Abstract
Background
At present, the flexible endoscopic evaluation of swallowing (FEES) is one of the most commonly used methods for the objective assessment of swallowing. This multicenter trial prospectively collected data on the safety of FEES and also assessed the impact of this procedure on clinical dysphagia management.
Methods
Patients were recruited in 23 hospitals in Germany and Switzerland from September 2014 to May 2017. Patient characteristics, professional affiliation of the FEES examiners (physicians or speech and language therapists), side-effects and cardiorespiratory parameters, severity of dysphagia and clinical consequences of FEES were documented.
Results
2401 patients, mean age 69.8 (14.6) years, 42.3% women, were included in the FEES-registry. The most common main diagnosis was stroke (61%), followed by Parkinson’s disease (6.5%). FEES was well tolerated by patients. Complications were reported in 2% of examinations, were all self-limited and resolved without sequelae and showed no correlation to the endoscopist’s previous experience. In more than 50% of investigations FEES led to changes of feeding strategies, in the majority of cases an upgrade of oral diet was possible.
Discussion
This study confirmed that FEES, even when performed by less experienced clinicians is a safe and well tolerated procedure and significantly impacts on the patients’ clinical course. Implementation of a FEES-service in different clinical settings may improve dysphagia care.
Trial registration
ClinicalTrials.gov NCT03037762, registered January 31st 2017.
Introduction
Neurogenic dysphagia is one of the most frequent and life-threatening symptoms of neurological disorders such as stroke, traumatic brain injury, Parkinson’s disease, dementia, multiple sclerosis, and different neuromuscular disorders [1–7]. In view of the demographic shift, especially with increasing numbers of very old people, these already alarming figures will further increase in the future since many underlying pathologies are age related. The clinical consequences of dysphagia are serious and, in general, directly linked to the patient’s overall prognosis. Irrespective of the underlying disease the set of typical complications comprises aspiration pneumonia, malnutrition and dehydration ultimately leading to an increase in mortality [8]. Apart from these medical issues, dysphagia has a significant impact on the psychological well-being of affected individuals and has been linked to social isolation, low mood and depression [9, 10].
Since the first description of Flexible Endoscopic Evaluation of Swallowing (FEES) was published in 1988 by Langmore and co-workers [11], this particular technique has turned into one of the most commonly used methods for the objective assessment of swallowing worldwide [12]. In terms of day-to-day practicality, the merits of FEES are that (i) it can be performed at the bedside, thus facilitating examination of severely motor-impaired, bedridden or uncooperative patients, for example in the intensive care unit or the stroke unit; (ii) follow-up examinations can be performed at short notice and, if necessary, frequently; and (iii) oropharyngeal secretion management and efficacy of clearing mechanisms, such as coughing and throat clearing, can be assessed simply and directly. In several studies FEES has been successfully applied in a wide range of specific disorders, such as stroke [13], traumatic brain injury [14], cerebral palsy [15], Parkinson’s disease and atypical Parkinsonian syndromes [16, 17], different types of dementia [4], amyotrophic lateral sclerosis [18], Kennedy’s disease [19], and head and neck cancer [20]. In addition, FEES is also being increasingly used in paediatrics [21], geriatrics [22] and intensive-care medicine [23, 24]. The growing interest in this technique is also reflected by the development of systematic educational curricula put forward by different medical societies. Remarkably, these curricula are not confined to a specific medical profession but are all designed as interdisciplinary concepts involving a variety of healthcare professionals being engaged in the management of dysphagia [25–27].
In spite of the increasing dissemination of FEES, there are only few studies that evaluate procedure related side effects and the clinical benefits related to providing this tool for objective dysphagia evaluation. This multicenter trial, the FEES-registry, therefore prospectively collected data on the safety of FEES and also assessed the impact this procedure had on dysphagia management in the studied patient cohort.
Patients and methods
Patients were prospectively recruited in 23 hospitals in Germany and Switzerland from September 2014 to May 2017. Trial sites were identified among those hospitals actively supporting the German FEES education initiative. Trial sites included 10 neurological departments, 9 rehabilitation facilities and 4 geriatric departments. Patients were considered eligible for this study if a FEES was scheduled during their treatment either within the in- or outpatient service. There were no in- or exclusion criteria with regards to the patients’ main diagnosis or treatment facility. The study protocol was approved by all involved ethics committees, and all patients or their legal representative provided written informed consent. The FEES-registry was registered as NCT03037762.
Patient characteristics
The following epidemiological and clinical variables were recorded: sex and age, main diagnosis, Barthel index [28] and the use of antithrombotics, antiplatelets or anticoagulation. Directly prior to FEES the Richmond Agitation and Sedation Scale (RASS) was scored [29]. In addition, using a previously established definition of so called “complex patients”, it was noted, whether the examination was particularly challenging, which was considered to be the case if patients showed a respiratory impairment (increased respiratory rate, need for oxygen supply), were restless (due to for example a movement disorder), had a limited understanding of the situation or a fluctuating vigilance, or had a tracheal cannula in place [25].
Professional affiliation of the examiner
The profession of the involved examiners was documented (either physician or speech-and-language therapist (SLT)). Their previous experience in performing FEES was categorized in < 30 FEES, 30–200 FEES, 201–500 FEES, or > 500 FEES.
Cardiorespiratory monitoring and side-effects
Where possible, heart rate and oxygen saturation were monitored during FEES and the following four values were noted: i) pretest, ii) highest value during FEES, iii) lowest value during FEES, iv) posttest. Blood pressure was measured twice, immediately prior and directly after FEES. Apart from that the following side-effects were noted: Epistaxis, laryngospasm, bradycardia, decrease of the level of consciousness (i.e. for example from alert to somnolent). After completion of the examination, patients were asked to rate the level of discomfort associated with FEES as “none”, “mild”, “moderate”, or “severe”.
Rating of feeding strategy and dysphagia
Prior to FEES the oral intake of patients was rated with the Functional Oral Intake Scale (FOIS) [30], which ranges from 1 (no oral intake) to 7 (total oral intake with no restrictions). Based on the FEES results, severity of swallowing dysfunction was classified according to a 4-grade dysphagia severity scale that has previously been developed and published [16, 31] (0 = no relevant dysphagia, 1 = mild dysphagia (premature spillage and/or residues, but no penetration/aspiration events), 2 = moderate dysphagia (penetration/aspiration events with one consistency), 3 = severe dysphagia (penetration/aspiration events with two or more consistencies)). In addition, based on the FEES findings and the global clinical situation a new FOIS score was defined with the difference between the FOIS-scores pre- and post-FEES reflecting the clinical impact of this examination. In addition, it was noted whether in patients with a tracheal cannula in place decannulation was recommended after FEES.
Statistical analysis
Statistical analyses were carried out with SPSS 25.0 for WINDOWS (SPSS Inc). The paired-samples t-test was used to compare pre- and post-test blood pressure, the repeated-measures ANOVA was used to compare oxygen saturation and heart rate prior, during and after FEES. Categorical data were analyzed using the chi-square test. For correlation analysis the Pearson-correlation coefficient was calculated.
Results
As summarized in Tables 1, 2401 patients were included in the FEES-registry. Mean age was 70 years and 42.2% were female. Mean RASS score was close to 0 and mean Barthel index was 35. Close to 19% of patients were on anticoagulation, about one third of patients received antiplatelets and more than 40% were treated with antithrombotic drugs. More than 45% of patients were rated as complex cases, most frequently cited conditions were disorientation (20.7%), presence of a tracheal cannula (18.6%), and fluctuating consciousness (16.2%). The most common main diagnosis of patients enrolled in this study was stroke (61%), followed by Parkinson’s disease (6.5%), CIP (5.6%), Motor-neuron disorders (3.1%) and dementia (2.7%). Non-neurological diseases were rare and constituted malignoma (2.0%), psychogenic dysphagia (1.4%), cervical spine surgery (0.8%), pneumonia (0.5%) and esophageal diseases (0.5%).
Table 1.
Epidemiological and clinical characteristics of the patient cohort. Numbers in brackets give the number of patients with complete datasets with regards to the specific items
| General characteristics (N = 2236) | |
|---|---|
| Age | 69.8 (14.6) |
| Female gender | 1013 (42.2) |
| Barthel | 35 (35.4)) |
| RASS | −0.1 (0.81) |
| Anticoagulation | 451 (18.8) |
| Anti-platelets | 796 (33.2) |
| Antithrombotic drugs | 1005 (41.9) |
| Specific characteristics (N = 2330) | |
| Complex patients | 1089 (45.4) |
| Respiratory problems | 279 (11.6) |
| Tracheal cannula | 447 (18.6) |
| Agitation | 161 (6.7) |
| Disorientation | 496 (20.7) |
| Fluctuating vigilance | 390 (16.2) |
| Main Diagnosis (N = 2401) | |
| Stroke | 1465 (61.0) |
| Stroke with Thrombolysis | 393 (26.8) |
| Parkinson’s Disease | 157 (6.5) |
| Critical-Illness Polyneuropathy | 135 (5.6) |
| Motorneuron Disorder | 75 (3.1) |
| Dementia | 64 (2.7) |
| Malignoma | 48 (2.0) |
| Movenent Disorders (other) | 41 (1.7) |
| Enzephalopathia | 37 (1.5) |
| Traumatic Brain Injury | 36 (1.5) |
| Meningitis/Enzephalitis | 36 (1.5) |
| Myasthenia gravis | 35 (1.5) |
| Immune-mediated neuropathy | 34 (1.4) |
| Psychogenic dysphagia | 34 (1.4) |
| Seizure | 33 (1.4) |
| Myopathy | 29 (1.2) |
| Cervical spine surgery | 20 (0.8) |
| Multiple Sclerosis | 18 (0.7) |
| Pneumonia | 13 (0.5) |
| Esophageal diseases | 12 (0.5) |
| Other/Missing | 79 (3.3) |
Most of the examinations were done in an acute care facility (70.5%), 20.5% of patients were enrolled in rehabilitation clinics and 9.0% were seen as outpatients (Table 2). Inpatients were examined at all levels of care, i.e. normal wards (46.6%), intermediate care units (31.1%) and intensive care units (22.4%) (Table 2). In nearly all FEES SLTs were involved (95.5%), 41.2% were done by a team of SLTs without involvement of other personnel, physicians took part in 58.8% of examinations. The majority of FEES was done by a highly experienced clinician; however, in 17.7% of cases the endoscopist had done less than 30 FEES before (Table 2). The mean examination time devoted to the endoscopic procedure was close to 10 min. This figure does not include the additional time needed for preparation of FEES, for writing the report, for communicating the findings within the treating team and for the cleaning procedure.
Table 2.
Features of the clinical context, in which FEES was carried out
| Setting (N = 2401) | |
| Outpatient service | 216 (9.0) |
| Acute care facility | 1692 (70.5) |
| Rehabilitation facility | 493 (20.5) |
| Level of care (for inpatients, N = 1735) | |
| Normal ward | 808 (46.6) |
| Intermediate care unit | 539 (31.1) |
| Intensive care unit | 388 (22.4) |
| Examiner’s profession (N = 2389) | |
| Physician involved | 1404 (58.8) |
| SLT involved | 2282 (95.5) |
| SLT alone | 985 (41.2) |
| Examiner’s experience (N = 2401) | |
| < 30 FEES | 420 (17.7) |
| 30–200 FEES | 609 (25.6) |
| 201–500 | 389 (16.4) |
| > 500 | 960 (40.4) |
| Examination time (min.) (N = 2362) | 9.84 (5.89) |
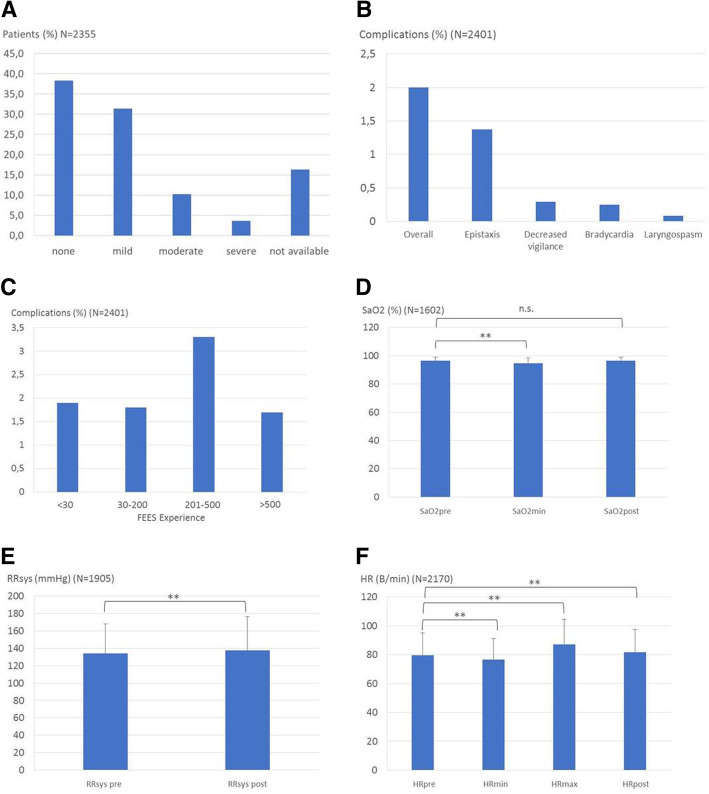
FEES was tolerated well by the patients with nearly 70% rating the procedure as not uncomfortable or mildly uncomfortable. 10.2% stated that FEES was moderately uncomfortable and 3.7% experienced severe discomfort with the remaining 16.3% not being able to provide a rating due to their underlying illness (Fig. 1A).
Fig. 1.
Tolerance, complications and alterations of cardiorespiratory parameters during FEES. a: Patients’ rating of FEES-associated discomfort ranging from none to severe; b: Incidence of complications; c: Incidence of complications in relation to the clinician’s FEES-experience. Numbers below columns give the number of FEES performed during prior training; d: Procedure-related changes of oxygen saturation (SaO2); E: Procedure-related changes of systolic blood pressure (RRsys); F: Procedure-related changes of heart rate (HR)
Complications were reported in 2% of examinations (Fig. 1B). In 33 cases (1.37%) epistaxis occurred, a decreased consciousness was noted in 7 patients (0.29%), 6 patients (0,25%) developed bradycardia and in 2 patients (0.08%) a laryngospasm was reported. All of these complications were self-limited and terminated within a few minutes without specific intervention. The incidence of complications was not related to the endoscopist’s experience. In fact, FEES done by endoscopists with a professional experience of 200–500 examinations featured the highest rate of complications, although without significant differences between groups (Fig. 1C). As shown in Fig. 1 D-F FEES was associated with significant changes in cardiorespiratory parameters. Thus, oxygen saturation dropped in mean by 1.8%, systolic blood pressure increased by 3.5 mmHg, and maximum heart rate increased by 7.4 bpm and minimum heart rate decreased by 3.2 bpm. The clinical impact of these alterations was however limited. Thus, post-intervention oxygen saturation and heart rate had nearly returned to the respective baseline-values and no associated complications were observed.
The 4-grade FEES-based dysphagia score correlated well with the FOIS score (Pearson correlation coefficient − 0.761, p < 0.001; Fig. 2). A dysphagia score of 0 corresponded to a FOIS score between 6 and 7, a dysphagia score of 1 to a FOIS score between 5 and 6, a dysphagia score of 2 to a FOIS score of close to 4, and a dysphagia score of 3 to a FOIS score between 2 and 3.
Fig. 2.
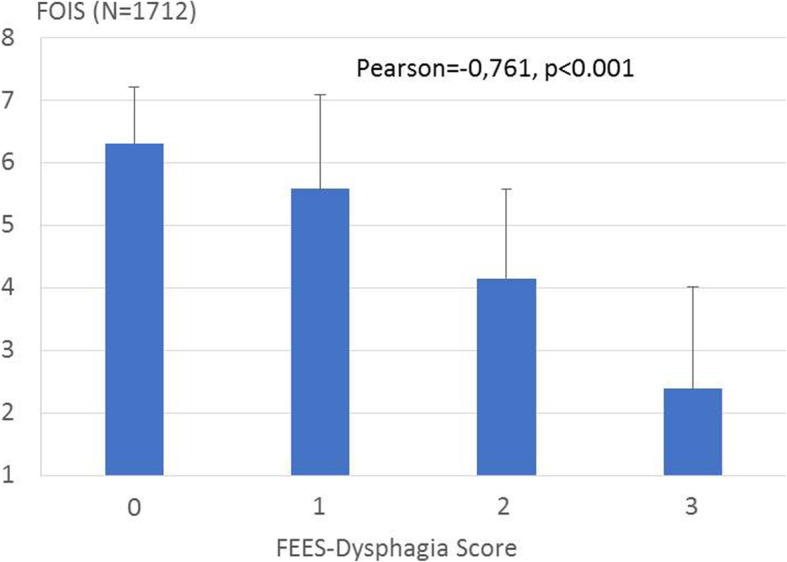
Correlation of the FEES-based dysphagia score with the Functional Oral Intake Score (FOIS)
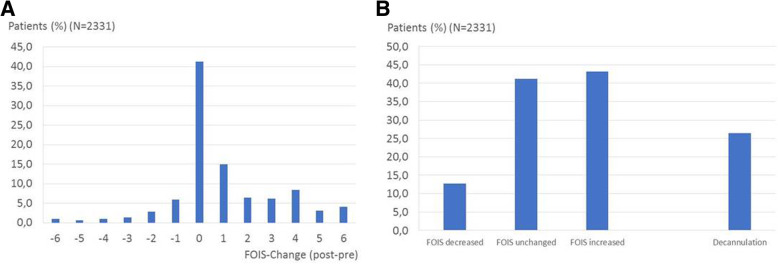
In more than 50% of cases FEES led to changes of feeding strategies (Fig. 3). Whereas in 43.2% of patients an upgrade of the oral diet was possible and in more than 20% of patients the FOIS scale increased by 3 or more points (Fig. 3A), oral diet needed to be restricted after FEES in 12.7% of patients. In the subgroup of tracheotomized patients decannulation was possible in more than 25% of them (Fig. 3B).
Fig. 3.
Changes of dysphagia management after FEES. a: Detailed changes of FOIS score after FEES. A positive value indicates an upgrade of the FOIS score after FEES, a negative value indicates more restrictive feeding strategy. b: Summary of FOIS changes and management of tracheotomised patients with regards to decannulation
Discussion
The FEES-registry assessed the safety and clinical impact of FEES in a prospective multicenter design across different levels of care facilities in a heterogeneous patient cohort. The study’s first main finding was that the procedure was safe and well tolerated, and complications, in particular laryngospasm, epistaxis and hypotensive episodes were very rare and always self-limited, thereby corroborating reports from the literature [32–36]. Secondly, this study showed that the incidence of procedure-related side-effects was not related to the endoscopist’s experience. Therefore, FEES seems to be safe even when performed by professionals with limited prior training. This result supports recently published formalized training curricula for FEES that suggest that after taking part in a dedicated workshop, conducting 60 supervised examinations and passing a practical test physicians and SLTs can safely perform this procedure [25–27]. Third, this trial showed that FEES was associated with discernible but clinically insignificant alterations of cardiovascular parameters. Interestingly, and in line with a smaller previous trial exclusively focusing on acute stroke patients [36], the recorded mild increases of heart rate and systolic blood pressure were clearly less pronounced than encountered during placement of nasogastric tubes in acute stroke patients with dysphagia [37]. In the latter scenario a mean increase of systolic blood pressure of 35 mmHg (as opposed to 3.5 mmHg in the present trial) and a mean increase of heart rate of 23 bpm (as opposed to 3.2 bpm in the present trial) were noted. Therefore, it may be concluded that the FEES procedure, even if examination times may be longer, is not as unpleasant as any procedure involving blind manipulation within the nostrils and the pharynx such as placing nasogastric tubes or nasotracheal suctioning. Fourth, this study showed that a simple FEES-based algorithm grading dysphagia severity according to efficiency and safety of swallowing with regards to different consistencies correlates well with the less swallowing specific FOIS score. In the past, this algorithm was used in patients with movement disorders [16, 31]. However, since the present multicenter trial has demonstrated that the algorithm (i) is readily applicable in different diagnostic groups and (ii) is able to grade dysphagia in a clinically meaningful way, it may be assumed that this FEES-score could be helpful in everyday patients’ care and might be useful as an endpoint in clinical studies devoted to the topic of neurogenic dysphagia [38]. Finally, the present study also collected data with regards to the impact of FEES on dysphagia management. In more than 50% of patients FEES led to changes in the feeding strategy. Furthermore, in more than 25% of the subgroup of 447 tracheotomized patients, decannulation was deemed safe based on FEES-findings. These results corroborate existing literature, which usually focused on specific patient cohorts. Thus, in a recent study recruiting stroke patients and adopting a pre-post-design, Bax and co-workers showed that providing FEES-service on a stroke unit reduced the incidence of post-stroke pneumonia and increased the proportion of patients leaving hospital on a regular diet [39]. Hafner et al. reported clinical consequences of using FEES in a critical care setting in recently extubated patients [23]. Based on FEES prolonged non-oral feeding was required in 49.7% of patients, in 6.3% a tracheostomy was performed, an oral diet was started in 30.7% and tracheostomies were closed in 22.9%. Evaluating swallowing function in tracheostomized neurointensive care patients with a FEES-based decannulation algorithm, Warnecke et al. demonstrated that safe decannulation was possible in more than 50% of patients, whereas only about 30% of them would have been decannulated based on clinical swallowing evaluation alone [40]. Taken together, these studies provide first evidence that implementation of a FEES-service in different clinical settings may improve dysphagia care.
The strengths of this prospective observational study are its multicentre design, the inclusion of a heterogeneous patient cohort and the specific documentation of different features of the examination setting and of the respective results. However, some limitations are apparent. First, trial sites were chosen among those hospitals actively supporting the German FEES education initiative. Therefore, it is conceivable that sites with a more advanced level of proficiency were chosen against less experienced centres, which may have introduced a bias into the findings. Second, the study did not include documentation of potentially eligible patients that for various reasons were not recruited in the end. Hence, a selection bias cannot fully be ruled out. Third, probably reflecting the usual distributions of different disease categories in a given patient collective, stroke was by far the most common disease, whereas other disorders were significantly rarer. Thus, the generalizability of the study’s conclusions may be limited to a certain extent. Fourth, the documentation of how FEES was performed in detail was for reasons of practicability limited. Therefore, for example, it was not recorded whether topical anesthesia had been used, a factor that may well have been related to patients’ comfort [41] Fifth, there was no central reading of FEES findings and, sixth, for some study items the proportion of missing data was rather high. Both of these aspects may have impacted the scientific validity of the study’s results. Finally, while this study showed that FEES was safe even in the hands of less experienced endoscopists, the quality of the examinations and the derived conclusions were not scrutinized and evaluated.
In conclusion, this study confirmed that FEES, even when performed by less experienced clinicians, is a safe procedure with only moderate associated alterations of cardiovascular parameters. FEES had a significant impact on dysphagia management and by adopting a simple FEES-based dysphagia score, FEES showed to provide a clinically meaningful assessment of overall dysphagia severity.
Acknowledgements
The authors are grateful to the German Society of Neurology, the German Stroke Society and the German Geriatric Society for supporting the FEES curriculum.
Competing of interests
The authors reported no conflicts of interest.
Funding
None.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available because secondary analysis needs to be conducted and published. Data will be available from the corresponding author on reasonable request after the project has been finished.
Abbreviations
- CIP
Critical Illness Polyneuropathy
- FEES
Flexible Endoscopic Evaluation of Swallowing
- FOIS
Functional Oral Intake Scale
Authors’ contributions
RD, CL, JG, BLP and TW designed the trial; all authors contributed to the acquisition of data; RD, SL, CL, BLP, RW and TW analysed and interpreted the data. RD and TW drafted the manuscript; all authors revised the manuscript critically for important intellectual content and gave final approval of the version submitted.
Ethics approval and consent to participate
The study protocol was originally approved by the ethics committee of the Westfälische Wilhelms University Münster (2014–370-f-S) on August 19th 2014 and was also approved by all other involved ethics committees. All patients or their legal representative provided written informed consent.
Consent for publication
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rainer Dziewas, Email: dziewas@uni-muenster.de.
Matthias auf dem Brinke, Email: m.aufdembrinke@asklepios.com.
Ulrich Birkmann, Email: Ulrich.Birkmann@johannes-krankenhaus.com.
Götz Bräuer, Email: goetz.braeuer@helios-gesundheit.de.
Kolja Busch, Email: kolja.busch@hospital-borken.de.
Franziska Cerra, Email: schumann.fran@gmail.com.
Renate Damm-Lunau, Email: r.damm@august-bier-klinik.de.
Juliane Dunkel, Email: dunkel@drk-nh.de.
Amelie Fellgiebel, Email: a.fellgiebel@august-bier-klinik.de.
Elisabeth Garms, Email: garms@nkw-bw.de.
Jörg Glahn, Email: Joerg.Glahn@muehlenkreiskliniken.de.
Sandra Hagen, Email: s.hagen@asklepios.com.
Sophie Held, Email: sophie.held@klinik-feldafing.de.
Christine Helfer, Email: christine.helfer@vivantes.de.
Mirko Hiller, Email: mirko.hiller@gmx.de.
Christina Horn-Schenk, Email: C.Horn-Schenk@marien-kh.de.
Christoph Kley, Email: Christoph.Kley@johannes-krankenhaus.com.
Nikolaus Lange, Email: Nikolaus.Lange@sozialstiftung-bamberg.de.
Sriramya Lapa, Email: Sriramya.Lapa@kgu.de.
Christian Ledl, Email: CLedl@Schoen-Kliniken.de.
Beate Lindner-Pfleghar, Email: beate.lindner-pfleghar@rku.de.
Marion Mertl-Rötzer, Email: mmertl-roetzer@schoen-kliniken.de.
Madeleine Müller, Email: M.Mueller@rehab.ch.
Hermann Neugebauer, Email: hermann.neugebauer@uni-ulm.de.
Duygu Özsucu, Email: Duygu.Oezsucu@vivantes.de.
Michael Ohms, Email: Michael.Ohms@hjk-muenster.de.
Markus Perniß, Email: m.perniss@ruppiner-kliniken.de.
Waltraud Pfeilschifter, Email: waltraud.pfeilschifter@kgu.de.
Tanja Plass, Email: Plass@nkw-bw.de.
Christian Roth, Email: Roth@drk-nh.de.
Robin Roukens, Email: Robin.Roukens@stmtk.de.
Tobias Schmidt-Wilcke, Email: Tobias.Schmidt-Wilcke@stmtk.de.
Beate Schumann, Email: bschumann@ukaachen.de.
Julia Schwarze, Email: j.schwarze@sprechsalon.de.
Kathi Schweikert, Email: k.schweikert@rehab.ch.
Holger Stege, Email: h.stege@ruppiner-kliniken.de.
Dirk Theuerkauf, Email: d.theuerkauf@asklepios.com.
Randall S. Thomas, Email: r.thomas@asklepios.com
Ulrich Vahle, Email: U.Vahle@marien-kh.de.
Nancy Voigt, Email: voigt@sachsenklinik.de.
Hermann Weber, Email: Hermann.Weber@sozialstiftung-bamberg.de.
Cornelius J. Werner, Email: cwerner@ukaachen.de
Rainer Wirth, Email: rainer.wirth@elisabethgruppe.de.
Ingo Wittich, Email: i.wittich@krankenhaus-tutzing.de.
Hartwig Woldag, Email: hartwig@woldag.de.
Tobias Warnecke, Email: tobias.warnecke@ukmuenser.de.
References
- 1.Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke - incidence, diagnosis, and pulmonary complications. Stroke; a Journal of Cerebral Circulation. 2005;36:2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- 2.Morgan AS, Mackay LE. Causes and complications associated with swallowing disorders in traumatic brain injury. The Journal of Head Trauma Rehabilitation. 1999;14:454–461. doi: 10.1097/00001199-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Kalf JG, de Swart BJ, Bloem BR, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson's disease: A meta-analysis. Parkinsonism & Related Disorders. 2012;18(4):311–315. doi: 10.1016/j.parkreldis.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Langmore SE, Olney RK, Lomen-Hoerth C, Miller BL. Dysphagia in patients with frontotemporal lobar dementia. Archives of Neurology. 2007;64(1):58–62. doi: 10.1001/archneur.64.1.58. [DOI] [PubMed] [Google Scholar]
- 5.Kühnlein P, Gdynia HJ, Sperfeld AD, Lindner-Pfleghar B, Ludolph AC, Prosiegel M, Riecker A. Diagnosis and treatment of bulbar symptoms in amyotrophic lateral sclerosis. Nature Clinical Practice. Neurology. 2008;4:366–374. doi: 10.1038/ncpneuro0853. [DOI] [PubMed] [Google Scholar]
- 6.Grob D, Arsura L, Brunner NG, Namba R. The course of myasthenia gravis and therapies affecting outcome. Annals of the New York Academy of Sciences. 1987;505:472–499. doi: 10.1111/j.1749-6632.1987.tb51317.x. [DOI] [PubMed] [Google Scholar]
- 7.Guan XL, Wang H, Huang HS, Meng L. Prevalence of dysphagia in multiple sclerosis: A systematic review and meta-analysis. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2015;36(5):671–681. doi: 10.1007/s10072-015-2067-7. [DOI] [PubMed] [Google Scholar]
- 8.Altmann KW, Yu GP, Schaffer SD. Consequence of dysphagia in the hospitalized patient: Impact on prognosis and hospital ressources. Archives of otolaryngology--head & neck surgery. 2010;136:784–789. doi: 10.1001/archoto.2010.129. [DOI] [PubMed] [Google Scholar]
- 9.Holland G, Jayasekeran V, Pendleton N, Horan M, Jones M, Hamdy S. Prevalence and symptom profiling of oropharyngeal dysphagia in a community dwelling of an elderly population: A self-reporting questionnaire survey. Diseases of the Esophagus. 2011;24(7):476–480. doi: 10.1111/j.1442-2050.2011.01182.x. [DOI] [PubMed] [Google Scholar]
- 10.Ekberg O, Hamdy S, Woisard V, Wuttge-Hannig A, Ortega P. Social and psychological burden of dysphagia: Its impact on diagnosis and treatment. Dysphagia. 2002;17(2):139–146. doi: 10.1007/s00455-001-0113-5. [DOI] [PubMed] [Google Scholar]
- 11.Langmore SE, Schatz K, Olsen N. Fiberoptic endoscopic examination of swallowing safety: A new procedure. Dysphagia. 1988;2:216–219. doi: 10.1007/BF02414429. [DOI] [PubMed] [Google Scholar]
- 12.Langmore SE. History of Fiberoptic endoscopic evaluation of swallowing for evaluation and Management of Pharyngeal Dysphagia: Changes over the years. Dysphagia. 2017;32(1):27–38. doi: 10.1007/s00455-016-9775-x. [DOI] [PubMed] [Google Scholar]
- 13.Lapa S, Luger S, Pfeilschifter W, Henke C, Wagner M, Foerch C. Predictors of dysphagia in acute pontine infarction. Stroke; a Journal of Cerebral Circulation. 2017;48(5):1397–1399. doi: 10.1161/STROKEAHA.116.015045. [DOI] [PubMed] [Google Scholar]
- 14.Leder SB. Fiberoptic endoscopic evaluation of swallowing in patients with acute traumatic brain injury. The Journal of Head Trauma Rehabilitation. 1999;14(5):448–453. doi: 10.1097/00001199-199910000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Bader CA, Niemann G. Dysphagia in children with cerebral palsy--fiberoptic-endoscopic findings. Laryngo- Rhino- Otologie. 2010;89(2):90–94. doi: 10.1055/s-0029-1237348. [DOI] [PubMed] [Google Scholar]
- 16.Warnecke T, Oelenberg S, Teismann I, Hamacher C, Lohmann H, Ringelstein EB. Dziewas R: Endoscopic characteristics and levodopa responsiveness of swallowing function in progressive supranuclear palsy. Movement Disorders. 2010;25:1239–1245. doi: 10.1002/mds.23060. [DOI] [PubMed] [Google Scholar]
- 17.Pflug C, Bihler M, Emich K, Niessen A, Nienstedt JC, Flugel T, Koseki JC, Plaetke R, Hidding U, Gerloff C, et al. Critical dysphagia is common in Parkinson disease and occurs even in early stages: A prospective cohort study. Dysphagia. 2018;33(1):41–50. doi: 10.1007/s00455-017-9831-1. [DOI] [PubMed] [Google Scholar]
- 18.Leder SB, Novella S, Patwa H. Use of fiberoptic endoscopic evaluation of swallowing (FEES) in patients with amyotrophic lateral sclerosis. Dysphagia. 2004;19(3):177–181. doi: 10.1007/s00455-004-0009-2. [DOI] [PubMed] [Google Scholar]
- 19.Warnecke T, Oelenberg S, Teismann I, Suntrup S, Hamacher C, Young P, Ringelstein EB. Dziewas R: Dysphagia in X-linked bulbospinal muscular atrophy (Kennedy disease) Neuromuscular Disorders. 2009;19:704–708. doi: 10.1016/j.nmd.2009.06.371. [DOI] [PubMed] [Google Scholar]
- 20.Deutschmann MW, McDonough A, Dort JC, Dort E, Nakoneshny S, Matthews TW. Fiber-optic endoscopic evaluation of swallowing (FEES): Predictor of swallowing-related complications in the head and neck cancer population. Head & Neck. 2013;35(7):974–979. doi: 10.1002/hed.23066. [DOI] [PubMed] [Google Scholar]
- 21.Willging JP, Thompson DM. Pediatric FEESST: Fiberoptic endoscopic evaluation of swallowing with sensory testing. Current Gastroenterology Reports. 2005;7(3):240–243. doi: 10.1007/s11894-005-0041-x. [DOI] [PubMed] [Google Scholar]
- 22.Leder SB, Suiter DM, Agogo GO, Cooney LM., Jr An epidemiologic study on ageing and dysphagia in the acute care geriatric-hospitalized population: A replication and continuation study. Dysphagia. 2016;31(5):619–625. doi: 10.1007/s00455-016-9714-x. [DOI] [PubMed] [Google Scholar]
- 23.Hafner G, Neuhuber A, Hirtenfelder S, Schmedler B. Eckel HE: Fiberoptic endoscopic evaluation of swallowing in intensive care unit patients. European Archives of Oto-Rhino-Laryngology. 2008;265:441–446. doi: 10.1007/s00405-007-0507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marian T, Dunser M, Citerio G, Kokofer A, Dziewas R. Are intensive care physicians aware of dysphagia? The MAD(ICU) survey results. Intensive Care Medicine. 2018;44(6):973–975. doi: 10.1007/s00134-018-5181-1. [DOI] [PubMed] [Google Scholar]
- 25.Dziewas R, Glahn J, Helfer C, Ickenstein G, Keller J, Ledl C, Lindner-Pfleghar B, D GN, Prosiegel M, Riecker A, et al. Flexible endoscopic evaluation of swallowing (FEES) for neurogenic dysphagia: Training curriculum of the German Society of Neurology and the German stroke society. BMC Medical Education. 2016;16:70. doi: 10.1186/s12909-016-0587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dziewas R, Glahn J, Helfer C, Ickenstein G, Keller J, Lapa S, Ledl C, Lindner-Pfleghar B, Nabavi D, Prosiegel M, et al. FEES for neurogenic dysphagia: Training curriculum of the German Society of Neurology and the German stroke society. Der Nervenarzt. 2014;85(8):1006–1015. doi: 10.1007/s00115-014-4114-7. [DOI] [PubMed] [Google Scholar]
- 27.Dziewas, R., Baijens, L., Schindler, A., Verin, E., Michou, E., & Clave, P. (2017). European Society for Swallowing Disorders FEES accreditation program for neurogenic and geriatric oropharyngeal dysphagia. Dysphagia. [DOI] [PMC free article] [PubMed]
- 28.Wade DT, Collin C. The Barthel ADL index: A standard measure of physical disability? International Disability Studies. 1988;10(2):64–67. doi: 10.3109/09638288809164105. [DOI] [PubMed] [Google Scholar]
- 29.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond agitation-sedation scale: Validity and reliability in adult intensive care unit patients. American Journal of Respiratory and Critical Care Medicine. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 30.Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Archives of Physical Medicine and Rehabilitation. 2005;86(8):1516–1520. doi: 10.1016/j.apmr.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 31.Warnecke T, Suttrup I, Schroder JB, Osada N, Oelenberg S, Hamacher C, Suntrup S, Dziewas R. Levodopa responsiveness of dysphagia in advanced Parkinson's disease and reliability testing of the FEES-levodopa-test. Parkinsonism & Related Disorders. 2016;28:100–106. doi: 10.1016/j.parkreldis.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 32.Aviv JE, Kaplan ST, Thomson JE, Spitzer J, Diamond B, Close LG. The safety of flexible endoscopic evaluation of swallowing with sensory testing (FEESST): An analysis of 500 consecutive evaluations. Dysphagia. 2000;15(1):39–44. doi: 10.1007/s004559910008. [DOI] [PubMed] [Google Scholar]
- 33.Cohen MA, Setzen M, Perlman PW. The safety of flexible endoscopic evaluation of swallowing with sensory testing in an outpatient otolaryngology setting. The Laryngoscope. 2003;113:21–24. doi: 10.1097/00005537-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Langmore SE. Endoscopic evaluation and treatment of swallowing disorders. New York. Stuttgart: Thieme; 2001. [Google Scholar]
- 35.Nacci A, Matteucci J, Romeo SO, Santopadre S, Cavaliere MD, Barillari MR, Berrettini S, Fattori B. Complications with Fiberoptic endoscopic evaluation of swallowing in 2,820 examinations. Folia phoniatrica et logopaedica : official organ of the International Association of Logopedics and Phoniatrics (IALP) 2016;68(1):37–45. doi: 10.1159/000446985. [DOI] [PubMed] [Google Scholar]
- 36.Warnecke T, Teismann I, Oelenberg S, Hamacher C, Ringelstein EB, Schabitz WR, Dziewas R. The safety of fiberoptic endoscopic evaluation of swallowing in acute stroke patients. Stroke; a Journal of Cerebral Circulation. 2009;40:482–486. doi: 10.1161/STROKEAHA.108.520775. [DOI] [PubMed] [Google Scholar]
- 37.Dziewas R, Schilling M, Konrad C, Stögbauer F, Lüdemann P. Placing nasogastric tubes in stroke patients with dysphagia: Efficiency and tolerability of the reflex-placement. Journal of Neurology, Neurosurgery, and Psychiatry. 2003;74:1429–1431. doi: 10.1136/jnnp.74.10.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dziewas R, Beck AM, Clave P, Hamdy S, Heppner HJ, Langmore SE, Leischker A, Martino R, Pluschinski P, Roesler A, et al. Recognizing the importance of dysphagia: Stumbling blocks and stepping stones in the twenty-first century. Dysphagia. 2017;32(1):78–82. doi: 10.1007/s00455-016-9746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bax L, McFarlane M, Green E, Miles A. Speech-language pathologist-led fiberoptic endoscopic evaluation of swallowing: Functional outcomes for patients after stroke. Journal of Stroke and Cerebrovascular Diseases : The Official Journal of National Stroke Association. 2014;23(3):e195–e200. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 40.Warnecke T, Suntrup S, Teismann IK, Hamacher C, Oelenberg S, Dziewas R. Standardized endoscopic swallowing evaluation for tracheostomy decannulation in critically ill neurologic patients. Critical Care Medicine. 2013;41(7):1728–1732. doi: 10.1097/CCM.0b013e31828a4626. [DOI] [PubMed] [Google Scholar]
- 41.O'Dea MB, Langmore SE, Krisciunas GP, Walsh M, Zanchetti LL, Scheel R, McNally E, Kaneoka AS, Guarino AJ, Butler SG. Effect of lidocaine on swallowing during FEES in patients with dysphagia. The Annals of Otology, Rhinology, and Laryngology. 2015;124(7):537–544. doi: 10.1177/0003489415570935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available because secondary analysis needs to be conducted and published. Data will be available from the corresponding author on reasonable request after the project has been finished.