. Anti-inflammatory effects of Hederacoside-C on Staphylococcus aureus induced inflammation via TLRs and their downstream signal pathway in vivo and in vitro. Microb Pathog. 2019;137:103767. doi: 10.1016/j.micpath.2019.103767 [DOI] [PubMed] [Google Scholar]
- 12.Wu H, Wang J, Zhang B, Fang L, Xu K, Liu R. CpG-ODN promotes phagocytosis and autophagy through JNK/P38 signal pathway in Staphylococcus aureus-stimulated macrophage. Life Sci. 2016;S1883492716. [DOI] [PubMed] [Google Scholar]
- 13.Zahoor A, Yang Y, Yang C, Akhtar M, Guo Y. Gas6 negatively regulates the Staphylococcus aureus -induced inflammatory response via TLR signaling in the mouse mammary gland. J Cell Physiol. 2020;235(10):7081–7093. doi: 10.1002/jcp.29604 [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y, Li H, Ding S, Wang Y. Autophagy inhibition promotes phagocytosis of macrophage and protects mice from methicillin-resistant staphylococcus aureus pneumonia. J Cell Biochem. 2018;119(6):4808–4814. [DOI] [PubMed] [Google Scholar]
- 15.Qiu S, Tao ZB, Tao L, Zhu Y. Melatonin induces mitochondrial apoptosis in osteoblasts by regulating the STIM1/cytosolic calcium elevation/ERK pathway. Life Sci. 2020;248:117455. doi: 10.1016/j.lfs.2020.117455 [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Peng X, Huang Y, Xiao Y, Wang Z, Zhan L. Propofol attenuates hypoxia/reoxygenation-induced apoptosis and autophagy in HK-2 cells by inhibiting JNK activation. Yonsei Med J. 2019;60(12):1195–1202. doi: 10.3349/ymj.2019.60.12.1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li B, Yang N, Shan Y, et al. Therapeutic potential of a designed CSalphabeta peptide ID13 in Staphylococcus aureus-induced endometritis of mice. Appl Microbiol Biotechnol. 2020:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muruganandan S, Gupta S, Kataria M, Lal J, Gupta PK. Mangiferin protects the streptozotocin-induced oxidative damage to cardiac and renal tissues in rats. Toxicology. 2002;176(3):165–173. doi: 10.1016/S0300-483X(02)00069-0 [DOI] [PubMed] [Google Scholar]
- 19.Wei Z, Yan L, Deng J, Deng J. Mangiferin protects rats against chronic bronchitis via regulating NF-kappaB (P65) and IkappaBalpha expression in mononuclear cells. Yao Hsüeh Hsüeh Pao. 2014;49:596. [PubMed] [Google Scholar]
- 20.Deng J. Effects of mangiferin on cytokines in rats with chronic bronchitis and expression of macrophage COX-2 in mice. China J Chin Mater Med. 2011;36:1348. [PubMed] [Google Scholar]
- 21.Jia L, Sun P, Gao H, et al. Mangiferin attenuates bleomycin-induced pulmonary fibrosis in mice through inhibiting TLR 4/p65 and TGF -β1/Smad2/3 pathway. J Pharm Pharmacol. 2019;71(6):1017–1028. doi: 10.1111/jphp.13077 [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Zhu -Y-Y, Wang L, et al. Mangiferin ameliorates fatty liver via modulation of autophagy and inflammation in high-fat-diet induced mice. Biomed Pharmacother. 2017;96:328–335. doi: 10.1016/j.biopha.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 23.Hou J, Zheng D, Xiao W, Li D, Ma J, Hu Y. Mangiferin enhanced autophagy via inhibiting mTORC1 pathway to prevent high glucose-induced cardiomyocyte injury. Front Pharmacol. 2018;9:383. doi: 10.3389/fphar.2018.00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suchal K, Malik S, Gamad N, et al. Mangiferin protect myocardial insults through modulation of MAPK/TGF-β pathways. Eur J Pharmacol. 2016;776:34–43. doi: 10.1016/j.ejphar.2016.02.055 [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Zhu X, Zhang L, et al. Up-regulation of glyoxalase 1 by mangiferin prevents diabetic nephropathy progression in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2013;721(1–3):355–364. doi: 10.1016/j.ejphar.2013.08.029 [DOI] [PubMed] [Google Scholar]
- 26.Ding LZ, Teng X, Zhang ZB, Zheng CJ, Chen SH. Mangiferin inhibits apoptosis and oxidative stress via BMP2/Smad-1 signaling in dexamethasone-induced MC3T3-E1 cells. Int J Mol Med. 2018;41:2517–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silbernagel KM, Lindberg KG. Petrifilm™ rapid S. aureus count plate method for rapid enumeration of Staphylococcus aureus in selected foods: Collaborative Study. J AOAC Int. 2019;5. [PubMed] [Google Scholar]
- 28.Zang H, Qian S, Li J, et al. The effect of selenium on the autophagy of macrophage infected by Staphylococcus aureus. Int Immunopharmacol. 2020;83:106406. doi: 10.1016/j.intimp.2020.106406 [DOI] [PubMed] [Google Scholar]
- 29.Sambol M, Ester K, Landgraf S, Mihaljević B, Cindrić M, Kralj M, Basarić N. Competing photochemical reactions of bis-naphthols and their photoinduced antiproliferative activity. Photochem Photobiol Sci. 2020;18(5):1197–1211. doi: 10.1039/C8PP00532J [DOI] [PubMed] [Google Scholar]
- 30.Fang L, Wu HM, Ding PS, Liu RY. TLR2 mediates phagocytosis and autophagy through JNK signaling pathway in Staphylococcus aureus-stimulated RAW264.7 cells. Cell Signal. 2014;26:806–814. doi: 10.1016/j.cellsig.2013.12.016 [DOI] [PubMed] [Google Scholar]
- 31.Kvansakul M. Viral infection and apoptosis. Viruses. 2017;9(12):356. doi: 10.3390/v9120356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Y, Zhu W, Wang Z, et al. Combinatorial microRNAs suppress hypoxia-induced cardiomyocytes apoptosis. Cell Physiol Biochem. 2015;37(3):921–932. doi: 10.1159/000430219 [DOI] [PubMed] [Google Scholar]
- 33.Wang S, Xia B, Qiao Z, et al. Tetramethylpyrazine attenuated bupivacaine-induced neurotoxicity in SH-SY5Y cells through regulating apoptosis, autophagy and oxidative damage. Drug Des Devel Ther. 2019;13:1187–1196. doi: 10.2147/DDDT.S196172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y, Fan Y, Hu Y, et al. α-Mangostin suppresses the de novo lipogenesis and enhances the chemotherapeutic response to gemcitabine in gallbladder carcinoma cells via targeting the AMPK/SREBP1 cascades. J Cell Mol Med. 2020;24(1):760–771. doi: 10.1111/jcmm.14785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19:1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang Y-J, Yang W-X. Kinesins in MAPK cascade: how kinesin motors are involved in the MAPK pathway? Gene. 2019;684:1–9. doi: 10.1016/j.gene.2018.10.042 [DOI] [PubMed] [Google Scholar]
- 37.Yang H, Bai W, Gao L, et al. Mangiferin alleviates hypertension induced by hyperuricemia via increasing nitric oxide releases. J Pharmacol Sci. 2018;137(2):154–161. doi: 10.1016/j.jphs.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 38.Mahavanakul W, Nickerson EK, Srisomang P, et al. Feasibility of modified surviving sepsis campaign guidelines in a resource-restricted setting based on a cohort study of severe S. aureus sepsis [corrected]. PLoS One. 2012;7(2):e29858. doi: 10.1371/journal.pone.0029858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guenther F, Stroh P, Wagner C, Obst U, Hansch GM. Phagocytosis of Staphylococci biofilms by polymorphonuclear Neutrophils: S. aureus and S. epidermidis differ with regard to their susceptibility towards the host defense. Int J Artif Organs. 2009;32(9):565–573. doi: 10.1177/039139880903200905 [DOI] [PubMed] [Google Scholar]
- 40.Nagasawa Y, Kiku Y, Sugawara K, et al. Staphylococcus aureus-specific IgA antibody in milk suppresses the multiplication of S. aureus in infected bovine udder. BMC Vet Res. 2019;15:286. doi: 10.1186/s12917-019-2025-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L, Wang CC. Inflammatory response of macrophages in infection. Hepatobiliary Pancreat Dis Int. 2014;13:138–152. doi: 10.1016/S1499-3872(14)60024-2 [DOI] [PubMed] [Google Scholar]
- 42.Nedeva C, Menassa J, Puthalakath H. Sepsis: inflammation is a necessary evil. Front Cell Dev Biol. 2019;7:108. doi: 10.3389/fcell.2019.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang T, Han F, Gao G, Liu M. Mangiferin exert cardioprotective and anti-apoptotic effects in heart failure induced rats. Life Sci. 2020;249:117476. doi: 10.1016/j.lfs.2020.117476 [DOI] [PubMed] [Google Scholar]
- 44.Saha S, Sadhukhan P, Sinha K, Agarwal N, Sil PC. Mangiferin attenuates oxidative stress induced renal cell damage through activation of PI3K induced Akt and Nrf-2 mediated signaling pathways. Biochem Biophys Rep. 2016;5:313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galluzzi L, Green DR. Autophagy-independent functions of the autophagy machinery. Cell. 2019;177(7):1682–1699. doi: 10.1016/j.cell.2019.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doherty J, Baehrecke EH. Life, death and autophagy. Nat Cell Biol. 2018;20(10):1110–1117. doi: 10.1038/s41556-018-0201-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ravanan P, Srikumar IF, Talwar P. Autophagy: the spotlight for cellular stress responses. Life Sci. 2017;188:53–67. doi: 10.1016/j.lfs.2017.08.029 [DOI] [PubMed] [Google Scholar]
- 48.Provost KA, Smith M, Miller-Larsson A, Gudleski GD, Sethi S, Ricciardolo FLM. Bacterial regulation of macrophage bacterial recognition receptors in COPD are differentially modified by budesonide and fluticasone propionate. PLoS One. 2019;14(1):e207675. doi: 10.1371/journal.pone.0207675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chistyakov DV, Azbukina NV, Astakhova AA, Polozhintsev AI, Sergeeva MG, Reiser G. Toll-like receptors control p38 and JNK MAPK signaling pathways in rat astrocytes differently, when cultured in normal or high glucose concentrations. Neurochem Int. 2019;131:104513. doi: 10.1016/j.neuint.2019.104513 [DOI] [PubMed] [Google Scholar]
- 50.Shaukat A, Yang C, Yang Y, et al. Ginsenoside Rb 1: a novel therapeutic agent in Staphylococcus aureus-induced acute lung injury with special reference to oxidative stress and apoptosis. Microb Pathog. 2020;143:104109. doi: 10.1016/j.micpath.2020.104109 [DOI] [PubMed] [Google Scholar]
- 51.Shaukat A, Guo Y-F, Jiang K, et al. Ginsenoside Rb1 ameliorates Staphylococcus aureus-induced acute lung injury through attenuating NF-κB and MAPK activation. Microb Pathog. 2019;132:302–312. doi: 10.1016/j.micpath.2019.05.003 [DOI] [PubMed] [Google Scholar]