Abstract
Purpose
The prevalence of extended-spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) has been increasing worldwide since the early 2000s. E. coli is found in 70–90% of community-acquired urinary tract infections (CA-UTIs). We performed a systematic literature review to determine the risk factors for CA-UTI caused by ESBL-EC.
Methods
We searched the MEDLINE, Cochrane Library, Embase and Web of Science databases without language or date restriction up to March 2019. Two independent reviewers selected studies with quantified risk factors for CA-UTI due to ESBL-EC, and assessed their quality using the Newcastle-Ottawa Scale.
Results
Among the 5,597 studies identified, 16 observational studies (n=12,138 patients) met the eligibility criteria. The included studies were performed in various countries, and 14/16 were published after 2012. The most relevant risk factors for CA-UTI due to ESBL-EC identified were prior use of antibiotics (odds ratio (OR) from 2.2 to 21.4), previous hospitalization (OR: 1.7 to 3.9), and UTI history (OR: 1.3 to 3.8). Two risk factors were related to environmental contamination: travelling abroad, and swimming in freshwater.
Conclusion
Our findings could allow adapting empiric antibiotic treatments according to the patient profile. Further studies are needed to quantify the relationships between CA-UTI due to ESBL-EC and the environment.
Keywords: multi-drug resistant bacteria, enterobacteria infection, community-acquired infection, risk factor, beta-lactam resistance, systematic review
Introduction
Extended-spectrum beta-lactamases (ESBL) provide resistance to most beta-lactam antibiotics. Their prevalence has been increasing since the early 2000s.1,2 This is a worldwide phenomenon, but higher resistance rates have been reported in developing countries. The prevalence is currently lower than 10% in Europe,3–5 but can reach 46% in some South Asian countries.6–8 In 2017, the World Health Organization (WHO) defined a list of priority antibiotic-resistant pathogens for research purposes in which ESBL-producing enterobacteria are the most critical group.9 Indeed, these bacteria are resistant to penicillin and third-generation cephalosporins, two antibiotics that are among the most used worldwide due to their broad spectrum of action and low toxicity. In addition, ESBL presence is frequently associated with resistance to fluoroquinolones.1,10,11
Urinary tract infections (UTIs) are among the most common bacterial infections in the community. The enterobacterium Escherichia coli (E. coli) is found in 70–90% of UTIs. The antibiotic treatment for these infections is most often empiric.12–14 Therefore, the identification of factors that increase the risk of UTIs caused by ESBL-producing E. coli is a major but crucial challenge15–17 in order to optimize empiric antibiotic treatments and to limit the spread of antibiotic resistance.
In 2018, Tenney et al18 published a systematic literature review on the risk factors of UTI caused by multidrug-resistant bacteria but without restriction on specific pathogens (ie, E. coli), mechanisms of resistance (ie, ESBL), or settings (ie, community). The most robust risk factors identified were previous use of antibiotics, urinary catheterization, previous hospitalization, and living in a nursing home. However, data extraction for this review was completed in February 2016 before the publication of the WHO list of priority pathogens. Therefore, we thought appropriate to perform an updated systematic review of the literature by focusing on multidrug-resistant enterobacteria because the WHO identified them as a priority for research purposes (ie, ESBL-producing enterobacteria), and as the most frequently found in UTIs (ie, E. coli). The spread of these multidrug-resistant bacteria in community settings is becoming a matter of concern because it is harder to control than in hospital settings. Therefore, we decided to focus our review on factors that have been found to be associated with the emergence of community-acquired UTI (CA-UTI) caused by ESBL-producing E. coli.
The aim of this systematic review was to identify risk factors of carrying ESBL-producing E. coli among patients with UTI in order to optimize their management in the community.
Methods
Study Protocol and Registration
The protocol of this systematic review was registered in the international prospective register of systematic reviews (PROSPERO) database (no. CRD 42018089205) in March 2018. Results were reported according to the PRISMA group recommendations.
Eligibility Criteria
Studies were eligible for inclusion if they reported factors associated with UTI that was caused by ESBL-producing E. coli (identified by a laboratory test) and that occurred in the community (ie, outside hospitals or medium- and long-stay centers). Hospital-based studies were included only if UTI was diagnosed no later than 48 hours after hospital admission and thus could not be considered a nosocomial infection.19
Studies could include adult or pediatric populations without age restriction. Studies on populations who required specialized antibiotic treatment (eg, immunosuppressed patients, patients with kidney graft, with malformation of the urinary tract) were excluded. Only studies on humans were included.
Sources of Information and Search Strategy
The following databases were searched with no date restriction until the end of March 2019: MEDLINE, Embase, Cochrane Library, and Web Of Science. No filter on date, location, or publication language was used in the search strategy. The initial search equation in MEDLINE was then adapted for each database (supplemental Table S1). A manual search was also performed in the conference archives of the American Society of Microbiology (ASM), the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), and the International Congress on Infectious Diseases (ICID). No standardized search strategy was used for the grey literature.
When publications were deemed relevant but the published data were insufficient, the authors were contacted by email to provide complementary data. In the absence of a reply, a reminder was sent.
The reference list of all selected publications was analyzed to identify (and include) new relevant articles.
Study Selection
Only studies published in English, German, French, and Spanish were included. Duplicates were identified and removed. Two researchers (VD, PD) independently screened each title and abstract. Then, the full text of all studies considered to be relevant was obtained. The same two researchers independently assessed these studies for inclusion; any disagreement was resolved by consensus. In the absence of consensus, a third researcher (SL) was consulted.
Data Collection and Collected Data
The two researchers (VD, PD) independently collected the following data from the selected studies using a standardized form: (i) study characteristics (title, authors, journal, year of publication, country, objective); (ii) methods (study type, duration, number and location of participating centers, diagnostic and microbiological criteria for community-acquired UTI due to ESBL-producing E. coli); (iii) population characteristics (number, sex, age, inclusion and exclusion criteria); (iv) endpoints (variables that could be potential risk factors); (v) results: odd ratios (OR) and 95% confidence interval (95% CI); (vi) funding sources, conflicts of interest.
Additional Analyses
When the association indicators were not provided by the authors, they were calculated with their 95% CI using the MedCalc® software20 based on the Altman method.21
Study Quality Assessment
Two researchers (VD, PD) independently assessed the methodological quality of the included studies.
Case-control studies were assessed using the Newcastle-Ottawa Scale (NOS)22. This scale allows assigning a numerical score to each study. It includes eight items that are classified according to three assessment criteria: selection, comparison, and exposure. For each item, several response options are possible. A star system is used to assess the study quality. A maximum of one star can be assigned to each item, with the exception of the comparability criterion where up to two stars can be assigned. The total score ranges between 0 and 9 stars. A high-quality study will have a high final score.
Cross-sectional studies were assessed using an adapted version of the NOS22,23 that includes only seven items classified according to three assessment criteria: selection, comparison, and results. Depending on the item, one or two stars can be assigned; the maximum score is 10 stars.
Results
Study Selection
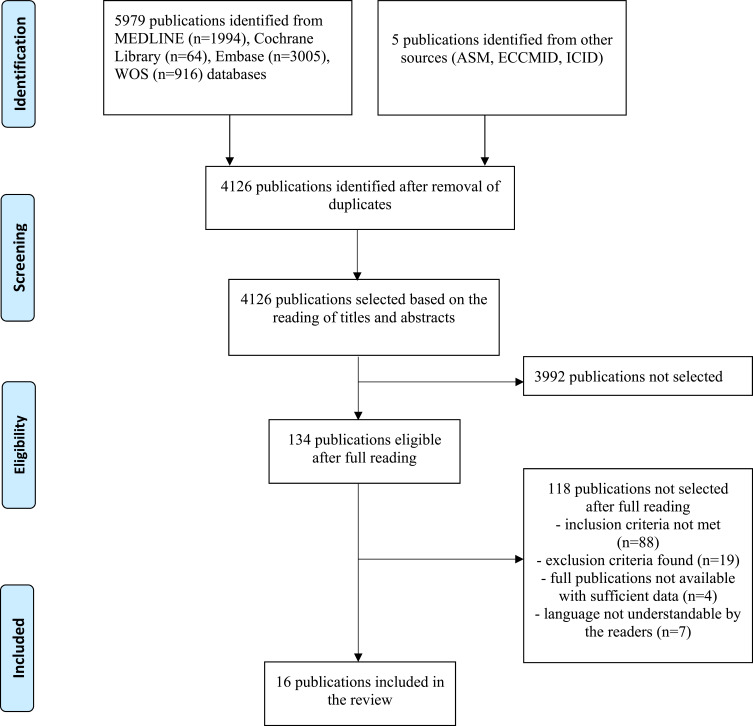
A total of 5,984 articles were found (Figure 1): 1,994 via MEDLINE, 64 via Cochrane Library, 3,005 via Embase, and 916 via World Of Science. Five articles were found by manual search of the ASM, ECCMID and ICID conference archives. After duplicate removal, 4,126 articles were eligible for inclusion. Based on the titles and abstracts, 134 articles were selected for full reading. Among the 15 authors contacted to obtain complementary data, four answered. After reading the retained articles, 88 did not meet the inclusion criteria, 19 included some exclusion criteria, 7 were not found in a language understandable by the authors of the review, and 4 were excluded for insufficient data. In total, 16 studies (n=12,138 patients) were included24–39.
Figure 1.
Study flowchart according to the PRISMA recommendations.
Characteristics of the Included Studies
All 16 studies were observational; 14 were case-control studies, and 2 were cross-sectional studies. Fourteen studies were published in peer-reviewed journals, and two corresponded to congress posters. Two studies were published between 2006 and 2009, and the others between 2012 and 2017.
These studies were carried out in Asia (n=5), Europe (n=8), South America (n=2) and North Africa (n=1), and concerned adult (n=11) and paediatric populations (n=3). Two studies did not provide any information on the patients’ age.
Only two studies concerned patients who were managed in non-hospital settings. The others included also inpatients (n=4), or concerned hospitalized patients in whom UTI was identified within 48 hours after hospital admission in all cases (n=10).
Study Quality
Nine studies had a score ≥8, five had intermediate scores (6 or 7), and two studies had a score ≤6 (Table 1).
Table 1.
Characteristics and Quality of the Included Studies
| Authors/Ref Country/Year | Study Type | Patients | Quality (Score) |
|---|---|---|---|
| Søgaard M et al38 Denmark, 2017 | Dual case-control study | Patients from general medicine departments n=7,170 UTI due to ESBL-producing E. coli n=339 Controls: UTI due to non-ESBL-producing E. coli n=3,390 |
9/9 |
| Hertz FB et al27 Denmark, 2015 |
Triple case-control study | Patients from general medicine departments with UTI due to E. coli n=449 ESBL-producing E. coli n=98 Controls: multi-sensitive n=177 |
9/9 |
| Søraas A et al35 Norway 2013 |
Case-control study | All patients with community-acquired UTI due to enterobacteria from 4 hospitals n=290 E. coli subgroup n=272 ESBL-producing E. coli n=95 non-ESBL-producing E. coli n=177 |
8/9 |
| Artero A et al31 Spain, 2017 | Case-control study | Patients >65 years, hospitalized for community-acquired acute pyelonephritis or urinary sepsis due to E. coli n=310 ESBL-producing E. coli n=85 non-ESBL-producing E. coli n=225 |
8/9 |
| Calbo E et al36 Spain, 2006 | Case-control study | Outpatients or emergency patients with UTI due to E. coli n=74 ESBL-producing E. coli n=19 Non-ESBL-producing E. coli n=55 |
8/9 |
| Azap OK et al37 Turkey, 2009 | Case-control study | Outpatients with UTI aged between 18 and 65 years n=510 E. coli subgroup n=464 ESBL-producing E. coli n=51 Non-ESBL-producing E. coli n=413 |
8/9 |
| Ozdogan FN et al33 Turkey, 2015 |
Case-control study | Outpatients with UTI due to E. coli aged >18 years n=200 ESBL-producing E. coli n=100 Non-ESBL-producing E. coli n=100 |
7/9 |
| Toumi A et al34 Tunisia, 2015 |
Case-control study | Patients aged >14 years hospitalized in infectious disease departments for community-acquired acute pyelonephritis due to E. coli n=484 ESBL-producing E. coli n=24 Non-ESBL-producing E. coli n=442 |
7/9 |
| Castillo-Tokumori F et al39 Peru, 2017 |
Case-control study | Outpatients with UTI due to E. coli aged >18 years n=172 ESBL-producing E. coli n=67 Non-ESBL-producing E. coli n=105 |
7/9 |
| Blanco Victor M et al24 Colombia, 2015 |
Case-control study | Emergency patients with UTI due to E. coli with no age restriction n=431 ESBL-producing E. coli n=54 Non-ESBL-producing E. coli n=377 |
7/9 |
| Savatmorigkorngul S et al29 Thailand, 2016 |
Cross-sectional study | Emergency patients with UTI due to E. coli aged >15 years n=408 ESBL-producing E. coli n=159 Non-ESBL-producing E. coli n=249 |
8/10 |
| Park SH et al30 South Korea, 2015 |
Case-control study | Patients hospitalized for community-acquired acute pyelonephritis due to E. coli aged >15 years n=300 ESBL-producing E. coli n=75 Non-ESBL-producing E. coli n=225 |
8/9 |
| Kang CI et al28 South Korea, 2012 |
Case-control study | Outpatients or emergency patients with infection due to E. coli aged >15 years n=140 Subgroup with UTI alone: ESBL-producing E. coli n=73 Non-ESBL-producing E. coli n=67 |
6/9 |
| Nisha KV et al25 India, 2017 |
Case-control study | Outpatient children with UTI due to E. coli aged 3 months to 18 years n=523 ESBL-producing E. coli n=196 Non-ESBL-producing E. coli n=327 |
8/9 |
| Pérez Heras I et al.26 Spain, 2017 |
Cross-sectional study | Emergency pediatric patients with UTI due to E. coli aged <14 years n=229 ESBL-producing E. coli n=21 Non-ESBL-producing E. coli n=208 |
4/10 |
| Fan NC et al.32 Taiwan, 2014 |
Case-control study | Inpatient children with community-acquired UTI due to E. coli aged <15 years n=312 ESBL-producing E. coli n=104 Non-ESBL-producing E. coli n=208 |
5/9 |
Risk Factors of ESBL-Producing E. coli Urinary Tract Infection
Results are presented in Table 2.
Table 2.
Identified ESBL-E coli Community-Acquired Urinary Tract Infection Risk Factors
| Risk Factors (Reference) | Adjusted OR | CI | Sample Size |
|---|---|---|---|
| Care related factors | |||
| Prior use of antibiotics | |||
| In the last 30 days27 | 1.8 | [1.0–3.1] | n=449 |
| [39] | 3.1 | [1.4–6.7] | n=172 |
| In the last 3 months34 | 4.0 | [1.6–10.0] | n=484 |
| In the previous year30 | 4.6 | [1.9–11.0] | n=300 |
| Time NS28 | 5.6 | [2.1–14.8] | n=140 |
| Prior use of broad spectrum antibiotic | |||
| Any, 31–365 days before index date38 | 0.9 | [0.5–1.7] | n=7,170 |
| Penicillin, 31–365 days before index date38 | 1.0 | [0.7–1.5] | n=7,170 |
| Prior use of beta-lactams | |||
| In the last 90 days35 | 4.5 | [1.8–11.0] | n=290 |
| [37] | 4.6 | [2.0–10.7] | n=510 |
| Prior use of penicillin | |||
| Any, time NS29 | 2.7 | [1.2–6.3] | n=408 |
| Prior use of cephalosporine | |||
| Cefuroxime, time NS36 | 21.4 | [5.4–85.2] | n=74 |
| 2GC, time NS33 | 3.9 | [1.8–8.5] | n=200 |
| Cephalosporin, time NS29 | 2.2 | [1.1–4.5] | n=408 |
| 3GC, time NS33 | 2.2 | [1.01–5.0] | n=200 |
| Prior use of Macrolide | |||
| Any, 31–365 days before index date38 | 1.5 | [1.1–2.2] | n=7,170 |
| Prior use of nitrofurantoin | |||
| 31–365 days before index date38 | 1.54 | [1.1–2.3] | n=7,170 |
| Prior use of fluoroquinolones | |||
| Any, in the last 30 days27 | 2.1 | [0.6–7.3] | n=449 |
| Any, in the last 90 days35 | 19.0 | [3.3–111.4] | n=290 |
| Any, time NS33 | 2.6 | [1.3–5.1] | n=200 |
| [28] | 9.9 | [2.2–44.6] | n=140 |
| Prior hospitalization | |||
| Any, in the last 30 days27 | 3.9 | [1.2–12.7] | n=449 |
| Any, in the last 3–12 months39 | 2.9 | [1.3–6.6] | n=172 |
| 1–2 hospitalizations, in the previous year38 | 1.7 | [1.3–2.3] | n=7,170 |
| > 3 hospitalizations, in the previous year38 | 3.9 | [2.6–5.8] | n=7,170 |
| Prior surgery, in the last 3–12 months39 | 2.8 | [1.9–8.0] | n=172 |
| History of UTIs | |||
| Any, in the previous year38 | 1.3 | [1.01–1.6] | n=7,170 |
| ≥3 episodes of UTI, in the previous year37 | 3.8 | [1.8–8.1] | n=510 |
| History of UTI due to E. coli, time NS29 | 3.4 | [1.8–6.7] | n=408 |
| Renal or urological disorder | |||
| History of recurrent acute pyelonephritis30 | 1.7 | [0.7–3.9] | n=300 |
| Recurrent acute pyelonephritis + history of diabetes30 | 4.2 | [1.3–16.9] | n=300 |
| Renal disease38 | 1.6 | [1.0–2.5] | n=7,170 |
| Urological abnormality34 | 3.5 | [1.0–11.5] | n=484 |
| Prior urinary catheterization29 | 3.3 | [1.7–6.6] | n=408 |
| History of prostatic disease37 | 9.6 | [2.1–44.8] | n=510 |
| Diabetes | |||
| [35] | 3.7 | [1.1–12.7] | n=290 |
| [34] | 3.0 | [1.1–8.0] | n=484 |
| [30] | 1.7 | [0.8–3.4] | n=300 |
| Prior medication | |||
| Prior immunosuppressive therapy38 | 1.5 | [1.1–2.1] | n=7,170 |
| Chronic treatment with corticosteroids39 | 24.3 | [2.4–246.9] | n=172 |
| Demographic characteristics | |||
| Male sex | |||
| [38] | 1.6 | [1.2–2.1] | n=7,170 |
| Age >55 years | |||
| [30] | 2.0 | [1.02–3.5] | n=300 |
| Citizenship | |||
| Northern Europe vs other countries38 | 0.4 | [0.2–0.7] | n=7,170 |
| Environmental factors | |||
| Travelling abroad (Asia, Middle East, Africa) | |||
| In the previous 6 weeks35 | 16.4 | [3.4–78.8] | n=290 |
| Same regions between 6 weeks and 2 years before35 | 2.2 | [1.1–4.3] | n=290 |
| Swimming in freshwater | |||
| [35] | 2.1 | [1.02–4.2] | n=290 |
| Number of fish meals per week | |||
| [35] | 0.6 | [0.5–0.9] | n=290 |
Prior Use of Antibiotic
Previous antibiotic intake was the most frequently identified risk factor for UTI due to ESBL-producing E. coli (n=12 studies) and was strongly associated with UTI occurrence in most of these studies (OR >4 in 8 of these 12 studies).
Four studies reported antibiotic intake without specifying the classes, with OR values ranging between 3.1 (95% CI: 1.4–6.7) and 5.6 (95% CI: 2.1–14.8) in adults.27,29,33,37 When looking at the antibiotic classes, beta-lactams (penicillin and cephalosporins) were found to be an independent risk factor in five studies, with ORs ranging between 2.2 (95% CI: 1.1–4.5) and 21.4 (95% CI: 5.4–85.2).29,33,35–37
Fluoroquinolones use was associated with higher risk of UTI in three studies.32,38,40 Søraas et al reported the use of fluoroquinolones as a major risk factor (OR: 19.0), but with very wide confidence intervals (95% CI: 3.3–111.4), but not Ozdogan et al (OR: 2.6, 95% CI: 1.3–5.1).
Nisha et al and Søgaard et al identified nitrofurantoin as a risk factor in children (OR: 11.5, 95% CI: 1.5–89.1) and in the general population (OR: 1.54, 95% CI: 1.05–2.26).
Prior Hospitalization
Prior hospitalization was identified as a risk factor in three good-quality studies (NOS score: 7 to 9). The OR values ranged between 1.7 (95% CI: 1.3–2.3) and 3.9 (95% CI: 1.2–12.7), depending on the number of prior hospitalizations and time to infection.27,38,39
History of UTIs
UTI history was identified as a risk factor in three (four according to Table 2) studies. The ORs ranged between 1.3 (95% CI: 1.0–1.6) and 3.8 (95% CI: 1.8–8.1), but the definition of “UTI history” was heterogenous: UTI during the previous year, ≥3 UTI episodes during the previous year, and recurrent acute pyelonephritis.29,37,38
Underlying Condition
Diabetes was a moderated risk factor for UTI due to ESBL-producing E. Coli (OR: 3.7 (95% CI: 1.1–12.7)).34 This risk factor was also reported in the study by Toumi et al in patients hospitalized for pyelonephritis (OR: 3.0, 95% CI: 1.1–8.0). Similarly, Park et al reported a synergistic effect of diabetes with recurrent acute pyelonephritis (OR: 4.2, 95% CI: 1.3–16.9).
Patient Care-Related Infections
Catheter-related UTI (OR: 3.3, 95% CI: 1.7–6.6),29 surgery 3–12 months before infection (OR: 2.7, 95% CI: 1.9–8.0),37 use of immunosuppressive treatments (OR: 1.5; 95% CI: 1.1–2.1),38 and chronic corticosteroid treatments (OR: 24.3; 95% CI: 2.4–246.9)39 were identified as risk factors for UTI due to ESBL-producing E. coli.
Two studies investigated a composite “care-related infection” risk factor. Artero et al, who studied patients older than 65 years, defined this risk as the presence of at least one of the following criteria: hospitalization in the previous 3 months, living in a nursing home, and administration of antibiotics in the previous 3 months (OR: 6.8, 95% CI: 3.2–14.3). Kang et al defined this risk factor differently (ie, hospitalization for at least 48 hours in the previous 90 days; having received haemodialysis, intravenous treatment, or wound care at home in the previous 30 days; living in a long-stay facility or in a nursing home) and obtained an OR of 6.8 (95% CI: 2.8–16.2).
Blanco Victor et al described a “complicated urinary tract infection” (pyelonephritis, functional or structural abnormality of the urinary tract, immunosuppression, UTI in men or in pregnant women) as a risk factor of UTI due to ESBL-producing E. coli (OR: 3.9, 95% CI: 1.1–13.9).
Demographic Factors
Age over 55 years (OR: 2.0, 95% CI: 1.0–3.5)30 and male sex (OR: 1.6, 95% CI: 1.2–2.1 in the higher quality study by Søgaard et al)38 also have been reported as risk factors for UTI due to ESBL-producing E. coli.
Other Factors
Søraas et al identified having travelled abroad (Asia, Middle East, Africa) in the previous 6 weeks as a major and independent risk factor (OR: 21; 95% CI: 4.5–97), as well as swimming in freshwater (OR: 2.1; 95% CI: 1.0–4.2).35
Discussion
The purpose of this systematic review was to identify risk factors of UTI caused by ESBL-producing E. coli. Our findings confirm the results of previous studies and systematic reviews. Indeed, previous use of antibiotics, hospitalization in the previous months, and history of UTIs were known risk factors.18 However, travelling to endemic areas seems also an important risk factor (OR: 16.4 [3.4–78.8]), according to the study by Søraas et al who showed that this risk is present for several months after travelling, and it decreases over time. Although this risk factor was identified only in this study, the risk of intestinal carriage of ESBL-producing E. coli following a trip in an endemic area, particularly Asia, has been already reported in the literature.40,41 However, additional studies are needed to confirm the importance of this risk factor.
The heterogeneity of the definitions of risk factors among studies is the main limitation of this review. For example, the risk factor “previous use of antibiotics” requires to specify the time between the previous use of antibiotics and UTI occurrence. However, among the 12 studies that investigated this factor, only five gave this information, and among them, three reported use of antibiotics in the three months before UTI. The same is true for “history of UTIs”. Previous UTI frequencies and characteristics varied among studies, thus making difficult the accurate description of this factor.
Most risk factors were related to patient healthcare. Two studies introduced a composite factor referred to as “care-related infection”. While the definition proposed by Kang et al was derived from the literature,42 the one used by Artero et al is questionable since it encompassed heterogeneous criteria such as hemodialysis and residence in nursing home without any obvious justification. In both cases, the clinical application of these factors appeared difficult because they are composed of risk factors that have very different ORs when studied individually as shown in our review. Moreover, only three studies were carried out exclusively in paediatric populations among whom two were of low quality (NOS score ≤5). Therefore, it is difficult to draw conclusions for this specific population.
Another limitation is that most studies were performed in hospital settings. This bias is caused by the difficulties of health data collection in the community, with patients and caregivers often scattered over a large territory. Although we included only studies on patients with UTI detected within the first 48h after hospital admission to exclude a nosocomial infection, patients recruited in hospital might not be representative of E coli BLSE-infected subjects in the community (co-morbidity, age …). More studies conducted in the community would enable to refute or confirm this bias. Nevertheless, this patient population highlights the risk of importing multi-resistant E. coli from the community to the hospital,43 and the importance of the rapid identification of potential carriers.
This systematic review identified two new risk factors: prior treatment with nitrofurantoin and swimming in freshwater. Prior treatment with nitrofurantoin was reported in two studies.25,38 However, Søgaard et al stressed the risk of possible confusion concerning this factor. Indeed, nitrofurantoin is used for the prevention of recurrent UTI that is also a risk factor of UTI in studies carried out in hospital settings.44,45 This might explain its identification as a risk factor of UTI caused by ESBL-producing E. coli. Moreover, the association reported by Nisha et al was among hospitalised children. So, this association should be interpreted with caution. Nevertheless, as nitrofurantoin is recommended as first-line therapy in the treatment of uncomplicated cystitis in most countries, other studies are needed to confirm that this antibiotic is (or not) an independent risk factor.
The other new risk factor identified by one study was swimming in freshwater. Environmental contamination, particularly of aquatic environments, by ESBL-producing E. coli has been already described in the literature.46–48 Our systematic review highlights the impact of this contamination on human health. Its presence in the environment makes more complex the implementation of models to predict infection or colonization by ESBL-producing E. coli. Several predictive models were proposed based on previously identified risk factors,49,50 essentially related to patient care and patient characteristics. The limits of these models are their medium sensitivity and their low external validity. Additional factors of environmental origin could be considered to improve the latter. Therefore, more studies are needed to precisely determine the environmental factors associated with the increased prevalence of UTIs caused by ESBL-producing E. coli in order to better manage them and to identify potential carriers. Moreover, local specificities should be taken into account to develop robust predictive models. Finally, studies on community patients should be promoted to allow a generalisation of the conclusions.
Funding Statement
There is no funding to report.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lee DS, Lee S-J, Choe H-S. Community-acquired urinary tract infection by Escherichia coli in the era of antibiotic resistance. Biomed Res Int. 2018;2018:7656752. doi: 10.1155/2018/7656752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaikh S, Fatima J, Shakil S, Rizvi SMD, Kamal MA. Antibiotic resistance and extended spectrum beta-lactamases: types, epidemiology and treatment. Saudi J Biol Sci. 2015;22(1):90–101. doi: 10.1016/j.sjbs.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chervet D, Lortholary O, Zahar J-R, Dufougeray A, Pilmis B, Partouche H. Antimicrobial resistance in community-acquired urinary tract infections in Paris in 2015. Med Mal Infect. 2018;48(3):188–192. doi: 10.1016/j.medmal.2017.09.013 [DOI] [PubMed] [Google Scholar]
- 4.Arana DM, Rubio M, Alós J-I. Evolution of antibiotic multiresistance in Escherichia coli and Klebsiella pneumoniae isolates from urinary tract infections: a 12-year analysis (2003–2014). Enferm Infecc Microbiol Clin. 2017;35(5):293–298. doi: 10.1016/j.eimc.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 5.Picozzi S, Ricci C, Gaeta M, et al. Do we really know the prevalence of multi-drug resistant Escherichia coli in the territorial and nosocomial population? Urol Ann. 2013;5(1):25–29. doi: 10.4103/0974-7796.106962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patwardhan V, Kumar D, Goel V, Singh S. Changing prevalence and antibiotic drug resistance pattern of pathogens seen in community-acquired pediatric urinary tract infections at a tertiary care hospital of North India. J Lab Physicians. 2017;9(4):264–268. doi: 10.4103/JLP.JLP_149_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balasubramanian S, Kuppuswamy D, Padmanabhan S, Chandramohan V, Amperayani S. Extended-spectrum beta-lactamase-producing community-acquired urinary tract infections in children: chart review of risk factors. J Glob Infect Dis. 2018;10(4):222–225. doi: 10.4103/0974-777X.246391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fatima S, Muhammad IN, Usman S, Jamil S, Khan MN, Khan SI. Incidence of multidrug resistance and extended-spectrum beta-lactamase expression in community-acquired urinary tract infection among different age groups of patients. Indian J Pharmacol. 2018;50(2):69–74. doi: 10.4103/ijp.IJP_200_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Essential medicines and health products. February 27, 2017. Available from:https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/. Accessed October6, 2020.
- 10.Livermore DM. Current epidemiology and growing resistance of gram-negative pathogens. Korean J Intern Med. 2012;27(2):128–142. doi: 10.3904/kjim.2012.27.2.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ur Rahman S, Ali T, Ali I, Khan NA, Han B, Gao J. The growing genetic and functional diversity of extended spectrum beta-lactamases. Biomed Res Int. 2018;2018:9519718. doi: 10.1155/2018/9519718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahlmeter G, Poulsen HO. Antimicrobial susceptibility of Escherichia coli from community-acquired urinary tract infections in Europe: the ECO·SENS study revisited. Int J Antimicrob Agents. 2012;39(1):45–51. doi: 10.1016/j.ijantimicag.2011.09.013 [DOI] [PubMed] [Google Scholar]
- 13.Tandogdu Z, Wagenlehner FME. Global epidemiology of urinary tract infections. Curr Opin Infect Dis. 2016;29(1):73–79. doi: 10.1097/QCO.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 14.Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. 2014;28(1):1–13. doi: 10.1016/j.idc.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Antimicrobial resistance: global report on surveillance [Internet]. 2014. Available from: https://apps.who.int/iris/handle/10665/112642. Accessed April18, 2019.
- 16.Review on Antimicrobial Resistance. Tackling drug-resistant infections globally: final report and recommendations [Internet]. 2016. Available from: https://amr-review.org/. Accessed April18, 2019.
- 17.World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics [Internet]. 2017. Available from: http://apps.who.int/medicinedocs/en/m/abstract/Js23171en/. Accessed April18, 2019.
- 18.Tenney J, Hudson N, Alnifaidy H, Li JTC, Fung KH. Risk factors for aquiring multidrug-resistant organisms in urinary tract infections: a systematic literature review. Saudi Pharm J. 2018;26(5):678–684. doi: 10.1016/j.jsps.2018.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costel EE, Mitchell S, Kaiser AB. Abbreviated surveillance of nosocomial urinary tract infections: a new approach. Infect Control. 1985;6(1):11–13. doi: 10.1017/S0195941700062433 [DOI] [PubMed] [Google Scholar]
- 20.Schoonjans F. MedCalc statistical software [Internet]. MedCalc Available from: https://www.medcalc.org/. Accessed April18, 2019.
- 21.Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall; 1991. [Google Scholar]
- 22.Wells G, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyzes. Ottawa Hospital Research Institute. 2009. [Google Scholar]
- 23.Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. doi: 10.1186/1471-2458-13-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanco VM, Maya JJ, Correa A, et al. [Prevalence and risk factors for extended-spectrum β-lactamase-producing Escherichia coli causing community-onset urinary tract infections in Colombia]. Enferm Infecc Microbiol Clin. 2016;34(9):559–565. doi: 10.1016/j.eimc.2015.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nisha KV, Veena SA, Rathika SD, Vijaya SM, Avinash SK. Antimicrobial susceptibility, risk factors and prevalence of bla cefotaximase, temoneira, and sulfhydryl variable genes among Escherichia coli in community-acquired pediatric urinary tract infection. J Lab Physicians. 2017;9(3):156–162. doi: 10.4103/0974-2727.208262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez Heras I, Sanchez-Gomez JC, Beneyto-Martin P, Ruano-de-Pablo L, Losada-Pinedo B. Community-onset extended-spectrum β-lactamase producing Escherichia coli in urinary tract infections in children from 2015 to 2016: prevalence, risk factors, and resistances. Medicine. 2017;96(50):e8571. doi: 10.1097/MD.0000000000008571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hertz FB, Schønning K, Rasmussen SC, et al. Epidemiological factors associated with ESBL- and non ESBL-producing E. coli causing urinary tract infection in general practice. Infect Dis (Lond). 2016;48(3):241–245. doi: 10.3109/23744235.2015.1103895 [DOI] [PubMed] [Google Scholar]
- 28.Kang C-I, Wi YM, Lee MY, et al. Epidemiology and risk factors of community onset infections caused by extended-spectrum β-lactamase-producing Escherichia coli strains. J Clin Microbiol. 2012;50(2):312–317. doi: 10.1128/JCM.06002-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savatmorigkorngul S, Poowarattanawiwit P, Sawanyawisuth K, Sittichanbuncha Y. Factors associated with extended spectrum β-lactamase producing escherichia coli in community-acquired urinary tract infection at hospital emergency department, Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 2016;47(2):227–233. [PubMed] [Google Scholar]
- 30.Park SH, Choi S-M, Lee D-G, et al. Impact of extended-spectrum β-lactamase production on treatment outcomes of acute pyelonephritis caused by Escherichia coli in patients without health care-associated risk factors. Antimicrob Agents Chemother. 2015;59(4):1962–1968. doi: 10.1128/AAC.04821-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Artero A, Esparcia A, Alberola J, Madrazo M, Nogueira JM, Eiros JM. Prospective cohort study of risk factors for extended-spectrum ß-lactamase-producing Escherichia coli urinary tract infections in elderly patients admitted to hospital. Int J Clin Pract. 2017;71:9. doi: 10.1111/ijcp.13001 [DOI] [PubMed] [Google Scholar]
- 32.Fan N-C, Chen -H-H, Chen C-L, et al. Rise of community-onset urinary tract infection caused by extended-spectrum β-lactamase-producing Escherichia coli in children. J Microbiol Immunol Infect. 2014;47(5):399–405. doi: 10.1016/j.jmii.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 33.Ozdogan FN, Demirdal T, Nemli SA. Risk factors for community acquired urinary tract infections caused by extended spectrum beta-lactamase (ESBL) producing Escherichia coli. In: European Congress of Clinical Microbiology & Infectious Diseases 2015 [Internet]; 2015; Copenhague, Danemark: ESCMID; Available from: https://www.escmid.org/escmid_publications/escmid_elibrary/. Accessed April2, 2019. [Google Scholar]
- 34.Toumi A, Hafsa M, Kadri Y. Risk factors for community-acquired acute pyelonephritis caused by extended-spectrum beta-lactamase-producing Escherichia coli in a university hospital in Tunisia. In: European Congress of Clinical Microbiology & Infectious Diseases 2015 [Internet]; 2015; Copenhague, Danemark: ESCMID; Available from: https://www.escmid.org/escmid_publications/escmid_elibrary/?q=toumi&id=2173&L=0&x=26&y=26. Accessed April2, 2019. [Google Scholar]
- 35.Søraas A, Sundsfjord A, Sandven I, Brunborg C, Jenum PA. Risk factors for community-acquired urinary tract infections caused by ESBL-producing enterobacteriaceae – a case-control study in a low prevalence country. PLoS One. 2013;8(7):e69581. doi: 10.1371/journal.pone.0069581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calbo E, Romaní V, Xercavins M, et al. Risk factors for community-onset urinary tract infections due to Escherichia coli harbouring extended-spectrum β-lactamases. J Antimicrob Chemother. 2006;57(4):780–783. doi: 10.1093/jac/dkl035 [DOI] [PubMed] [Google Scholar]
- 37.Azap OK, Arslan H, Serefhanoğlu K, et al. Risk factors for extended-spectrum beta-lactamase positivity in uropathogenic Escherichia coli isolated from community-acquired urinary tract infections. Clin Microbiol Infect. 2010;16(2):147–151. doi: 10.1111/j.1469-0691.2009.02941.x [DOI] [PubMed] [Google Scholar]
- 38.Søgaard M, Heide-Jørgensen U, Vandenbroucke JP, Schønheyder HC, Vandenbroucke-Grauls CMJE. Risk factors for extended-spectrum β-lactamase-producing Escherichia coli urinary tract infection in the community in Denmark: a case-control study. Clin Microbiol Infect. 2017;23(12):952–960. doi: 10.1016/j.cmi.2017.03.026 [DOI] [PubMed] [Google Scholar]
- 39.Castillo-Tokumori F, Irey-Salgado C, Málaga G. Worrisome high frequency of extended-spectrum beta-lactamase-producing Escherichia coli in community-acquired urinary tract infections: a case-control study. Int J Infect Dis. 2017;55:16–19. doi: 10.1016/j.ijid.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 40.Tham J, Odenholt I, Walder M, Andersson L, Melander E. Risk factors for infections with extended-spectrum beta-lactamase-producing Escherichia coli in a county of southern Sweden. Infect Drug Resist. 2013;6:93–97. doi: 10.2147/IDR.S46290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arcilla MS, van Hattem JM, Haverkate MR, et al. Import and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis. 2017;17(1):78–85. doi: 10.1016/S1473-3099(16)30319-X [DOI] [PubMed] [Google Scholar]
- 42.Friedman ND, Kaye KS, Stout JE, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137(10):791–797. doi: 10.7326/0003-4819-137-10-200211190-00007 [DOI] [PubMed] [Google Scholar]
- 43.Pitout JDD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159–166. doi: 10.1016/S1473-3099(08)70041-0 [DOI] [PubMed] [Google Scholar]
- 44.Al-Otaibi FE, Bukhari EE. Clinical and laboratory profiles of urinary tract infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in a tertiary care center in central Saudi Arabia. Saudi Med J. 2013;34(2):171–176. [PubMed] [Google Scholar]
- 45.Briongos-Figuero LS, Gómez-Traveso T, Bachiller-Luque P, et al. Epidemiology, risk factors and comorbidity for urinary tract infections caused by extended-spectrum beta-lactamase (ESBL)-producing enterobacteria. Int J Clin Pract. 2012;66(9):891–896. doi: 10.1111/j.1742-1241.2012.02991.x [DOI] [PubMed] [Google Scholar]
- 46.Köck R, Daniels-Haardt I, Becker K, et al. Carbapenem-resistant Enterobacteriaceae in wildlife, food-producing, and companion animals: a systematic review. Clin Microbiol Infect. 2018;24(12):1241–1250. doi: 10.1016/j.cmi.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 47.Lazarus B, Paterson DL, Mollinger JL, Rogers BA. Do human extraintestinal Escherichia coli infections resistant to expanded-spectrum cephalosporins originate from food-producing animals? A systematic review. Clin Infect Dis. 2015;60(3):439–452. doi: 10.1093/cid/ciu785 [DOI] [PubMed] [Google Scholar]
- 48.Jørgensen SB, Søraas AV, Arnesen LS, Leegaard TM, Sundsfjord A, Jenum PA. A comparison of extended spectrum β-lactamase producing Escherichia coli from clinical, recreational water and wastewater samples associated in time and location. PLoS One. 2017;12(10):e0186576. doi: 10.1371/journal.pone.0186576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tumbarello M, Trecarichi EM, Bassetti M, et al. Identifying patients harboring extended-spectrum-beta-lactamase-producing Enterobacteriaceae on hospital admission: derivation and validation of a scoring system. Antimicrob Agents Chemother. 2011;55(7):3485–3490. doi: 10.1128/AAC.00009-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson SW, Anderson DJ, May DB, Drew RH. Utility of a clinical risk factor scoring model in predicting infection with extended-spectrum β-lactamase-producing enterobacteriaceae on hospital admission. Infect Control Hosp Epidemiol. 2013;34(4):385–392. doi: 10.1086/669858 [DOI] [PMC free article] [PubMed] [Google Scholar]