Abstract
Mesenchymal stem cells (MSCs) are promising for cartilage regeneration, but readily undergo terminal differentiation. The aim of this study was two-fold: a) investigate physiochemical cues from a cartilage-mimetic hydrogel under dynamic compressive loading on MSC chondrogenesis and hypertrophy and b) identify whether Smad signaling and p38 MAPK signaling mediate hypertrophy during MSC chondrogenesis. Human MSCs were encapsulated in photoclickable poly(ethylene glycol) hydrogels containing chondroitin sulfate and RGD, cultured under dynamic compressive loading or free swelling for three weeks, and evaluated by qPCR and immunohistochemistry. Loading inhibited hypertrophy in the cartilage-mimetic hydrogel indicated by a reduction in pSmad 1/5/8, Runx2, and collagen X proteins, while maintaining chondrogenesis by pSmad 2/3 and collagen II proteins. Inhibiting pSmad 1/5/8 under free swelling culture significantly reduced collagen X protein, similar to the loading condition. Chondroitin sulfate was necessary for load-inhibited hypertrophy and correlated with enhanced S100A4 expression, which is downstream of the osmotic responsive transcription factor NFAT5. Inhibiting p38 MAPK under loading reduced S100A4 expression, and upregulated Runx2 and collagen X protein. Findings from this study indicate that chondroitin sulfate with dynamic loading create physiochemical cues that support MSC chondrogenesis and attenuate hypertrophy through Smad 1/5/8 inhibition and p38 MAPK upregulation.
Keywords: Mesenchymal stem cells, chondrogenesis, hypertrophy, chondroitin sulfate, hydrogel, dynamic loading
1. Introduction
Human bone-marrow derived mesenchymal stem cells (hMSCs) are a promising cell source for articular cartilage tissue engineering due to their proliferation capacity and chondrogenic potential. Although promising, MSCs are known to undergo endochondral ossification in vivo [1]. This process occurs during development through condensation of MSCs and initiation of chondrogenic differentiation, but which is followed by terminal differentiation, hypertrophy, and eventually formation of bone [2]. During hypertrophy, the collagen II-rich cartilage extracellular matrix (ECM) is replaced by collagen X, a protein that binds calcium and induces mineralization of the cartilage matrix [3]. This process raises concern for the clinical translation of MSCs, leading to inferior cartilage repair and eventually apoptosis, vascularization, and ossification.
One promising strategy to improve cartilage tissue engineering with MSCs is to exploit their surrounding environment by recapitulating aspects of native cartilage within a scaffold. Hyaline cartilage is comprised predominantly of collagen II fibers and aggrecan monomers that aggregate along strands of hyaluronic acid. Aggrecan is made up of many chains of sulfated glycosaminoglycans, including chondroitin sulfate, which is responsible for the high fixed charged density in cartilage. Analogs of these ECM molecules, such hyaluronic acid and chondroitin sulfate, have been modified to enable their incorporation into MSC-laden hydrogels and shown to enhance chondrogenesis over purely synthetic hydrogels [4–11]. Hydrogels made from a combination of collagen II and hyaluronic acid have also been shown to support chondrogenesis of MSCs [12]. The cell adhesion peptide RGD, which is found in fibronectin and critical to initial stages of chondrogenesis [13–16], has been added into hydrogels either at low concentrations [17] or through degradable tethers [16] and shown to enhance cartilage ECM deposition by MSCs. However, maintaining a stable chondrogenic phenotype that prevents hypertrophy remains a challenge. Studies from our group and others have reported terminal differentiation and hypertrophy of chondrogenically differentiating MSCs encapsulated in hydrogels, evidenced by the co-expression of collagen II and collagen X proteins [7,10,18–20].
A few studies have reported that combinations of ECM analogs and/or mechanical stimulation can reduce expression of hypertrophic markers. For example, introducing chondroitin sulfate or sulfate groups into a hyaluronic acid hydrogel led to decreased expression of hypertrophic markers collagen X and matrix metalloproteinase (MMP) 13, and reduced calcium deposition by MSCs [21,22]. Dynamic compressive loading of a hyaluronic acid hydrogel led to higher collagen and GAG contents, while reducing the expression of hypertrophic markers in MSCs [23] and in vitro pre-conditioning of a hyaluronic acid hydrogel with dynamic loading prior to implantation improved cartilage repair in a rat osteochondral defect [24]. Additionally, cyclic hydrostatic pressure has been reported to reduce hypertrophic markers of MSCs embedded in an agarose hydrogel without the need for ECM analogs [25]. Studies from our group have reported that dynamic compressive loading of MSCs encapsulated in a synthetic poly(ethylene glycol) (PEG) hydrogel containing chondroitin sulfate and RGD inhibited collagen X protein expression, but in a strain rate dependent manner [26]. Collectively, these and other studies indicate that chemical and/or mechanical cues are important regulators of MSC hypertrophy.
Understanding the mechanisms that mediate hypertrophy during MSC chondrogenesis has been the focus of intense research [27]. The Smad pathway has been identified as one such signaling pathway that is critical in chondrogenesis [28–32]. Specifically, Smad2/3 signaling is necessary for the expression of the transcription factor Sox9, a master regulator of chondrogenesis that controls collagen II expression [33,34] and is also thought to be important in preventing hypertrophy [35,36]. In contrast, Smad1/5/8 signaling appears to be necessary in early chondrogenesis during development [37] and during chondrogenesis of adult MSCs [38]. However, its long-term expression has been linked to hypertrophic markers and endochondral ossification [38–41]. In vitro chondrogenesis is induced by TGF-β through activation of Smad2/3 signaling [42,43], but TGF-β is also a potent enhancer of Smad1/5/8 signaling [44,45]. Thus, it is not surprising that in vitro studies report a hypertrophic phenotype during MSC chondrogenesis with TGF-β [10,21,46]. In articular cartilage explants with phenotypically stable chondrocytes, phosphorylated (i.e., active) Smad2/3 was observed while phosphorylated Smad1/5/8 was not detected [47]. These studies suggest that a stable chondrogenic phenotype is achieved when Smad1/5/8 is turned off [38]. Questions remain regarding the regulation of Smad2/3 and Smad1/5/8 signaling and whether cues from the local environment may be important in this regulation.
One intracellular signaling molecule that is involved in translating extracellular stimuli, such as mechanical forces, osmolarity, and growth factors, from the local environment into cellular responses is p38 mitogen-activated protein kinase (MAPK) [48,49]. P38 MAPK has been shown to be a positive regulator of MSC chondrogenesis [50,51]. For example, inhibiting p38 MAPK in MSCs downregulated expression of the chondrogenic markers Sox9, collagen II and aggrecan, but enhanced expression of Runx2 [52], an osteogenic transcription factor linked to hypertrophy [53]. There is evidence of crosstalk between p38 MAPK and Smad signaling, whereby inhibiting p38 MAPK led to a decrease in phosphorylated Smad2/3 and downregulated chondrogenic genes [28]. Thus, p38 MAPK may be one mechanism involved in the translation of external stimuli to affect Smad signaling and ultimately chondrogenesis.
This study hypothesized that physiochemical cues within a cartilage-mimetic hydrogel subjected to dynamic compressive loading inhibit MSC hypertrophy via Smad signaling and p38 MAPK signaling. We utilized our cartilage-mimetic hydrogel [26] containing crosslinked PEG as the base chemistry and to which ECM analogs of chondroitin sulfate and RGD are incorporated (Fig. 1A). Herein, the hydrogel was designed from the facile photoclickable thiol:norbornene reaction [54,55]. To test this hypothesis, we performed three studies (Fig. 1B). We first confirmed that MSCs encapsulated in cartilage-mimetic hydrogel, resulted in inhibition of MSC hypertrophy under loading [26]. Next, the role of Smad1/5/8 signaling in mediating load-inhibited MSC hypertrophy was investigated. Finally, the importance of chondroitin sulfate including tis role in hyperosmolarity and p38 MAPK signaling was investigated. Overall findings from this study provide evidence that physiochemical cues that mimic the native cartilage environment, notably chondroitin sulfate coupled with dynamic compression, are able to inhibit MSC hypertrophy and support a stable chondrogenic phenotype through SMAD 1/5/8 inhibition and p38 MAPK upregulation.
Figure 1.

A. Schematic of the formation of the cartilage-mimetic hydrogel using the photoclickable thiol-norbornene reaction between multi-arm norbornene functionalized monomers and thiol containing crosslinkers and extracellular matrix mimetics of chondroitin sulfate and RGD. B. Experimental design of the three studies performed in this work.
2. Materials and Methods
2.1. Macromer Synthesis
The monomer, 8-arm PEG functionalized with norbornene (PEG-NB), was synthesized as described previously [56]. Briefly, PEG-amine (8-arm, 10kDA) was dissolved in dimethylformamide (DMF) and reacted with 5-norbornene-2-carboxylic acid at 4 molar excess with n,n-diisopropylethylamine (DIEA) and 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolog[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU) under argon, overnight, and at room temperature. The product was precipitated in cold diethyl ether and vacuum filtered, purified via dialysis, and recovered by lyophilization. The percent conjugation of norbornene to each arm of the 8-arm PEG was determined to be ~100% by 1HNMR by comparing the area under the peak for the allylic hydrogen closest to the norbornene hydrocarbon group (δ=3.1 – 3.2 ppm) to the area under the peak representing the methyl groups in the PEG backbone (δ=3.4 – 3.85 ppm).
Thiolated chondroitin sulfate (ChS-SH) was synthesized following methods described previously [6]. Briefly, chondroitin sulfate (ChS, Sigma Aldrich) was dissolved in water, with excess dithiobis(propanoic dihydrazide) (DTP) (2 moles DTP: 1 mole ChS repeat unit). The pH was adjusted to 4.75 by the addition of 1 M HCl. Excess 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDCI) (2 moles EDCI: 1 mole ChS repeat unit) was added to the ChS and DTP solution and reacted overnight. The reaction was stopped by raising the pH to 7 with the addition of 1 M NaOH. Excess of dithiothreitol (DTT) (6.5 moles DTT: 1 mole ChS repeat unit) was added to the solution and the pH adjusted to 8.8 with 1 M NaOH. The reaction was carried out for 24 hours to reduce the thiols of the DTP. The final product (ChS-SH) was purified and recovered by dialysis against 0.3 mM HCl, centrifuged to remove any particulates and the supernatant was lyophilized. Conjugation of thiol groups to ChS was determined to be 15% by 1HNMR by comparing the area under the peak for the two side chain methylenes of DTP (δ=2.5 – 2.6 and 2.6 – 2.8 ppm) to the area under the peak representing the methyl protons of the acetyl amine side chain (δ=1.8–2.0 ppm), indicating that there are approximately 7 thiol groups per molecule of ChS assuming a molecular weight of 22kDa [57].
2.2. Mesenchymal Stem Cell (MSC) Culture
Adult human mesenchymal stem cells (hMSCs) (donor 1: 24 year old female, donor 2: 24 year old female, donor 3: 25 year old female) were purchased at passage 1 from Texas A&M Cell Distribution Center. The hMSCs were expanded in growth media (20% fetal bovine serum (FBS, Atlanta Biologicals), 50 U ml−1 penicillin, 50 mg ml−1 streptomycin, 20 mg ml−1 gentamicin, and 5 ng ml−1 fibroblast growth factor-basic (bFGF) (Invitrogen) in low glucose Dulbecco’s modified Eagle media (DMEM, Invitrogen). The hMSCs were seeded at 3000 cells cm−2 and grown under standard culture conditions (37°C with 5% CO2 in a humid environment) to 80% confluency and passaged. hMSCs at passage three were used in all studies.
2.3. Hydrogel Formation and MSC Encapsulation
Hydrogels were fabricated via photopolymerization from a precursor solution. The cartilage-mimetic hydrogels were prepared from a precursor solution of 9% (g/g) PEG-NB, 1% (g/g) ChS-SH, 0.1 mM CRGDS (Genscript), 2.14 (g/g) PEG dithiol (1kDa, Sigma-Aldrich). Hydrogels were also formed without ChS (referred to as -ChS condition) from a precursor solution of 9% (g/g) PEG-NB, 0.1 mM CRGDS, 2.67% (g/g) PEG dithiol (1kDa). All precursor solutions were sterile-filtered (0.22 μm filter) and polymerized with 0.05% (g/g) photoinitiator Irgacure 2959 (I2959, BASF) in phosphate buffered saline (PBS, pH 7.4) with 352 nm light at 5 mW cm−2 for 8 minutes. hMSCs were encapsulated in hydrogels by combining 10 million cells per ml of precursor solution followed by photopolymerization. The hMSC-laden constructs (5mm in diameter and 2mm in height) were cultured in chondrogenic differentiation medium of 1% ITS+ Premix, 100 nM dexamethasone, 2.5 ng ml−1 TGF-β3, 50 μg ml−1 l-ascorbic acid 2-phosphate, 50 U ml−1 penicillin, 50 mg ml−1 streptomycin, and 10 mg ml−1 gentamicin in high glucose Dulbecco’s modified Eagle media. The constructs were cultured under standard culture conditions (37°C with 5% CO2 in a humid environment). A lower concentration of TGF-β3 was used to slow chondrogenesis and enable the effects of the hydrogel and dynamic mechanical loading environments to be investigated on chondrogenesis [10,26].
2.4. Acellular Hydrogel Characterization
Acellular hydrogels were characterized to determine the introduction of fixed negative charged into the hydrogel by incorporating ChS. These hydrogels were formed without RGD. PEG hydrogels were formed from a precursor solution of 9% (g/g) PEG-NB and 2.67% (g/g) PEG dithiol (1kDa). PEG hydrogels with ChS (PEG+ChS Hydrogel) were formed from a precursor solution of 9% (g/g) PEG-NB, 1% (g/g) ChS-SH, and 2.4 (g/g) PEG dithiol (1kDa).
The hydrogels were swollen to equilibrium in PBS for 24 hours. The incorporation of ChS-SH into the hydrogel was confirmed using toluidine blue, which stains negatively charged glycosaminoglycans. PEG and PEG+ChS hydrogels were stained with toluidine blue solution (0.1% toluidine blue, 7% ethanol, 0.1% NaCl in PBS, pH<2.5) for 24 hours, rinsed for 48 hours in deionized H2O (diH2O) and imaged using light microscopy (Zeiss Pascal, Olympus DP70 100x magnification). The percent water at equilibrium was also determined for the cartilage mimetic hydrogels when swollen to equilibrium in either diH2O (n=10) or PBS (n=10) for 24 hours. At which time, the swollen mass was measured. After mass measurements were taken, all hydrogels were placed in diH2O for two additional days, which was changed three times per day for two days. This process was done to remove salts from the PBS. The hydrogels were then lyophilized, and the dry polymer mass was measured. The fixed charged density in mEq per volume of solvent in the hydrogel was estimated from the amount of ChS incorporated into the hydrogel, assuming an average molecular weight of ChS as ~48,700 g/mol [5], and knowing there are two moles of equivalent charge per repeat unit.
2.5. Experimental Conditions
Three studies were performed (Fig. 1B). In all studies, the hMSC-laden hydrogels were cultured for one week under free swelling conditions. At which time, one set of hydrogels was continued under free swelling conditions while the remainder was placed in a custom-built bioreactor [58,59] for an additional two weeks. In the bioreactor, the hydrogels were subjected to intermittent unconfined dynamic compressive strain applied daily for 1 hour at 5% peak-to-peak strain (2.5% amplitude strain) and 1Hz in a sinusoidal waveform followed by 23 hours of rest at a minimal tare strain of 0.5%. Hydrogels were cultured individually in wells of a 24 well plate with two milliliters of chondrogenic medium per well. The medium was replaced every other day for the duration of the study. Study 1 (donor 1) used the cartilage-mimetic PEG hydrogels with and without dynamic loading. Study 2 (donor 2) used the cartilage-mimetic PEG hydrogels and the small molecule dorsomorphin (Biomol International) that inhibits the kinase domains of ALK 1,2, 3, and 6. Dorsomorphin (10 μM [38]) was added fresh to the culture medium during each medium exchange from days 7 to 21 for a subset of hydrogels in the free swelling condition. Study 3 (donor 3) used the cartilage-mimetic and hydrogels without ChS and the small molecule SB203580 (Sigma Aldrich), which inhibits p38 MAP Kinase. SB203580 (3 μM [52]) was added fresh to the culture medium during each medium exchange from days 7 to 21 for a subset of hydrogels in the free swelling and loading conditions.
2.6. Gene Expression by qPCR
At prescribed time points, hMSC-laden hydrogels (n=3) were removed from culture and homogenized (TissueLyzer II, Qiagen) at 30Hz for 10 minutes in RNA lysis buffer. RNA was extracted from the hydrogels using MicroElute Total RNA Kit per manufacturer (Omega). RNA was transcribed to cDNA using a high capacity reverse transcription kit per manufacturer (Applied Biosystems). Quantitative PCR (qPCR) was performed with Fast SYBR Green Master Mix (Applied Biosystems) and a 7500 Fast Real-time PCR Machine (Applied Biosystems). Gene expression data are reported as the relative expression of the gene of interest (GOI) to the housekeeping gene (HKG) L30 and calculated from delta Ct values using the true PCR efficiencies (E) [60]. Relative expression (RE) was determined by
Normalized gene expression where the control was pre-encapsulated hMSCs was determined by
ΔCt is the difference in Ct values between the control, as defined, and the sample. PCR efficiency (E) for each set of primers was determined from serial dilutions of cDNA and the slope between Ct values of 15–25 following methods described in [60]. The genes of interest (GOI) included SOX9, RUNX2, ACAN, COL2A1, COL10A1, NFAT5, and S100A4 and the housekeeping gene (HKG) was L30. Their primer sequence and efficiency are given in Table 1.
Table 1.
Primer Sequences and Efficiency for qPCR Analysis
| Gene | Forward Sequence | Reverse Sequence | Efficiency |
|---|---|---|---|
| L30 | 5’-TTAGCGGCTGCTGTTGGTT-3’ | 5’-TCCAGCGACTTTTTCGTCTTC-3’ | 94% |
| SOX9 | 5’-TGACCTATCCAAGCGCATTACCA-3’ | 5’-ATCATCCTCCACGCTTGCTCTGAA-3’ | 95% |
| ACAN | 5’-AGTATCATCGTCCCAGAATCTAGCA-3’ | 5’-AATGCAGAGGTGGTTTCACTCA-3’ | 88% |
| COL2A1 | 5’-CAACACTGCCAACGTCCAGAT-3’ | 5’-TCTTGCAGTGGTAGGTGATGTTCT-3’ | 102% |
| RUNX2 | 5’-TTGGCCTGGTGGTGTCATTA-3’ | 5’-GAGTCCTTCTGTGGCATGCA-3’ | 98% |
| COL10A1 | 5’-TTTTGCTGCTAGTATCCTTGAACT-3’ | 5’-ACCTCTAGGGCCAGAAGGAC-3’ | 87% |
| NFAT5 | 5’-GGGTCAAACGACGAGATTGTG-3’ | 5’-TTGTCCGTGGTAAGCTGAGAA-3’ | 101% |
| S100A4 | 5’-GTCCACCTTCCACAAGTACTCG-3’ | 5’-TCATCTGTCCTTTTCCCAAG-3’ | 98% |
2.7. Immunohistochemistry (IHC)
At prescribed time points, hMSC-laden hydrogels (n=3) were removed from culture and processed for IHC. hMSC-laden hydrogels were fixed overnight at 4°C in 4% paraformaldehyde and transferred to 30% sucrose solution for storage at 4°C. Hydrogels were dehydrated following standard methods, embedded in paraffin, and sectioned (10 μm). For certain primary antibodies, sections were pre-treated with enzyme as follows: 2000 U ml−1 hyaluronidase for anti-collagen II, 1 mg ml−1 protease followed by 1 mg ml−1 pepsin, and lastly 0.25% trypsin in 1mM EDTA for anti-collagen X, antigen retrieval followed by chondroitinase ABC (100 mU ml−1) and keratinase I (1 U ml−1) followed by hyaluronidase (2000 U ml−1) for anti-aggrecan, and no enzyme pre-treatment was used for anti-Runx2. Anti-pSmad2/3 and anti-pSmad1/5/8 were pretreated with Retrievagen A (BD Pharmingen) for antigen retrieval. Following permeabilization and blocking, sections were treated overnight at 4°C with the primary antibody: 1:50 anti-collagen II (US Biological, C7510–20F), 1:5 anti-aggrecan (US Biological, A1059–53F), 1:50 anti-collagen X (Abcam, ab49945), 1:50 anti-Runx2 (Abcam, ab23981), and 1:50 anti-pSmad2/3 (Santa Cruz Biotechnology, sc-11769) and anti-pSmad1/5/8 (Santa Cruz Biotechnology, sc-12353) in blocking solution. Sections were subsequently treated for two hours with goat anti-mouse IgG or goat anti-rabbit IgG labelled Alexa Fluor 488 (1:100) or Alexa Flour 546 (1:100) and the nuclei counterstained with DAPI.
Select conditions were quantified either by the fraction of cells that stained positive for a given protein or by the relative intensity. Representative confocal microscopy images (n=4 images per hydrogel, n=4 hydrogels per condition) were selected and processed using NIH ImageJ. For each image, the total number of nuclei was counted as an indication of cell number. For collagen II and collagen X proteins, the intensity threshold was adjusted using ImageJ to remove background fluorescence. The number of nuclei that were associated with positive staining for the protein was counted. Data are reported as a fraction of positively stained cells (i.e., positive cells/total number of cells) for a given protein. For pSmad2/3 and pSmad1/5/8 proteins, the total intensity of the stain per image was determined. Using ImageJ, the fluorescent channels were split, and the channel representing the protein was evaluated for intensity by measuring the average intensity of the image and subtracting the background intensity (measured in an area where no cells are present) for each image. The intensity was divided by the number of nuclei in the image (DAPI) and the data are reported as average intensity per nuclei.
2.8. Statistical Analysis
Data are presented as mean with standard deviation parenthetically in the text or as error bars in the figures. Minitab 17, IBM SPSS, and Real-Statistics add-in for Excel were used for statistical analysis. A student’s unpaired t-test was used to determine differences in the water content of acellular hydrogel as a function of swelling solvent. For study 1, differences in gene expression and quantitative IHC for culture condition (free swelling and loading) for a given gene or protein under a given culture condition were assessed by an unpaired t-test. For study 2, quantitative IHC analysis was analyzed using a two-way ANOVA (α=0.05) with culture time (days 7, 14 and 21) and culture condition (free swelling, loading, and free swelling+dorsormorphin) as factors. For study 3, differences in gene expression were analyzed using a 3-way ANOVA with hydrogel (cartilage-mimetic hydrogel +/−ChS), culture condition (free swelling and loading) and treatment (+/− SB203580) as factors. If two or more two-way interactions or three-way interaction were statistically significant, follow-up tests two-way ANOVA (α=0.05) tests were performed. For the day 7 time point with only +/− ChS, the data were analyzed using an unpaired t-test. A Tukey’s post-hoc test was used to test pair-wise comparisons. Statistical significance was determined as p<0.05.
3. Results
3.1. Characterization of cartilage-mimetic hydrogels
The incorporation of covalently linked chondroitin sulfate (via thiolated ChS) into the PEG hydrogel was confirmed in hydrogels formed via photopolymerization, but without CRGDS. After equilibrium swelling and allowing diffusion of any unreacted thiolated ChS, the presence of ChS was confirmed by toluidine blue, which stains negatively charged molecules (Fig. 2A). No staining was observed in the PEG hydrogel (Fig. 2A). The fixed charge density was estimated to be 0.0026 (0.0001) mEq/ml based on the amount of ChS that was incorporated into the hydrogel during hydrogel formation. While these hydrogels are predominantly water with >93% by mass water, the amount of water imbibed in the hydrogel at equilibrium was slightly, but significantly (p=0.042), higher when swollen in deionized water compared to a saline solution of PBS, where the fixed negative charges are shielded (Fig. 2B). The later reduces the charge-induced effects on swelling.
Figure 2.
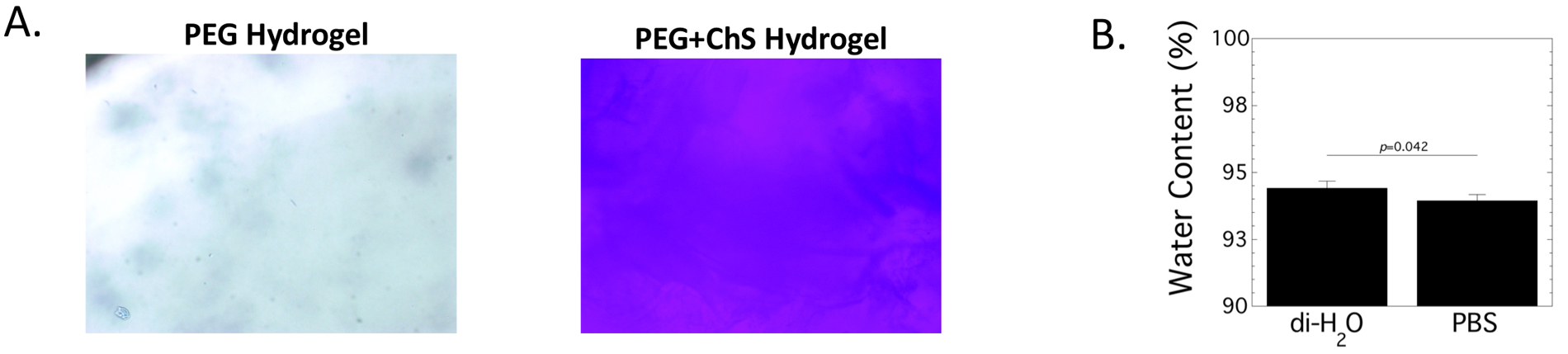
Characterization of incorporated thiolated-chondroitin sulfate (ChS) into the PEG hydrogel. A. The incorporation of ChS was confirmed by toluidine blue which stains negatively charged molecules blue. Brightfield microscopy images of PEG only and PEG-ChS hydrogels are shown (100x magnification). B. The water content of PEG-ChS hydrogels swollen in diH2O or phosphate buffered saline (PBS) for 24 hours. Data are reported as mean with standard deviation as error bar (n=10).
3.2. Assessment of chondrogenesis of hMSCs in the cartilage-mimetic hydrogel under dynamic loading
Chondrogenesis in the cartilage-mimetic hydrogel was evaluated at day 21 by gene expression and IHC (Fig. 3) in Study 1 (Fig. 1B). At the gene level, SOX9, ACAN and COL2A1 were evaluated as markers for chondrogenesis and RUNX2 and COL10A1 were evaluated as markers for hypertrophy. Gene expression for each gene was normalized to a control of pre-encapsulated hMSCs (Fig. 3A). SOX9, ACAN, RUNX2 and COL10A1 levels were not affected by dynamic loading. Dynamic loading led to a reduction (p<0.001) in COL2A1 levels, but its expression remained above the control.
Figure 3.
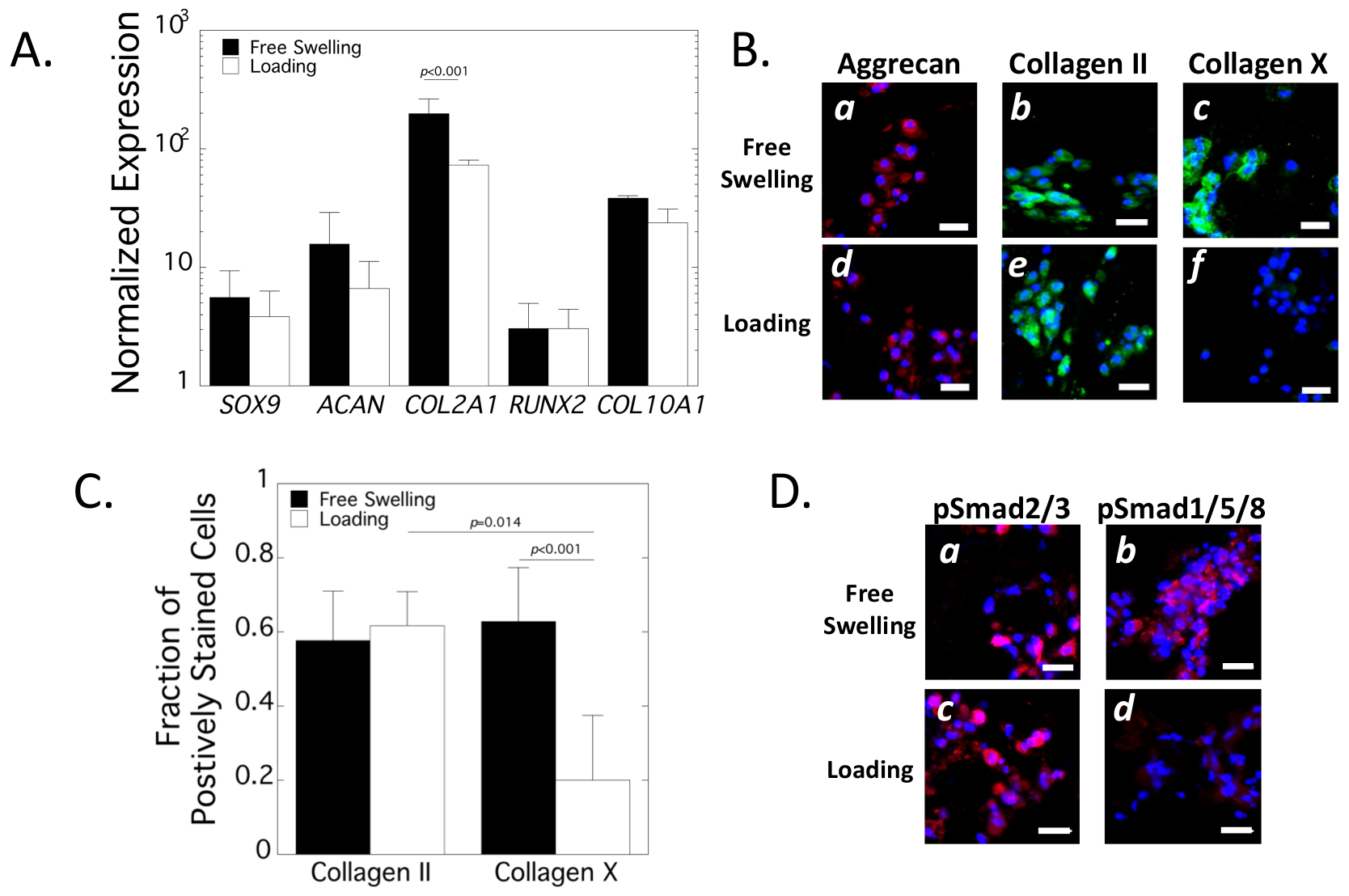
Assessment of chondrogenesis in the cartilage-mimetic hydrogels under loading. A. Normalized gene expression of encapsulated hMSCs at day 21 of free swelling (black bars) and loading (white bars) culture conditions. Relative expression was normalized to pre-encapsulated hMSCs. Data are mean with standard deviation as error bars (n=3). B. Representative confocal microscopy images for aggrecan (red), collagen II (green) and collagen X (green) at day 21 under free swelling (a-c) and loading (d-f) culture conditions. Nuclei are counterstained blue. Scale bar = 20 μm. C. Quantitative analysis of the fraction of positively stained cells for collagen II and collagen X at day 21 in free swelling (black bars) and loading (white bars) culture condition. Data are mean with standard deviation as error bars (n= 4 for IHC). D. Representative confocal microscopy images for pSmad2/3 (red) and pSmad1/5/8 (red) at day 21 under free swelling (a-b) and loading (c-d) culture conditions. Nuclei are counterstained blue. Scale bar = 20 μm.
At the protein level, aggrecan and collagen II were evaluated as markers of chondrogenesis and collagen X was evaluated as a marker of hypertrophy (Fig. 3B). Aggrecan and collagen II were detected in the immediate region surrounding the encapsulated cells regardless of culture condition. Approximately 60% of the encapsulated cells stained positive for collagen II deposition was not affected by culture condition. Collagen X protein was detected in the immediate region surrounding the encapsulated cells under free swelling conditions with ~60% of cells staining positive for collagen X. Dynamic loading led to a seventy percent reduction (p<0.001) in the number of cells that stained positive for collagen X (Fig. 3C). There were fewer (p=0.014) cells staining positively for collagen X than collagen II. The active (i.e., phosphorylated) forms of Smad2/3 (pSmad2/3) and Smad1/5/8 (pSmad1/5/8) were also evaluated qualitatively by IHC (Fig. 3D). pSmad2/3 was localized cellularly and present in both culture conditions. pSmad1/5/8 was also localized cellularly and present under free swelling, but minimally detectable under loading. Collectively, these results demonstrate that hMSCs in the cartilage-mimetic hydrogel underwent chondrogenesis evident at the gene and protein levels. Dynamic loading maintained chondrogenesis with pSmad2/3 and cartilage-ECM deposition, but downregulated hypertrophic markers associated with pSmad1/5/8 and collagen X.
3.3. The Role of Smad 1/5/8 in hMSC chondrogenesis and hypertrophy in the cartilage-mimetic hydrogel
The role of Smad1/5/8 signaling in hMSC chondrogenesis and hypertrophy was assessed by introducing the small molecule dorsomorphin in the culture media of the free swelling constructs and comparing to no treatment under free swelling and dynamic loading (Study 2, Fig. 1B). The presence of pSmad2/3 and pSmad1/5/8 was evaluated by IHC (Fig. 4A) and the corresponding quantitative assessment is reported as intensity per nuclei at days 14 and 21 (Fig. 4B). Neither culture time nor culture condition affected pSmad2/3. Culture condition (p<0.001), but not culture time (p=0.056) affected pSmad1/5/8. There was a significant interaction (p=0.018) between the two factors. Dynamic loading inhibited (p<0.001) pSmad1/5/8 compared to free swelling at both time points. Dorsomorphin also inhibited (p< 0.001) pSmad1/5/8 at both time points compared to the free swelling condition with no treatment. There were no differences in pSmad1/5/8 between dorsomorphin treatment under free swelling and dynamic loading. These results confirm that dorsomorphin in the cartilage-mimetic hydrogel cultured under free swelling inhibits pSmad1/5/8 without affecting pSmad2/3.
Figure 4.

Assessment of SMAD signaling in the cartilage-mimetic hydrogel under loading and with dorsomorphin, an inhibitor of pSmad1/5/8. A. Representative confocal microscopy images at day 14 and day 21 for pSmad2/3 (red) and pSmad1/5/8 (red) under free swelling (a-b,g-h), loading (c-d, i-j), and free swelling with dorsomorphin (e-f, k-l). Nuclei are counterstained blue. Scale bar = 20 μm. C. Quantitative analysis of pSmad2/3 and pSmad1/5/8 intensity per nuclei at day 14 (black bars) and day 21 (white bars) for the different culture conditions. Data are mean with standard deviation as error bars (n=4 hydrogels). Results from two-way ANOVA with pairwise comparisons are provided.
Chondrogenesis and markers of hypertrophy were evaluated by gene expression as a function of culture time (days 7,14, and 21) and culture condition (Fig 5A). SOX9 levels were not affected by culture time or culture condition. ACAN levels were affected by culture condition (p=0.03) and there was a significant interaction (p=0.04) between the factors. Under dynamic loading, ACAN levels increased with culture time and was significant (p=0.01) from days 7 to 21. Contrarily, ACAN levels remains constant under free swelling culture conditions regardless of dorsomorphin treatment and were lower (p=0.01) than the levels under dynamic loading. Culture time (p=0.002) and culture condition (p=0.009) affected COL2A1 levels. Under dynamic loading, COL2A1 levels increased with culture time and was significant (p=0.003) from days 7 to 21. Under free swelling, the mean expression increased with time, but was not statistically significant. COL2A1 levels remained constant with dorsomorphin treatment over time and were significantly lower (p=0.004) than the levels under dynamic loading. The expression profiles for RUNX2 and COL10A1 mirrored that of COL2A1 expression profile. RUNX2 levels increased (p=0.02) from days 7 to 21 under dynamic loading and at day 21 were higher (p=0.027) than the levels with dorsomorphin treatment. For COL10A1, there were no significant pairwise comparisons. Taken together, none of the genes investigated were affected during the first week of treatment (day 7 to 14) with either dynamic loading or dorsomorphin. However, by day 21 and two weeks of dynamic loading, ACAN and COL2A1 but also RUNX2 and COL10A1 were up-regulated. The up-regulation was not observed with dorsomorphin treatment. These results indicate that load-inhibition of hypertrophy does not occur at the level of transcription.
Figure 5.

Assessment of chondrogenesis in the cartilage-mimetic hydrogel under loading and with dorsomorphin, an inhibitor of pSmad1/5/8. A. Normalized gene expression of encapsulated hMSCs as a function of culture time for free swelling (circle, black line), loading (square, green line), and free swelling with dorsomorphin (diamond, blue line). Relative expression was normalized to pre-encapsulated hMSCs. Data are mean with standard deviation as error bars (n=3). Results from two-way ANOVA are provided. B. Representative confocal microscopy images at day 21 for collagen II (green), Runx2 (green), and collagen X (green) under free swelling (a,d,g), loading (b,e,h), and free swelling with dorsomorphin (c,f,i). Nuclei are counterstained blue. Scale bar = 20 μm. C. Quantitative analysis of the fraction of positively stained cells for collagen II and collagen X at day 21 in free swelling (black bars) and loading (white bars) culture condition. Data are mean with standard deviation as error bars (n=4). Results from two-way ANOVA with pairwise comparisons are provided.
Chondrogenesis and markers of hypertrophy were also evaluated by IHC as a function of culture time (days 14 and 21) and culture condition. Representative confocal microscopy images for collagen II, Runx2 and collagen X are provided for day 21 (Fig. 5B) along with the corresponding quantitative assessment for collagen II and X reported as the fraction of positively stained cells at days 14 and 21 (Fig. 5C). Collagen II protein was present in all culture conditions at day 21 with greater ~95% of the cells staining positive for collagen II at days 14 and 21. The fraction of cells stained for collagen II was not affected by culture time or culture condition. The fraction of cells stained for collagen X was affected by culture condition (p=0.0013), but not culture time. Under free swelling, cells stained positive for Runx2 and collagen X at day 21 with ~75–80% of cells stained positive for collagen X at days 14 and 21. It is worth noting that although the sections were stained separately for collagen II and X, the high number of cells staining positive for both collagens indicate that the many of the cells express both collagen II and X. Under dynamic loading and with dorsomorphin, there was an apparent reduction in staining for Runx2 and collagen X. Specifically, the number of cells expressing collagen X protein was ~2-fold lower under dynamic loading (p=0.02) and under dorsomorphin (p=0.03) at day 14, with no difference between the two treatments. By day 21, the number of cells expressing collagen X remained ~2–3-fold lower, but this reduction was only significant under dynamic loading. These results confirm load-inhibited hypertrophy in the cartilage mimetic hydrogel without affecting chondrogenesis and demonstrate that dorsomorphin treatment under free swelling recapitulates the inhibition of hypertrophy implicating pSmad1/5/8 signaling.
3.4. The role of ChS and p38 MAPK on chondrogenesis under free swelling and dynamic loading
To determine the role of ChS and its associated negative charges in the load-inhibited hypertrophy, hMSC differentiation was in the cartilage mimetic hydrogel was compared to a hydrogel without ChS. Using the small molecule inhibitor SB203580, p38 MAPK was also investigated for its involvement in mechanical loading and osmolarity. Representative images for pSmad2/3 and collagen II are shown in Fig. 6A and 6B, respectively. There was positive staining for pSmad2/3 in the cartilage mimetic hydrogels regardless of loading and SB203580. This observation was mirrored by Collagen II staining, which was present throughout. Without ChS, pSmad 2/3 was present in all conditions, but appeared to be reduced when compared to the cartilage mimetic hydrogels with ChS, especially under dynamic loading. Collagen II was present in the hydrogels without ChS when cultured under free swelling, but was minimally detectable under loading. Representative images for pSmad1/5/8, Runx2, and collagen X are shown in Fig. 6C, 6D, and 6E, respectively. In the cartilage mimetic hydrogel, pSmad1/5/8 was present under free swelling conditions. There was consistently minimal staining for pSmad1/5/8 under loading, but which was recovered by SB203580 treatment. These observations were mirrored by Runx2 and collagen X staining. Without ChS, pSmad1/5/8, Runx2, and collagen X were present under all conditions, regardless of loading and regardless of SB203580.
Figure 6.

Assessment of chondrogenesis in the cartilage-mimetic hydrogel under loading and with SB203580, an inhibitor of p38 signaling. Representative confocal microscopy images at day 21 for A. pSmad2/3 (red), B. collagen II (green), C. pSmad1/5/8 (red), D. Runx2 (green), and E. collagen X (green). Experimental conditions included with (a,c,e,f) and without (b,d,f,h) chondroitin sulfate (ChS) in the cartilage-mimetic hydrogel, free swelling without (a-b) and with (e-f) SB203580, and loading without (c-d) and with (f-h) SB203580. Nuclei are counterstained blue. Scale bar = 20 μm.
NFAT5 is an osmolarity-responsive binding protein that mediates the transcriptional activation of ion transporters including s100a4, a calcium binding protein (Fig. 7A). Thus, expression of S100A4 is a direct indication of NFAT5 transcriptional activity. NFAT5 and S100A4 were investigated at the gene level (Fig. 7B and 7C, respectively). NFAT5 levels were initially higher (p=0.006) in the cartilage mimetic hydrogel with ChS after seven days of free swelling culture when compared with hydrogels without ChS. At day 21, NFAT5 levels were affected by the presence of ChS in the hydrogel (p=0.03) and dynamic loading (p=0.018) with lower expressions in the hydrogels without ChS and under loading. There were no significant effects with the inhibitor SB203580. Moreover, no significant pairwise comparisons were identified. S100A4 expression levels mirrored that of NFAT5, but the differences were more significant. Initially after seven days of free swelling culture, S100A4 expression levels were higher (p=0.02) in the cartilage mimetic hydrogel with ChS. A three-way ANOVA revealed significant two-way interactions between ChS (+/− ChS) and loading (+/− dynamic loading) (p=0.0001) and between ChS and inhibitor (+/− SB203580) (p=0.029). Therefore, two-way ANOVAs were performed followed by post-hoc analyses and pairwise comparisons are reported. Under free swelling conditions, there were no significant differences due to ChS or SB203580. Under dynamic loading, S100A4 levels were higher in the cartilage mimetic hydrogel with ChS than without ChS. With SB203580, loading also elevated S100A4, but to levels that were less than the no SB203580 treatment. Without ChS, S100A4 levels were significantly down-regulated by dynamic loading and also lower than with ChS under loading. This result was not dependent on SB203580. Overall, these results indicate that ChS is required for the load-inhibited hypertrophy in the cartilage mimetic hydrogel and that p38 MAPK is involved. Moreover, the presence of ChS up-regulates osmotic responsive genes but only under dynamic loading, implicating dynamic changes in hyperosmolarity as a potential contributing factor to the load- inhibited hypertrophy.
Figure 7.

Assessment of hyperosmotic genes in the cartilage-mimetic hydrogel under loading and with SB203580, an inhibitor of p38 signaling. A. Schematic of hyperosmolarity effects on NFAT5 transcription factor and its downstream control of S100A4. B. Relative gene expression of encapsulated hMSCs at day 7 and day 21 in cartilage-mimetic hydrogel with and without chondroitin sulfate (ChS), SB203580, and loading. Data are mean with standard deviation as error bars (n=3). Three-way ANOVA results for the main effects are provided for NFAT5 at day 21. Pairwise comparisons are provided for S100A4 at day 21.
4. Discussion
This study confirms that a cartilage-mimetic PEG hydrogel containing ECM analogs of ChS and RGD and prepared from the thiol:norbornene photoclick reaction scheme supports hMSC chondrogenesis and inhibits hypertrophy under dynamic compressive loading, a finding that supports our previous work [26]. The load-induced inhibition of hypertrophy during hMSC chondrogenesis was mediated by a down-regulation in Smad1/5/8 signaling and the presence of negatively charged ChS via p38 MAPK signaling. Taken together, findings from this study identified ChS, which when combined with dynamic compressive loading, as a potent physiochemical cue that maintains stable hMSC chondrogenesis.
This study demonstrates that Smad1/5/8 signaling is necessary for hMSCs to undergo hypertrophy during chondrogenesis when embedded in the cartilage mimetic hydrogel. Under free swelling culture conditions, hypertrophy was evident by the positive staining for Runx2 and collagen X protein. Smad2/3 signaling along with collagen II protein expression remained prevalent in the cartilage mimetic hydrogel, confirming that the hMSCs underwent terminal differentiation. It is well known that BMP receptors (i.e., ALK2, 3, and 6) activate Smad1/5/8 signaling. However, TGFβ has been shown to bind promiscuously to these receptors resulting in TGFβ-activated Smad1/5/8 signaling concomitant with Smad2/3 signaling [61]. Others have reported that TGFβ, including TGFβ3, which is often used to induce chondrogenesis of MSCs, readily leads to terminal differentiation of MSCs in pellet culture evident by positive staining for collagen X [62]. These findings suggest that while the hydrogel was designed with cartilage-ECM cues based on chondroitin sulfate and low concentrations of the RGD cell adhesion peptide, this environment was not sufficient to prevent terminal differentiation.
Under dynamic compressive loading, Smad1/5/8 signaling was down regulated along with Runx2 and collagen X protein expression indicating load-inhibited hypertrophy. On the other hand, Smad2/3 signaling, cartilage-specific genes, and collagen II protein expression remained high and similar to free swelling conditions. Our results indicate that dynamic loading does not affect the chondrogenic capabilities of the MSCs in the cartilage mimetic hydrogel, but inhibits hypertrophy. Our findings are in agreement with studies reporting high expression of pSmad1/5/8 under free swelling, but a reduction under dynamic compression for MSCs seeded in a porous scaffold and undergoing chondrogenesis [63]. Smad2/3 dominated signaling has been known to protect articular cartilage and block chondrocyte terminal differentiation [63]. Other studies have reported that mechanical loading promotes the phosphorylation of Smad2/3, which suppresses hypertrophy [47,64,65]. In this study, dynamic loading did not appear to affect pSmad2/3 signaling, which may suggest that the cartilage mimetic hydrogel with TGFβ3 was the primary driver of pSmad2/3 signaling. However, the prevalence of pSmad2/3 was insufficient to prevent hypertrophy. Inhibition of pSmad1/5/8 by either dynamic loading or dorsomorphin treatment was necessary to inhibit hypertrophy. Overall, downregulation in Smad1/5/8 by dynamic loading, which results in a shift to Smad2/3 dominated signaling, maintained a stable chondrogenic phenotype while inhibiting hypertrophy.
The protein collagen X has been shown to be critically involved in the early events of cartilage hypertrophy, which lead to endochondral ossification [66]. Thus, collagen X at the protein level was used as a marker for MSC chondrocyte. However, despite a significant reduction in the hypertrophic markers at the protein level by dynamic loading, expression at the gene level was similar to the free swelling conditions. At day 14, there was also no affect in the gene levels with dorsomorphin. Contrarily, downregulation of pSmad1/5/8 was evident by day 14 along with a reduction in the protein expression of the hypertrophic markers of Runx2 and collagen X under dynamic loading and with dorsomorphin. These findings suggest a differential role in the translational control over hypertrophy. Studies have reported that the type of Runx2 mRNA (type I vs II) can lead to variable translation efficiencies and even repression of its translation [67]. Interestingly type II has been found in osteoblasts and terminal hypertrophic chondrocytes, while type I is found in early differentiating cells [68,69]. In addition, others have also reported expression of COL10A1, but no detectable collagen X protein [70], which is consistent with our findings here and also findings from our prior work [26]. Although the exact mechanisms involved in the translational control of Runx2 and collagen X remain to be elucidated, our findings suggest a role for Smad1/5/8 signaling in this process. The high mRNA levels of these hypertrophic genes may suggest that hypertrophy could be readily activated upon the removal of loading and/or by the activation of the BMP receptors. Further investigations are needed to better understand the mechanisms which control the translation of hypertrophic proteins.
The inclusion of ChS into the hydrogel was necessary to the achieve load-inhibited hMSC hypertrophy in the cartilage mimetic hydrogel. In the absence of ChS, dynamic loading appeared to inhibit collagen II protein expression, while maintaining expression of Runx2 and collagen X. This finding is consistent with our previous reports, which showed that PEG alone, i.e. without ChS or RGD, led to load-induced inhibition of collagen II protein, but had no effect on collagen X protein [18]. These results suggest that RGD is not mediating hypertrophy in the hydrogels and instead the hMSCs may have a propensity for terminal differentiation. ChS, on the other hand, introduces fixed negative charges into the hydrogel which attract mobile cations from the media and increase the local osmolarity within the hydrogel [71]. Although the fixed negative charge density introduced into the hydrogel by 1% ChS is lower than that reported for cartilage [59], the presence of these charges will lead to a higher osmolarity than hydrogels without ChS. Several studies have reported positive effects of hyperosmotic conditions on chondrocytes and chondrogenically differentiating MSCs, leading to increased ECM synthesis [71–74]. Under hyperosmotic culture conditions, the osmosensitive transcription factor NFAT5 is upregulated [75–77]. NFAT5 activation induces the expression of osmoprotective genes including the osmolyte transporter s100a4, which aids in cell adaption to hyperosmolarity [75]. Thus, an upregulation of S100A4 is a direct indication of NFAT5 activity [78]. In this study, NFAT5 and S100A4 expressions were up-regulated early by day 7 in the hydrogels containing ChS, which is attributed to the elevated osmolarity with ChS. However, longer-term (i.e., day 21) as the hMSCs differentiate and deposit their own ECM, these two genes were similar regardless of the presence of ChS in the free swelling condition. Moreover, hypertrophy was prevalent. These findings suggest that simply introducing ChS into the cartilage mimetic hydrogel and the local hyperosmotic environment, under static (i.e., free swelling) conditions, is insufficient to affect hypertrophy.
On the contrary, dynamic loading had a much greater effect on the hydrogels with ChS leading to higher S100A4 expression. Dynamic loading of a charged environment induces dynamic changes in osmolarity, which we reported has a greater positive effect on chondrocytes over a static hyperosmotic environment [71]. Therefore, we hypothesize that the combination of ChS and dynamic mechanical loading lead to dynamic changes in osmolarity which, in turn, inhibit hypertrophy and maintain chondrogenesis. In support, the signaling molecule p38 MAPK has been reported to be highly responsive to extracellular stimuli including osmotic stress [79,80] and mechanical loading [52,81]. Inhibiting p38 signaling abrogated ChS’s load-inhibited hypertrophy in the cartilage mimetic hydrogels as shown by a prevalence of Runx2 and collagen X protein. In addition, S100A4 was down-regulated when p38 MAPK was inhibited to levels that were similar to the free swelling conditions. These results suggest that dynamic changes in osmolarity is likely playing an important role in mediating hypertrophy and that p38 signaling is involved.
Interestingly in the hydrogels without ChS, dynamic mechanical loading inhibited S100A4 expression and this result did not appear to be dependent on p38 signaling. NFAT5 has been reported to regulate SOX9 [75], a master regulator of collagen II [82]. The inhibition of S100A4 expression, which indicates a downregulation in NFAT5 activity, could explain the downregulation in collagen II protein expression in the hydrogels without ChS under dynamic mechanical loading. The exact signaling mechanism involved in the load-induced inhibition of collagen II in hydrogels without ChS remains to be elucidated.
There are several limitations of this study. This study focused primarily on the effect of physiochemical cues on two pathways that are known to be important in chondrogenesis, Smad1/5/8 and p38 MAPK signaling. However, we recognize that other signaling mechanisms are likely to be involved in regulating hypertrophy during MSC chondrogenesis and thus future studies are warranted. Additionally, NFAT activity was indirectly assessed through its transcriptional regulation of the gene, S100A4. Future studies will need to investigate the role of NFAT activity in response to dynamic changes in osmolarity. Stable hydrogels were employed to minimize confounding effects that result from changes to the hydrogel chemistry and mechanical properties. The nature of non-degradable crosslinks limits ECM deposition to the pericellular space and prevents further elaboration into the extracellular space [83], which is observed in this study. Future studies using degradable crosslinks combined with dynamic mechanical loading are needed to confirm that a stable chondrogenic phenotype can be achieved long-term and to evaluate whether the biomimetic hydrogel can also enhance ECM deposition or is primarily playing a role in inhibiting hypertrophy. Finally, this study did not delineate the contribution of negative charges that result from cell-secreted aggrecan as the pericellular matrix is formed during chondrogenesis. Cartilage has an unusually high osmolarity and ex vivo studies have identified a range in osmolarity that is beneficial to chondrocytes [84]. We therefore hypothesize that the negative charges from the hydrogel with ChS combined with the nascent PCM is within the physiologically relevant range, but remains low in the hydrogels without ChS. Further studies are needed to test this hypothesis.
5. Conclusions
This study demonstrates that dynamic mechanical loading of hMSC encapsulated in a cartilage mimetic hydrogel inhibits terminal differentiation of chondrogenically differentiating and supports a stable chondrogenic phenotype. The presence of chondroitin sulfate is responsible for the load-induced inhibition of hypertrophy, which occurs by inhibiting Smad1/5/8 signaling and up-regulating p38 signaling. The resulting dynamic changes in osmolarity that arises due to the incorporation of fixed negative charges by the chondroitin sulfate and dynamic compressive loading are in part responsible for the load-induced inhibition of hypertrophy. This study provides new insights into how external cues from the hydrogel and environment can enhance and maintain stable chondrogenesis of hMSCs.
Acknowledgments
The research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institute of Health under the award #1R01AR069060. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors also acknowledge support from a Department of Education’s Graduate Assistantship in Areas of National Need fellowship to EAA.
Footnotes
Data Availability
Raw and/or processed data are available upon request.
References
- [1].Farrell E, Both SK, Odörfer KI, Koevoet W, Kops N, O’Brien FJ, de Jong RJB, Verhaar JA, Cuijpers V, Jansen J, Erben RG, van Osch GJ, In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells, BMC Musculoskelet. Disord 12 (2011) 31. doi: 10.1186/1471-2474-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W, Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice, Arthritis Rheum. 54 (2006) 3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- [3].Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU, In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells, Exp. Cell Res 238 (1998) 265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- [4].Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J, Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells, Matrix Biol. 27 (2008) 12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- [5].Bryant SJ, Davis-Arehart KA, Luo N, Shoemaker RK, Arthur JA, Anseth KS, Synthesis and Characterization of Photopolymerized Multifunctional Hydrogels: Water-Soluble Poly(Vinyl Alcohol) and Chondroitin Sulfate Macromers for Chondrocyte Encapsulation, Macromolecules. 37 (2004) 6726–6733. doi: 10.1021/ma0499324. [DOI] [Google Scholar]
- [6].Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD, Disulfide cross-linked hyaluronan hydrogels, Biomacromolecules. 3 (2002) 1304–1311. [DOI] [PubMed] [Google Scholar]
- [7].Bian L, Hou C, Tous E, Rai R, Mauck RL, Burdick JA, The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy, Biomaterials. 34 (2013) 413–421. doi: 10.1016/j.biomaterials.2012.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Burdick JA, Prestwich GD, Hyaluronic acid hydrogels for biomedical applications, Adv. Mater. Deerfield Beach Fla 23 (2011) H41–56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Highley CB, Prestwich GD, Burdick JA, Recent advances in hyaluronic acid hydrogels for biomedical applications, Curr. Opin. Biotechnol 40 (2016) 35–40. doi: 10.1016/j.copbio.2016.02.008. [DOI] [PubMed] [Google Scholar]
- [10].Steinmetz NJ, Bryant SJ, Chondroitin sulfate and dynamic loading alter chondrogenesis of human MSCs in PEG hydrogels, Biotechnol. Bioeng 109 (2012) 2671–2682. doi: 10.1002/bit.24519. [DOI] [PubMed] [Google Scholar]
- [11].Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J, Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells, Matrix Biol. 27 (2008) 12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- [12].Calderon L, Collin E, Velasco-Bayon D, Murphy M, O’Halloran D, Pandit A, Type II collagen-hyaluronan hydrogel--a step towards a scaffold for intervertebral disc tissue engineering, Eur. Cell. Mater 20 (2010) 134–148. [DOI] [PubMed] [Google Scholar]
- [13].Connelly JT, García AJ, Levenston ME, Inhibition of in vitro chondrogenesis in RGD-modified three-dimensional alginate gels, Biomaterials. 28 (2007) 1071–1083. doi: 10.1016/j.biomaterials.2006.10.006. [DOI] [PubMed] [Google Scholar]
- [14].Tavella S, Bellese G, Castagnola P, Martin I, Piccini D, Doliana R, Colombatti A, Cancedda R, Tacchetti C, Regulated expression of fibronectin, laminin and related integrin receptors during the early chondrocyte differentiation, J. Cell Sci 110 (1997) 2261–2270. [DOI] [PubMed] [Google Scholar]
- [15].Salinas CN, Cole BB, Kasko AM, Anseth KS, Chondrogenic differentiation potential of human mesenchymal stem cells photoencapsulated within poly(ethylene glycol)-arginine-glycine-aspartic acid-serine thiol-methacrylate mixed-mode networks, Tissue Eng. 13 (2007) 1025–1034. doi: 10.1089/ten.2006.0126. [DOI] [PubMed] [Google Scholar]
- [16].Salinas CN, Anseth KS, The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities, Biomaterials. 29 (2008) 2370–2377. doi: 10.1016/j.biomaterials.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim IL, Khetan S, Baker BM, Chen CS, Burdick JA, Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues, Biomaterials. 34 (2013) 5571–5580. doi: 10.1016/j.biomaterials.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Steinmetz NJ, Bryant SJ, The effects of intermittent dynamic loading on chondrogenic and osteogenic differentiation of human marrow stromal cells encapsulated in RGD-modified poly(ethylene glycol) hydrogels, Acta Biomater. 7 (2011) 3829–3840. doi: 10.1016/j.actbio.2011.06.031. [DOI] [PubMed] [Google Scholar]
- [19].Bian L, Zhai DY, Tous E, Rai R, Mauck RL, Burdick JA, Enhanced MSC Chondrogenesis Following Delivery of TGF-β3 from Alginate Microspheres within Hyaluronic Acid Hydrogels In Vitro and In Vivo, Biomaterials. 32 (2011) 6425–6434. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kim IL, Mauck RL, Burdick JA, Hydrogel design for cartilage tissue engineering: A case study with hyaluronic acid, Biomaterials. 32 (2011) 8771–8782. doi: 10.1016/j.biomaterials.2011.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhu M, Feng Q, Sun Y, Li G, Bian L, Effect of cartilaginous matrix components on the chondrogenesis and hypertrophy of mesenchymal stem cells in hyaluronic acid hydrogels, J. Biomed. Mater. Res. B Appl. Biomater 105 (2017) 2292–2300. doi: 10.1002/jbm.b.33760. [DOI] [PubMed] [Google Scholar]
- [22].Feng Q, Lin S, Zhang K, Dong C, Wu T, Huang H, Yan X, Zhang L, Li G, Bian L, Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy, Acta Biomater. 53 (2017) 329–342. doi: 10.1016/j.actbio.2017.02.015. [DOI] [PubMed] [Google Scholar]
- [23].Bian L, Zhai DY, Zhang EC, Mauck RL, Burdick JA, Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels, Tissue Eng. Part A 18 (2012) 715–724. doi: 10.1089/ten.TEA.2011.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lin S, Lee WYW, Feng Q, Xu L, Wang B, Man GCW, Chen Y, Jiang X, Bian L, Cui L, Wei B, Li G, Synergistic effects on mesenchymal stem cell-based cartilage regeneration by chondrogenic preconditioning and mechanical stimulation, Stem Cell Res. Ther 8 (2017) 221. doi: 10.1186/s13287-017-0672-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Carroll SF, Buckley CT, Kelly DJ, Cyclic hydrostatic pressure promotes a stable cartilage phenotype and enhances the functional development of cartilaginous grafts engineered using multipotent stromal cells isolated from bone marrow and infrapatellar fat pad, J. Biomech 47 (2014) 2115–2121. doi: 10.1016/j.jbiomech.2013.12.006. [DOI] [PubMed] [Google Scholar]
- [26].Aisenbrey EA, Bryant SJ, Mechanical loading inhibits hypertrophy in chondrogenically differentiating hMSCs within a biomimetic hydrogel, J. Mater. Chem. B 4 (2016) 3562–3574. doi: 10.1039/C6TB00006A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhong L, Huang X, Karperien M, Post JN, The Regulatory Role of Signaling Crosstalk in Hypertrophy of MSCs and Human Articular Chondrocytes, Int. J. Mol. Sci 16 (2015) 19225–19247. doi: 10.3390/ijms160819225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Watanabe H, de Caestecker MP, Yamada Y, Transcriptional Cross-talk between Smad, ERK1/2, and p38 Mitogen-activated Protein Kinase Pathways Regulates Transforming Growth Factor-β-induced Aggrecan Gene Expression in Chondrogenic ATDC5 Cells, J. Biol. Chem 276 (2001) 14466–14473. doi: 10.1074/jbc.M005724200. [DOI] [PubMed] [Google Scholar]
- [29].Hatakeyama Y, Nguyen J, Wang X, Nuckolls GH, Shum L, Smad Signaling in Mesenchymal and Chondroprogenitor Cells, JBJS. 85 (2003) 13–18. [DOI] [PubMed] [Google Scholar]
- [30].Fujii M, Takeda K, Imamura T, Aoki H, Sampath TK, Enomoto S, Kawabata M, Kato M, Ichijo H, Miyazono K, Roles of Bone Morphogenetic Protein Type I Receptors and Smad Proteins in Osteoblast and Chondroblast Differentiation, Mol. Biol. Cell 10 (1999) 3801–3813. doi: 10.1091/mbc.10.11.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sakou T, Onishi T, Yamamoto T, Nagamine T, Sampath TK, ten Dijke P, Localization of Smads, the TGF-β Family Intracellular Signaling Components During Endochondral Ossification, J. Bone Miner. Res 14 (1999) 1145–1152. doi: 10.1359/jbmr.1999.14.7.1145. [DOI] [PubMed] [Google Scholar]
- [32].Itoh S, Itoh F, Goumans M-J, ten Dijke P, Signaling of transforming growth factor-β family members through Smad proteins, Eur. J. Biochem 267 (2000) 6954–6967. doi: 10.1046/j.1432-1327.2000.01828.x. [DOI] [PubMed] [Google Scholar]
- [33].Furumatsu T, Ozaki T, Asahara H, Smad3 activates the Sox9-dependent transcription on chromatin, Int. J. Biochem. Cell Biol 41 (2009) 1198–1204. doi: 10.1016/j.biocel.2008.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Furumatsu T, Tsuda M, Taniguchi N, Tajima Y, Asahara H, Smad3 Induces Chondrogenesis through the Activation of SOX9 via CREB-binding Protein/p300 Recruitment, J. Biol. Chem 280 (2005) 8343–8350. doi: 10.1074/jbc.M413913200. [DOI] [PubMed] [Google Scholar]
- [35].Yang X, Chen L, Xu X, Li C, Huang C, Deng C-X, TGF-β/Smad3 Signals Repress Chondrocyte Hypertrophic Differentiation and Are Required for Maintaining Articular Cartilage, J. Cell Biol 153 (2001) 35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li T-F, Darowish M, Zuscik MJ, Chen D, Schwarz EM, Rosier RN, Drissi H, O’Keefe RJ, Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation, J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res 21 (2006) 4–16. doi: 10.1359/JBMR.050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lorda-Diez CI, Montero JA, Diaz-Mendoza MJ, Garcia-Porrero JA, Hurle JM, Defining the Earliest Transcriptional Steps of Chondrogenic Progenitor Specification during the Formation of the Digits in the Embryonic Limb, PLOS ONE. 6 (2011) e24546. doi: 10.1371/journal.pone.0024546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hellingman CA, Davidson ENB, Koevoet W, Vitters EL, van den Berg WB, van Osch GJVM, van der Kraan PM, Smad signaling determines chondrogenic differentiation of bone-marrow-derived mesenchymal stem cells: inhibition of Smad1/5/8P prevents terminal differentiation and calcification, Tissue Eng. Part A 17 (2011) 1157–1167. doi: 10.1089/ten.TEA.2010.0043. [DOI] [PubMed] [Google Scholar]
- [39].Retting KN, Song B, Yoon BS, Lyons KM, BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation, Dev. Camb. Engl 136 (2009) 1093–1104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Leboy P, Grasso-Knight G, D’Angelo M, Volk S, Lian JV, Drissi H, Stein G, Adams SL, Smad-Runx interactions during chondrocyte maturation, 2001. [PubMed]
- [41].Chen G, Deng C, Li Y-P, TGF-β and BMP Signaling in Osteoblast Differentiation and Bone Formation, Int. J. Biol. Sci 8 (2012) 272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang W, Rigueur D, Lyons KM, TGFβ Signaling in Cartilage Development and Maintenance, Birth Defects Res. Part C Embryo Today Rev 102 (2014) 37–51. doi: 10.1002/bdrc.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].van der Kraan PM, van den Berg WB, Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration?, Osteoarthritis Cartilage. 20 (2012) 223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- [44].Keller B, Yang T, Chen Y, Munivez E, Bertin T, Zabel B, Lee B, Interaction of TGFβ and BMP Signaling Pathways during Chondrogenesis, PLOS ONE. 6 (2011) e16421. doi: 10.1371/journal.pone.0016421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Karamboulas K, Dranse HJ, Underhill TM, Regulation of BMP-dependent chondrogenesis in early limb mesenchyme by TGFβ signals, J Cell Sci. 123 (2010) 2068–2076. doi: 10.1242/jcs.062901. [DOI] [PubMed] [Google Scholar]
- [46].Li Z, Kupcsik L, Yao S-J, Alini M, Stoddart MJ, Mechanical load modulates chondrogenesis of human mesenchymal stem cells through the TGF-β pathway, J. Cell. Mol. Med 14 (2010) 1338–1346. doi: 10.1111/j.1582-4934.2009.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Madej W, van Caam A, Blaney Davidson EN, van der Kraan PM, Buma P, Physiological and excessive mechanical compression of articular cartilage activates Smad2/3P signaling, Osteoarthritis Cartilage. 22 (2014) 1018–1025. doi: 10.1016/j.joca.2014.04.024. [DOI] [PubMed] [Google Scholar]
- [48].Oeztuerk-Winder F, Ventura J-J, The many faces of p38 mitogen-activated protein kinase in progenitor/stem cell differentiation, Biochem. J 445 (2012) 1–10. doi: 10.1042/BJ20120401. [DOI] [PubMed] [Google Scholar]
- [49].Cuenda A, Rousseau S, p38 MAP-Kinases pathway regulation, function and role in human diseases, Biochim. Biophys. Acta BBA - Mol. Cell Res 1773 (2007) 1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- [50].Li J, Zhao Z, Liu J, Huang N, Long D, Wang J, Li X, Liu Y, MEK/ERK and p38 MAPK regulate chondrogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF-beta1/Smads pathway, Cell Prolif. 43 (2010) 333–343. doi: 10.1111/j.1365-2184.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yu B, Yu D, Cao L, Zhao X, Long T, Liu G, Tang T, Zhu Z, Simulated microgravity using a rotary cell culture system promotes chondrogenesis of human adipose-derived mesenchymal stem cells via the p38 MAPK pathway, Biochem. Biophys. Res. Commun 414 (2011) 412–418. doi: 10.1016/j.bbrc.2011.09.103. [DOI] [PubMed] [Google Scholar]
- [52].Li J, Zhao Z, Yang J, Liu J, Wang J, Li X, Liu Y, p38 MAPK mediated in compressive stress-induced chondrogenesis of rat bone marrow MSCs in 3D alginate scaffolds, J. Cell. Physiol 221 (2009) 609–617. doi: 10.1002/jcp.21890. [DOI] [PubMed] [Google Scholar]
- [53].Komori T, Runx2, a multifunctional transcription factor in skeletal development, J. Cell. Biochem 87 (2002) 1–8. doi: 10.1002/jcb.10276. [DOI] [PubMed] [Google Scholar]
- [54].Fairbanks BD, Schwartz MP, Halevi AE, Nuttelman CR, Bowman CN, Anseth KS, A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene Photopolymerization, Adv. Mater. Deerfield Beach Fla 21 (2009) 5005–5010. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Roberts JJ, Bryant SJ, Comparison of photopolymerizable thiol-ene PEG and acrylate-based PEG hydrogels for cartilage development, Biomaterials. 34 (2013) 9969–9979. doi: 10.1016/j.biomaterials.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Skaalure SC, Chu S, Bryant SJ, An enzyme-sensitive PEG hydrogel based on aggrecan catabolism for cartilage tissue engineering, Adv. Healthc. Mater 4 (2015) 420–431. doi: 10.1002/adhm.201400277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].da Cunha AL, de Oliveira LG, Maia LF, de Oliveira LFC, Michelacci YM, de Aguiar JAK, Pharmaceutical grade chondroitin sulfate: Structural analysis and identification of contaminants in different commercial preparations, Carbohydr. Polym 134 (2015) 300–308. doi: 10.1016/j.carbpol.2015.08.006. [DOI] [PubMed] [Google Scholar]
- [58].Nicodemus GD, Bryant SJ, The role of hydrogel structure and dynamic loading on chondrocyte gene expression and matrix formation, J. Biomech 41 (2008) 1528–1536. doi: 10.1016/j.jbiomech.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Villanueva I, Gladem SK, Kessler J, Bryant SJ, Dynamic loading stimulates chondrocyte biosynthesis when encapsulated in charged hydrogels prepared from poly(ethylene glycol) and chondroitin sulfate, Matrix Biol. J. Int. Soc. Matrix Biol 29 (2010) 51–62. doi: 10.1016/j.matbio.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pfaffl MW, A new mathematical model for relative quantification in real-time RT–PCR, Nucleic Acids Res. 29 (2001) e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Goumans MJ, Mummery C, Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice., Int. J. Dev. Biol 44 (2000) 253–265. [PubMed] [Google Scholar]
- [62].Mueller MB, Fischer M, Zellner J, Berner A, Dienstknecht T, Prantl L, Kujat R, Nerlich M, Tuan RS, Angele P, Hypertrophy in Mesenchymal Stem Cell Chondrogenesis: Effect of TGF-beta Isoforms and Chondrogenic Conditioning, Cells Tissues Organs. 192 (2010) 158–166. doi: 10.1159/000313399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhang T, Wen F, Wu Y, Goh GSH, Ge Z, Tan LP, Hui JHP, Yang Z, Cross-talk between TGF-beta/SMAD and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic compression, Biomaterials. 38 (2015) 72–85. doi: 10.1016/j.biomaterials.2014.10.010. [DOI] [PubMed] [Google Scholar]
- [64].Bougault C, Aubert-Foucher E, Paumier A, Perrier-Groult E, Huot L, Hot D, Duterque-Coquillaud M, Mallein-Gerin F, Dynamic Compression of Chondrocyte-Agarose Constructs Reveals New Candidate Mechanosensitive Genes, PLoS ONE. 7 (2012). doi: 10.1371/journal.pone.0036964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mouw JK, Connelly JT, Wilson CG, Michael KE, Levenston ME, Dynamic Compression Regulates the Expression and Synthesis of Chondrocyte-Specific Matrix Molecules in Bone Marrow Stromal Cells, STEM CELLS. 25 (2007) 655–663. doi: 10.1634/stemcells.2006-0435. [DOI] [PubMed] [Google Scholar]
- [66].Chan D, Jacenko O, Phenotypic and biochemical consequences of collagen X mutations in mice and humans, Matrix Biol. 17 (1998) 169–184. doi: 10.1016/s0945-053x(98)90056-7. [DOI] [PubMed] [Google Scholar]
- [67].Xiao Z-S, Simpson LG, Quarles LD, IRES-dependent translational control of Cbfa1/Runx2 expression., J. Cell. Biochem 88 (2003) 493–505. doi: 10.1002/jcb.10375. [DOI] [PubMed] [Google Scholar]
- [68].Enomoto H, Enomoto-Iwamoto M, Iwamoto M, Nomura S, Himeno M, Kitamura Y, Kishimoto T, Komori T, Cbfa1 is a positive regulatory factor in chondrocyte maturation, J. Biol. Chem 275 (2000) 8695–8702. doi: 10.1074/jbc.275.12.8695. [DOI] [PubMed] [Google Scholar]
- [69].Sudhakar S, Li Y, Katz MS, Elango N, Translational regulation is a control point in RUNX2/Cbfa1 gene expression., Biochem. Biophys. Res. Commun 289 (2001) 616–622. doi: 10.1006/bbrc.2001.6033. [DOI] [PubMed] [Google Scholar]
- [70].Mwale F, Stachura D, Roughley P, Antoniou J, Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation, J. Orthop. Res 24 (2006) 1791–1798. doi: 10.1002/jor.20200. [DOI] [PubMed] [Google Scholar]
- [71].Farnsworth NL, Mead BE, Antunez LR, Palmer AE, Bryant SJ, Ionic osmolytes and intracellular calcium regulate tissue production in chondrocytes cultured in a 3D charged hydrogel, Matrix Biol. 40 (2014) 17–26. doi: 10.1016/j.matbio.2014.08.002. [DOI] [PubMed] [Google Scholar]
- [72].Hall AC, Urban JP, Gehl KA, The effects of hydrostatic pressure on matrix synthesis in articular cartilage, J. Orthop. Res. Off. Publ. Orthop. Res. Soc 9 (1991) 1–10. doi: 10.1002/jor.1100090102. [DOI] [PubMed] [Google Scholar]
- [73].Urban JPG, THE CHONDROCYTE: A CELL UNDER PRESSURE, Rheumatology. 33 (1994) 901–908. doi: 10.1093/rheumatology/33.10.901. [DOI] [PubMed] [Google Scholar]
- [74].Villanueva I, Bishop NL, Bryant SJ, Medium osmolarity and pericellular matrix development improves chondrocyte survival when photoencapsulated in poly(ethylene glycol) hydrogels at low densities, Tissue Eng. Part A 15 (2009) 3037–3048. doi: 10.1089/ten.TEA.2009.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Caron MMJ, van der Windt AE, Emans PJ, van Rhijn LW, Jahr H, Welting TJM, Osmolarity determines the in vitro chondrogenic differentiation capacity of progenitor cells via nuclear factor of activated T-cells 5, Bone. 53 (2013) 94–102. doi: 10.1016/j.bone.2012.11.032. [DOI] [PubMed] [Google Scholar]
- [76].van der Windt AE, Haak E, Das RH, Kops N, Welting TJ, Caron MM, van Til NP, Verhaar JA, Weinans H, Jahr H, Physiological tonicity improves human chondrogenic marker expression through nuclear factor of activated T-cells 5 in vitro, Arthritis Res. Ther 12 (2010) R100. doi: 10.1186/ar3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Tomita M, Reinhold MI, Molkentin JD, Naski MC, Calcineurin and NFAT4 Induce Chondrogenesis, J. Biol. Chem 277 (2002) 42214–42218. doi: 10.1074/jbc.C200504200. [DOI] [PubMed] [Google Scholar]
- [78].Küper C, Beck F-X, Neuhofer W, NFAT5-mediated expression of S100A4 contributes to proliferation and migration of renal carcinoma cells, Front. Physiol 5 (2014). doi: 10.3389/fphys.2014.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tew SR, Hardingham TE, Regulation of SOX9 mRNA in human articular chondrocytes involving p38 MAPK activation and mRNA stabilization, J. Biol. Chem 281 (2006) 39471–39479. doi: 10.1074/jbc.M604322200. [DOI] [PubMed] [Google Scholar]
- [80].Matta C, Zakany R, Calcium signalling in chondrogenesis: implications for cartilage repair, Front. Biosci. Sch. Ed 5 (2013) 305–324. [DOI] [PubMed] [Google Scholar]
- [81].Li J, Wang J, Zou Y, Zhang Y, Long D, Lei L, Tan L, Ye R, Wang X, Zhao Z, The influence of delayed compressive stress on TGF-β1-induced chondrogenic differentiation of rat BMSCs through Smad-dependent and Smad-independent pathways, Biomaterials. 33 (2012) 8395–8405. doi: 10.1016/j.biomaterials.2012.08.019. [DOI] [PubMed] [Google Scholar]
- [82].Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B, SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene., Mol. Cell. Biol 17 (1997) 2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Nicodemus GD, Skaalure SC, Bryant SJ, Gel structure has an impact on pericellular and extracellular matrix deposition, which subsequently alters metabolic activities in chondrocyte-laden PEG hydrogels, Acta Biomater. 7 (2011) 492–504. doi: 10.1016/j.actbio.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Urban JPG, Hall AC, Gehl KA, Regulation of Matrix Synthesis Rates By the Ionic and Osmotic Environment of Articular Chondrocytes, J. Cell. Physiol 154 (1993) 262–270. [DOI] [PubMed] [Google Scholar]


