Abstract
Background
Palbociclib is a specific inhibitor of cyclin-dependent kinases 4 and 6 that is approved for the treatment of advanced or metastatic breast cancer patients. Despite a good toxicity profile in pivotal trials, where asymptomatic neutropenia was the main adverse effect, its wider use in clinical practice may show less prevalent but serious toxicities.
Case Presentation
Here, we describe a case of pneumonitis due to palbocicblib. A 57-year-old female with breast cancer with bone metastasis presented dyspnea at rest 3 months after beginning treatment with palbociclib and letrozole. Palbociclib-induced pneumonitis was considered the most probable cause after ruling out all alternatives, and the patient was successfully treated with steroids and showed complete remission.
Conclusions
In summary, we present a well-documented case report of pneumonitis related to palbociclib. However, the mechanism of toxicity is still unknown, and there are as yet no reliable biomarkers to predict toxicity with cyclin-dependent kinase 4/6 inhibitors. In this case report, we alert physicians about new drugs that can provoke old toxicities.
Keywords: Case report, Palbociclib, Pneumonitis, Drug-related pneumonitis, Lung toxicity
Established Facts
Cyclin-dependent kinase 4/6 inhibitors such as palbociclib have already been approved in metastatic breast cancer patients.
The median progression-free survival is about 24 months in the first-line treatment in metastatic breast cancer patients.
Neutropenia G3 was seen in about 80% of the patients, mostly asymptomatic without any infection symptomatology.
Lung toxicity such as drug-related pneumonitis has been described in other cancer drugs in metastatic breast cancer patients.
Pivotal trials and drug information of palbociclib do not inform about pneumonitis related to palbociclib.
Novel Insights
Pneumonitis is an acute, life-threatening toxicity.
Pneumonitis related to palbociclib is not well documented in the literature and may lead to underdiagnosis.
Other possible causes, such as infection, pulmonary thromboembolism, and cardiac dysfunction, have to be discarded before the diagnosis.
The mechanism of toxicity is still not known, but it is reversible by stopping CDK 4/6 inhibitor treatment and initiate corticotherapy.
Multidisciplinary approach is needed to conclude the final diagnosis.
Background
Palbociclib is a highly specific inhibitor of cyclin-dependent kinases (CDK) 4 and 6. CDK are a large family of serine-threonine kinases involved in the regulation of cell-cycle progression [1]. Cyclin D binds both CDK4 and CDK6 and induces hyperphosphorylation of the retinoblastoma protein, causing progression of tumor cells by promoting the transcription factor E2F that regulates progression from the G1 to the S phase. Both CDK4 and CDK6 are thus key regulators of cell division, and their inhibition controls cell growth and suppresses tumor activity [2]. Palbociclib has been approved for the treatment of hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative, advanced, or metastatic breast cancer in combination with either an aromatase inhibitor as initial hormonal therapy in postmenopausal women or fulvestrant in women with disease progression following hormonal therapy [3].
Two pivotal phase III randomized trials [4, 5] have shown a benefit in progression-free survival (PFS) for palbociclib plus hormonal therapy. The PALOMA-2 randomized patients in a 2:1 ratio to receive either palbociclib-letrozole or placebo-letrozole. The median PFS was 24.8 months in the palbociclib-letrozole arm, compared to 14.5 months in the placebo-letrozole arm (p < 0.001) [4]. The PALOMA-3 trial [5], comparing palbociclib-fulvestrant and placebo-fulvestrant in patients who had progressed on previous endocrine therapy, also showed a benefit in PFS for palbociclib (9.5 vs. 4.6 months; p < 0.001).
Other CDK 4/6 inhibitors have also been approved in combination with hormonal therapy in breast cancer, including ribociclib [6, 7, 8] and abemaciclib [9, 10].
In clinical trials, CKD 4/6 inhibitors have shown good tolerability. The most common adverse reaction to palbociclib in the PALOMA trials [4, 5] was neutropenia. Other common toxicities included leukopenia, anemia, thrombocytopenia, stomatitis, alopecia, rash, and fatigue. However, it is not reported any lung toxicity in palbociclib information sheet. The MONARCH 2 trial of abemaciclib reported two deaths due to pneumonitis [10], and the palbociclib risk management plan includes pneumonitis as a potential risk [11]. However, pneumonitis was not observed in either of the PALOMA trials, although clinical trials include only a limited number of patients, usually without comorbidities. Long-term safety is unknown and less prevalent adverse effects can only be identified when the drug has been used in clinical practice.
In fact, only a few cases of palbociclib-related pneumonitis have been reported. A meta-analysis [12] of 8,906 patients with different cancers enrolled in 470 phase I trials found that only 6 patients with pneumonitis had received CDK 4/6 inhibitors. In a single-center study of 100 patients, one case of pneumonitis was observed in a 72-year-old female [13]. A single case report described a 52-year-old woman receiving palbociclib who developed pneumonitis [14]. These cases of pneumonitis may have been palbociclib-related, but no diagnosis of exclusion was performed to rule out alternatives. Here, we present a case of pneumonitis in a patient receiving palbociclib where a diagnosis of exclusion based on the Spanish Pharmacovigilance System Algorithm [15], a modification of the Karch and Lasagna algorithm [16], indicated that the pneumonitis was treatment related.
Case Presentation
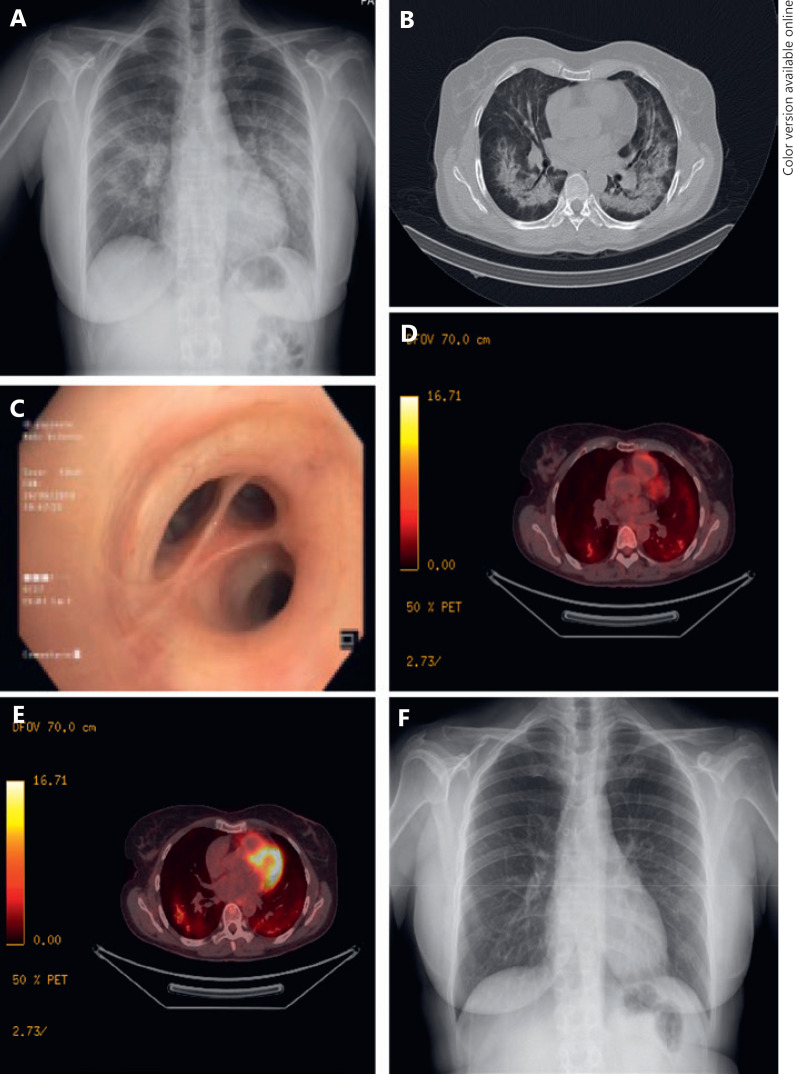
A 57-year-old, Caucasian, female ex-smoker with no prior history of pulmonary disease was diagnosed with high-grade infiltrating ductal carcinoma (estrogen receptors, 99%; progesterone receptors 5%; HER2-negative; Ki67 index 30%, stage IV due to confirmed bone involvement). She received palbociclib 125 mg/day in 4-week cycles (3 weeks on, 1 week off) plus letrozole 2.5 mg/day continuously and zoledronic acid 4 mg/every 28 days, with no other concomitant treatments. After 3 months of treatment, she came to the emergency department of our hospital with a 1-week history of progressive shortness of breath accompanied by nonproductive cough and fever (maximum 37.7°C). On physical examination, the patient was afebrile and eupnoeic; pulmonary auscultation showed no remarkable findings. Pulse oximetry was normal but dropped below 90% with minimum effort. Blood analysis showed an elevation of acute-phase reactants, without leukocytosis or neutropenia. A chest X-ray showed bilateral pulmonary infiltrates (Fig. 1A). Empirical antibiotic therapy with ceftriaxone and levofloxacin was initiated without clinical improvement. Blood culture and urine antigen test of L. pneumophila and S. pneumoniae were negative. A computed tomography (CT) of the chest revealed extensive ground glass opacities throughout both lungs (Fig. 1B) but no signs of pulmonary embolism. The CT pattern suggested a differential diagnosis between infectious causes and drug-induced toxicity, and the patient was hospitalized for further study. She underwent a positron emission tomography-CT with a bilateral inflammatory pattern and a bronchoscopy (Fig. 1C) where the bronchoalveolar lavage (BAL) showed lymphocytosis (55%) with a CD4/CD8 ratio of 0.18. Cultures for bacteria, fungus, mycobacteria, and virus in BAL and bronchial aspirates were negative. Cytology showed no malignancies, and a 2D echocardiogram showed no cardiac dysfunction.
Fig. 1.
Radiological and pulmonological tests performed on our patient. A X-ray performed when the patient was admitted to the Emergency Department, showing a diffuse bilateral consolidation pattern. B CT performed during hospital admission, showing dense infiltrates in both lungs and areas with ground-glass attenuation. C Bronchoscopy showing no morphological alterations. D, E Positron-emission tomography-CT revealing a bilateral inflammatory pattern. F X-ray performed after 6 weeks with corticoid therapy showing complete resolution of the pulmonary infiltrations.
The patient's clinical presentation and the negative cultures argued against an infectious cause, while the radiological findings and the predominance of CD8 in the BAL suggested that drug-induced pulmonary toxicity was likely. We confirmed this in accordance with the Spanish Pharmacovigilance System Algorithm [15], which assesses the causality of an adverse reaction by assigning points to different factors (Table 1). The total number of points obtained in our patient was six, indicating a probable drug-related toxicity. Therefore, palbociclib was stopped, and she was treated with prednisolone (2 mg/kg/day), showing clinical improvement within 72 h. Six weeks later, the patient presented a normal clinical exploration without any symptomatology, with a complete radiologic resolution. Corticoid therapy was slowly reduced, and the symptomatology did not recur. Due to pulmonary toxicity grade 4 attributed to palbociclib, treatment was not restarted. The patient has been treated with letrozol in monotherapy for 6 months without disease progression or associated toxicity.
Table 1.
Diagnosis of exclusion of other causes of pneumonitis in our patient, based on the Spanish Pharmacovigilance System Algorithm (SPSA) [15]
| SPSA factor | Our patient | Points assigned |
|---|---|---|
|
Chronology Did the adverse event occur after drug exposure? |
Yes |
2 |
|
Criteria from the literature Have other cases been reported? |
Yes Two cases [13, 14] |
1 |
|
Outcome of the adverse reaction after drug discontinuation Did the patient improve when the drug was stopped? |
Yes Dyspnea disappeared within 4 days |
2 |
|
Rechallenge Was treatment resumed after improvement? |
No | 0 |
|
Other possible causes Were other causes ruled out? |
Yes Negative cultures showed no infection |
1 |
|
Contributory factors Are there other factors favoring a causal relationship? |
No | 0 |
|
Investigations Is there any evidence of drug concentration in a biological fluid or biopsy? |
No No analyses were performed |
0 |
| Total number of points | 6 | |
Conclusions
In summary, while palbociclib-related pneumonitis remains a rare event, with an unknown mechanism of action, it is crucial for physicians to be alert to this risk in spite of the good toxicity profile reported in clinical trials.
The association between pulmonary disease and antineoplastic drugs is well known, although its pathogenesis is complex, multifactorial, and dependent on different mechanisms [17, 18]. Although 14 metabolites have been identified in palbociclib, none have toxicity potential [19]. With no biomarkers currently available to predict toxicity, the identification of palbociclib-induced toxicity must rely on a diagnosis of exclusion to rule out pulmonary infections, lymphangitis carcinomatosis, and cardiogenic pulmonary edema. Algorithms [15, 16, 20] for assessing the causality of an adverse event can help unify the varying clinical impressions of different observers and lead to a final empirical diagnosis. The role of the clinical pharmacologist is critical to the correct implementation of these algorithms when determining the cause of suspected drug reactions in clinical practice.
Statement of Ethics
The patient gave consent to publish the manuscript and signed a consent form.
Disclosure Statement
E.F.: travel grant: Pfizer, Roche, Lilly, and Novartis. M.M.: travel grant: Celgene and Kern; consultant or advisory role: Novartis, Pfizer, Roche, and Kern; speakers bureau: Novartis. B.C.: travel grant: Roche, BMS, Eisai, Merck, and Pierre-Fabre; consultant or advisory role: BMS, Roche, Merck, and Eisai. V.Q.: travel grant: BMS, Roche, Novartis, and Pfizer; consultant or advisory role: Kern; speakers bureau: Roche and Eisai. M.R.: travel grant: Pfizer, MSD; consultant or advisory role: Tesaro, AstraZeneca, and Roche. L.L., C.P.-M., D.Q., I.T., I.G., C.C., and E.B. have no conflicts of interest to disclose.
Funding Sources
This study received no outside funding.
Author Contributions
E.F., L.L., C.P.-M., D.Q., I.G., M.M., I.T., B.C., C.C., M.R., E.B., and V.Q. contributed to writing and designing the study and gave final approval to publish the manuscript.
Acknowledgements
We thank all professionals who treated the patient and helped formulate the final diagnosis.
References
- 1.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004 Nov;3((11)):1427–38. [PubMed] [Google Scholar]
- 2.Chen P, Lee NV, Hu W, Xu M, Ferre RA, Lam H, et al. Spectrum and Degree of CDK Drug Interactions Predicts Clinical Performance. Mol Cancer Ther. 2016 Oct;15((10)):2273–81. doi: 10.1158/1535-7163.MCT-16-0300. [DOI] [PubMed] [Google Scholar]
- 3.Beaver JA, Amiri-Kordestani L, Charlab R, Chen W, Palmby T, Tilley A, et al. FDA Approval: Palbociclib for the Treatment of Postmenopausal Patients with Estrogen Receptor-Positive, HER2-Negative Metastatic Breast Cancer. Clin Cancer Res. 2015 Nov;21((21)):4760–6. doi: 10.1158/1078-0432.CCR-15-1185. [DOI] [PubMed] [Google Scholar]
- 4.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016 Nov;375((20)):1925–36. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, Bartlett CH, Schnell P, DeMichele AM, Loi S, Ro J, et al. Palbociclib in Combination With Fulvestrant in Women With Hormone Receptor-Positive/HER2-Negative Advanced Metastatic Breast Cancer: Detailed Safety Analysis From a Multicenter, Randomized, Placebo-Controlled, Phase III Study (PALOMA-3) Oncologist. 2016 Oct;21((10)):1165–75. doi: 10.1634/theoncologist.2016-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med. 2016 Nov;375((18)):1738–48. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 7.Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018 Jul;19((7)):904–15. doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 8.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J Clin Oncol. 2018 Aug;36((24)):2465–72. doi: 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 9.Dickler MN, Tolaney SM, Rugo HS, Cortés J, Diéras V, Patt D, et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2- Metastatic Breast Cancer. Clin Cancer Res. 2017 Sep;23((17)):5218–24. doi: 10.1158/1078-0432.CCR-17-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sledge GW, Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol. 2017 Sep;35((25)):2875–84. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 11. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003853/WC500217198.pdf.
- 12.Yonemori K, Hirakawa A, Kawachi A, Kinoshita F, Okuma H, Nishikawa T, et al. Drug induced interstitial lung disease in oncology phase I trials. Cancer Sci. 2016 Dec;107((12)):1830–6. doi: 10.1111/cas.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong J, Cho M, Yu KW, Waisman J, Yuan Y, Mortimer J. A single institution experience with palbociclib toxicity requiring dose modifications. Breast Cancer Res Treat. 2018 Apr;168((2)):381–7. doi: 10.1007/s10549-017-4606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahsan I, Malik F, Jafri S. Palbociclib Related Pnemotoxicity: A Rare Side Effect. Am J Respir Crit Care Med. 2017;195:A5546. [Google Scholar]
- 15.Aguirre C, García M. [Causality assessment in reports on adverse drug reactions. Algorithm of Spanish pharmacovigilance system] Med Clin (Barc) 2016 Nov;147((10)):461–4. doi: 10.1016/j.medcli.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Karch FE, Lasagna L. Toward the operational identification of adverse drug reactions. Clin Pharmacol Ther. 1977 Mar;21((3)):247–54. doi: 10.1002/cpt1977213247. [DOI] [PubMed] [Google Scholar]
- 17.Omarini C, Thanopoulou E, Johnston SR. Pneumonitis and pulmonary fibrosis associated with breast cancer treatments. Breast Cancer Res Treat. 2014 Jul;146((2)):245–58. doi: 10.1007/s10549-014-3016-5. [DOI] [PubMed] [Google Scholar]
- 18.Vahid B, Marik PE. Pulmonary complications of novel antineoplastic agents for solid tumors. Chest. 2008 Feb;133((2)):528–38. doi: 10.1378/chest.07-0851. [DOI] [PubMed] [Google Scholar]
- 19.Chavan BB, Tiwari S, G S, Nimbalkar RD, Garg P, R S, et al. In vitro and in vivo metabolic investigation of the Palbociclib by UHPLC-Q-TOF/MS/MS and in silico toxicity studies of its metabolites. J Pharm Biomed Anal. 2018 Aug;157:59–74. doi: 10.1016/j.jpba.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981 Aug;30((2)):239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]