Abstract
Introduction
Stroke and its long-term consequences pose major challenges for the lives of those affected and healthcare systems. Neurological rehabilitation therefore primarily attempts to improve function in order to increase independence in activities of daily living, and to enable social participation. There is only scarce data on dynamics of functional recovery after patients discharge from inpatient neurological rehabilitation. Even less is known about the patient’s perspective on long-term recovery from stroke. The Interdisciplinary Platform for Rehabilitation Research and Innovative Care of Stroke Patients (IMPROVE) aims to address this knowledge gap by providing new insights into the dynamics and extent of functional recovery from stroke beyond inpatient rehabilitation treatment.
Methods
We provide the protocol for an observational, longitudinal, multicenter study conducted in an Universitary Stroke Center in cooperation with five Neurological Rehabilitation Centers in Northern Germany. Patients who suffered from ischemic or hemorrhagic stroke will be enrolled by the end of inpatient rehabilitation and followed up to 1 year. In addition, a group of chronic stroke patients and a group of craniocerebral trauma patients will be enrolled as a comparison group. Data on stroke characteristics, vascular risk factors, co-morbidities, social support, and demographics will be recorded. Comprehensive clinical evaluation will be performed at baseline, three, six, and twelve months after enrollment. The assessments and scores used reflect the three components of the International Classification of Functioning, Disability and Health (ICF), some of them are tests regularly used in rehabilitation settings. Tests of motor function, cognition, and mood are included, as are tests of self-reported health-related quality of life. Primary outcome measure is a hand motor score, built by the sum of the hand items of the Fugl-Meyer Assessment as an objective measurement of hand function at 12 months after enrollment. Predictors of the primary outcome will be analyzed using linear regression analysis.
Perspective
The results of IMPROVE will inform about the long-term dynamics of functional stroke recovery after patients’ discharge from inpatient rehabilitation and will provide insights into the association of clinical and demographic factors with recovery of function.
Trial registration
The protocol is registered at ClinicalTrials.gov (NCT04119479).
Keywords: Stroke, Rehabilitation, Functional recovery, Hand motor function, International classification of functioning, Disability and health
Introduction
Cerebrovascular diseases, such as stroke, are among the greatest challenges in healthcare [23, 24]. In Germany, about 196,000 first-ever strokes occur every year. In addition, about 66,000 recurrent strokes take place [21]. In the context of the demographic development with an ageing population, the number of stroke patients is expected to increase significantly in the coming years [14]. In parallel with the increasing incidence and decreasing mortality, however, it is above all the impairments caused by a stroke that are of great interest to health care practitioners and other health care actors [33, 41].
Stroke, the second leading cause of death worldwide, represents one of the main reasons for long-term disability [45]. In high-income countries, stroke is even the most common cause of acquired impairment in adulthood, and some of those affected are confronted with dramatic changes in patients’ everyday lives [17, 43]. Data on long-term disability and recovery after stroke vary widely and depend on various influencing factors, like age or type of stroke [6, 46]. However, motor impairments after stroke are found in about 80% of all cases [27], which are associated with persisting disability and dependency in over 30% [12].
The importance of neuro-rehabilitative research becomes obvious in regard to these numbers [5]. The main aim of neurological rehabilitation is to improve function and independence in activities of daily living, and to enable social participation [12]. The understanding of rehabilitation must not only be based on the underlying disease mechanisms, but must above all be based on the consequences of the disease according to the International Classification of Functioning, Disability and Health [44]. This concept divides the disease consequences into three main areas: (a) damage to body functions and structure (e.g. paresis of the hand), (b) activity (e.g. learning activities, exercise of skills) and (c) participation (e.g. participation in professional and social life). In addition to pathophysiological classification, this system offers the possibility of classifying and specifically treating patients with regard to their functional state of health, the degree of limitation and their social effects.
Stroke research is often focused on the acute treatment phase as well as on the first weeks of rehabilitation – usually the phase of inpatient rehabilitation [40]. Data on long-term disability and recovery after stroke is limited. Few studies have explored functionality based on the ICF conceptual model [38].
In stroke patients, a wide range of individual variability of recovery extent and rate can be observed clinically. However, it is still open which factors contribute to functional recovery. Detailed data describing the long-term development of stroke patients after being discharged from rehabilitation programs are rare. To date, it is not fully described how stable the effects of rehabilitation are and how much clinical improvement can be seen in the time period following inpatient rehabilitation, especially with regard to ICF functionality. Even less is known about the patients’ perspective on long-term recovery from stroke.
This lack of data is also due to a missing link between research institutions and rehabilitation centers [19, 39]. Therefore, the IMPROVE-platform was established as a research collaboration between an Universitary Stroke Center, i.e., the department of neurology at the University Medical Center Hamburg-Eppendorf (UKE) and five rehabilitation clinics, to address the mentioned questions in future research projects.
This first study of the IMPROVE platform is a multicenter observational prospective study to investigate the main factors influencing the functional recovery of stroke patients with regard to the ICF-dimensions in a long-term view with a focus on hand function.
Methods
Aim of the study
The overall goal of this study is to identify and understand factors that influence the course of recovery following focal brain lesions within the framework of standard neurorehabilitation in Germany. In this observational longitudinal study, the long-term recovery processes of stroke patients will be investigated. The influence of motor function, cognition, care situation, depression, fatigue as well as information and knowledge about the disease on functional recovery, participation, autonomy and quality of life will be assessed. The assessment of motor function focuses on hand-function, which represents a crucial function for independence and participation in activities of daily living. The current state of subjective as well as objective parameters is surveyed. The development of hand function 1 year after rehabilitation and the main factors influencing it will be analyzed. Ideally, the results obtained can be used to identify opportunities and improvement potentials for future rehabilitation strategies. More targeted planning and individualized therapy courses could be results of this development.
Study description and study design
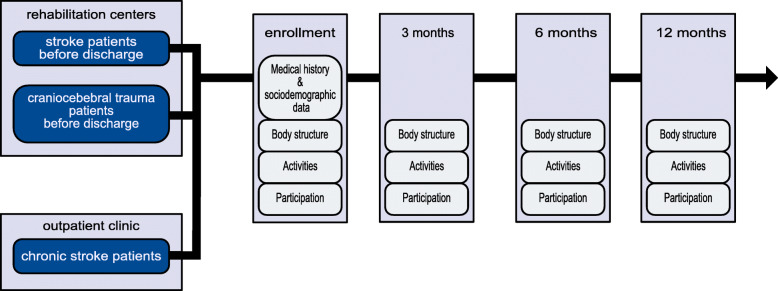
IMPROVE is an observational, longitudinal, multicenter study in an Universitary Stroke Center and five rehabilitation centers in northern Germany (NCT04119479). The applied set of assessments includes self-reported outcome measures as well as objective scores representing the ICF dimensions body function and structure, activity and participation. Initial measures are conducted during the end of inpatient rehabilitation in the cooperating study centers. Follow-up examinations take place three, six and 12 months after inclusion (Fig. 1). Measures include standardized state of the art motor and cognitive function assessments carried out by trained health care specialists. In addition, questionnaires are provided covering a number of stroke specific health care topics. If available, routine magnetic resonance imaging data from the acute care phase is collected as well. Before inclusion, patients are informed thoroughly about the study structure and the ability to opt-out at any given point. Data collection is carried out according to European data protection law.
Fig. 1.
Stroke patients with an ischemic or hemorrhagic stroke with a still remaining deficit (mRS ≥1) during the end of inpatient rehabilitation are included in the study. In addition, a group of patients with craniocerebral trauma from the rehabilitation centers as well as a group of chronic stroke patients is recruited as a comparison group. All groups undergo the same examinations and ICF-orientated assessments
The IMPROVE research platform
For this research project, rehabilitation centers suitable for implementation were sought and approached. The general study structure was discussed in preparatory meetings and developed together with the participating centers. In the first months of 2017, the last adjustments to the study design were made, particularly with regard to the selection of assessors.
For the implementation in clinical everyday life of the rehabilitation clinics, an intensive exchange with the cooperation clinics took place via visits and various meetings. Task force meetings are hold, which are attended by one representative of each of the partner clinics. In addition, joint cooperation meetings involving the local medical study management and representatives of the therapy areas of all clinics are performed. At the same time, there are regular telephone and E-mail exchanges, covering individual questions and assessment contents. A monthly IMPROVE newsletter is set up to inform all participants about current activities and the current recruitment status.
An initial standardized on-site training was given to the staff in each of the participating rehabilitation clinics, covering the topics inclusion, documentation, the exact performance of assessments and the exchange of data between the rehabilitation clinic and UKE. Only trained personnel are allowed to carry out the study in order to ensure a standardized implementation. A test run of one to two patients per clinic was initially carried out for quality assurance and in order to integrate the study into the processes of the clinics. If new members join the study team, they are also trained in the IMPROVE standards. An electronic Case Report Form is set up for the IMPROVE study. All follow-up examinations are carried out at the UKE by the same trained study team. In order to enable all patients to participate, travel costs are reimbursed or transport to the study center is organized.
Study arms
Three groups are included in the study. Mainly subacute stroke patients with an ischemic or hemorrhagic stroke at the end of their inpatient rehabilitation period. Only patients with a still remaining deficit are included. In one rehabilitation center, craniocerebral trauma patients at the end of the inpatient rehabilitation are recruited as a second group. Additionally, a cohort of chronic stroke patients is recruited by the post stroke clinic of the UKE Neurology department. The chronic stroke group as well as the group of craniocerebral trauma patients represent a comparison group. All groups undergo the same examinations.
Eligibility criteria
Patients admitted to the cooperating rehabilitation centers following cerebral infarction and/or cerebral hemorrhage (ICD 10 I61-I69) are screened by a physician for eligibility. Patients from the rehabilitation centers are included at the end of rehabilitation phases C and D according to the criteria of the Bundesarbeitsgemeinschaft für Rehabilitation [3].
Inclusion criteria are:
Ischemic or hemorrhagic stroke according to ICD 10 I61-I69
Patients in or after completion of rehabilitation phases C and D according to BAR criteria
Age ≥ 18
Sufficient knowledge of German
Existing declaration of consent
Deficit still existing (modified Rankin score of at least 1 at inclusion)
Exclusion criteria are:
Need for care prior stroke
Subarachnoid hemorrhage, craniocerebral trauma, transient ischemic attack as primary diagnosis
Severe pre-existing psychiatric disease
Participation in follow-up examination not possible
For the chronic stroke group as well as the craniocerebral trauma group the same inclusion/exclusion criteria are applied. For the chronic stroke group patients are included in an outpatient setting after completing inpatient rehabilitation. For the craniocerebral trauma group patients with craniocerebral trauma (ICD 10 S06.1-S06.9) as a primary diagnosis are included.
Sample size estimation
As this is an explorative observational study, no classical calculation of sample sizes is eligible. Based on the annual treatment numbers of the participating clinics, an initial estimate of 300 inclusions was made. Eighteen months after the beginning of the study we performed a sample size calculation based on the available data on recovery of the hand function in the Stroke Impact Scale (SIS) and the dropout rate of the patients included until then. Based on these data we estimated that we would need N = 225 to be able to identify a difference with Cohens d = 0.3. The recruitment goal could be reduced to 225 patients.
Outcome measures
The primary outcome measure is a hand motor score built by the sum of the hand items of the Fugl-Meyer upper limb motor score as an objective measurement of hand function after 1 year [15]. This subdomain covers seven performance tasks rated in a three-point scale from 0 = cannot perform, 1 = performs partially and 2 = performs fully. The seven tasks include Finger flexion (1), Finger extension (2), Extension of metocarpophalangeal joints, flexion of proximal and distal interphalangeal joints (3), Thumb adduction (4), Thumb opposition (5), Grasp cylinder (6), Grasp tennis ball (7). The maximal possible score is 14.
Further outcome measures include assessments and scores that are regularly used in rehabilitation settings and are designed to reflect the three components of the International Classification of Functioning, Disability and Health (Tables 1, 2 and 3).
Table 1.
Assessments used in the study reflecting the component body structure of the International Classification of Functioning, Disability and Health
| Body structure | |
|---|---|
| National Institutes of Health Stroke Scale | The National Institute of Health Stroke Scale is a score system to quantify the impairment caused by a stroke. The sum of the values from the investigations results in a maximum of 42 points. The higher the score, the more extensive the stroke [7]. |
| Fugl-Meyer Assessment (upper extremity) | The section motor function of upper limb is one of five domains, a three-point scale is used for rating performance as 0 = cannot perform, 1 = performs partially and 2 = performs fully, maximal possible score: 66 points [15]. |
| Grip and pinch strength | A dynamometer is used to measure grip strength and a pinch gauge to measure pinch force. |
| Montreal Cognitive Assessment | The Montreal Cognitive Assessment is a screening assessment for detecting cognitive impairment, a maximum of 30 points (no restrictions) can be achieved [30]. |
| Line Bisection Test | The line bisection test is a test to detect the presence of unilateral spatial neglect. To complete the test, the middle of several horizontal lines is estimated and marked [1, 2]. |
| Bells Test | The Bells test is a cancellation task used for quantitative and qualitative evaluation of visual neglect. Patients are asked to find bells that are distributed pseudo-randomly among distractive stimuli [16]. |
| Aphasia Test | Standardized test for differential diagnosis Aphasia - no aphasia [26]. |
| Apraxia Screen of TULIA | The Apraxia Screen from TULIA is a short assessment to diagnose apraxia with 12 hand movements, dichotomous scale: 0 = not fulfilled, 1 = fulfilled motion task based on the comprehensive standardized Test for Upper-Limp Apraxia (TULIA) [42]. |
| ASKU self-efficacy short form | A 3-items scale for the measurement of self-efficacy [4]. |
Table 2.
Assessments used in the study reflecting the component activities of the International Classification of Functioning, Disability and Health
| Activities | |
|---|---|
| modified Rankin Scale | The modified Rankin scale (mRS) is a standardized measure that describes the extent of disability after a stroke. It ranges from 0 = no symptoms to 6 = death due to stroke [8, 34]. |
| Timed Up and Go Test | The Timed “Up and Go” test is a clinical test to assess a patient’s mobility and risk of falling [32]. |
| Nine Hole Peg Test | The Nine Hole Peg Test is a timed measure of fine manual dexterity where the patient is instructed to first take nine pegs out of a container and subsequently place them back into the empty holes of the container as quickly as possible [29]. |
| Barthel Index | The Barthel Index is an ordinal scale that accounts for the patient’s autonomy and need for care. It covers essential activities of daily living and ranges from 0 (total dependency) to 100 (independent) [37, 28]. |
| Fatigue scale for motor function and cognition | Fatigue scale for motor and cognitive functions, an assessment of fatigue, containing two subscales (mental and physical fatigue), ranging from 20 = no fatigue at all to 100 = severest grade of fatigue [31]. |
| ICHOM-Questionnaire | A standard Set of Patient-reported Outcome Measurements for stroke from The International Consortium for Health Outcomes Measurements including the PROMIS-10 [35]. |
| AUDIT C | The Alcohol Use Disorders Identification Test (AUDIT) alcohol consumption questions. A Screening Test for Problem Drinking [9]. |
| Fagerström Test | A 6-items questionnaire for nicotine dependence [13]. |
Table 3.
Assessments used in the study reflecting the components participation of the International Classification of Functioning, Disability and Health
| Participation | |
|---|---|
| Stroke impact scale (SIS) | Measurement of subjective stroke-specific health status, 64 items in eight domains, domain scores range between 0 and 100, with higher scores represent better health status [11]. Since this questionnaire includes questions about stroke impact in the last 4 weeks, it is applicable starting from the first follow-up after 3 months. |
| Index for measuring restrictions on participation (IMET) | The Index of measurement of participation restrictions (IMET) records patient-related participation as a self-evaluation tool, on a scale from 0 = no impairment to 10 = no more activity possible [10]. |
| Patient Health Questionnaire 4 | The Patient Health Questionnaire 4 is a screening tool for diagnosing depression and includes questions on the nine DSM-IV criteria for the diagnosis of major depression [25]. |
| Patient reported health status (EQ-5D) | The EQ-5D questionnaire is a standardized, generic measure of health-related quality of life. It is a self-administered questionnaire [22, 20]. |
| Return to work | A questionnaire developed by the research group (including questions on occupation and lifestyle). |
| ZAPA | Questionnaire assessing satisfaction with outpatient care with focus on patient participation [36]. |
In addition, medical history is recorded at each examination time point. Questionnaires about health care structure (ZAPA) [36], level of information and information needs and sociodemographic information [18] are included.
Certain data regarding sociodemographic information and clinical characteristics of stroke are collected at the first examination. Questionnaires which are just applicable for patients, which have left the hospital environment (like the SIS) are added to the assessments starting from the first follow-up after 3 months.
If available, magnetic resonance imaging from the acute phase are acquired and stroke volume and location is analyzed using semi-automatic segmentation software.
Contacts
The study is conducted as a multicenter study by the Department of Neurology of the University Medical Center Hamburg-Eppendorf in collaboration with the departments for neurological rehabilitation of RehaCentrum Hamburg, MEDICLIN Klinikum Soltau, Klinikum Bad Bramstedt, VAMED Klinik Geesthacht and VAMED Rehaklinik Damp. The study is funded by the University Medical Center Hamburg-Eppendorf and the Deutsche Rentenversicherung Nord.
Perspective
While stroke rehabilitation research often focuses on the acute treatment phase and the time period of inpatient rehabilitation, we designed a study to investigate the long-term development of stroke patients after discharge from the rehabilitation clinic. Recruitment to the study was started in July 2017. The estimated primary completion date is July 2020.
The data of this study will provide an insight into the long-time course of stroke rehabilitation. The data collected have the potential to identify factors that influence recovery from stroke in the long term. This can be the basis for further treatment improvements in stroke rehabilitation by addressing potentially unknown factors and thus individualize therapy paths.
Besides that, investigating the long-term course of chronic stroke patients will give an important information about the recovery potential of these patients in a standard care setting. Quality of life at the end of the rehabilitation as well as changes in quality of life over 1 year will be investigated. Data on patients’ stroke-related health literacy and information needs over 1 year will be analyzed.
Overall, this study can contribute to a better understanding of the long-term course of stroke patients, thereby help health professionals dealing with stroke with an earlier prognosis and maybe provide data for patient-individualized treatment-protocols in the future.
With this study, the IMPROVE-Platform is established as an interdisciplinary platform for rehabilitation research for stroke patients. This first study addressing the actual practice of neurorehabilitation will be the basis for building new research hypotheses, which than can be tested in future studies within the IMPROVE-Platform. This platform connects experts from research and clinical practice of neurorehabilitation and thereby forms a network to build up future research projects in the area of neurorehabilitation.
Acknowledgements
Not applicable.
Abbreviations
- IMPROVE
The Interdisciplinary Platform for Rehabilitation Research and Innovative Care of Stroke Patients
- ICF
International Classification of Functioning, Disability and Health
- mRS
Modified Rankin Scale
- UKE
University Medical Center Hamburg-Eppendorf
- ICD
International Classification of Diseases
- BAR
Bundesarbeitsgemeinschaft für Rehabilitation
- SIS
The Stroke Impact Scale
- ZAPA
Questionnaire assessing satisfaction with outpatient care with focus on patient participation - “Fragebogen zur Zufriedenheit in der ambulanten Versorgung – Schwerpunkt Patientenbeteiligung (ZAPA)”
- TULIA
Test for Upper-Limb Apraxia
- ASKU
Short scale for the measurement of self-efficacy – “Allgemeine Selbstwirksamkeit Kurzskala”
- ICHOM
The International Consortium for Health Outcomes Measurements
- PROMIS-10
Patient-reported Outcomes Measurements Information System 10-Question Short Form
- AUDIT C
Alcohol Use Disorders Identification Test
- SIS
Stroke impact scale
- IMET
The Index of measurement of participation restrictions
- EQ-5D
European Quality of Life 5 Dimensions
Authors’ contributions
GBi contributed to the planning of the study, acquisition of data and wrote the manuscript; SW and TI contributed to the planning of the study, acquisition of data and revised the manuscript critically for important intellectual content; CB, GBe, AM, AN, KO, OP, MP and JS contributed to acquisition of data and revised the manuscript critically for important intellectual content; GT and CG conceptualized the study, drafted the study design and revised the manuscript critically for important intellectual content; All authors approved the final version and take responsibility for the accuracy and integrity of the study.
Funding
The study is funded by the University Medical Center Hamburg-Eppendorf and the Deutsche Rentenversicherung Nord.
Availability of data and materials
The data will be deposited on a protected server of the University Medical Centre Hamburg-Eppendorf. Access is strongly regulated even for study personnel. Owing to the difficulty of de-identification (routine care, qualitative data, etc.), individual participant data will not be shared publicly. Upon reasonable request that includes a methodologically sound proposal for the usage of data that is also approved by the responsible review committee data may be shared.
Ethics approval and consent to participate
The study is carried out following the Helsinki Declaration of the World Medical Association and according to the principles of good clinical and good scientific practice. Study participation is voluntary and can be withdrawn at any time. Written informed consent will be obtained prior to participation, if applicable. Patients will be fully informed about aims, procedures, data collection and the use of collected data in this study. Rejecting participation has no negative consequences for patients. No foreseeable risk results from participating. No compassionate use is carried out. No invasive intervention is conducted. This study is registered at clinicaltrial.gov (NCT04119479). Principles of data protection will be kept. Approval of the local ethics committee (ethical boards of the medical associations Hamburg, Schleswig-Holstein, Niedersachsen) has been obtained (Approval: PV5483). The results of this study will be disseminated via peer-reviewed journals. Deviations from protocol, i.e. inclusion, recruitment and procedure will be discussed.
Consent for publication
Not applicable.
Competing interests
GBi, SW, TI, CB, Gbe, AM, AN, KO, OP, MP, JS have no conflict of interest. CG reports personal fees from Amgen, Bayer Vital, Bristol-Myers Squibb, Boehringer Ingelheim, Sanofi Aventis, Abbott, and Prediction Biosciences outside the submitted work. GT reports receiving consulting fees from Acandis, grant support, and lecture fees from Bayer, lecture fees from Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, and Daiichi Sankyo, and consulting fees and lecture fees from Stryker outside the submitted work.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Azouvi P, Bartolomeo P, Beis J-M, Perennou D, Pradat-Diehl P, Rousseaux M. A battery of tests for the quantitative assessment of unilateral neglect. Restorative Neurology and Neuroscience. 2006;24(4–6):273–285. [PubMed] [Google Scholar]
- 2.Azouvi P, Samuel C, Louis-Dreyfus A, Bernati T, Bartolomeo P, Beis JM, et al. Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;73(2):160–166. doi: 10.1136/jnnp.73.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.BAR . Arbeitshilfe für die Rehabilitation von Schlaganfallpatienten. Schriftenreihe der Bundesarbeitsgemeinschaft für Rehabilitation (Heft 4) 1998. [Google Scholar]
- 4.Beierlein C, Kovaleva A, Kemper CJ, Rammstedt B. ASKU - Allgemeine Selbstwirksamkeit Kurzskala. With assistance of Leibniz Institut für Psychologische Information und Dokumentation (ZPID) 2012. [Google Scholar]
- 5.Bernhardt J, Borschmann K, Boyd L, Thomas Carmichael S, Corbett D, Cramer SC, et al. Moving rehabilitation research forward: Developing consensus statements for rehabilitation and recovery research. International journal of stroke : official journal of the International Stroke Society. 2016;11(4):454–458. doi: 10.1177/1747493016643851. [DOI] [PubMed] [Google Scholar]
- 6.Boyd LA, Hayward KS, Ward NS, Stinear CM, Rosso C, Fisher RJ, et al. Biomarkers of stroke recovery: Consensus-based Core recommendations from the stroke recovery and rehabilitation roundtable. Neurorehabilitation and Neural Repair. 2017;31(10–11):864–876. doi: 10.1177/1545968317732680. [DOI] [PubMed] [Google Scholar]
- 7.Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke. 1989;20(7):864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 8.Bruno A, Shah N, Lin C, Close B, Hess DC, Davis K, et al. Improving modified Rankin scale assessment with a simplified questionnaire. Stroke. 2010;41(5):1048–1050. doi: 10.1161/STROKEAHA.109.571562. [DOI] [PubMed] [Google Scholar]
- 9.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Ambulatory care quality improvement project (ACQUIP). Alcohol Use Disorders Identification Test. Archives of Internal Medicine. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 10.Deck R, Mittag O, Hüppe A, Muche-Borowski C, Raspe H. Index zur Messung von Einschränkungen der Teilhabe (IMET) - Erste Ergebnisse eines ICF-orientierten Assessmentinstruments. 2007. pp. 113–120. [Google Scholar]
- 11.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30(10):2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 12.Dworzynski K, Ritchie G, Playford ED. Stroke rehabilitation: Long-term rehabilitation after stroke. Clinical Medicine. 2015;15(5):461–464. doi: 10.7861/clinmedicine.15-5-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerstrom test for cigarette dependence. Nicotine & Tobacco Research : official journal of the Society for Research on Nicotine and Tobacco. 2012;14(1):75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- 14.Foerch C, Misselwitz B, Sitzer M, Steinmetz H, Neumann-Haefelin T. The projected burden of stroke in the German federal state of Hesse up to the year 2050. Deutsches Arzteblatt International. 2008;105(26):467–473. doi: 10.3238/arztebl.2008.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scandinavian Journal of Rehabilitation Medicine. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 16.Gauthier L, Dehaut F, Joanette Y. The bells test: a quantitative and qualitative test for visual neglect. 1989. [Google Scholar]
- 17.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: A report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanefeld U, Hoffmeyer-Zlotnik JHP. Demographische Standards. Ausgabe 2010. 5., überarb. und erw. Aufl. Wiesbaden: Statistisches Bundesamt (Statistik und Wissenschaft, 17); 2010. [Google Scholar]
- 19.Hempler I, Woitha K, Thielhorn U, Farin E. Post-stroke care after medical rehabilitation in Germany: a systematic literature review of the current provision of stroke patients. BMC Health Services Research. 2018;18(1):468. doi: 10.1186/s12913-018-3235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heuschmann P, Busse O, Wagner M, Endres M, Villringer A, Röther J, et al. Schlaganfallhäufigkeit und Versorgung von Schlaganfallpatienten in Deutschland. Akt Neurol. 2010;37(07):333–340. doi: 10.1055/s-0030-1248611. [DOI] [Google Scholar]
- 22.Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: A multi-country study. Quality of Life Research : An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation. 2013;22(7):1717–1727. doi: 10.1007/s11136-012-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotseva K, Gerlier L, Sidelnikov E, Kutikova L, Lamotte M, Amarenco P, Annemans L. Patient and caregiver productivity loss and indirect costs associated with cardiovascular events in Europe. European Journal of Preventive Cardiology. 2019;26(11):1150–1157. doi: 10.1177/2047487319834770. [DOI] [PubMed] [Google Scholar]
- 24.Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: Findings from the global burden of disease study 2010. The Lancet Global Health. 2013;1(5):e259–e281. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroenke, K., Spitzer, R. L., Williams, J. B. W., & Löwe, B. (2009). An ultra-brief screening scale for anxiety and depression: The PHQ-4. Psychosomatics, 50(6), 613–621. 10.1016/S0033-3182(09)70864-3. [DOI] [PubMed]
- 26.Kroker C. Aphasie-Schnelltest (AST). Ein standardisierter Test für die Differentialdiagnose ; Aphasie - keine Aphasie - Dysarthrie in der Akutphase. 3. Idstein: Schulz-Kirchner (Das Gesundheitsforum); 2006. [Google Scholar]
- 27.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: A systematic review. The Lancet Neurology. 2009;8(8):741–754. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 28.Mahoney F, Barthel DW. Functional evaluation: The Bathel index. A simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Maryland State Medical Journal. 1965;14:61–65. [PubMed] [Google Scholar]
- 29.Mathiowetz V, Weber K, Kashman N, Volland G. Adult norms for the nine hole peg test of finger dexterity. The Occupational Therapy Journal of Research. 1985;5(1):24–38. doi: 10.1177/153944928500500102. [DOI] [Google Scholar]
- 30.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 31.Penner IK, Raselli C, Stöcklin M, Opwis K, Kappos L, Calabrese P. The fatigue scale for motor and cognitive functions (FSMC): Validation of a new instrument to assess multiple sclerosis-related fatigue. Multiple sclerosis (Houndmills, Basingstoke, England) 2009;15(12):1509–1517. doi: 10.1177/1352458509348519. [DOI] [PubMed] [Google Scholar]
- 32.Podsiadlo D, Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 33.Pollock A, George S, Bridget, Fenton M, Firkins L. Top 10 research priorities relating to life after stroke--consensus from stroke survivors, caregivers, and health professionals. International Journal of Stroke: Official Journal of the International Stroke Society. 2014;9(3):313–320. doi: 10.1111/j.1747-4949.2012.00942.x. [DOI] [PubMed] [Google Scholar]
- 34.Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scottish Medical Journal. 1957;2(5):200–215. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- 35.Salinas J, Sprinkhuizen SM, Ackerson T, Bernhardt J, Davie C, George MG, et al. An international standard set of patient-centered outcome measures after stroke. Stroke. 2016;47(1):180–186. doi: 10.1161/STROKEAHA.115.010898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scholl, I., Hölzel, L., Härter, M., Dierks, M.-L., Bitzer, E.-M., & Kriston, L. (2011). Fragebogen zur Zufriedenheit in der ambulanten Versorgung – Schwerpunkt Patientenbeteiligung (ZAPA). Klin. Diagnostik u. Evaluation, 4(1), 50–62.
- 37.Schönle PW. Der Frühreha-Barthelindex (FRB)--eine frührehabilitationsorientierte Erweiterung des Barthelindex. Die Rehabilitation. 1995;34(2):69–73. [PubMed] [Google Scholar]
- 38.Silva SM, Corrêa FI, Faria CDC d M, Buchalla CM, Silva PF d C, Corrêa JCF. Evaluation of post-stroke functionality based on the international classification of functioning, disability, and health: A proposal for use of assessment tools. Journal of Physical Therapy Science. 2015;27(6):1665–1670. doi: 10.1589/jpts.27.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stinear, C. M. (2016). Stroke rehabilitation research needs to be different to make a difference. F1000Research, 5. 10.12688/f1000research.8722.1. [DOI] [PMC free article] [PubMed]
- 40.Teasell RW, Fernandez M, Manuel, McIntyre A, Mehta S. Rethinking the continuum of stroke rehabilitation. Archives of Physical Medicine and Rehabilitation. 2014;95(4):595–596. doi: 10.1016/j.apmr.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Ullberg T, Zia E, Petersson J, Norrving B. Perceived unmet rehabilitation needs 1 year after stroke: An observational study from the Swedish stroke register. Stroke. 2016;47(2):539–541. doi: 10.1161/STROKEAHA.115.011670. [DOI] [PubMed] [Google Scholar]
- 42.Vanbellingen T, Kersten B, van Hemelrijk B, van de Winckel A, Bertschi M, Müri R, et al. Comprehensive assessment of gesture production: A new test of upper limb apraxia (TULIA) European Journal of Neurology. 2010;17(1):59–66. doi: 10.1111/j.1468-1331.2009.02741.x. [DOI] [PubMed] [Google Scholar]
- 43.Warlow, C. (2008). Stroke. Practical management (3rd ed.). Malden: Blackwell Pub Print ISBN:9781405127660.
- 44.WHO . The international classification of functioning, disability and health. Geneva: World Health Organization; 2001. [Google Scholar]
- 45.WHO. (2014). Global status report on noncommunicable diseases 2014. Geneva: World Health Organization Available online at https://www.who.int/nmh/publications/ncd-status-report-2014/en/.
- 46.Winovich DT, Longstreth WT, Arnold AM, Varadhan R, Al Hazzouri Z, Adina, Cushman M, et al. Factors associated with ischemic stroke survival and recovery in older adults. Stroke. 2017;48(7):1818–1826. doi: 10.1161/STROKEAHA.117.016726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be deposited on a protected server of the University Medical Centre Hamburg-Eppendorf. Access is strongly regulated even for study personnel. Owing to the difficulty of de-identification (routine care, qualitative data, etc.), individual participant data will not be shared publicly. Upon reasonable request that includes a methodologically sound proposal for the usage of data that is also approved by the responsible review committee data may be shared.