Abstract
Background
Cryptosporidium is a protozoan parasite which is a common cause of gastroenteritis worldwide. In developing countries, it is one of the most important causes of moderate to severe diarrhoea in young children; in industrialised countries it is a cause of outbreaks of gastroenteritis associated with drinking water, swimming pools and other environmental sources and a particular concern in certain immunocompromised patient groups, where it can cause severe disease. However, over recent years, longer-term sequelae of infection have been recognised and a number of studies have been published on this topic. The purpose of this systematic review was to examine the literature in order to better understand the medium- to long-term impact of cryptosporidiosis.
Methods
This was a systematic review of studies in PubMed, ProQuest and Web of Science databases, with no limitations on publication year or language. Studies from any country were included in qualitative synthesis, but only those in industrialised countries were included in quantitative analysis.
Results
Fifteen studies were identified for qualitative analysis which included 3670 Cryptosporidium cases; eight studies conducted in Europe between 2004–2019 were suitable for quantitative analysis, including five case-control studies. The most common reported long-term sequelae were diarrhoea (25%), abdominal pain (25%), nausea (24%), fatigue (24%) and headache (21%). Overall, long-term sequelae were more prevalent following infection with Cryptosporidium hominis, with only weight loss and blood in stool being more prevalent following infection with Cryptosporidium parvum. Analysis of the case-control studies found that individuals were 6 times more likely to report chronic diarrhoea and weight loss up to 28 months after a Cryptosporidium infection than were controls. Long-term abdominal pain, loss of appetite, fatigue, vomiting, joint pain, headache and eye pain were also between 2–3 times more likely following a Cryptosporidium infection.
Conclusions
This is the first systematic review of the long-term sequelae of cryptosporidiosis. A better understanding of long-term outcomes of cryptosporidiosis is valuable to inform the expectations of clinicians and their patients, and public health policy-makers regarding the control and prevention of this infection.
Systematic review registration PROSPERO Registration number CRD42019141311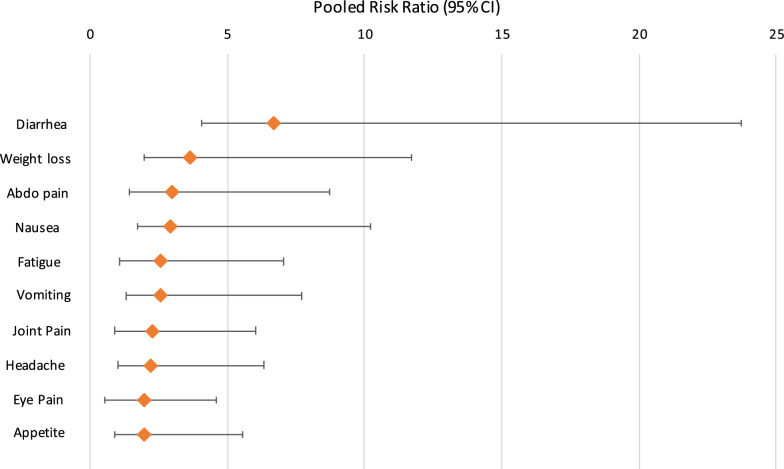
Keywords: Cryptosporidiosis, Sequelae, Cryptosporidium hominis, Cryptosporidium parvum
Background
Cryptosporidiosis is a clinical disease, typically affecting the intestinal tract of humans and animals who have ingested the protozoan parasite Cryptosporidium in its oocyst (infective) stage [1]. Transmission of Cryptosporidium occurs predominantly via the faecal-oral route, or through consumption of contaminated food or water and therefore the prevalence of human Cryptosporidium infections is higher in low-resource settings [2]. However, Cryptosporidium infections are not infrequent in industrialized countries [3], with large outbreaks being reported in Sweden [4], the USA [5, 6] and the UK [7] following contamination of public water supplies.
While asymptomatic carriage is possible [8, 9], human cryptosporidiosis typically presents as an acute, gastroenteritis-like illness characterized by profuse, watery diarrhoea, frequently accompanied by abdominal pain/cramps, vomiting and weight loss, as well as more non-specific symptoms such as fatigue, low-grade fever, nausea and muscle weakness [10]. In immunocompetent hosts, cryptosporidiosis is generally self-limiting; however, disease severity can be influenced by host factors, such as age, immune status and nutritional status, as well as pathogen factors e.g. Cryptosporidium species and subtype [11].
Alongside ongoing interest in the acute symptomology of human cryptosporidiosis, there is also growing evidence to suggest that, rather like some bacterial causes of gastroenteritis and giardiasis [12–14], Cryptosporidium infection may have longer-term health consequences. Seven studies [15–21], with follow-up periods ranging from 2 months to 3 years, have investigated numerous potential post-Cryptosporidium infection sequelae including diarrhoea, abdominal pain, vomiting, loss of appetite, irritable bowel syndrome (IBS) [21], joint pain and fatigue, while case reports document incidences of reactive arthritis [22–24], Reiter’s syndrome [25], acute pancreatitis [26, 27] and haemolytic uremic syndrome [28], in the context of Cryptosporidium infection. There is also some emerging evidence, recently reviewed, of a possible association between cryptosporidiosis and cancer [29].
Due to resource limitations, public health professionals currently face the challenge of identifying and prioritising specific infectious diseases whose quantified burden of disease estimates justify the allocation of interventions and funding for research [30]. The Global Enteric Multicentre Study [31] identified Cryptosporidium as the second most common cause of moderate-to-severe diarrhoea (MSD; defined as diarrhoeal disease with presence of the suggestive features of sunken eyes, wrinkled skin, hospitalization, receipt of intravenous hydration, or dysentery) in children less than 2 years-old within sub-Saharan Africa and south Asia, while in 2016, the European Network for Foodborne Parasites (Euro-FBP) ranked Cryptosporidium spp. as the second highest priority foodborne parasite in northern and western Europe, and the eighth highest priority in eastern and south-western Europe [30]. However, actual burden of disease estimates for Cryptosporidium still vary widely [11] and it remains difficult to quantify the true burden of cryptosporidiosis, as current estimates only account for the morbidity and mortality associated with the acute illness, while the potential contributions of long-term manifestations are not included [32, 33]. A recent study from the Netherlands [2] found that long-term manifestations contributed nearly 10% of the total Disability-Adjusted Life Years (DALYs) and costs when included in burden of disease models for Cryptosporidium, suggesting a higher public heath burden and cost than previously estimated.
Accurate estimations of the burden of disease associated with Cryptosporidium will inform decisions regarding the allocation of diagnostic, surveillance and interventional measures to prevent and control Cryptosporidium infections. Due to the potential morbidity and mortality associated with long-term sequelae of human cryptosporidiosis, an accurate estimation of the proportion of cases that develop such sequelae is needed to quantify true burden of disease estimates for Cryptosporidium.
The objectives of this review were: (i) estimate the proportion of people that self-report health sequelae post-Cryptosporidium infection; (ii) estimate the risk of specific sequelae following Cryptosporidium infection; and (iii) explore potential risk factors associated with developing sequelae following Cryptosporidium infection in industrialised countries.
Methods
Search strategy
We searched for studies in PubMed, ProQuest and Web of Science databases, with no limitations on publication year or language. The reference lists from relevant papers identified during our electronic searches were also reviewed for additional relevant papers which may warrant inclusion in our review. Search terms were initially developed and piloted using PubMed and, to ensure consistency, the same search terms were used when searching ProQuest and Web of Science databases. Databases were searched using the following keywords: Cryptosporid*, Complications, Sequel*, Post-infecti*, Long term and Chronic. The full electronic search strategies are documented in Additional file 1: Table S1. The review was registered with PROSPERO, registration number CRD42019141311.
Selection of studies
All citations identified using the final search strategies were exported to Mendeley® reference managing software for organisation and removal of duplicates. The titles and abstracts of the remaining articles were screened for relevance by one reviewer (BC), after which, the remaining articles were independently screened by two reviewers (BC and APD) to ensure consistent application of the pre-determined inclusion/exclusion criteria (Additional file 1: Table S1).
Studies from any country were included in qualitative synthesis, but only those in industrialised countries were included in quantitative analysis. An industrialised country was defined using Organisation for Economic Co-operation & Development (OECD) membership.
Final inclusion of studies was decided by consensus, with any conflicts being reviewed by a third reviewer (RMC). The full text was retrieved and reviewed for articles where the title and abstract had been deemed relevant by reviewers.
Data extraction
Data were extracted from eligible studies and collated in a Microsoft Word document. We recorded post-Cryptosporidium infection health sequelae data as reported in the individual papers (e.g. prevalence, cumulative incidence, etc.). Relative risks or odds ratios were recorded where data were available. We also extracted the following study characteristics from each paper if available: name of authors, year of publication, study location/setting, study design, year(s) of study, study duration and duration of follow-up, number of included study participants, participation rate, study population demographics (including age and gender distributions), Cryptosporidium species data, the diagnostic method to ascertain Cryptosporidium infection and the types of sequelae reported. Additionally, where available, data on the incidence/prevalence of post-infectious IBS following Cryptosporidium infection and the IBS diagnostic criterion applied were collected.
Quality assessment
The methodological quality of the included studies was assessed using the Newcastle-Ottawa Scale (NOS) for nonrandomised studies [34]. NOS was used to score studies using three domains: (i) the selection of the study groups; (ii) the comparability of the groups; and (iii) the determination of either the exposure or outcome of interest in case-control or cohort studies, respectively. Scores ranged between 5–8 (Additional file 1: Table S2).
Statistical analysis
The proportion of Cryptosporidium cases that developed specific sequelae was calculated by dividing the number of individuals developing a sequela by the total number of Cryptosporidium cases. Where data were available from two or more appropriate studies, we used a random-effects meta-analysis model to obtain pooled estimates of prevalence for the outcomes of interest (i.e. sequelae) across the eligible studies. For this analysis, a study could be included more than once if sequelae data were reported longitudinally at different time periods. Data analyses were performed using Meta XI [35].
Assessment of heterogeneity and reporting biases
Forest plots and the I2 statistic were used to assess heterogeneity between the studies. Values of 0–40%, 30–60%, 50–90% and 75–100% were interpreted as; might not be important, may represent moderate heterogeneity, may represent substantial heterogeneity and considerable heterogeneity, respectively [36]. Funnel plots were used to assess for publication bias and small-study effects.
Stratified analysis was performed for the following subgroups; time (less than 6 months post-infection and more than 6 months post-infection) and Cryptosporidium sp. (e.g. C. parvum vs C. hominis).
Results
Data synthesis
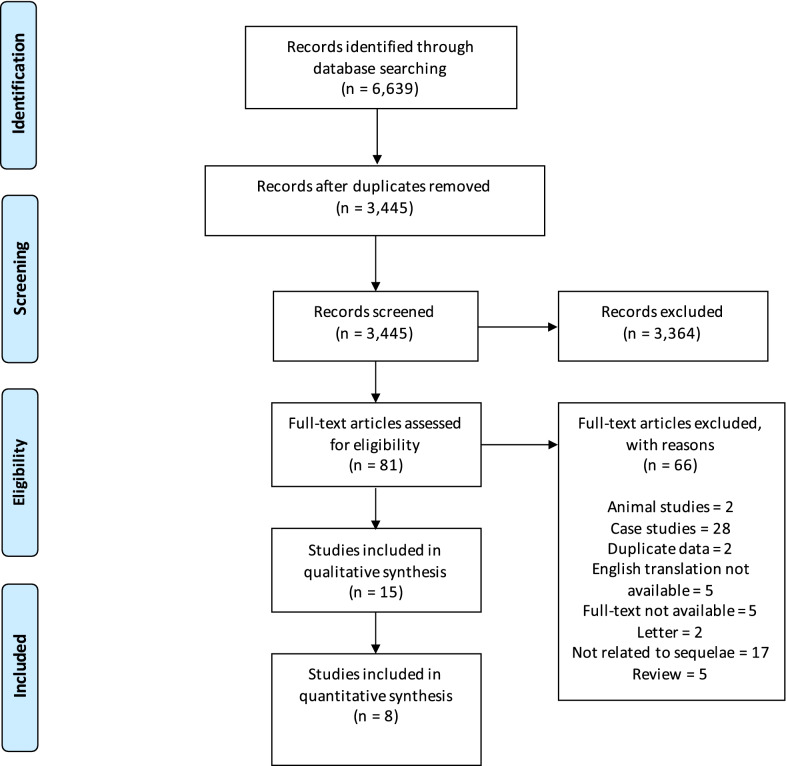
The number of papers identified, included and excluded is presented according to the requirements of the PRISMA statement [37] in Fig. 1. Fifteen studies were identified for qualitative synthesis and eight of these were identified as being set in industrialised countries and of sufficient quality for additional quantitative synthesis.
Fig. 1.
PRISMA flowchart
The qualitative synthesis is shown in Table 1. Quantitative synthesis results are shown in Tables 2, 3 and 4 and Figs. 2 and 3.
Table 1.
Fifteen studies included in the qualitative synthesis
| References/location | Study setting, design and duration of follow-up | Cryptosporidium spp. | Sample size | Range of age/sex | Main findings |
|---|---|---|---|---|---|
|
Agnew et al. [38] Fortaleza, Brazil |
Urban slum Nested case-control study of a cohort of young children Cases (diagnosed cryptosporidiosis): 453 ± 49 (15–1167) days Controls: 436 ± 53 (0–1165) days |
Unidentified | 43 cases; 43 controls |
Age of cases (months): 11 ± 0.9 (range: 3–26) Age of controls (months): 11 ± 0.9 (4–27) Cases: 63% boys Controls: 40% boys |
Children who had an episode of symptomatic Cryptosporidium infection had a significantly increased diarrhoeal disease burden (days of diarrhoea/child-year) compared with that for controls both before (39.3 ± 7 vs 21.3 ± 5 days, respectively; P < 0.04) and after (46.1 ± 9 vs 13.9 ± 5 days, respectively; P < 0.04) the diagnosis of Cryptosporidium infection In the post-Cryptosporidium period, case-children who were < 1 year of age had significantly more episodes of diarrhoea than their controls and significantly more episodes of diarrhoea than in their pre-Cryptosporidium period (data not shown; P ≤ 0.001 and P < 0.05, respectively) Before Cryptosporidium infection, 8 case-children, who were ≤ 1 year-old and had no diarrhoeal illnesses, had height-for-age Z-scores identical to matched controls. However, after Cryptosporidium infection, these case-children had significant decline in height-for-age Z-scores which were not seen in the matched controls (P < 0.005 for pre-infection vs post-infection case-children) It is not known whether the increase in post-Cryptosporidium diarrhoeal disease burden was due solely to the impact of infection with Cryptosporidium, or if a similar phenomenon would also be seen with other serious enteric infections (e.g. rotavirus or enteroaggregative Escherichia coli) |
|
Ajjampur et al. [39] Vellore, South India |
Semi-urban slum Prospective birth cohort study 3 years |
Unidentified |
40/116 children who consented to take part in the study were identified as having had cryptosporidial diarrhoea, 66 of them had giardial diarrhoea and 22 had both 32 with no documented episodes of cryptosporidial or giardial diarrhoea were also recruited |
Mean (± SD) age of the children during assessment was 3.51 ± 0.38 years Median (IQR) for age at the first documented cryptosporidial episodes were 1.29 (0.81–2.05) years 55.2% males |
Children with cryptosporidial diarrhoea had a mean (SD) social quotient (SQ) of 118.70 (35.01) (P = 0.714) Children with cryptosporidial diarrhoea did not have significantly lower IQ scores than those without a past history of cryptosporidial diarrhoea (mean IQ 100.12, SD 17.28) In the univariate analysis, a past history of any protozoan diarrhoea, either giardial or cryptosporidial, was not a significant predictor of stunting or being underweight Cryptosporidial diarrhoea was not associated with poor IQ, SQ or physical growth |
|
Berkman et al. [40] Lima, Peru |
Periurban shanty town Prospective birth cohort Follow-up birth to 2 years with cognitive function at 9 years |
Unidentified |
Cognitive assessment completed in 143 children 77 (54%) had at least one episode of Cryptosporidium infection |
Follow-up birth to 2 years with cognitive function at 9 years. Estimated median age at onset of first Cryptosporidium infection was 16.1 months 76 (53%) males |
No association between Cryptosporidium infection and cognitive test scores according to the number of episodes, incidence and prevalence, and symptomatic infections |
|
*Carter et al. [21] UK (Wales) |
Sporadic community cases Prospective case cohort study 12 months |
C. parvum (n = 121) C. hominis (n = 79) C. parvum and C. hominis (n = 2) Other species (n = 3) |
515 eligible; 205 participated |
42 (20%): 6 months-4 years 63 (31%): 5–17 years 100 (49%): 18 years or over 60.6% female at baseline 58.2% female at 3 months 66.3% female at 12 months |
12 months follow-up: over a third of cases reported persistent abdominal pain and diarrhoea, 28% reported joint pain and 26% reported fatigue At both 3 and 12 months, the proportion reporting fatigue and abdominal pain after C. hominis infection was statistically significantly greater than after C. parvum Overall, 10% of cases had sufficient symptoms to meet IBS diagnostic criteria. A further 27% met all criteria except 6 months’ duration and another 23% had several features of IBS but did not fulfil strict Rome III criteria. There was no significant difference between C. parvum and C. hominis infection with regard to PI-IBS |
|
Delahoy et al. [41] Kenya |
Rural community Prospective, age-stratified, health facility-based matched case-control study of children with MSD ~ 60 days (acceptable range 50–90 days) |
Unidentified | Among the 1778 MSD case children enrolled, Cryptosporidium was identified in 195 cases (11.0%) |
46%: 0–11 months 27%: 12–23 months 25%: 24–59 months 56% male |
At follow-up, Cryptosporidium- positive cases had increased odds of being stunted (adjusted odds ratio, aOR: 1.65, 95% CI: 1.06–2.57), underweight (aOR: 2.08, 95% CI: 1.34–3.22), or wasted (aOR: 2.04, 95% CI: 1.21–3.43), and had significantly larger negative changes in height- and weight-for-age z-scores from enrollment |
|
Guerrant et al. [42] Fortaleza, Brazil |
Urban slum Prospective cohort study 6–9 years |
Unidentified | 26 children; 9 Cryptosporidium infections (6 with diarrhoea, 3 without diarrhoea) |
26 children (12 boys and 14 girls) Age range: 6.5–9 years |
Cryptosporidium infections (seen in 9/26 children) in the first 2 years of life were correlated with a 2-fold increase in episodes of diarrhoea at 0–2 years of age (P = 0.017, by 2-sample t-test) Fitness scores in children with early childhood Cryptosporidium were 10% lower than in controls (9.0 vs 10.0; P = 0.008, by 2-sample t-test) Adjusting for Cryptosporidium removed both the significance of the correlation between diarrhoea and fitness and between Cryptosporidium and fitness |
|
*Hunter et al. [15] UK (Northwest of England and Wales) |
Sporadic community cases and controls Case-control study 2 months |
C. parvum (n = 50) C. hominis (n = 61) Unidentified (n = 124) |
235 case patients; 232 control subjects |
Age range: 0–89 years Control subjects were significantly older than case patients (χ2 = 8.574, P = 0.0034) 49% of case patients and 46% of control subjects were male |
40% of case patients reported recurrence of intestinal symptoms after resolution of the acute stage of illness Reports of joint pain (odds ratio, OR: 2.8), eye pains (OR: 2.44), recurrent headache (OR: 2.10), dizzy spells (OR: 1.69), and fatigue (OR: 3.0) were significantly more common in case patients than in control subjects, but only in people who had experienced C. hominis infection |
|
*Igloi et al. [15] Netherlands |
Sporadic community cases and controls Case-crossover and cryptosporidiosis case control study 4 months |
C. parvum (n = 216) C. hominis (n = 92) |
308 cases |
Median age: 26 years (range: 1–80) 58% were female |
Compared to before illness, cases were significantly more likely to report dizziness (OR: 2.25), headache (OR: 2.15), fatigue (OR: 2.04), weight loss (OR: 1.82), diarrhoea (OR: 1.50), abdominal pain (OR: 1.38) or joint pain (OR: 1.84). However, symptoms of joint pain and headache occurred among cases after illness at a rate that was not significantly different from that observed in the general population There were no significant differences in post-infection symptom occurrence between C. hominis and C. parvum |
|
*Insulander et al. [19] Stockholm County, Sweden |
Sporadic community cases Prospective cryptosporidiosis case cohort study 25–36 months |
C. parvum (n = 111) C. hominis (n = 65) Other species (n = 17) |
271 cases |
Median age: 32 years (range: 1–73 years) 126 male and 145 female |
After 25–36 months follow-up: 15% reported intermittent diarrhoea (8/53), 9% reported abdominal pain (5/53), 8% reported myalgia/arthralgia (4/53), 4% reported fatigue (2/53) There was no difference in frequency of persisting symptoms between patients infected with C. parvum or C. hominis |
|
Korpe et al. [43] Bangladesh |
Peri-urban slum Prospective birth cohort study 2 years |
C. hominis (n = 220) C. parvum (n = 8) C. parvum and C. hominis (n = 5) Other species (n = 5) Unidentified (n = 154) |
392 children |
Birth to 24 months of age 55% male |
Children with Cryptosporidium spp. infection had a greater than 2-fold increased risk of severe stunting at age two compared to uninfected children (OR: 2.69, 95% CI 1.17–6.15, P = 0.019) independent of sex, income, maternal body-mass index, maternal education and weight for age adjusted z-score (WAZ) at birth |
|
*Lilja et al. [20] Ostersund, Sweden |
Outbreak cohort and controls Case-control study 28 months |
C. hominis | 215 cases; 344 non-cases |
Median age of cases: 41 (range: 3–79) years Median age of non-cases: 56 (range: 3–95) years 57% of cases and 55% of controls were women |
48% of cases reported symptoms at follow-up, most commonly headache, fatigue, abdominal pain, and nausea Compared to non-cases, the cases were more likely to report watery diarrhoea, abdominal pain, stiff joints, joint pain, joint discomfort, fatigue, nausea, and headache at follow-up after adjusting for age and sex The likelihood of cases reporting symptoms at follow-up differed between age groups: joint pain (OR: 13.2, 95% CI: 2.8–61.9) and nausea (OR: 2.7, 95% CI: 1.2–6.0) were associated only with the 16–40-year age group; diarrhoea (OR: 3.9, 95% CI: 1.1–14.3) was associated only with the > 65-year age group; and headache (OR: 4.0, 95% CI: 1.3–13.1) was associated only with the 6–15-year-old age group |
|
Phillips et al. [44] London, UK |
Sporadic urban community and traveller community cases Retrospective cohort Variable |
Unidentified | 123 children | Not specified |
50% of children excreting only Cryptosporidium had diarrhoea lasting over 21 days; in 8% of cases diarrhoea continued for over 6 months. 23% of cases had weight below the third centile and a further 9% had failure to thrive. Most cases (63%) of chronic diarrhoea occurred in the first two years of life. A mild to moderate enteropathy was present in all 9 children undergoing a small intestinal biopsy and 7 showed the presence of Cryptosporidium adhering to villous epithelium. All patients eventually recovered spontaneously Although a greater proportion of patients with mixed infections had weight below the 3rd percentile (8/21) this was not significantly different to those with Cryptosporidium alone (11/61) |
|
*Rehn et al. [17] Ostersund and Skelleftea, Sweden |
Community outbreak cases and controls Case-control study 11 months |
C. hominis |
Östersund: 872 (310 cases) Skellefteå: 743 (149 cases) |
Östersund: Median age of cases: 32 years (range: 1–93) Skellefteå: Median age of cases: 34 years (range: 2–92) Östersund: 310 (38%) cases, 138 (45 %) were male Skellefteå study: 149 (22%) cases, 73 (49 %) were |
Outbreak cases were more likely to report diarrhoea (Östersund OR: 3.3, 95% CI: 2.0–5.3. Skellefteå OR: 3.6, 95% CI: 2.0–6.6), watery diarrhoea (Östersund OR: 3.4, 95% CI: 1.9–6.3. Skellefteå OR: 2.8, 95% CI: 1.5–5.1) abdominal pain (Östersund OR: 2.1, 95% CI: 1.4–3.3, Skellefteå OR: 2.7, 95% CI: 1.5–4.6) and joint pain (Östersund OR: 2.0, 95% CI: 1.2–3.3, Skellefteå OR: 2.0, 95% CI: 1.1–3.6) at follow-up compared to non-cases |
|
*Stiff et al. [18] UK (mainly northern England) |
Community outbreak cases Prospective cohort study 12 months |
C. parvum | 197 invited; 54 took part |
Mean age: 41.8 years 14 males and 40 females |
12 months follow up: participants self-reported weight loss (31%), abdominal pain (38%), diarrhoea (33%), eye pain (9 %), joint pain (33 %), fatigue (22 %) and symptoms consistent with irritable bowel syndrome (IBS) (28 %). Two people were medically diagnosed with IBS |
|
*Widerstrom et al. [4] Östersund, Sweden |
Community outbreak cases and controls Case-control study 2 months |
C. hominis | 1524 eligible; 1044 (69.2%) responded |
Median age: 44 years (range: 0–98 years) 481 male (46.1%) and 563 female (53.9%) |
Most common symptoms among case-patients were episodes of diarrhoea > 3 times daily (89.0%), watery diarrhoea (84.3%), abdominal cramps (78.8%), fatigue (73.1%), nausea (63.9%), and headache (57.1%) Muscle or joint aches, which were reported less frequently in Östersund than in other studies The median duration of diarrhoea, the level of attack rates in different age groups, and recurrence rate of diarrhoea corresponded to findings in other outbreaks |
Note: Eight studies which were included in the quantitative synthesis are marked by an asterisk (*)
Table 2.
Pooled estimates for the prevalence of post-Cryptosporidium sequelae using a random effects model
| Sequelae | No. of studies | Pooled estimate (%) (95% CI) | Cochran Q | P-value |
|---|---|---|---|---|
| Diarrhoea | 13 | 25 (10–44) | 1382.71 | < 0.001 |
| Abdominal pain | 13 | 25 (13–39) | 575.30 | < 0.001 |
| Joint pain | 13 | 15 (12–19) | 63.28 | < 0.001 |
| Fatigue | 13 | 24 (13–37) | 477.50 | < 0.001 |
| Vomiting | 10 | 8 (5–12) | 72.61 | < 0.001 |
| Headache | 10 | 21 (12–33) | 271.21 | < 0.001 |
| Eye pain | 10 | 10 (7–14) | 46.66 | < 0.001 |
| Loss of appetite | 9 | 19 (14–24) | 51.32 | < 0.001 |
| Weight loss | 9 | 13 (7–20) | 97.98 | < 0.001 |
| Nausea | 8 | 24 (11–40) | 263.50 | < 0.001 |
| Blood in stool | 7 | 3 (2–6) | 17.26 | 0.01 |
| Dizzy spells | 6 | 8 (5–12) | 13.24 | 0.02 |
| Fever | 5 | 13 (4–25) | 51.28 | < 0.001 |
| Blurred vision | 5 | 6 (4–8) | 5.19 | 0.27 |
| IBS | 3 | 11 (6–16) | 0.11 | 0.95 |
Note: Studies were included more than once if outcomes were reported at more than one interval
Table 3.
Pooled prevalence of post-Cryptosporidium sequelae estimated by a random effects model, according to clinical manifestation by time period post-infection
| Sequelae | No. of studies | Pooled estimate (%) (95% CI) |
Cochranʼs Q | P-value |
|---|---|---|---|---|
| < 6 months | ||||
| Diarrhoea | 5 | 43 (12–77) | 532.73 | < 0.001 |
| Abdominal pain | 5 | 41 (16–68) | 278.61 | < 0.001 |
| Loss of appetite | 4 | 26 (21–32) | 8.11 | 0.04 |
| Nausea | 3 | 37 (59–69) | 82.86 | < 0.001 |
| Fatigue | 5 | 39 (17–63) | 227.41 | < 0.001 |
| Weight loss | 4 | 22 (19–26) | 3.20 | 0.36 |
| Fever | 3 | 15 (2–33) | 37.77 | < 0.001 |
| Vomiting | 5 | 9 (3–16) | 47.83 | < 0.001 |
| Joint pain | 5 | 18 (15–21) | 5.75 | 0.22 |
| Headache | 5 | 21 (5–42) | 215.84 | < 0.001 |
| Dizzy spells | 4 | 9 (5–14) | 10.81 | 0.01 |
| Eye pain | 5 | 9 (4–15) | 35.88 | < 0.001 |
| Blurred vision | 3 | 5 (3–7) | 2.10 | 0.35 |
| Blood in stool | 3 | 4 (3–6) | 0.00 | 1 |
| > 6 months | ||||
| Diarrhoea | 8 | 16 (13–22) | 31.14 | < 0.001 |
| Abdominal pain | 8 | 16 (9–25) | 71.17 | < 0.001 |
| Loss of appetite | 5 | 14 (10–18) | 8.68 | 0.07 |
| Nausea | 5 | 18 (13–25) | 19.47 | < 0.001 |
| Fatigue | 8 | 16 (9–26) | 80.85 | < 0.001 |
| Weight loss | 5 | 6 (4–9) | 6.50 | 0.16 |
| Fever | 2 | 10 (3–21) | 2.57 | 0.11 |
| Vomiting | 5 | 7 (3–12) | 21.53 | < 0.001 |
| Joint pain | 8 | 14 (9–19) | 45.27 | < 0.001 |
| Headache | 5 | 22 (12–34) | 52.38 | < 0.001 |
| Dizzy spells | 2 | 6 (2–11) | 0.04 | 0.84 |
| Eye pain | 5 | 12 (7–14) | 8.84 | 0.07 |
| Blurred vision | 2 | 9 (4–14) | 0.01 | 0.94 |
| Blood in stool | 4 | 3 (0–6) | 11.78 | 0.01 |
Table 4.
Pooled risk ratio of individual post-Cryptosporidium sequelae
| Sequelae | No. of studies | Pooled RR (95% CI) |
Cochranʼs Q | P-value |
|---|---|---|---|---|
| Diarrhoea | 5 | 6.7 (2.63–17.03) | 105.37 | < 0.001 |
| Abdominal pain | 5 | 2.99 (1.56–5.72) | 107.10 | < 0.001 |
| Loss of appetite | 4 | 1.98 (1.48–2.63) | 8.03 | 0.05 |
| Nausea | 3 | 2.89 (1.15–7.30) | 56.77 | < 0.001 |
| Fatigue | 5 | 2.56 (1.47–4.48) | 108.43 | < 0.001 |
| Weight loss | 4 | 3.65 (1.66–8.03) | 22.83 | < 0.001 |
| Vomiting | 5 | 2.56 (1.27–5.15) | 32.38 | < 0.001 |
| Joint pain | 5 | 2.26 (1.35–3.77) | 34.30 | < 0.001 |
| Headache | 5 | 2.23 (1.22–4.09) | 97.31 | < 0.001 |
| Eye pain | 5 | 1.98 (1.09–3.59) | 28.90 | < 0.001 |
Abbreviation: RR, risk ratio
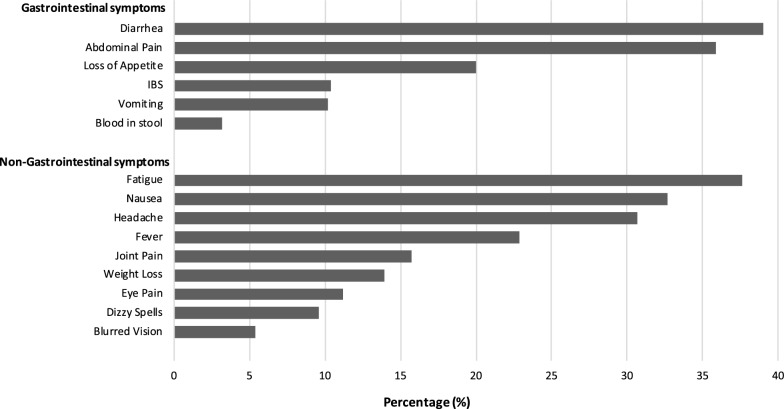
Fig. 2.
Reported sequelae up to 36 months post-Cryptosporidium infection
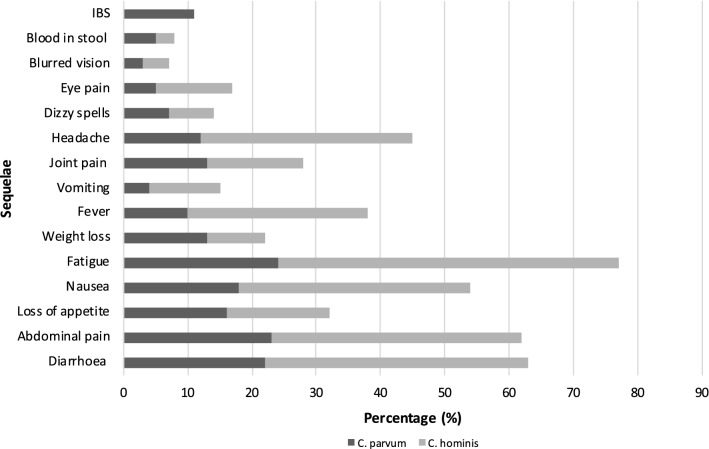
Fig. 3.
Reported sequelae up to 36 months post-Cryptosporidium infection by species (%)
Qualitative synthesis
Electronic searching returned 1251 PubMed, 2161 ProQuest and 3227 Web of Science abstracts. After removal of duplicates, screening and assessment, 15 articles were suitable for inclusion in the qualitative synthesis and the data extracted from these studies are summarized in Table 1.
The 15 shortlisted studies included 3670 Cryptosporidium cases. The studies comprised 8 cohort studies and 7 case-control studies. Seven studies were conducted in children, with the remaining 8 studies including both adults and children. The length of duration of follow-up ranged from 2 months to 9 years. Studies were conducted in South America (3 studies), Africa (1 study), South Asia (2 studies) and Europe (9 studies) and were all based in a community setting. The selected studies were published between 1992 and 2019. The studies investigated a range of potential sequelae; diarrhoea (3 studies), developmental delay (2 studies), stunting of growth (4 studies) and multiple gastrointestinal and non-gastrointestinal symptoms (8 studies).
Quantitative synthesis
Adequate information to estimate post-Cryptosporidium infection sequelae was available in 8 of the 15 studies [4, 15–21]. The pooled estimates for each of the sequelae are shown in Table 2. Data for each individual sequela are available in Additional file 2.
The eight studies were conducted in Europe between 2004 and 2019; four in Sweden, three in the UK and one in the Netherlands. The sequelae investigated were mostly gastrointestinal, with some non-gastrointestinal symptoms such as joint pain and eye pain and most recruited cases were adults. This was in contrast to studies in non-industrialised countries which focused on growth, nutrition and cognitive detriment in children.
The most frequently investigated sequelae are listed in Table 2 and included diarrhoea, abdominal pain, vomiting, fatigue, joint pain, eye pain and headache.
The most common reported long-term sequelae were diarrhoea (25%), abdominal pain (25%), nausea (24%), fatigue (24%) and headache (21%). The distribution of gastrointestinal manifestations and non-gastrointestinal manifestations reported is shown in Fig. 2.
Subgroup analysis
Table 3 shows the pooled estimates for the prevalence of post-Cryptosporidium sequelae by time period post-infection. With the exception of eye pain and headache, all sequelae were more frequently reported within 6 months of Cryptosporidium infection.
In all eight studies included in the quantitative analysis, species identification of Cryptosporidium had been performed. Four were outbreak cohort follow-up studies so contained only one species (three contained C. hominis cases exclusively and one contained C. parvum exclusively). The other four studies contained both species; one of these four also contained a small number of other species (17/271 cases), but because of the low numbers, these have not been considered here. Figure 3 shows the pooled estimates for the prevalence of post-Cryptosporidium sequelae by Cryptosporidium species. Overall, long-term sequelae were more prevalent following infection with C. hominis, with only weight loss and blood in stool being more prevalent following infection with C. parvum. IBS was reported in 11% of cases, however, it should be noted that data for this outcome were only available from 2 studies, one of which only studied C. parvum cases.
Sequelae risk
Five of the 8 qualitative synthesis studies included a control group. A limited evaluation of risk of individual sequelae using the five case-control studies available was undertaken [4, 15, 17, 19, 20]. Data were available for 10 sequelae (Table 4).
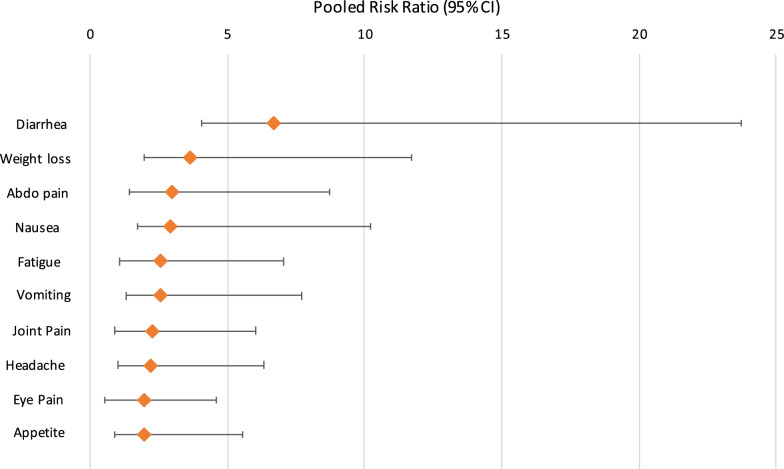
Individuals were 6 times more likely to report chronic diarrhoea and weight loss up to 28 months after a Cryptosporidium infection than controls. Long-term abdominal pain, loss of appetite, fatigue, vomiting, joint pain, headache and eye pain were also 2–3 times more likely following a Cryptosporidium infection (Fig. 4).
Fig. 4.
Pooled risk ratio of individual post-Cryptosporidium sequelae showing 95% confidence intervals
To view the PRISMA checklist relating to this work, please see Additional file 3.
Discussion
Of the 15 studies investigating long-term sequelae, just over half were set in industrialised countries. In contrast to those in non-industrialised settings, these involved mainly adult cases, with the inclusion of some children. Half were outbreak cohort studies, with the rest involving sporadic community cases. Studies from non-industrialised countries involved exclusively children, reflecting the greater clinical importance and recognition of paediatric infection in such settings. In industrialised countries there is more focus on detecting sporadic community cases of cryptosporidiosis in all age groups, partly in order to facilitate early detection of community outbreaks, for example from drinking water, swimming pools, or other environmental sources. The studies in non-industrialised countries also differed in that the children were recruited and tested as part of the specific studies, whilst the studies in industrialised countries relied on cases initially diagnosed routinely.
The eight studies suitable for inclusion in the quantitative analysis were all carried out in just three countries in Europe (UK, Sweden and the Netherlands), where species data are routinely generated and all except one [11] were relatively recent, dated between 2013–2019. In many non-industrialised countries, or in earlier European studies, species identification would not be routinely performed, and this is reflected in the study data. The geographical reach of the eight studies is somewhat limited, since they were all located in northwest Europe. Only five were case-control studies, and of these, only two included both C. hominis and C. parvum, with the other three limited to studying C. hominis alone following outbreaks. Since the bulk of the cryptosporidiosis burden is found in low-income countries, there is a need in future to conduct similar quantitative evaluations using data from developing countries, where obtaining suitable data may be more challenging.
There were some limitations to this review. The role of genotype in long-term outcomes could not be explored. Typing was undertaken by gp60 sequencing in three of the studies but was either not analysed with symptoms data [16], or was an outbreak where all had the same subtype [4, 18]. There were insufficient data to compare between studies. Another limitation was that since not all cases were necessarily tested for all gastrointestinal pathogens, or the results of such tests were not stated, long-term sequelae identified cannot be proven to be Cryptosporidium-specific and not due to other infectious agents.
Most of the studies examined quantitatively were concentrated on adult individuals, whereas cryptosporidiosis is commonest in young children. This over-representation of adults results from the fact that several of the studies followed large waterborne outbreaks involving many adults, rather than sporadic cases. Identifying and defining sometimes rather non-specific sequelae is more difficult in very young children. However, a study by Carter et al. [21] of sporadic cases did include children, and in fact this study found that the proportion developing IBS or IBS-like symptoms was higher in children than in adults, with 78% reporting it among 5–17 years-old and 63% at 6 months to 4 years-old.
The results indicate that sequelae are frequently reported after cryptosporidiosis lasting up to at least 2 years. Only one study investigated cases for longer, up to 36 months [11]. For both main infecting species, sequelae occur, but there are differences in the frequency of each depending on the species. Following the publication of the first study in 2004 [15], the evidence base surrounding post-Cryptosporidium infection sequelae has continued to expand [16–21]. Gastrointestinal sequelae such as continuing diarrhoea, nausea and abdominal pain appear particularly common, each reported by around a quarter of cases up to 36 months post-infection, with analysis of the case-control studies finding that persistent diarrhoea is around six times more likely than in controls and weight loss over three times more likely over 28 months. Fatigue and headache were also commonly reported and occurred in the case-control studies between two-three times more commonly in cases than controls over the same time period. Overall, the most commonly reported long-term sequelae were diarrhoea (25%), abdominal pain (25%), nausea (24%), fatigue (24%) and headache (21%). Where it was investigated, there was evidence that symptoms meeting the definition for IBS were described just over 10% of cases up to 36 months.
Conclusions
This is the first systematic review of the long-term sequelae of cryptosporidiosis. The proportion of cases self-reporting sequelae post-infection has been estimated and estimates of risk of specific sequelae presented. Risk factors for sequelae were less well identified. A better understanding of the long-term outcomes of cryptosporidiosis is valuable to inform the expectations of clinicians and their patients and public health policy makers regarding the control and prevention of this infection.
Supplementary information
Additional file 1: Table S1. Full electronic search strategies. Table S2. Newcastle-Ottawa quality assessment scale.
Additional file 2. Data for individual sequelae.
Acknowledgements
Not applicable.
Authors’ contributions
BC undertook the literature search and screened the titles and abstracts for relevance initially after which they were independently screened by BC and APD to ensure consistent application of the pre-determined inclusion/exclusion criteria. Final inclusion of studies was decided by consensus, with any conflicts being reviewed by RMC. BC wrote the first draft and AD and RC added to and reviewed it. All authors read and approved the final manuscript.
Funding
This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its additional files.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bethan L. Carter, Email: bethanlc25@gmail.com
Rachel M. Chalmers, Email: rachel.chalmers@wales.nhs.uk
Angharad P. Davies, Email: angharad.p.davies@swansea.ac.uk
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13071-020-04308-7.
References
- 1.Chalmers RM, Davies AP. Minireview: clinical cryptosporidiosis. Exp Parasitol. 2010;124:138–146. doi: 10.1016/j.exppara.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Monge S, Pijnacker R, Van Pelt W, Franz E, Kortbeek LM, Mangen MJJ. Accounting for long-term manifestations of Cryptosporidium spp. infection in burden of disease and cost-of-illness estimations, the Netherlands (2013–2017) PLoS ONE. 2019;14:1–16. doi: 10.1371/journal.pone.0213752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Efstratiou A, Ongerth JE, Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks—an update 2011–2016. Water Res. 2017;114:14–22. doi: 10.1016/j.watres.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 4.Widerström M, Schönning C, Lilja M, Lebbad M, Ljung T, Allestam G, et al. Large outbreak of Cryptosporidium hominis infection transmitted through the public water supply, Sweden. Emerg Infect Dis. 2014;20:581–589. doi: 10.3201/eid2004.121415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacKenzie WR, Schell WL, Blair KA, Addiss DG, Peterson DE, Hoxie NJ, et al. Massive outbreak of waterborne Cryptosporidium infection in Milwaukee, Wisconsin: recurrence of illness and risk of secondary transmission. Clin Infect Dis. 1995;21:57–62. doi: 10.1093/clinids/21.1.57. [DOI] [PubMed] [Google Scholar]
- 6.DeSilva MB, Schafer S, Kendall Scott M, Robinson B, Hills A, Buser GL, et al. Communitywide cryptosporidiosis outbreak associated with a surface water-supplied municipal water system—Baker City, Oregon, 2013. Epidemiol Infect. 2016;14:274–284. doi: 10.1017/S0950268815001831. [DOI] [PubMed] [Google Scholar]
- 7.Chalmers RM, Robinson G, Elwin K, Elson R. Analysis of the Cryptosporidium spp. and gp60 subtypes linked to human outbreaks of cryptosporidiosis in England and Wales, 2009 to 2017. Parasit Vectors. 2009;2019(12):95. doi: 10.1186/s13071-019-3354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies AP, Campbell B, Evans MR, Bone A, Roche ACR. Asymptomatic carriage of protozoan parasites in children in day care centers in the United Kingdom. Pediatr Infect Dis J. 2009;28:838–840. doi: 10.1097/INF.0b013e31819d646d. [DOI] [PubMed] [Google Scholar]
- 9.Reh L, Muadica AS, Köster PC, Balasegaram S, Verlander NQ, Chércoles ER, et al. Substantial prevalence of enteroparasites Cryptosporidium spp., Giardia duodenalis and Blastocystis sp. in asymptomatic schoolchildren in Madrid, Spain, November 2017 to June 2018. Euro Surveill. 2019;24:1900241. doi: 10.2807/1560-7917.ES.2019.24.43.1900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehsan MA, Akter M, Ahammed M, Ali MA, Ahmed MU. Prevalence and clinical importance of Cryptosporidium and Giardia in humans and animals. Bangladesh J Vet Med. 2016;14:109–122. [Google Scholar]
- 11.Shirley DT, Moonah SN, Kotloff KL. Burden of disease from cryptosporidiosis. Curr Opin Infect Dis. 2015;25:555–563. doi: 10.1097/QCO.0b013e328357e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halliez MCBA. Extra-intestinal and long term consequences of Giardia duodenalis infections. World J Gastroenterol. 2013;19:8974–8985. doi: 10.3748/wjg.v19.i47.8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litleskare S, Rortveit G, Eide GE, Hanevik K, Langeland NWK. Prevalence of irritable bowel syndrome and chronic fatigue 10 years after Giardia infection. Clin Gastroenterol Hepatol. 2018;16:1064–1072. doi: 10.1016/j.cgh.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Mørch K, Hanevik K, Rivenes AC, Bødtker JE, Næss H, Stubhaug B, et al. Chronic fatigue syndrome 5 years after giardiasis: differential diagnoses, characteristics and natural course. BMC Gastroenterol. 2013;13:28. doi: 10.1186/1471-230X-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter PR, Hughes S, Woodhouse S, Nicholas R, Syed Q, Chalmers RM, et al. Health sequelae of human cryptosporidiosis in immunocompetent patients. Clin Infect Dis. 2004;39:504–510. doi: 10.1086/422649. [DOI] [PubMed] [Google Scholar]
- 16.Insulander M, Silverlås C, Lebbad M, Karlsson L, Mattsson JG, Svenungsson B. Molecular epidemiology and clinical manifestations of human cryptosporidiosis in Sweden. Epidemiol Infect. 2013;141:1009–1020. doi: 10.1017/S0950268812001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rehn M, Wallensten A, Widerström M, Lilja M, Grunewald M, Stenmark S, et al. Post-infection symptoms following two large waterborne outbreaks of Cryptosporidium hominis in northern Sweden, 2010–2011. BMC Public Health. 2015;15:529. doi: 10.1186/s12889-015-1871-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stiff RE, Davies AP, Mason BW, Hutchings HA, Chalmers RM. Long-term health effects after resolution of acute Cryptosporidium parvum infection: a 1-year follow-up of outbreak-associated cases. J Med Microbiol. 2017;66:1607–1611. doi: 10.1099/jmm.0.000609. [DOI] [PubMed] [Google Scholar]
- 19.Iglói Z, Mughini-Gras L, Nic Lochlainn L, Barrasa A, Sane J, Mooij S, et al. Long-term sequelae of sporadic cryptosporidiosis: a follow-up study. Eur J Clin Microbiol Infect Dis. 2018;37:1377–1384. doi: 10.1007/s10096-018-3268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lilja M, Widerström M, Lindh J. Persisting post-infection symptoms 2 years after a large waterborne outbreak of Cryptosporidium hominis in northern Sweden. BMC Res Notes. 2018;11:625. doi: 10.1186/s13104-018-3721-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter BL, Stiff RE, Elwin K, Hutchings HA, Mason BW, Davies AP, et al. Health sequelae of human cryptosporidiosis—a 12-month prospective follow-up study. Eur J Clin Microbiol Infect Dis. 2019;38:1709–1717. doi: 10.1007/s10096-019-03603-1. [DOI] [PubMed] [Google Scholar]
- 22.Hay E, Winfield W, McKendrick MW. Reactive arthritis associated with Cryptosporidium enteritis. Br Med J (Clin Res Ed) 1987;295:248. doi: 10.1136/bmj.295.6592.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shepherd RC, Smail PJ, Sinha GP. Reactive arthritis complicating cryptosporidial infection. Arch Dis Child. 1989;64:743–744. doi: 10.1136/adc.64.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sing A, Bechtold S, Heesemann J, Belohradsky BH, Schmidt H. Reactive arthritis associated with prolonged cryptosporidial infection. J Infect. 2003;47:181–184. doi: 10.1016/s0163-4453(03)00035-5. [DOI] [PubMed] [Google Scholar]
- 25.Cron RQ, Sherry DD. Reiter’s syndrome associated with cryptosporidial gastroenteritis. J Rheumatol. 1995;22:1962–1963. [PubMed] [Google Scholar]
- 26.Hawkins SP, Thomas RP, Teasdale C. Acute pancreatitis: a new finding in Cryptosporidium enteritis. Br Med J (Clin Res Ed) 1987;294:483–484. doi: 10.1136/bmj.294.6570.483-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norby SM, Bharucha AE, Larson MV, Temesgen Z. Acute pancreatitis associated with Cryptosporidium parvum enteritis in an immunocompetent man. Clin Infect Dis. 2008;27:223–224. doi: 10.1086/517686. [DOI] [PubMed] [Google Scholar]
- 28.Printza N, Sapountzi E, Dotis J, Papachristou F. Hemolytic uremic syndrome related to Cryptosporidium infection in an immunocompetent child. Pediatr Int. 2013;55:788–790. doi: 10.1111/ped.12127. [DOI] [PubMed] [Google Scholar]
- 29.Kalantari N, Gorgani-Firouzjaee T, Ghaffari S, Bayani M, Ghaffari T, Chehrazi M. Association between Cryptosporidium infection and cancer: a systematic review and meta-analysis. Parasitol Int. 2020;74:101979. doi: 10.1016/j.parint.2019.101979. [DOI] [PubMed] [Google Scholar]
- 30.Bouwknegt M, Devleesschauwer B, Graham H, Robertson LJ, van der Giessen JW, The Euro-Fbp Workshop participants Prioritisation of food-borne parasites in Europe, 2016. Euro Surveill. 2018;23:17-00161. doi: 10.2807/1560-7917.ES.2018.23.9.17-00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 32.Cassini A, Colzani E, Pini A, Mangen MJ, Plass D, McDonald SA, et al. Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): results from the burden of communicable diseases in Europe study, European Union and European Economic Area countries, 2009 to 2013. Euro Surveill. 2018;23:17–00454. doi: 10.2807/1560-7917.ES.2018.23.16.17-00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mangen MJJ, Bouwknegt M, Friesema IHM, Haagsma JA, Kortbeek LM, Tariq L, et al. Cost-of-illness and disease burden of food-related pathogens in the Netherlands, 2011. Int J Food Microbiol. 2015;196:84–93. doi: 10.1016/j.ijfoodmicro.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 34.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2012. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.%0A. Accessed 4 June 2020.
- 35.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67:974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. London: The Cochrane Collaboration; 2011. http://handbook-5-1.cochrane.org/.
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agnew DG, Lima AA, Newman RD, Wuhib T, Moore RD, Guerrant RL, et al. Cryptosporidiosis in northeastern Brazilian children: association with increased diarrhea morbidity. J Infect Dis. 1998;177:754–760. doi: 10.1086/514247. [DOI] [PubMed] [Google Scholar]
- 39.Ajjampur SSR, Koshy B, Venkataramani M, Sarkar R, Joseph AA, Jacob KS, et al. Effect of cryptosporidial and giardial diarrhoea on social maturity, intelligence and physical growth in children in a semi-urban slum in south India. Ann Trop Paediatr. 2011;31:205–212. doi: 10.1179/1465328111Y.0000000003. [DOI] [PubMed] [Google Scholar]
- 40.Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359:564–571. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- 41.Delahoy MJ, Omore R, Ayers TL, Schilling KA, Blackstock AJ, Ochieng JB, et al. Clinical, environmental, and behavioral characteristics associated with Cryptosporidium infection among children with moderate-to-severe diarrhea in rural western Kenya, 2008–2012: the Global Enteric Multicenter Study (GEMS) PLoS Negl Trop Dis. 2018;12:e0006640. doi: 10.1371/journal.pntd.0006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg. 1999;61:707–713. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- 43.Korpe PS, Haque R, Gilchrist C, Valencia C, Niu F, Lu M, et al. Natural history of cryptosporidiosis in a longitudinal study of slum-dwelling Bangladeshi children: association with severe malnutrition. PLoS Negl Trop Dis. 2016;10:e0004564. doi: 10.1371/journal.pntd.0004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillips AD, Thomas AG, Walker-Smith JA. Cryptosporidium, chronic diarrhoea and the proximal small intestinal mucosa. Gut. 1992;33:1057–1061. doi: 10.1136/gut.33.8.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Full electronic search strategies. Table S2. Newcastle-Ottawa quality assessment scale.
Additional file 2. Data for individual sequelae.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its additional files.