Abstract
Osteosarcoma (OS) is the most common primary bone malignancy and responsible for considerable morbidity and mortality due to its high rates of pulmonary metastasis. Although neoadjuvant chemotherapy has improved 5-year survival rates for patients with localized OS from 20% to over 65%, outcomes for those with metastasis remain dismal. In addition, therapeutic regimens have not significantly improved patient outcomes over the past four decades, and metastases remains a primary cause of death and obstacle in curative therapy. These limitations in care have given rise to numerous works focused on mechanisms and novel targets of OS pathogenesis, including tumor niche factors. OS is notable for its hallmark production of rich extracellular matrix (ECM) of osteoid that goes beyond simple physiological growth support. The aberrant signaling and structural components of the ECM are rich promoters of OS development, and very recent works have shown the specific pathogenic phenotypes induced by these macromolecules. Here we summarize the current developments outlining how the ECM contributes to OS progression and metastasis with supporting mechanisms. We also illustrate the potential of tumorigenic ECM elements as prognostic biomarkers and therapeutic targets in the evolving clinical management of OS.
Keywords: Extracellular matrix, Osteosarcoma, Metastasis, Prognostic biomarker, Therapeutic target
Background
Osteosarcoma (OS) is the most common primary bone malignancy and disproportionately affects those in childhood and adolescence [1]. Before the widespread use of chemotherapy in the 1970s, surgical resection was the primary treatment modality available to OS patients [2]. Adjuvant chemotherapy has since dramatically improved the prognosis for OS patients, with the five-year survival rate increased from 20% to approximately 55 to 70% in patients with localized disease [3, 4]. However, in cases of metastatic lesions, the five-year survival rate remains dismal at less than 20% [5]. Targeting and preventing metastasis has thus been a significant obstacle in OS treatment, and recent publications have highlighted various novel treatment strategies to that end. The dysregulation and aberrant remodeling of extracellular matrix (ECM) has gained considerable attention for its promise in pathogenic targeting and predictive value.
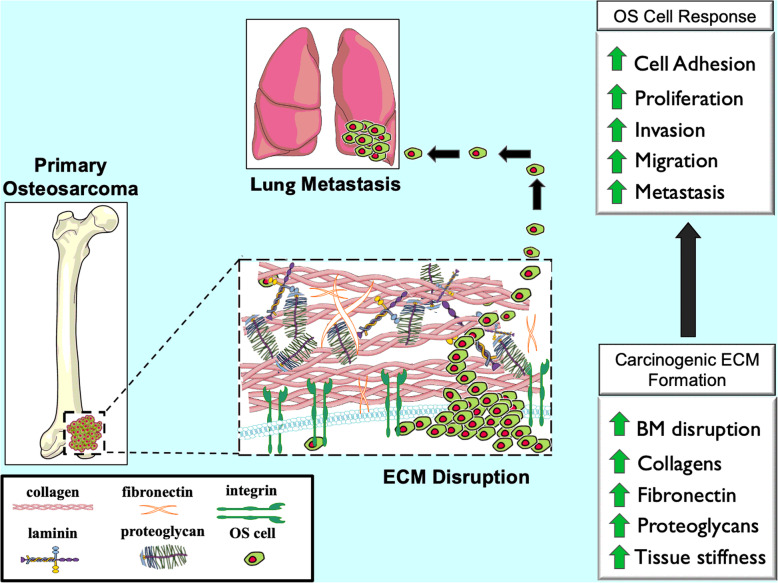
Very recently, the tumor microenvironment (TME) has gained prominence outside of its traditional role of cellular support as a veritable contributor to cancer progression and metastasis [6]. The TME, consists of a complex arrangement of blood vessels, fibroblasts, immune cells, endothelial cells, signaling molecules, extracellular vesicles and most importantly, the ECM. The ECM forms a three-dimensional acellular network of macromolecules which provide the necessary structural and biochemical support of its cellular constituents [7–9]. In addition to its function as a supportive framework, the ECM regulates most cellular behaviors, including communication, migration, adhesion, proliferation, and differentiation [10–12]. Furthermore, when aberrant, these functions are hijacked and form a specific ECM remodeling profile that enables metastatic dissemination of cancer cells [13, 14]. These features of ECM transformation have been reported in OS development and progression, a tumor with a characteristically robust ECM [15, 16]. For OS, the generation of pathogenic osteoid matrix and other ECM components enables a supportive scaffold for rapid tumor progression [17, 18] (Fig. 1).
Fig. 1.
ECM changes in OS progression and metastasis. The primary components of ECM in normal bone are significantly changed in osteosarcoma (OS). Due to activated fibroblasts, cancer cells, collagen deposition, fibronectin, and other ECM components, ECM production is dramatically increased which results in a stiffer stroma and more aggressive phenotype. The basement membrane surrounding the primary tumor site is broken down by ECM remodeling enzymes allowing for OS cells from the primary tumor to undergo hematogenous spread where they frequently seed the lung
In this review, we summarize the most recent discoveries of ECM contribution to OS progression and metastasis. We also detail the various ECM components that have shown preclinical and clinical promise as prognostic predictors and therapeutic targets in OS.
ECM components and their function in OS
The ECM is primarily composed of collagen, fibronectin, laminin, and proteo- glycan which shape and maintain tissue vitality [7, 19, 20]. In the pathological state of cancer, however, ECM cultivates tumorigenesis and metastasis in malignancies such as OS [21–25] (Table 1).
Table 1.
The ECM components involved in OS
| ECM protein | Expression in OS | Roles in OS | References | |
|---|---|---|---|---|
| Collagens | Collagen I | Increase | Invasion and metastasis | [26] |
| Collagen III | Increase | Chemotherapy resistance | [27] | |
| Collagen IV | Increase | Angiogenesis | [28] | |
| Collagen V | Increase | Adhesion | [29] | |
| Collagen XVIII | Decrease | Anti-angiogenesis | [30, 31] | |
| Cell growth and Metastasis | [32] | |||
| Fibronectin | Increase | Adhesion | [33–35] | |
| Chemotherapy resistance | [36] | |||
| Metastasis | [37, 38] | |||
| Invasion | [39] | |||
| Laminins | Increase | Adhesion | [40] | |
| Invasion | [41, 42] | |||
| Proteoglycans | Biglycan | Increase | Cell growth | [43, 44] |
| Decorin | Decrease | Migration | [45] | |
| Cell growth | [46] | |||
| Lumican | Increase | Cell growth | [47] | |
| Adhesion | [48] | |||
| Versican | Increase | Migration and invasion | [49] | |
| HA | Increase | Proliferation and invasion | [50–52] | |
| Cell apoptosis | [53] | |||
| Metastasis | [54, 55] | |||
Collagens
Collagens are the main organic components of the ECM and represents approximately 30% of the total protein mass of the human body [56]. The collagen superfamily includes 28 members, each with their own unique three polypeptide chains assembled into a final triple helix structure [57, 58]. Several collagens have been investigated in OS, including collagens I, III, IV, V, and XVIII. The exact changes these collagens undergo are of considerable interest in OS progression especially given their abundance to the OS stroma.
Collagen I
Collagen I is composed of two alpha 1 chains and one alpha 2 chain, which are encoded by the COL1A1 and COL1A2 genes, respectively [59]. Collagen I is a rich ECM component and found in connective tissues such as bone, tendon, and ligament [60]. Elevated concentrations of collagen I metabolites have been found in untreated OS patients’ serum [61], and supplementation with exogenous collagen I has shown to increase the synthesis and activation of MMP-2 in OS cell lines [62]. This is of interest, as MMP-2 alone has been shown to promote OS progression, invasion, and migration [26]. Additionally, MMP2 activity is significantly increased in those OS patients with poor response to chemotherapy [63].
Collagen III
Collagen III is composed of three identical peptide chains encoded by the COL3A1 gene and is found throughout cortical bone [64, 65]. A significantly higher level of COL3A1 mRNA expression has been observed in chemoresistant patients compared to those with a more favorable response to therapy [27]. Furthermore, overexpression of COL3A1 in methotrexate-resistant OS cell lines significantly reduces apoptosis via the activity of miR-29abc, a miRNA in the miR-29 family [27].
Collagen IV
Collagen IV is a heterotrimer composed of three different α chains from alpha 1 to alpha 6 [66]. These chains are encoded by the COL4A1- COL4A6 genes. Collagen IV is a major constituent of basement membranes in the ECM and is heavily involved in interaction with other cellular components [67]. In a combined culture system with a 3D OS cell line and 2D endothelial cell line, the endothelial cells formed a vascular network expressing collagen IV. These networks infiltrated the nearby tumor spheroids with tubular structures. These results support the role of collagen IV in regulating OS angiogenesis, a well-known feature of tumor proliferation [28].
Collagen V
Collagen V exists as an alpha1, alpha2, and alpha3 heterotrimer which are encoded by COL5A1, COL5A2, and COL5A3 genes, respectively [68]. While collagen V is a relatively minor component of the ECM, it has critical roles in matrix organization alongside collagen I [69]. Together, the deposition and cross-linking of collagen I and collagen V are the principal components of cultured OS cell ECM [70]. Collagen V is especially important in the cell contact and interactions of OS, as the peptides derived from the basic segment of the alpha 3 chain of collagen V form adhesive qualities [29].
Collagen XVIII
Collagen XVIII contains 10 collagenous domains encoded by the COL18A1 gene [71]. This collagen is a component of basement membranes in the ECM, with structural properties of both collagen and proteoglycan [72]. Proteolytic cleavage within the C-terminal domain of collagen XVIII releases a fragment, endostatin, with anti-angiogenic effects [73]. Endostatin is important in the progression of various tumors, including OS [74, 75]. As angiogenesis is important for OS progression and metastasis, researchers elected to analyze the effects of endostatin in an orthotopic OS model. Results were positive, as their endostatin anti-angiogenic therapy significantly reduced the postoperative progression of pulmonary metastasis [30, 31]. Another study showed a combination of endostatin with Adriamycin produced synergistic inhibition of tumor growth and pulmonary metastases in an orthotopic OS model [32].
Fibronectin
Fibronectin is an adhesive glycoprotein of the ECM composed of two polypeptides which bind integrins, collagen, fibrin, heparin, and proteoglycans [76, 77]. It forms a multidimensional fibrillar matrix with partial control of cell adhesion, migration, and differentiation [78–80]. Abundant expression of fibronectin is apparent in OS cell lines [81]. The heparin-binding domain of fibronectin affects cell adhesion and spreading of OS cells by cooperating with the central cell-binding domain of fibronectin [33]. Significant upregulation of fibronectin had been detected in chemo- resistant OS cell lines [36].
Fibronectin displays various functional motifs that interact with integrins, which are the most common transmembrane receptors and regulate its function [82–84]. The integrin structure is formed by heterodimers of α and β subunits which penetrate the cell membrane and form several cytoplasmic domains [85]. The binding of fibronectin with integrins represents a crucial step in OS progression and metastasis [37, 38]. In a recent in vitro work, integrins were shown to be involved not only in cell adhesion but also in the binding and assembly of exogenous fibronectin [34]. Selective down-regulation of integrins resulted in the decreased deposition of fibronectin within the ECM and subsequently reduced overall OS cell spreading and adhesion [39]. Conversely, upregulation of integrins enhanced adhesiveness of OS cells to fibronectin [35].
Laminins
Laminins are components of the basement membrane in ECM and are constructed of heterotrimeric glycoproteins with alpha, beta, and gamma chain subunits [86, 87]. They interact with their respective cancer cell receptors whereby they promote angiogenesis, invasion, and metastasis [88]. Laminins have demonstrated to enhance cell adhesion in OS cell lines [40], with high laminin-adherent OS cells showing notably more invasiveness than their low laminin-adherent counterparts [41]. In a work which implemented a 3D OS cell line model, a matrix supplemented with laminin led to an increased invasion of OS cells into the surrounding acellular bone marrow environment [42].
Proteoglycans
Proteoglycans are heavily dispersed throughout the ECM and are composed of glycosylated proteins with a protein core and covalently attached glycosaminoglycan (GAG) chains [89, 90]. The GAGs are major regulators of metastasis in various cancers [91–93]. Hyaluronic acid (HA), also known as hyaluronan or hyaluronate, is another macromolecule that belongs to the GAG family. HA is abundant in most tissues and has unique properties as a result of its variable covalent bonding and core proteins [90, 94]. And although HA is not a true proteoglycan, it possesses similar biological functions. It is synthesized on the cytoplasmic membrane and is directly secreted into the ECM [95]. Based on the core protein and GAG chain properties, proteoglycans are classified into one of three groups, including small leucine-rich proteoglycans (SLRPs), modular proteoglycans, and cell-surface proteoglycans [90]. Overall, these unique variants have roles in ECM communication, tumor angiogenesis, progression, and metastasis [96, 97].
SLRPs
SLRPs have relatively short protein cores with a central domain of leucine-rich repeats [98]. The SLRP family is divided into five classes according to structure and includes classes I to V [99, 100]. Functionally, these proteins regulate ECM organization and cell behavior [101]. Of the SLRP family members, biglycan, decorin, and lumican have been investigated in OS.
Biglycan is a class I SLRP encoded by the BGN gene which promotes proliferation and differentiation in OS cells [43, 102]. A mechanistic study has revealed biglycan enhances OS cell growth through the low-density lipoprotein receptor- related protein 6 /β-catenin/IGF-I receptor signaling pathway [44].
Decorin is another class I SLRP and a small pericellular matrix proteoglycan with a structure closely related to biglycan. That is where their similarities end, however, as its presence negatively correlates with oncogenesis. Decorin inhibits OS cell migration through its glycosaminoglycan side chains [45, 103]. Ectopic expression of decorin significantly decreased OS cell growth through the induction of cyclin-dependent kinase inhibitor P21 [46].
Lumican is a class II SLRPs and encoded by the LUM gene [104]. It positively correlates with OS cell differentiation and inversely correlated with growth [47]. In a subsequent study, lumican was shown to regulate OS cell adhesion by modulating transforming growth factor beta-2 activity [48].
Modular proteoglycans
Modular proteoglycans are multidomain motif proteins with a highly glycosylated structure [105]. They are subdivided into families of HA-binding, lectin-binding, and non-HA-binding proteoglycans [90, 96]. The four proteoglycans versican, aggrecan, neurocan, and brevican constitute the family of HA-binding proteoglycans [106]. Versican is notable for its ability to regulate cellular processes including adhesion, proliferation, apoptosis, and invasion [107, 108]. High expression of versican has been found in OS tissues relative to normal tissues [49]. Its expression is up-regulated by transforming growth factor-beta 1 (TGFß1), which leads to enhanced OS cell migration and invasion [49].
Ha
As previously stated, HAs have similar functions to proteoglycans [94]. They exist in all tissues and are abundant in bone [109]. In addition to their structural importance, HAs have strong roles in cancer cell differentiation, proliferation, and migration when aberrantly expressed [94]. HA promotes OS cell proliferation and invasion by initiating intracellular signal transduction [50]. In a work where HA accumulation was selectively inhibited, there was a substantial decrease in OS cell proliferation, motility, and invasiveness [51]. The inhibition of HA can also reduce cell viability and induce apoptosis in OS cells [53]. At the microscopic level, cells interact with HA through cell surface receptors, which initiates their actions. The cluster of differentiation 44 (CD44) is a well-known cell membrane receptor for HA. When HA is bound to CD44, it regulates cell-cell interactions, cell adhesion, and migration [110]. The HA-CD44 pathway increases tumor aggression and drug resistance as well as influencing the cancer stem cell phenotype through promoting stem-cell gene expression, progression, and metastasis [111]. Of note, the expression of CD44 is significantly higher in metastatic and recurrent OS patient tumor specimens compared to primary tumor tissues [54]. Therapeutically, the proliferation and spheroid formation of OS cells is inhibited in 3-D culture when CD44 is silenced [52]. In an orthotopic mouse model of OS, injection with CD44 overexpressing OS cells resulted in increased primary tumor formation and lung metastasis, which was dependent on the HA to CD44 interaction [55].
Signaling pathways responsible for ECM remodeling in OS
The function of ECM is derived from its diverse composition of macromolecules, proteases, inhibitors, and their respective downstream signaling pathways [112]. Within the ECM of OS, matrix metalloproteinases (MMPs) and heparinases regulate several pathways responsible for progression and metastasis (Fig. 2).
Fig. 2.
Schematic of signaling pathways involved the ECM remodeling in OS. The ECM is dynamically remodeled by multiple proteases and signaling pathways. In OS, MMP-2, MMP-9, and MMP-13 function via PI3K/Akt and ERK associated signaling pathways. Heparanase also participates in ECM remodeling, as it cleaves the ECM by heparan sulfate degradation, thus promoting OS cell invasion and metastasis
MMPs
MMPs are proteolytic enzymes that degrade surrounding ECM components, release active growth factors, and promote tumor angiogenesis [113]. Elevated levels of MMP-2, MMP-9, and MMP-13 exist in OS (Fig. 2) and contribute to cell migration, invasion, and metastasis. The upstream PI3K/Akt signaling pathway promotes the expression of MMP-2 and MMP-9, thus degrading ECM and enabling OS cell invasion and metastasis [114, 115]. The extracellular signal-regulated kinase (ERK) signaling pathway also upregulates MMP-2 and MMP-9 and migratory activity of OS cells [116, 117]. Another MMP, MMP-13, causes turnover of ECM collagens and proteoglycans and directly correlates with OS progression. In one recent study, plasminogen activator inhibitor-1 was shown to upregulate the expression of MMP-13 and promote invasion and lung metastasis in an OS mouse model [118].
Heparanase
Heparanase is an endo-β-D-glucuronidase that cleaves heparan sulfate chains in the ECM, thus releasing heparan sulfate-binding angiogenic factors and allowing for tumor cell migration, invasion, and metastasis [119, 120] (Fig. 2). Previous works have shown the down-regulation of heparanase significantly reduces OS cell proliferation and invasion [121, 122]. Additionally, OS patient tissues with more heparanase correlate with higher microvessel density and rates of pulmonary metastasis [122].
ECM components as prognostic biomarkers in OS
Although the adoption of neoadjuvant chemotherapy in OS has significantly improved patient survival since its implementation several decades ago, outcomes have since plateaued. The personalized and immunotherapies that have shown great promise in several cancers have had less favorable results for OS, likely due in part to its heterogeneity between patients. There is, therefore, an urgent need for prognostic biomarkers which allow for the delineation of patients according to their unique tumor microenvironments and response patterns, so that their therapeutic regimens can be tailored accordingly. In response, there has been an expansion of works investigating the components of the ECM, some of which have been found to play vital roles in cancer progression, metastasis, and clinical outcomes [123–125]. An emergence of clinical data has revealed various collagens to correlate with the clinical stage, metastasis, and outcomes [126]. The correlation between ECM makeup with clinical stage and prognosis in OS are summarized (Table 2). Several noteworthy examples exist, including the expression of collagen triple helix repeat containing 1 (CTHRC1) protein in OS. It has significantly higher expression compared to adjacent normal tissue controls, and predicts a poor prognosis of OS patients [127]. Functionally, CTHRC1 is a secretory protein known to regulate vascular remodeling and bone formation [128]. A collagen I (COL1A1) polymorphism is associated with OS susceptibility and death [129]. Fibronectin is overexpressed in OS specimens compared to osteochondroma as well as other tissues [130, 131]. Additionally, overexpression of fibronectin in OS tissues is associated with a poorer chemo- therapeutic response, distant metastasis, and shorter overall survival [130, 131]. In short, these works support high fibronectin expression as an underlying mechanism of aggressive clinical behavior in OS. A higher level of CD44 expression in OS tissues is apparent in patients with shorter survival and those with an unfavorable response to neoadjuvant chemotherapy [54]. Furthermore, CD44 expression is predictive of poor survival, metastasis, recurrence, and drug resistance in patients with OS [132, 133].
Table 2.
ECM as prognostic predictors in OS
| ECM components | Expression in OS | Prognostic value | References | |
|---|---|---|---|---|
| Collagens | CTHRC1 | High | Shorter survival time | [116] |
| COL1A1 | High | Shorter survival time | [118] | |
| Fibronectin | High | Metastasis, poor response to chemotherapy, and shorter survival time | [119, 120] | |
| Proteoglycans | CD44 | High | Poor response to chemotherapy | [54] |
| Metastasis, recurrence and shorter survival time | [121, 122] | |||
ECM components as potential therapeutic targets in OS
The ECM is pivotal in OS pathogenesis, especially in tumor cell migration and invasion. Targeting the regulatory and responsible molecules within the ECM has thus been explored as a novel strategy for OS treatment (Table 3).
Table 3.
ECM as therapeutic targets in OS
| Therapeutic target | Functions | References | |
|---|---|---|---|
| Collagens | COL3A1 | Methotrexate resistance, apoptosis | [27] |
| Tumstatin | Cell proliferation, apoptosis | [124] | |
| Endostatin | Metastases | [125] | |
| Fibronectin | Fibronectin | Doxorubicin sensitivity | [36] |
| Integrins | Cell proliferation, metastasis | [129] | |
| Proteoglycans | Decorin | Cell invasion, metastasis | [130] |
| CD44 | Doxorubicin sensitivity | [54] | |
Collagen targets
Overexpression of COL3A1 can decrease apoptosis and promote methotrexate resistance in OS cell lines. The precise targeting of COL3A1 is therefore a promising and personalized strategy for overcoming methotrexate resistance in candidate OS tumors [27]. The antiangiogenic protein fragment tumstatin, which is cleaved from collagen, is the non-collagenous domain of the alpha 3 chain in collagen IV shown to inhibit cell proliferation and induce cell apoptosis in OS cell lines [134]. Mechanistically, this occurs through the phosphorylation of p65NF-κB and its subsequent nuclear translocation [135]. Tumstatin has therefore become of interest in the treatment of OS [135]. Endostatin combined with other chemotherapy has been evaluated in an OS clinical trial, with results showing a significant reduction in angiogenesis, metastasis, and an increased event-free survival rate [136]. Overall, endostatin-targeting angiogenesis-based therapy has yielded positive results for OS patients at the clinical trial level.
Fibronectin targets
The fibronectin and integrin families within the ECM regulate a diverse array of cellular functions crucial for proliferation, progression, and metastasis [137]. Therapeutically, fibronectin inhibition greatly increases OS sensitivity to doxorubicin in vitro. Similarly, fibronectin knockdown decreases the tumor growth rate and can even resensitize OS to doxorubicin in orthotopic OS models [36]. Consequently, targeting fibronectin has become a promising treatment for doxorubicin-resistant OS [36]. As the main receptor of fibronectin, integrins are also proposed targets of cancer treatment. Several studies have shown inhibition of integrin or its downstream effectors to block many of the major hallmarks of cancer [137–139]. Additionally, selective knockdown of integrins significantly inhibits OS growth and lung metastasis, and an exogenous reintroduction of integrins can restore cell proliferation and lung metastasis in xenograft models of OS [140]. As pulmonary metastasis is the major cause of patient death in OS, these findings are especially promising and warrant future works.
Proteoglycans targets
In a murine OS model, significantly fewer pulmonary metastases and longer survival times were observed in mice treated with decorin, a matrix proteoglycan. The works of these investigations support decorin as a potential therapeutic target in the prevention of lung metastasis in OS [141]. As previously described, CD44 is important in OS progression. Furthermore, it is the direct target of miR-199a-3p, which is a significantly downregulated miRNA in OS [142, 143]. As a therapeutic strategy, overexpression of miR-199a-3p significantly inhibits CD44 expression in OS cell lines, with transfection also increasing chemosensitivity. Taken together, these results support targeting CD44 to reduce pulmonary metastasis and increase OS clinical outcomes [54].
Conclusion and future perspectives
In addition to is physiologic importance in structural and biochemical support, the ECM has gained increased recognition for its carcinogenic roles, including in the progression and metastasis of OS. The various components of the ECM including collagens, fibronectin, laminins, and proteoglycans may contribute to OS progression and metastasis through distinct and intertwining mechanisms. It is therefore important to further study and validate the ECM components, cellular receptors, and associated signaling pathways in OS synergistically and as components of the primary tumor tissue. Novel culture systems will be especially important in this endeavor, as resembling the in vivo tumor microenvironment with in vitro customizability, such as with 3D cell culture, will be necessary to accurately model extracellular matrix and growth. Overall, the ECM components have shown promise as clinical biomarkers and therapeutic targets in OS, and warrant a continued evaluation in preclinical models as well as future clinical trials.
Acknowledgements
Not applicable.
Abbreviations
- OS
Osteosarcoma
- TME
Tumor microenvironment
- ECM
Extracellular matrix
- GAG
Glycosaminoglycan
- HA
Hyaluronic acid
- SLRPs
Small leucine-rich proteoglycans
- CTHRC1
Collagen triple helix repeat containing 1
- MMPs
Matrix metalloproteinases
Authors’ contributions
Conception of the work: JC, ZC and ZD. Drafting of the article: JC and DD. Preparing figures: JC and FJH. Critical revision of the article: ZC and ZD. Final approval: JC, DD, FJH, ZC and ZD.
Funding
This work was supported, in part, by the Department of Orthopaedic Surgery at UCLA. Dr. Cui is supported by the Natural Science Foundation of Hunan (2018JJ3468), Science and Technology Innovation Program in Human Province (2018SK2077) and a scholarship from the China Scholarship Council (201808430243).
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors of this paper have approved the final version of the manuscript.
Competing interests
The authors declare that there are no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhiwei Chen, Email: czw9923@sina.com.
Zhenfeng Duan, Email: ZDuan@mednet.ucla.edu.
References
- 1.Sampson VB, Yoo S, Kumar A, Vetter NS, Kolb EA. MicroRNAs and potential targets in osteosarcoma: review. Front Pediatr. 2015;3:69. doi: 10.3389/fped.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcove RC, Mike V, Hajek JV, Levin AG, Hutter RV. Osteogenic sarcoma under the age of twenty-one. A review of one hundred and forty-five operative cases. J Bone Joint Surg Am. 1970;52(3):411–423. [PubMed] [Google Scholar]
- 3.Shin SH, Jeong HJ, Han I, Cho HS, Kim HS. Osteosarcoma and chondrosarcoma of the shoulder: site-specific comparative analysis. Orthopedics. 2013;36(2):e179–e185. doi: 10.3928/01477447-20130122-20. [DOI] [PubMed] [Google Scholar]
- 4.Sampo M, Koivikko M, Taskinen M, Kallio P, Kivioja A, Tarkkanen M, Bohling T. Incidence, epidemiology and treatment results of osteosarcoma in Finland - a nationwide population-based study. Acta Oncol. 2011;50(8):1206–1214. doi: 10.3109/0284186X.2011.615339. [DOI] [PubMed] [Google Scholar]
- 5.Guo J, Reddick WE, Glass JO, Ji Q, Billups CA, Wu J, Hoffer FA, Kaste SC, Jenkins JJ, Ortega Flores XC, et al. Dynamic contrast-enhanced magnetic resonance imaging as a prognostic factor in predicting event-free and overall survival in pediatric patients with osteosarcoma. Cancer. 2012;118(15):3776–3785. doi: 10.1002/cncr.26701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michel G, Tonon T, Scornet D, Cock JM, Kloareg B. The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in eukaryotes. New Phytol. 2010;188(1):82–97. doi: 10.1111/j.1469-8137.2010.03374.x. [DOI] [PubMed] [Google Scholar]
- 10.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(Pt 24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3(12):a005058. [DOI] [PMC free article] [PubMed]
- 12.Hynes RO. The extracellular matrix: not just pretty fibrils. Science (New York, NY) 2009;326(5957):1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15(1):35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehnman M, Larsson O. Microenvironmental targets in sarcoma. Front Oncol. 2015;5:248. doi: 10.3389/fonc.2015.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raimondi L, De Luca A, Gallo A, Costa V, Russelli G, Cuscino N, Manno M, Raccosta S, Carina V, Bellavia D, et al. Osteosarcoma cell-derived exosomes affect tumor microenvironment by specific packaging of microRNAs. Carcinogenesis. 2020;41(5):666–77. [DOI] [PubMed]
- 17.Zheng Y, Wang G, Chen R, Hua Y, Cai Z. Mesenchymal stem cells in the osteosarcoma microenvironment: their biological properties, influence on tumor growth, and therapeutic implications. Stem Cell Res Ther. 2018;9(1):22. doi: 10.1186/s13287-018-0780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borovski T, De Sousa EMF, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. 2011;71(3):634–639. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- 19.Singh B, Fleury C, Jalalvand F, Riesbeck K. Human pathogens utilize host extracellular matrix proteins laminin and collagen for adhesion and invasion of the host. FEMS Microbiol Rev. 2012;36(6):1122–1180. doi: 10.1111/j.1574-6976.2012.00340.x. [DOI] [PubMed] [Google Scholar]
- 20.Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol. 2014;802:31–47. doi: 10.1007/978-94-007-7893-1_3. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Wei L, Yu J, Li G, Zhang X, Wang A, He Y, Li H, Yin D. Targeting of the beta6 gene to suppress degradation of ECM via inactivation of the MAPK pathway in breast adenocarcinoma cells. Oncol Rep. 2014;32(5):1787–1795. doi: 10.3892/or.2014.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saviola AJ, Burns PD, Mukherjee AK, Mackessy SP. The disintegrin tzabcanin inhibits adhesion and migration in melanoma and lung cancer cells. Int J Biol Macromol. 2016;88:457–464. doi: 10.1016/j.ijbiomac.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Yang B, Tang F, Zhang B, Zhao Y, Feng J, Rao Z. Matrix metalloproteinase-9 overexpression is closely related to poor prognosis in patients with colon cancer. World J Surg Oncol. 2014;12:24. doi: 10.1186/1477-7819-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gou X, Chen H, Jin F, Wu W, Li Y, Long J, Gong X, Luo M, Bi T, Li Z, et al. Expressions of CD147, MMP-2 and MMP-9 in laryngeal carcinoma and its correlation with poor prognosis. Pathol Oncol Res. 2014;20(2):475–481. doi: 10.1007/s12253-013-9720-3. [DOI] [PubMed] [Google Scholar]
- 25.Li TY, Xu LY, Wu ZY, Liao LD, Shen JH, Xu XE, Du ZP, Zhao Q, Li EM. Reduced nuclear and ectopic cytoplasmic expression of lysyl oxidase-like 2 is associated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma. Hum Pathol. 2012;43(7):1068–1076. doi: 10.1016/j.humpath.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Korpi JT, Hagstrom J, Lehtonen N, Parkkinen J, Sorsa T, Salo T, Laitinen M. Expression of matrix metalloproteinases-2, −8, −13, −26, and tissue inhibitors of metalloproteinase-1 in human osteosarcoma. Surg Oncol. 2011;20(1):e18–e22. doi: 10.1016/j.suronc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Xu W, Li Z, Zhu X, Xu R, Xu Y. miR-29 family inhibits resistance to methotrexate and promotes cell apoptosis by targeting COL3A1 and MCL1 in osteosarcoma. Med Sci Monit. 2018;24:8812–8821. doi: 10.12659/MSM.911972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaddad H, Kuchler-Bopp S, Fuhrmann G, Gegout H, Ubeaud-Sequier G, Schwinte P, Bornert F, Benkirane-Jessel N, Idoux-Gillet Y. Combining 2D angiogenesis and 3D osteosarcoma microtissues to improve vascularization. Exp Cell Res. 2017;360(2):138–145. doi: 10.1016/j.yexcr.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi K, Matsuo N, Sumiyoshi H, Fujimoto N, Iyama KI, Yanagisawa S, Yoshioka H. Pro-alpha3(V) collagen chain is expressed in bone and its basic N-terminal peptide adheres to osteosarcoma cells. Matrix Biol. 2005;24(4):283–294. doi: 10.1016/j.matbio.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Dutour A, Monteil J, Paraf F, Charissoux JL, Kaletta C, Sauer B, Naujoks K, Rigaud M. Endostatin cDNA/cationic liposome complexes as a promising therapy to prevent lung metastases in osteosarcoma: study in a human-like rat orthotopic tumor. Mol Ther. 2005;11(2):311–319. doi: 10.1016/j.ymthe.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Kaya M, Wada T, Nagoya S, Yamashita T. Prevention of postoperative progression of pulmonary metastases in osteosarcoma by antiangiogenic therapy using endostatin. J Orthop Sci. 2007;12(6):562–567. doi: 10.1007/s00776-007-1179-1. [DOI] [PubMed] [Google Scholar]
- 32.Xu H, Niu X, Zhang Q, Hao L, Ding Y, Liu W, Yao L. Synergistic antitumor efficacy by combining adriamycin with recombinant human endostatin in an osteosarcoma model. Oncol Lett. 2011;2(5):773–778. doi: 10.3892/ol.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang W, Park S, Jang JH. Kinetic and functional analysis of the heparin-binding domain of fibronectin. Biotechnol Lett. 2008;30(1):55–59. doi: 10.1007/s10529-007-9522-3. [DOI] [PubMed] [Google Scholar]
- 34.Fogerty FJ, Akiyama SK, Yamada KM, Mosher DF. Inhibition of binding of fibronectin to matrix assembly sites by anti-integrin (alpha 5 beta 1) antibodies. J Cell Biol. 1990;111(2):699–708. doi: 10.1083/jcb.111.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang RS, Chiang HS, Tang CH, Yeh CS, Huang TF. Rhodostomin inhibits thrombin-enhanced adhesion of ROS 17/2.8 cells through the blockade of alphavbeta3 integrin. Toxicon. 2005;46(4):387–393. doi: 10.1016/j.toxicon.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Kun-Peng Z, Chun-Lin Z, Xiao-Long M, Lei Z. Fibronectin-1 modulated by the long noncoding RNA OIP5-AS1/miR-200b-3p axis contributes to doxorubicin resistance of osteosarcoma cells. J Cell Physiol. 2019;234(5):6927–6939. doi: 10.1002/jcp.27435. [DOI] [PubMed] [Google Scholar]
- 37.Gvozdenovic A, Boro A, Meier D, Bode-Lesniewska B, Born W, Muff R, Fuchs B. Targeting alphavbeta3 and alphavbeta5 integrins inhibits pulmonary metastasis in an intratibial xenograft osteosarcoma mouse model. Oncotarget. 2016;7(34):55141–55154. doi: 10.18632/oncotarget.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Z, Schwenzer A, Rupp T, Murdamoothoo D, Vegliante R, Lefebvre O, Klein A, Hussenet T, Orend G. Tenascin-C promotes tumor cell migration and metastasis through integrin alpha9beta1-mediated YAP inhibition. Cancer Res. 2018;78(4):950–961. doi: 10.1158/0008-5472.CAN-17-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boregowda RK, Krovic BM, Ritty TM. Selective integrin subunit reduction disrupts fibronectin extracellular matrix deposition and fibrillin 1 gene expression. Mol Cell Biochem. 2012;369(1–2):205–216. doi: 10.1007/s11010-012-1383-y. [DOI] [PubMed] [Google Scholar]
- 40.Min SK, Kang HK, Jang DH, Jung SY, Kim OB, Min BM, Yeo IS. Titanium surface coating with a laminin-derived functional peptide promotes bone cell adhesion. Biomed Res Int. 2013;2013:638348. doi: 10.1155/2013/638348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yudoh K, Matsui H, Kanamori M, Ohmori K, Yasuda T, Tsuji H. Characteristics of high and low laminin-adherent Dunn osteosarcoma cells selected by adhesiveness to laminin. Correlation between invasiveness through the extracellular matrix and pulmonary metastatic potential. Tumour Biol. 1996;17(6):332–340. doi: 10.1159/000217997. [DOI] [PubMed] [Google Scholar]
- 42.Pavlou M, Shah M, Gikas P, Briggs T, Roberts SJ, Cheema U. Osteomimetic matrix components alter cell migration and drug response in a 3D tumour-engineered osteosarcoma model. Acta Biomater. 2019;96:247–257. doi: 10.1016/j.actbio.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 43.Ungefroren H, Cikos T, Krull NB, Kalthoff H. Biglycan gene promoter activity in osteosarcoma cells is regulated by cyclic AMP. Biochem Biophys Res Commun. 1997;235(2):413–417. doi: 10.1006/bbrc.1997.6801. [DOI] [PubMed] [Google Scholar]
- 44.Aggelidakis J, Berdiaki A, Nikitovic D, Papoutsidakis A, Papachristou DJ, Tsatsakis AM, Tzanakakis GN. Biglycan regulates MG63 osteosarcoma cell growth through a LPR6/beta-catenin/IGFR-IR signaling Axis. Front Oncol. 2018;8:470. doi: 10.3389/fonc.2018.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merle B, Durussel L, Delmas PD, Clezardin P. Decorin inhibits cell migration through a process requiring its glycosaminoglycan side chain. J Cell Biochem. 1999;75(3):538–546. [PubMed] [Google Scholar]
- 46.Santra M, Mann DM, Mercer EW, Skorski T, Calabretta B, Iozzo RV. Ectopic expression of decorin protein core causes a generalized growth suppression in neoplastic cells of various histogenetic origin and requires endogenous p21, an inhibitor of cyclin-dependent kinases. J Clin Invest. 1997;100(1):149–157. doi: 10.1172/JCI119507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikitovic D, Berdiaki A, Zafiropoulos A, Katonis P, Tsatsakis A, Karamanos NK, Tzanakakis GN. Lumican expression is positively correlated with the differentiation and negatively with the growth of human osteosarcoma cells. FEBS J. 2008;275(2):350–361. doi: 10.1111/j.1742-4658.2007.06205.x. [DOI] [PubMed] [Google Scholar]
- 48.Nikitovic D, Chalkiadaki G, Berdiaki A, Aggelidakis J, Katonis P, Karamanos NK, Tzanakakis GN. Lumican regulates osteosarcoma cell adhesion by modulating TGFbeta2 activity. Int J Biochem Cell Biol. 2011;43(6):928–935. doi: 10.1016/j.biocel.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Li F, Li S, Cheng T. TGF-beta1 promotes osteosarcoma cell migration and invasion through the miR-143-versican pathway. Cellular Physiol Biochem. 2014;34(6):2169–2179. doi: 10.1159/000369660. [DOI] [PubMed] [Google Scholar]
- 50.Tofuku K, Yokouchi M, Murayama T, Minami S, Komiya S. HAS3-related hyaluronan enhances biological activities necessary for metastasis of osteosarcoma cells. Int J Oncol. 2006;29(1):175–183. [PubMed] [Google Scholar]
- 51.Nishida Y, Knudson W, Knudson CB, Ishiguro N. Antisense inhibition of hyaluronan synthase-2 in human osteosarcoma cells inhibits hyaluronan retention and tumorigenicity. Exp Cell Res. 2005;307(1):194–203. doi: 10.1016/j.yexcr.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu T, Yan Z, Liu Y, Choy E, Hornicek FJ, Mankin H, Duan Z. CRISPR-Cas9-mediated silencing of CD44 in human highly metastatic osteosarcoma cells. Cellular Physiol Biochem. 2018;46(3):1218–1230. doi: 10.1159/000489072. [DOI] [PubMed] [Google Scholar]
- 53.Hosono K, Nishida Y, Knudson W, Knudson CB, Naruse T, Suzuki Y, Ishiguro N. Hyaluronan oligosaccharides inhibit tumorigenicity of osteosarcoma cell lines MG-63 and LM-8 in vitro and in vivo via perturbation of hyaluronan-rich pericellular matrix of the cells. Am J Pathol. 2007;171(1):274–286. doi: 10.2353/ajpath.2007.060828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao Y, Feng Y, Shen JK, Lin M, Choy E, Cote GM, Harmon DC, Mankin HJ, Hornicek FJ, Duan Z. CD44 is a direct target of miR-199a-3p and contributes to aggressive progression in osteosarcoma. Sci Rep. 2015;5:11365. doi: 10.1038/srep11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gvozdenovic A, Arlt MJ, Campanile C, Brennecke P, Husmann K, Li Y, Born W, Muff R, Fuchs B. CD44 enhances tumor formation and lung metastasis in experimental osteosarcoma and is an additional predictor for poor patient outcome. J Bone Miner Res. 2013;28(4):838–847. doi: 10.1002/jbmr.1817. [DOI] [PubMed] [Google Scholar]
- 56.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20(1):33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3(1):a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bella J, Hulmes DJ. Fibrillar Collagens. Subcell Biochem. 2017;82:457–490. doi: 10.1007/978-3-319-49674-0_14. [DOI] [PubMed] [Google Scholar]
- 59.Exposito JY, Valcourt U, Cluzel C, Lethias C. The fibrillar collagen family. Int J Mol Sci. 2010;11(2):407–426. doi: 10.3390/ijms11020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brodsky B, Persikov AV. Molecular structure of the collagen triple helix. Adv Protein Chem. 2005;70:301–339. doi: 10.1016/S0065-3233(05)70009-7. [DOI] [PubMed] [Google Scholar]
- 61.Wiklund T, Blomqvist C, Risteli L, Risteli J, Karaharju E, Elomaa I. Type I and type III collagen metabolites in adult osteosarcoma patients. Br J Cancer. 1996;73(1):106–109. doi: 10.1038/bjc.1996.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elenjord R, Allen JB, Johansen HT, Kildalsen H, Svineng G, Maelandsmo GM, Loennechen T, Winberg JO. Collagen I regulates matrix metalloproteinase-2 activation in osteosarcoma cells independent of S100A4. FEBS J. 2009;276(18):5275–5286. doi: 10.1111/j.1742-4658.2009.07223.x. [DOI] [PubMed] [Google Scholar]
- 63.Kunz P, Sahr H, Lehner B, Fischer C, Seebach E, Fellenberg J. Elevated ratio of MMP2/MMP9 activity is associated with poor response to chemotherapy in osteosarcoma. BMC Cancer. 2016;16:223. doi: 10.1186/s12885-016-2266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuivaniemi H, Tromp G. Type III collagen (COL3A1): gene and protein structure, tissue distribution, and associated diseases. Gene. 2019;707:151–171. doi: 10.1016/j.gene.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keene DR, Sakai LY, Burgeson RE. Human bone contains type III collagen, type VI collagen, and fibrillin: type III collagen is present on specific fibers that may mediate attachment of tendons, ligaments, and periosteum to calcified bone cortex. J Histochem Cytochem. 1991;39(1):59–69. doi: 10.1177/39.1.1983874. [DOI] [PubMed] [Google Scholar]
- 66.Chioran A, Duncan S, Catalano A, Brown TJ, Ringuette MJ. Collagen IV trafficking: the inside-out and beyond story. Dev Biol. 2017;431(2):124–133. doi: 10.1016/j.ydbio.2017.09.037. [DOI] [PubMed] [Google Scholar]
- 67.Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71(5):357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gelse K, Poschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55(12):1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 69.Mak KM, Png CY, Lee DJ. Type V collagen in health, disease, and fibrosis. Anat Rec (Hoboken) 2016;299(5):613–629. doi: 10.1002/ar.23330. [DOI] [PubMed] [Google Scholar]
- 70.Fernandes RJ, Harkey MA, Weis M, Askew JW, Eyre DR. The post-translational phenotype of collagen synthesized by SAOS-2 osteosarcoma cells. Bone. 2007;40(5):1343–1351. doi: 10.1016/j.bone.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Passos-Bueno MR, Suzuki OT, Armelin-Correa LM, Sertie AL, Errera FI, Bagatini K, Kok F, Leite KR. Mutations in collagen 18A1 and their relevance to the human phenotype. An Acad Bras Cienc. 2006;78(1):123–131. doi: 10.1590/s0001-37652006000100012. [DOI] [PubMed] [Google Scholar]
- 72.Heljasvaara R, Aikio M, Ruotsalainen H, Pihlajaniemi T. Collagen XVIII in tissue homeostasis and dysregulation - lessons learned from model organisms and human patients. Matrix Biol. 2017;57-58:55–75. doi: 10.1016/j.matbio.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 73.Marneros AG, Olsen BR. Physiological role of collagen XVIII and endostatin. FASEB J. 2005;19(7):716–728. doi: 10.1096/fj.04-2134rev. [DOI] [PubMed] [Google Scholar]
- 74.Ackley BD, Crew JR, Elamaa H, Pihlajaniemi T, Kuo CJ, Kramer JM. The NC1/endostatin domain of Caenorhabditis elegans type XVIII collagen affects cell migration and axon guidance. J Cell Biol. 2001;152(6):1219–1232. doi: 10.1083/jcb.152.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abd El-Rehim DM, Osman NA. Expression of a disintegrin and metalloprotease 8 and endostatin in human osteosarcoma: implication in tumor progression and prognosis. J Egypt Natl Canc Inst. 2015;27(1):1–9. doi: 10.1016/j.jnci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 76.Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol. 2014;15(12):771–785. doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pietrocola G, Rindi S, Nobile G, Speziale P. Purification of human plasma/cellular Fibronectin and Fibronectin fragments. Methods Mol Biol. 2017;1627:309–324. doi: 10.1007/978-1-4939-7113-8_20. [DOI] [PubMed] [Google Scholar]
- 78.Wierzbicka-Patynowski I, Schwarzbauer JE. The ins and outs of fibronectin matrix assembly. J Cell Sci. 2003;116(Pt 16):3269–3276. doi: 10.1242/jcs.00670. [DOI] [PubMed] [Google Scholar]
- 79.Henderson B, Nair S, Pallas J, Williams MA. Fibronectin: a multidomain host adhesin targeted by bacterial fibronectin-binding proteins. FEMS Microbiol Rev. 2011;35(1):147–200. doi: 10.1111/j.1574-6976.2010.00243.x. [DOI] [PubMed] [Google Scholar]
- 80.Cseh B, Fernandez-Sauze S, Grall D, Schaub S, Doma E, Van Obberghen-Schilling E. Autocrine fibronectin directs matrix assembly and crosstalk between cell-matrix and cell-cell adhesion in vascular endothelial cells. J Cell Sci. 2010;123(Pt 22):3989–3999. doi: 10.1242/jcs.073346. [DOI] [PubMed] [Google Scholar]
- 81.Kilian O, Dahse R, Alt V, Zardi L, Rosenhahn J, Exner U, Battmann A, Schnettler R, Kosmehl H. Expression of EDA+ and EDB+ fibronectin splice variants in bone. Bone. 2004;35(6):1334–1345. doi: 10.1016/j.bone.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 82.Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kechagia JZ, Ivaska J, Roca-Cusachs P. Integrins as biomechanical sensors of the microenvironment. Nat Rev Mol Cell Biol. 2019;20(8):457–473. doi: 10.1038/s41580-019-0134-2. [DOI] [PubMed] [Google Scholar]
- 84.Bokel C, Brown NH. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev Cell. 2002;3(3):311–321. doi: 10.1016/s1534-5807(02)00265-4. [DOI] [PubMed] [Google Scholar]
- 85.Nermut MV, Green NM, Eason P, Yamada SS, Yamada KM. Electron microscopy and structural model of human fibronectin receptor. EMBO J. 1988;7(13):4093–4099. doi: 10.1002/j.1460-2075.1988.tb03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hamill KJ, Kligys K, Hopkinson SB, Jones JC. Laminin deposition in the extracellular matrix: a complex picture emerges. J Cell Sci. 2009;122(Pt 24):4409–4417. doi: 10.1242/jcs.041095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Developmental Dynamics. 2000;218(2):213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 88.Patarroyo M, Tryggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin Cancer Biol. 2002;12(3):197–207. doi: 10.1016/S1044-579X(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 89.Hynes RO, Naba A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2012;4(1):a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schaefer L, Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 2010;339(1):237–246. doi: 10.1007/s00441-009-0821-y. [DOI] [PubMed] [Google Scholar]
- 91.Sasisekharan R, Shriver Z, Venkataraman G, Narayanasami U. Roles of heparan-sulphate glycosaminoglycans in cancer. Nat Rev Cancer. 2002;2(7):521–528. doi: 10.1038/nrc842. [DOI] [PubMed] [Google Scholar]
- 92.Afratis N, Gialeli C, Nikitovic D, Tsegenidis T, Karousou E, Theocharis AD, Pavao MS, Tzanakakis GN, Karamanos NK. Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 2012;279(7):1177–1197. doi: 10.1111/j.1742-4658.2012.08529.x. [DOI] [PubMed] [Google Scholar]
- 93.Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med. 2011;15(5):1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4(7):528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 95.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91(1):221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iozzo RV, Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu L, Tang L, Zhang L. Proteoglycans as miscommunication biomarkers for cancer diagnosis. Prog Mol Biol Transl Sci. 2019;162:59–92. doi: 10.1016/bs.pmbts.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 98.Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol. 2001;11(6):725–732. doi: 10.1016/s0959-440x(01)00266-4. [DOI] [PubMed] [Google Scholar]
- 99.Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283(31):21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Igwe JC, Gao Q, Kizivat T, Kao WW, Kalajzic I. Keratocan is expressed by osteoblasts and can modulate osteogenic differentiation. Connect Tissue Res. 2011;52(5):401–407. doi: 10.3109/03008207.2010.546536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen S, Birk DE. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 2013;280(10):2120–2137. doi: 10.1111/febs.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Traupe H, van den Ouweland AM, van Oost BA, Vogel W, Vetter U, Warren ST, Rocchi M, Darlison MG, Ropers HH. Fine mapping of the human biglycan (BGN) gene within the Xq28 region employing a hybrid cell panel. Genomics. 1992;13(2):481–483. doi: 10.1016/0888-7543(92)90279-2. [DOI] [PubMed] [Google Scholar]
- 103.Pulkkinen L, Alitalo T, Krusius T, Peltonen L. Expression of decorin in human tissues and cell lines and defined chromosomal assignment of the gene locus (DCN) Cytogenet Cell Genet. 1992;60(2):107–111. doi: 10.1159/000133314. [DOI] [PubMed] [Google Scholar]
- 104.Chakravarti S, Stallings RL, SundarRaj N, Cornuet PK, Hassell JR. Primary structure of human lumican (keratan sulfate proteoglycan) and localization of the gene (LUM) to chromosome 12q21.3-q22. Genomics. 1995;27(3):481–488. doi: 10.1006/geno.1995.1080. [DOI] [PubMed] [Google Scholar]
- 105.Iozzo RV, Murdoch AD. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 1996;10(5):598–614. [PubMed] [Google Scholar]
- 106.Aspberg A. The different roles of aggrecan interaction domains. J Histochem Cytochem. 2012;60(12):987–996. doi: 10.1369/0022155412464376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harten IA, Kaber G, Agarwal KJ, Kang I, Ibarrientos SR, Workman G, Chan CK, Nivison MP, Nagy N, Braun KR, et al. The synthesis and secretion of versican isoform V3 by mammalian cells: a role for N-linked glycosylation. Matrix Biol. 2020;89:27–42. [DOI] [PMC free article] [PubMed]
- 108.Ricciardelli C, Sakko AJ, Ween MP, Russell DL, Horsfall DJ. The biological role and regulation of versican levels in cancer. Cancer Metastasis Rev. 2009;28(1–2):233–245. doi: 10.1007/s10555-009-9182-y. [DOI] [PubMed] [Google Scholar]
- 109.Liang J, Jiang D, Noble PW. Hyaluronan as a therapeutic target in human diseases. Adv Drug Deliv Rev. 2016;97:186–203. doi: 10.1016/j.addr.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feng S, Wu ZX, Zhao Z, Liu J, Sun K, Guo C, Wang H, Wu Z. Engineering of bone- and CD44-dual-targeting redox-sensitive liposomes for the treatment of Orthotopic osteosarcoma. ACS Appl Mater Interfaces. 2019;11(7):7357–7368. doi: 10.1021/acsami.8b18820. [DOI] [PubMed] [Google Scholar]
- 111.Caon I, Bartolini B, Parnigoni A, Carava E, Moretto P, Viola M, Karousou E, Vigetti D, Passi A. Revisiting the hallmarks of cancer: the role of hyaluronan. Semin Cancer Biol. 2020;62:9–19. doi: 10.1016/j.semcancer.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 112.Sanderson RD, Bandari SK, Vlodavsky I. Proteases and glycosidases on the surface of exosomes: newly discovered mechanisms for extracellular remodeling. Matrix Biol. 2019;75-76:160–169. doi: 10.1016/j.matbio.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science (New York, NY) 2009;324(5935):1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kuan YH, Huang FM, Li YC, Chang YC. Proinflammatory activation of macrophages by bisphenol A-glycidyl-methacrylate involved NFκB activation via PI3K/Akt pathway. Food Chem Toxicol. 2012;50(11):4003–4009. doi: 10.1016/j.fct.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 115.Felx M, Guyot MC, Isler M, Turcotte RE, Doyon J, Khatib AM, Leclerc S, Moreau A, Moldovan F. Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NF-kappaB in human osteosarcoma. Clin Sci (Lond) 2006;110(6):645–654. doi: 10.1042/CS20050286. [DOI] [PubMed] [Google Scholar]
- 116.Poudel B, Kim DK, Ki HH, Kwon YB, Lee YM, Kim DK. Downregulation of ERK signaling impairs U2OS osteosarcoma cell migration in collagen matrix by suppressing MMP9 production. Oncol Lett. 2014;7(1):215–218. doi: 10.3892/ol.2013.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheng G, Gao F, Sun X, Bi H, Zhu Y. Paris saponin VII suppresses osteosarcoma cell migration and invasion by inhibiting MMP-2/9 production via the p38 MAPK signaling pathway. Mol Med Rep. 2016;14(4):3199–3205. doi: 10.3892/mmr.2016.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hirahata M, Osaki M, Kanda Y, Sugimoto Y, Yoshioka Y, Kosaka N, Takeshita F, Fujiwara T, Kawai A, Ito H, et al. PAI-1, a target gene of miR-143, regulates invasion and metastasis by upregulating MMP-13 expression of human osteosarcoma. Cancer Med. 2016;5(5):892–902. doi: 10.1002/cam4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vlodavsky I, Beckhove P, Lerner I, Pisano C, Meirovitz A, Ilan N, Elkin M. Significance of heparanase in cancer and inflammation. Cancer Microenviron. 2012;5(2):115–132. doi: 10.1007/s12307-011-0082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng L, Jiang G, Mei H, Pu J, Dong J, Hou X, Tong Q. Small RNA interference-mediated gene silencing of heparanase abolishes the invasion, metastasis and angiogenesis of gastric cancer cells. BMC Cancer. 2010;10:33. doi: 10.1186/1471-2407-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fan L, Wu Q, Xing X, Liu Y, Shao Z. Targeted silencing of heparanase gene by small interfering RNA inhibits invasiveness and metastasis of osteosarcoma cells. J Huazhong Univ Sci Technolog Med Sci. 2011;31(3):348–352. doi: 10.1007/s11596-011-0379-2. [DOI] [PubMed] [Google Scholar]
- 122.Luo C, Yang Z, Wang L. Heparanase participates in the growth and invasion of human U-2OS osteosarcoma cells and its close relationship with hypoxia-inducible factor-1α in osteosarcoma. Neoplasma. 2010;57(6):562–571. [PubMed] [Google Scholar]
- 123.Todd JR, Ryall KA, Vyse S, Wong JP, Natrajan RC, Yuan Y, Tan AC, Huang PH. Systematic analysis of tumour cell-extracellular matrix adhesion identifies independent prognostic factors in breast cancer. Oncotarget. 2016;7(39):62939–62953. doi: 10.18632/oncotarget.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alfano M, Canducci F, Nebuloni M, Clementi M, Montorsi F, Salonia A. The interplay of extracellular matrix and microbiome in urothelial bladder cancer. Nature Reviews Urology. 2016;13(2):77–90. doi: 10.1038/nrurol.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lim SB, Tan SJ, Lim WT, Lim CT. An extracellular matrix-related prognostic and predictive indicator for early-stage non-small cell lung cancer. Nat Commun. 2017;8(1):1734. doi: 10.1038/s41467-017-01430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu S, Xu H, Wang W, Li S, Li H, Li T, Zhang W, Yu X, Liu L. The role of collagen in cancer: from bench to bedside. J Transl Med. 2019;17(1):309. doi: 10.1186/s12967-019-2058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu Y, Abulimiti N, Wang C. Collagen triple Helix repeat containing 1 expression in osteosarcoma: a new predictor of prognosis. Ann Clin Lab Sci. 2018;48(3):338–344. [PubMed] [Google Scholar]
- 128.Park EH, Kim S, Jo JY, Kim SJ, Hwang Y, Kim JM, Song SY, Lee DK, Koh SS. Collagen triple helix repeat containing-1 promotes pancreatic cancer progression by regulating migration and adhesion of tumor cells. Carcinogenesis. 2013;34(3):694–702. doi: 10.1093/carcin/bgs378. [DOI] [PubMed] [Google Scholar]
- 129.He M, Wang Z, Zhao J, Chen Y, Wu Y. COL1A1 polymorphism is associated with risks of osteosarcoma susceptibility and death. Tumour Biol. 2014;35(2):1297–1305. doi: 10.1007/s13277-013-1172-6. [DOI] [PubMed] [Google Scholar]
- 130.Shi K, Wang SL, Shen B, Yu FQ, Weng DF, Lin JH. Clinicopathological and prognostic values of fibronectin and integrin alphavbeta3 expression in primary osteosarcoma. World J Surg Oncol. 2019;17(1):23. doi: 10.1186/s12957-019-1566-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Na KY, Bacchini P, Bertoni F, Kim YW, Park YK. Syndecan-4 and fibronectin in osteosarcoma. Pathology. 2012;44(4):325–330. doi: 10.1097/PAT.0b013e328353447b. [DOI] [PubMed] [Google Scholar]
- 132.Xiao Z, Wan J, Nur AA, Dou P, Mankin H, Liu T, Ouyang Z. Targeting CD44 by CRISPR-Cas9 in multi-drug resistant osteosarcoma cells. Cell Physiol Biochem. 2018;51(4):1879–1893. doi: 10.1159/000495714. [DOI] [PubMed] [Google Scholar]
- 133.Kim CK, Oh S, Kim SJ, Leem SH, Heo J, Chung SH. Correlation of IGF1R expression with ABCG2 and CD44 expressions in human osteosarcoma. Genes Genomics. 2018;40(4):381–388. doi: 10.1007/s13258-017-0639-z. [DOI] [PubMed] [Google Scholar]
- 134.Hamano Y, Kalluri R. Tumstatin, the NC1 domain of alpha3 chain of type IV collagen, is an endogenous inhibitor of pathological angiogenesis and suppresses tumor growth. Biochem Biophys Res Commun. 2005;333(2):292–298. doi: 10.1016/j.bbrc.2005.05.130. [DOI] [PubMed] [Google Scholar]
- 135.Wang Y, Yin RF, Teng JS. Tumstatin induces apoptosis and stimulates phosphorylation of p65NF-kappaB in human osteoblastic osteosarcoma Saos-2 cells. Oncol Rep. 2016;35(6):3403–3408. doi: 10.3892/or.2016.4762. [DOI] [PubMed] [Google Scholar]
- 136.Xu M, Xu CX, Bi WZ, Song ZG, Jia JP, Chai W, Zhang LH, Wang Y. Effects of endostar combined multidrug chemotherapy in osteosarcoma. Bone. 2013;57(1):111–115. doi: 10.1016/j.bone.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 137.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5(10):816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 139.Intarajak T, Udomchaiprasertkul W, Bunyoo C, Yimnoon J, Soonklang K, Wiriyaukaradecha K, Lamlertthon W, Sricharunrat T, Chaiwiriyawong W, Siriphongpreeda B, et al. Genetic Aberration Analysis in Thai Colorectal Adenoma and Early-Stage Adenocarcinoma Patients by Whole-Exome Sequencing. Cancers (Basel). 2019;11(7):977. [DOI] [PMC free article] [PubMed]
- 140.Li R, Shi Y, Zhao S, Shi T, Zhang G. NF-kappaB signaling and integrin-beta1 inhibition attenuates osteosarcoma metastasis via increased cell apoptosis. Int J Biol Macromol. 2019;123:1035–1043. doi: 10.1016/j.ijbiomac.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 141.Shintani K, Matsumine A, Kusuzaki K, Morikawa J, Matsubara T, Wakabayashi T, Araki K, Satonaka H, Wakabayashi H, Iino T, et al. Decorin suppresses lung metastases of murine osteosarcoma. Oncol Rep. 2008;19(6):1533–1539. [PubMed] [Google Scholar]
- 142.Duan Z, Choy E, Harmon D, Liu X, Susa M, Mankin H, Hornicek F. MicroRNA-199a-3p is downregulated in human osteosarcoma and regulates cell proliferation and migration. Mol Cancer Ther. 2011;10(8):1337–1345. doi: 10.1158/1535-7163.MCT-11-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang L, Lyer AK, Yang X, Kobayashi E, Guo Y, Mankin H, Hornicek FJ, Amiji MM, Duan Z. Polymeric nanoparticle-based delivery of microRNA-199a-3p inhibits proliferation and growth of osteosarcoma cells. Int J Nanomedicine. 2015;10:2913–2924. doi: 10.2147/IJN.S79143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.