Abstract
BACKGROUND:
Aspirin (ASA) anti-platelet therapy is mandated with left ventricular assist devices (LVADs) to prevent hemocompatibility-related adverse events (HRAEs). However, the optimal dose of ASA with HeartMate 3 (HM3) LVAD is unknown.
METHODS:
In an exploratory analysis of HM3-supported patients in the MOMENTUM 3 study (NCT02224755), 2 groups were analyzed: usual-dose (325 mg) and low-dose (81 mg) ASA with anti-coagulation targeted to an international normalized ratio of 2.0 to 3.0. Exclusion criteria included patients not receiving either ASA 81 mg or 325 mg, those with HRAEs ≤7 days after device implantation, and those receiving >1 anti-platelet agent. The primary end-point was survival free from HRAEs (non-surgical bleeding, pump thrombosis, stroke, and peripheral arterial thromboembolic events) at 2 years.
RESULTS:
Overall, 321 HM3 patients (usual-dose: n = 141, low-dose: n = 180) were included in this analysis. Usual-dose group patients were younger (57 ± 13 vs 60 ± 12 years, p = 0.035) and less often assigned destination therapy (55% vs 67%, p = 0.029) than low-dose ASA. At 2 years, a similar proportion of patients in the usual- and low-dose groups (43.4% vs 45.3%, p = 0.94) met the primary endpoint. There were no differences in survival free from hemorrhagic (usual-dose: 54.4% vs low-dose: 51.7%, p = 0.42) or thrombotic (usual-dose: 76.8% vs low-dose: 75.7%, p = 0.92) events.
CONCLUSIONS:
Usual- and low-dose ASA revealed similar rates of bleeding and thrombotic events in HM3 LVAD-supported patients within the MOMENTUM 3 trial. Whether ASA therapy provides any meaningful therapeutic effect in patients treated by the HM3 LVAD remains to be determined.
Keywords: left ventricular assist device, aspirin, bleeding, thrombosis, hemocompatibility
Left ventricular assist device (LVAD) therapy improves hemodynamics and enhances survival in advanced heart failure, yet the abnormal circulation induced by continuous-flow rotary pumps is associated with platelet dysregulation and acquired von Willebrand syndrome.1–3 Aspirin (ASA) anti-platelet administration has remained a cornerstone of anti-thrombotic therapy during LVAD support. During continuous-flow LVAD support, platelet function is already influenced by shear stress and possibly acquired von Willebrand syndrome. It remains unknown whether the addition of ASA is beneficial or simply confers bleeding tendency.4 In the absence of compelling clinical evidence and outcome data, there is marked clinical variation in the dosing of ASA-based anti-platelet therapy. Hemocompatibility-related adverse event (HRAE) profiles vary between different LVADs, and whether the improved HRAE profile observed with HeartMate 3 (HM3) pump in clinical trials requires a reappraisal of the use of ASA therapy has not been previously addressed.5 HM3 patients within the MOMENTUM 3 clinical trial were prescribed varying doses ranging from usual-dose (325 mg) to low-dose (81 mg) ASA along with oral anti-coagulation using a vitamin K antagonist (warfarin) with a target international normalized ratio (INR) of 2 to 3. We examined the impact of these 2 distinct ASA dose regimens on HRAEs during HM3 support in a post-hoc analysis of the MOMENTUM 3 trial.
Methods
Study population
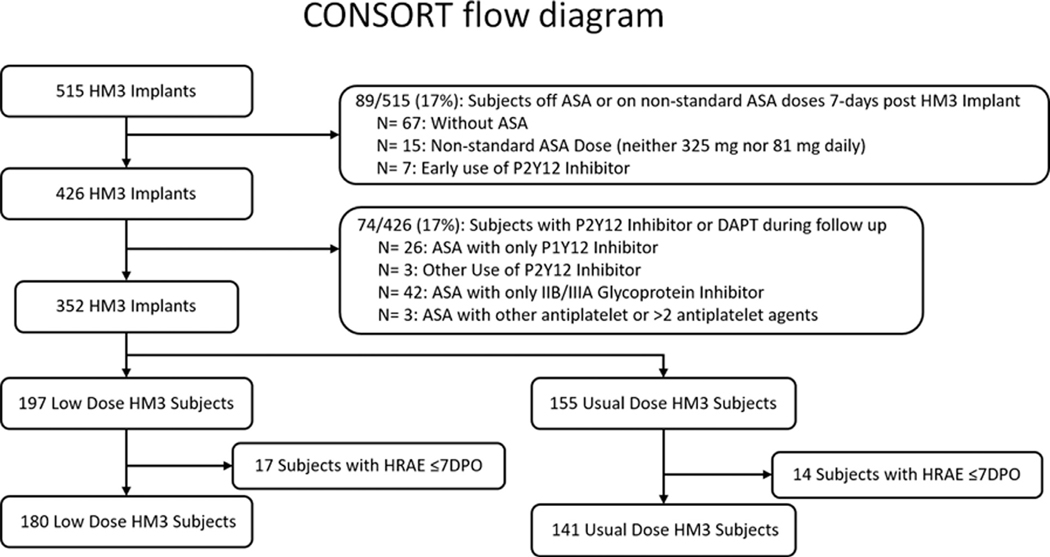
MOMENTUM 3 is a prospective, multicenter, non-blinded, randomized clinical trial comparing the HM3 and HeartMate II (HMII) LVADs in patients with advanced-stage heart failure. Trial enrollment was conducted between September 2014 and August 2018. Details of the trial design have been published previously.6 This post-hoc analysis focuses on the association of usual- or low-dose ASA on HRAEs exclusively in patients implanted with the HM3 device. Additional exclusion criteria included patients receiving ASA other than a dose of 81 mg or 325 mg daily at 7 days post-implant, those receiving >1 anti-platelet agent or a P2Y12 inhibitor, and those with an outcome or an HRAE ≤7 days after HM3 implantation. The remaining patients were categorized into 2 groups on the basis of daily ASA dose as usual dose (325 mg) or low dose (81 mg) at 7 days after HM3 implantation (Figure 1). ASA dose assignment was independently determined by treating clinicians at the implanting center and was not specified by the trial protocol, which allowed dosing in the 81 to 325 mg range. Concurrent anti-thrombotic therapy in the MOMENTUM 3 trial included anti-coagulation with warfarin to a targeted INR of 2.0 to 3.0 IU. The institutional review board at each participating center approved the protocol and all patients or their legally authorized representative provided written informed consent.
Figure 1. Consort diagram showing the derivation of usual-dose (325 mg) and low-dose (81 mg) ASA groups with HM3.
ASA, aspirin; DAPT, dual anti-platelet therapy; DPO, days post op; HM3, HeartMate 3; HRAE, hemocompatibility-related adverse event.
Data collection
Data were collected at baseline, HM3 implantation, and post-implant at 1 day, 7 days, discharge, 1 month, 3 months, 6 months, and then every 6 months until the 2-year follow-up period was completed. Adverse event data were collected as they occurred.
End-points
The primary end-point of this analysis was comparison of survival free from an HRAE at 2 years after HM3 implantation between patients on usual-dose ASA and those on low-dose ASA. HRAEs consisted of gastrointestinal or non-surgical bleeding episodes, stroke (hemorrhagic or ischemic), and thromboembolic events including suspected or confirmed pump thrombosis and arterial thromboembolism with or without end-organ involvement. Secondary end-points included survival free from hemorrhagic or thrombotic events analyzed separately between patients on usual-dose ASA and those on low dose at 2 years. Venous thromboembolism was excluded.
Stratification and sensitivity analyses
Stratification analyses were conducted for age and intention-to-treat categories. Patients <60 and ≥60 years old (59 was the mean age of the overall cohort) were separately assessed for survival free from an HRAE between those on usual- and low-dose ASA (a cut point of 60 was near the overall cohort mean and to balance strata). Similarly, patients assigned as bridge to transplantation or bridge to candidacy or as destination therapy (DT) were compared for survival free from an HRAE between those on usual- or low-dose ASA. To limit exclusion bias, a sensitivity analysis was performed including all patients without HRAE ≤7 days post-implant who were on ASA at 7 days post-implant and were grouped as (1) ≥325 mg vs <325 mg and (2) >81 mg vs ≤81 mg. This sensitivity analysis included patients with dual anti-platelet therapy, P2Y12 inhibitor use, or non-standard ASA doses at 7 days post-implant but excluded patients without ASA at 7 days post-implant.
Statistical analysis
Continuous data are presented as mean ± SD or median as appropriate and compared using Student’s t-test, unless otherwise specified. Categorical data are presented as percent and events per patient year for recurrent events and were compared by the chi-square test or Poisson regression, unless otherwise specified. Baseline demographics were compared between patients on usual-dose and low-dose ASA. Survival free from HRAEs was calculated by Kaplan—Meier methods and the difference between patients on usual- or low- dose ASA was determined by log-rank test. Analyses were landmarked at day 7, and censoring was done for heart transplantation or withdrawal. Multivariable Cox proportional regression was used to calculate adjusted hazard ratios (aHRs). Baseline covariables with a bivariate p < 0.1, age and intention to treat, were included in the multivariable model. In addition, sex, which may be related to HRAEs during LVAD support,7 was also included in the model. P < 0.05 was considered statistically significant. Statistical analyses were performed with SAS software, version 9.4 (SAS Institute).
Results
Patient characteristics
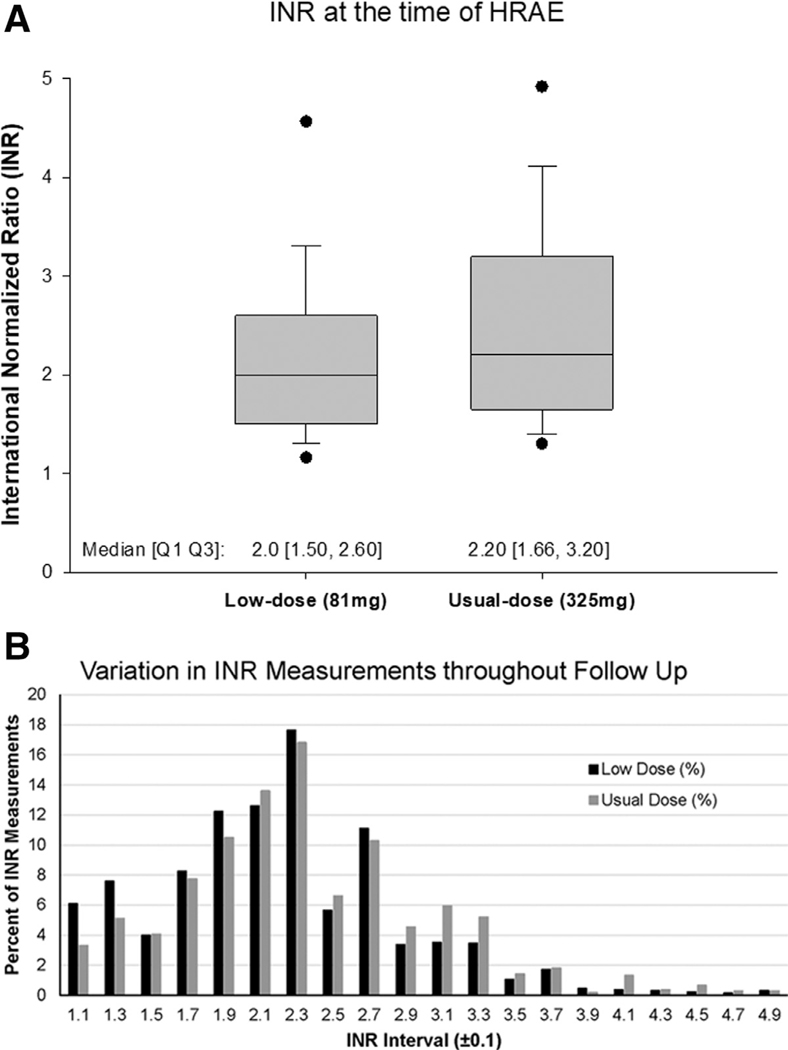
The study group comprised 321 patients who met the inclusion and exclusion criteria (Figure 1). There were 257 (80%) men, 115 (36%) with an ischemic etiology of heart failure, 143 (45%) with diabetes, 209 (65%) white, and 32 (10%) with a history of stroke. Before LVAD implant, 50% of all the patients in both groups were on ASA and in these patients, dose of ASA at baseline (before pump implant) was most often 81 mg daily (low dose: 94%, usual dose: 93%). After LVAD implant, within the cohort, 141 patients were on usual-dose (325 mg) ASA and 180 received low dose (81 mg). Patients in the usual-dose group were younger (57 ± 13 vs 60 ± 12 years, p = 0.035) and less often implanted as DT (55% vs 67%, p = 0.029) compared with those receiving low-dose ASA. The remaining baseline clinical characteristics, as listed in Table 1, were similar between both groups. INR at the time of HRAEs was clinically similar between usual- and low-dose groups (2.20 IU, interquartile range [IQR]: 1.66–3.20 vs 2.00 IU, IQR: 1.50–2.60, p = 0.054, Figure 2a). Moreover, INR at the time of hemorrhagic events (2.20 IU, IQR: 1.64–3.25 vs 2.00 IU, IQR: 1.51–2.60; p = 0.064) or thrombotic events (1.94 IU, IQR: 1.69–2.50 vs 1.65 IU, IQR: 1.50–2.00; p = 0.34) was similar between usual- and low-dose groups, respectively.
Table 1.
Baseline Clinical Characteristics of Patients With HeartMate 3 LVAD on Usual and Low Dose Aspirin
| Baseline characteristics | Low-Dose aspirin 81 mg/dayn = 180 | Usual-Dose aspirin 325 mg/dayN = 141 | p-value |
|---|---|---|---|
| Age (mean) | 60.3 ± 12.1 (180) | 57.3 ± 12.7 (141) | 0.04 |
| Age (median [Q1—Q3]) | 64 (53.5, 69) | 59 (49, 67) | |
| Men | 78.3% (141/180) | 82.3% (116/141) | 0.38 |
| Race—white | 65.6% (118/180) | 64.5% (91/141) | 0.85 |
| Ischemic cause of heart failure | 37.2% (67/180) | 34.0% (48/141) | 0.56 |
| Intravenous inotropic agents | 83.9% (151/180) | 84.4% (119/141) | 0.90 |
| Intra-aortic balloon pump | 12.2% (22/180) | 14.2% (20/141) | 0.60 |
| Serum creatinine (mg/dl) | 1.32 ± 0.42 (180) | 1.35 ± 0.42 (141) | 0.59 |
| Serum sodium (mmol/liter) | 135.4 ± 4.4(180) | 135.3 ± 3.9 (141) | 0.88 |
| Mean arterial pressure (mm Hg) | 79.4 ± 11.3 (138) | 78.3 ± 9.7 (99) | 0.45 |
| INTERMACS profiles | |||
| 1–2 | 34.4% (62/180) | 31.9% (45/141) | 0.63 |
| 3–7 | 65.6% (118/180) | 68.1% (96/141) | |
| Intent Goal of Pump Support | |||
| BTT/BTC | 32.8% (59/180) | 44.7% (63/141) | 0.03 |
| DT | 67.2% (121/180) | 55.3% (78/141) | |
| Diabetes | 40.6% (73/180) | 49.6% (70/141) | 0.10 |
| History of bleeding | 0.0% (0/180) | 0.7% (1/141) | 0.44a |
| History of stroke | 10.0% (18/180) | 9.9% (14/141) | 0.98 |
| History of atrial fibrillation | 42.8% (77/180) | 44.0% (62/141) | 0.83 |
| Baseline platelet count (103/μl) | 206.9 ± 64.3 (180) | 205.3 ± 62.1 (141) | 0.83 |
BTC, bridge to candidacy; BTT, bridge to transplantation; DT, destination therapy; LVAD, left Ventricular Assist Device.
Fisher’s exact test.
Figure 2. Comparison of INR at (a) the time of HRAE and (b) throughout the study follow-up period in HM3 patients on usual-dose (325 mg) and low-dose (81 mg) ASA.
ASA, aspirin; HM3, HeartMate 3; HRAE, hemocompatibility-related adverse event; INR, international normalized ratio.
Figure 2b further shows that INR distributions between ASA groups were similar during the study follow-up period.
End-points
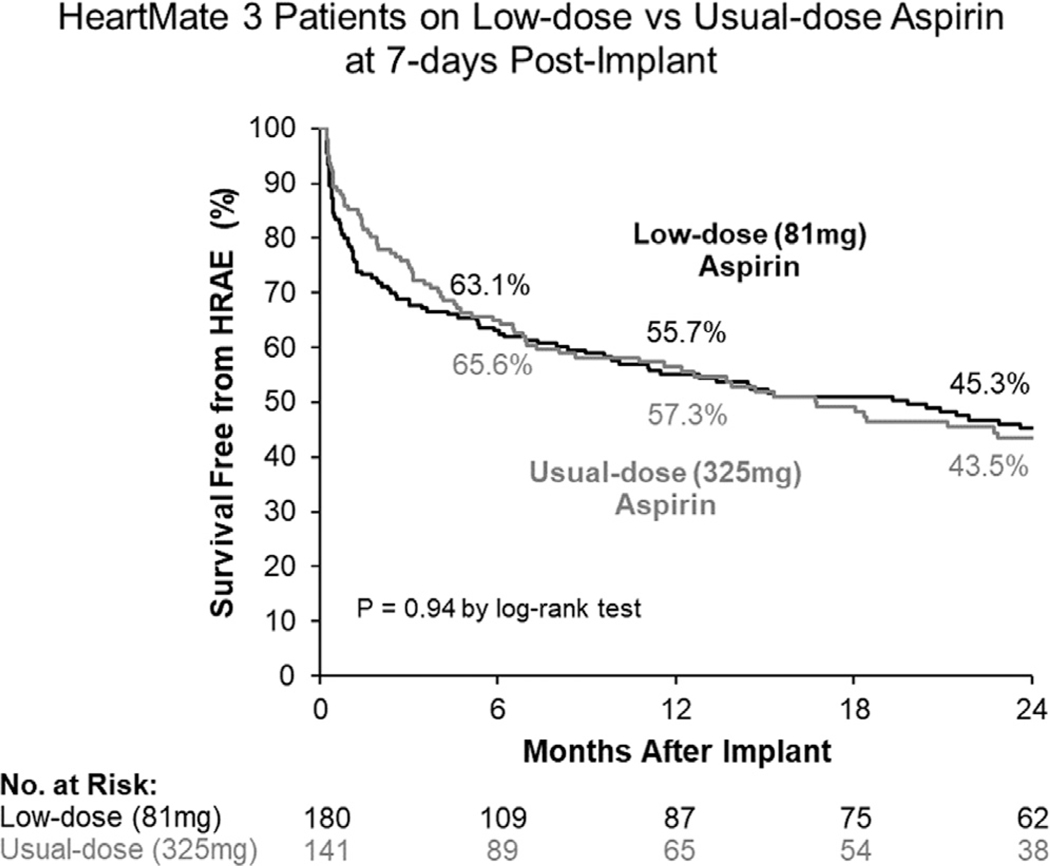
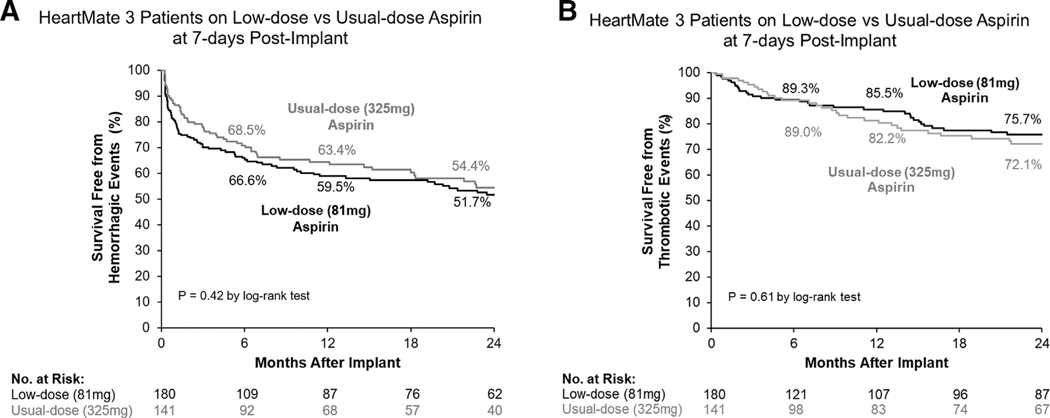
No patients were lost to follow-up over a 2-year period and no end-point data were missed. The primary end-point was similar between groups as nearly equivalent proportion of patients remained free of HRAEs on usual- (43.5%) and low-dose ASA (45.3%, p = 0.94, Figure 3). After adjustment for covariates, the likelihood of an HRAE remained similar for those on usual- (aHR: 1.00, 95% CI: 0.72–1.40, p = 0.99) and low-dose ASA (Table 2). There was no difference in freedom from hemorrhagic events between patients on usual-dose ASA (54.4%) and those on low-dose ASA (51.7%, p = 0.42, Figure 4a) at 2 years. This yielded an equivalent probability of incurring a bleeding event on usual-dose ASA in comparison with low-dose (aHR: 1.00, 95% CI: 0.71–1.41, p = 0.99). Freedom from thrombotic events was also comparable between patients on usual-dose ASA (72.1%) and those on low-dose (75.7%, p = 0.61, Figure 4b) at 2 years, and the likelihood of these events was similar between groups (usual-dose ASA: aHR: 1.04, 95% CI: 0.63–1.72, p = 0.88 vs low-dose ASA).
Figure 3. Cumulative survival free from HRAEs during HM3 support in patients on usual-dose (325 mg) and low-dose (81 mg) ASA.
ASA, aspirin; HM3, HeartMate 3; HRAE, hemocompatibility-related adverse event.
Table 2.
Multivariable Cox Modeling of Adverse Events (Low-dose versus Usual-dose Aspirin).
| Low dose 81 mg/day N = 180 | Usual dose 325 mg/day N = 141 | p-value | Low dose 81 mg/dayPt - yr = 267 | Usual dose 325 mg/dayPt - yr = 218 | p-value | Adjustedhazard ratio (95% CI)a | Favors usual dose | Favors low dose | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Percent (Patients) | EPPY (Events) | p-value | ||||||||
| HRAE | 46% (82) | 43% (61) | 0.73 | 0.63 (167) | 0.57 (123) | 0.40 | 1.00 [0.72, 1.40] | 0.99 | ||
| Thrombotic events | 3.9% (7) | 5.0(7) | 0.78 | 0.03 (9) | 0.04 (8) | 0.86 | 1.04 [0.63, 1.72] | 0.88 | ||
| Hemorrhagic events | 44% (80) | 40% (57) | 0.50 | 0.59 (158) | 0.53 (115) | 0.36 | 1.00 [0.71, 1.41] | 0.99 | ||
| Mortality | 17.2% (31) | 18.4% (26) | 0.77 | 0.12 (31) | 0.12 (26) | 0.91 | 1.05 [0.62, 1.80] | 0.85 | ||
| Overall bleeding | 42% (76) | 40% (56) | 0.731 | 0.57 (151) | 0.49 (107) | 0.27 | 1.00 [0.71, 1.42] | 0.99 | ||
| Requiring surgery | 5% (9) | 4% (6) | 0.80 | 0.03 (9) | 0.03 (7) | 0.93 | 1.06 [0.35, 3.22] | 0.92 | ||
| GI bleeding | 28% (50) | 22% (31) | 0.25 | 0.35 (93) | 0.27 (59) | 0.13 | 1.04 [0.66, 1.63] | 0.87 | ||
| Stroke | 6.1% (11) | 7.8% (11) | 0.66 | 0.05 (12) | 0.06 (14) | 0.36 | 1.01 [0.43, 2.40] | 0.97 | ||
| Ischemic | 2.2% (4) | 3.5% (5) | 0.51 | 0.02 (5) | 0.03 (6) | 0.52 | 1.10 [0.25, 4.91] | 0.90 | ||
| Hemorrhagic | 3.9% (7) | 5.7% (8) | 0.60 | 0.03 (7) | 0.04 (8) | 0.51 | 1.03 [0.33, 3.20] | 0.96 | ||
| Disabling | 3.3% (6) | 3.5% (5) | 1.00 | 0.03 (7) | 0.03 (7) | 0.70 | 1.15 [0.32, 4.14] | 0.83 | ||
| Non-disabling | 2.8% (5) | 5.0% (7) | 0.38 | 0.02 (5) | 0.03 (7) | 0.35 | 1.14 [0.29, 4.52] | 0.85 | ||
| 1.0 | ||||||||||
Adjusted hazard ratio covariates: age, intended goal of support (BTT/DT), sex
Figure 4. Cumulative survival free from (a) hemorrhagic and (b) thrombotic events during HM3 support in patients on usual-dose (325 mg) and low-dose (81 mg) ASA.
ASA, aspirin; HM3 HeartMate 3.
Risk of specific hemocompatibility-related adverse events and death
The likelihood of gastrointestinal bleeding was similar between patients on usual- and low-dose ASA (22% vs 28%, respectively, aHR: 1.04, 95% CI: 0.66–1.63, p = 0.87). A total of 8 (5.9%) patients experienced a hemorrhagic stroke on usual-dose in comparison with 7 (3.9%) on low-dose ASA (aHR: 1.03, 95% CI: 0.33–3.20, p = 0.96). Ischemic strokes occurred in 5 (3.5%) patients on usual-dose and in 4 (2.2%) on low-dose ASA (aHR: 1.10, 95% CI: 0.25–4.91, p = 0.90). As listed in Table 2, there were no differences in the risk of disabling stroke, non-disabling stroke, peripheral arterial thromboembolism, and device thrombosis between the ASA groups. In addition, 2-year mortality was similar between the usual- (18.4%) and the low-dose ASA (17.2%) groups (aHR: 1.05, 95% CI: 0.62 −1.80, p = 0.85).
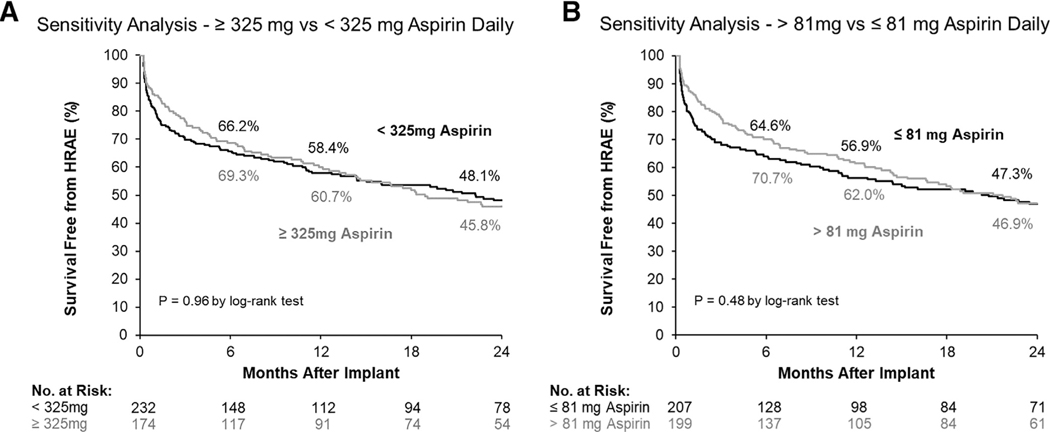
Stratification and sensitivity analyses
When patients with dual anti-platelet therapy, P2Y12 inhibitor use, or non-standard doses at 7 days post-implant were included, no difference in survival free from HRAE was noted between groups (Figure 5a: ≥325 mg vs <325 mg, p = 0.96; Figure 5b: >81 mg vs ≤81 mg, p = 0.48). Between usual- and low-dose ASA regimens, no difference in freedom from HRAEs was noted in sub-populations on the basis of age (age <60 years, p = 0.53; age ≥60 years, p = 0.40) or indication for use (DT p = 0.25; bridge to transplantation/bridge to candidacy p = 0.24).
Figure 5. Cumulative survival free from HRAEs in HM3 patients on (a) ASA ≥325 mg daily vs ASA <325 mg daily and on (b) ASA ≤81 mg daily vs greater than 81 mg daily of ASA.
ASA, aspirin; HM3, HeartMate 3; HRAE, hemocompatibility-related adverse event.
Discussion
Our principal findings in this post-hoc analysis of the MOMENTUM 3 trial within the HM3 study group are as follows: First, there was no difference in survival free of either hemorrhagic or thrombotic adverse events between patients receiving usual- or low-dose ASA. Second, specific sub-types of HRAEs including ischemic and hemorrhagic strokes, gastrointestinal bleeding, thromboembolism, and device thrombosis occurred at a similar rate between the 2 ASA regimens. Finally, there was no difference in overall survival between ASA groups. These findings were unlikely to be confounded by a variation in warfarin anti-coagulation because there were no clinically significant differences in the INR between the ASA groups at the time of HRAEs, and overall INR distribution was similar throughout follow-up. Furthermore, although patients on low-dose ASA were older and more often implanted as DT, no difference in the likelihood of HRAEs was present even after adjustment for the clinical covariates. ASA dose was prescribed at the discretion of the treating clinician and use of the low-dose (81 mg daily) in patients designated as DT may have been as a result of a perceived higher risk for bleeding or related to center-specific practices. It is noted that patients on usual- or low-dose ASA did not differ with respect to history of bleeding before HM3 implantation or pre-LVAD ASA dose.
Differing anti-thrombotic regimens and ASA dosing strategies have been evaluated in LVADs that preceded HM3, including HMII and HVAD pumps. In a retrospective comparison during HMII support, usual-dose ASA, in comparison with low-dose, was associated with a higher risk of bleeding that was not offset by lower thrombotic events.8 In the US-TRACE (Study of Reduced Anti-Coagulation/Anti-platelet Therapy in Patients with the HeartMate II) study, reduction in anti-thrombotic therapy (62% of which was cessation of anti-platelet alone or both the anti-platelet and warfarin) in response to bleeding was associated with a persistence of bleeding events during HMII support.9 However, in comparison with previous studies with concurrent ASA administration, the European TRACE study reported that during HMII pump support, reduced anti-thrombotic therapy with vitamin K antagonist monotherapy led to a lower rate of bleeding.10 Importantly, pump thromboses and ischemic strokes with warfarin monotherapy were not higher than in previous studies with the use of both ASA and vitamin K antagonists.10 In contrast, the ENDURANCE trial noted that usual-dose ASA (325 mg) was required to decrease the risk of thrombotic events (ischemic strokes) in comparison with lower-dose ASA during HVAD support, underscoring that anti-thrombotic therapy requires individualization to the device type during mechanical circulatory support.11
Investigating the burden of HRAEs with reduced anti-platelet therapy during HM3 support, Consolo et al12 reported their findings from a multicenter, observational study conducted in Italy. In this study, HM3-implanted patients with a post-operative bleeding event or an HAS-BLED score ≥4 (n = 7) were discharged on oral anti-coagulation alone with an INR goal of 2–2.5, whereas those with an HAS-BLED score <4 received ASA 100 mg daily in addition to a vitamin K antagonist (INR goal: 2–2.5). During a follow-up period of 645 (range: 431–802) days, no thrombotic events were observed in either group, yet 39% of patients on ASA experienced bleeding events whereas no bleeding was observed in patients off ASA with a therapeutic INR.12 In another retrospective analysis of 43 patients with 1-year follow-up post-HM3 implant, no thrombotic events occurred after discontinuation of ASA.13 In the HM3 ELEVATE registry, which recommended use of lower-dose (81–100 mg) ASA, 2-year major bleeding (32%) and stroke (9%) events were comparable to either usual- or low-dose ASA groups in our cohort.14,15
The preceding studies provide increasing rationale for investigating the potentially beneficial role of reducing anti-platelet therapy to decrease bleeding events during HM3 pump support. Although our study specifically excluded patients off ASA, the lack of association between ASA dose and HRAEs as well as between individual component bleeding and thrombotic events could also point to a limited role of ASA therapy during HM3 support. Such a hypothesis would be consistent with mechanistic studies showing reduced efficacy of ASA for decreasing platelet activation during the high shear conditions of mechanical circulatory support.16 Collectively, these results call into question whether ASA therapy provides any meaningful therapeutic effect in HM3-treated patients and highlight the need to definitively determine the clinical necessity of ASA therapy with ongoing vitamin K antagonist during HM3 support. The prospective, randomized, double-blind, placebo-controlled, international ARIES HM3 (anti-platelet Removal and Hemocompatibility Events with the HM3 Pump) trial will provide a definitive answer to this question (https://clinicaltrials.gov/ct2/show/NCT04069156).
Limitations
This investigation has several limitations. In this unplanned analysis of the MOMENTUM 3 trial, groups on usual-dose or low-dose ASA may not have been large enough to achieve adequate power to detect differences in HRAEs. However, because of a nearly equivalent likelihood of HRAEs between the ASA groups, the probability of a type II error is low. Next, during the course of the trial, patients may have changed their ASA dose or even stopped ASA at the discretion of treating physician. Thus, our findings represent the effects of originally intended therapy and do not reflect longitudinal use of ASA therapy. Selection bias as a result of confounding by indication may also have occurred in the primary analysis, which excluded patients not receiving either ASA 81 mg daily or 325 mg daily. To reduce this exclusion bias, a sensitivity analysis was done to include more HM3 patients with intermediate dose of ASA regimens, and no difference in the occurrence of HRAEs was detected. Finally, this study does not sufficiently account for the clinical rationale for choosing a usual- or low-dose ASA strategy in each patient.
Conclusions
In summary, in this exploratory analysis of the MOMENTUM 3 trial, usual- (325 mg daily) or low-dose (81 mg daily) ASA revealed similar rates of survival free of HRAEs and specific bleeding or thrombotic events in HM3 LVAD-supported patients. Thus, both usual- and low-dose ASA may have similar safety and efficacy during HM3 support. Whether ASA may be completely avoided in patients treated by the HM3 LVAD remains undetermined.
Acknowledgments
Disclosure statement
O.S. is supported by the National Institutes of Health (K23HL145140, UL1TR001073). Outside of the submitted work, P.C.C. reports research grants from Abbott and consultant (non-financial support) for Abbott. M.R.M. reports general conflicts to include consulting relationships with Abbott (paid to Brigham and Women’s Hospital), Medtronic, Janssen, Mesoblast, Portola, Bayer, NupulseCV, FineHeart, Leviticus, Triple Gene, and Baim Institute for Clinical Research. M.R.M. is also Editor in Chief of the Journal of Heart and Lung Transplantation; however, the writing does not constitute the official stance of the journal nor the society, the International Society for Heart and Lung Transplantation, that it represents. N.U. reports grant support, consulting fees, and honoraria from Abbott and Medtronic, and serves on the advisory boards for Leviticus Cardio and Livemetric/Cormetric. D.J.G. reports personal fees from Abbott outside the submitted work. J.C. reports grants from Abbott Medical and personal fees from Medtronic outside the submitted work. J.M.C. is supported by grants from Abbott Medical and has received personal fees from Medtronic, Abbott, Bristol-Myers Squibb/Pfizer, and Portola outside the submitted work. S.N. reports receiving personal fees from Abbott (consultant and speaker) and Medtronic (consultant) outside the submitted work. Outside of the submitted work, N.M. reports receiving personal fees from Abbott, Medtronic, SynCardia, and Carmat. A.B. reports receiving personal fees from Abbott (consultant) and Abiomed (advisory board), grant support and personal fees from TandemLife (consultant and advisory board) outside the submitted work. D.C. and P.S. were employees of Abbott during the conduct of this study. Outside the submitted work, U.P.J. reports serving as a consultant for Abbott
The MOMENTUM 3 Study (NCT02224755) was sponsored and funded by Abbott (Chicago, IL).
References
- 1.Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg 2010;90:1263–9. [DOI] [PubMed] [Google Scholar]
- 2.Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol 2010;56:1207–13. [DOI] [PubMed] [Google Scholar]
- 3.Ashbrook M, Walenga JM, Schwartz J, et al. Left ventricular assist device-induced coagulation and platelet activation and effect of the current anticoagulant therapy regimen. Clin Appl Thromb Hemost 2013;19:249–55. [DOI] [PubMed] [Google Scholar]
- 4.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res 2003;110:255–8. [DOI] [PubMed] [Google Scholar]
- 5.Uriel N, Colombo PC, Cleveland JC, et al. Hemocompatibility-related outcomes in the MOMENTUM 3 trial at 6 months: a randomized controlled study of a fully magnetically levitated pump in advanced heart failure. Circulation 2017;135:2003–12. [DOI] [PubMed] [Google Scholar]
- 6.Heatley G, Sood P, Goldstein D, et al. Clinical trial design and rationale of the multicenter study of MagLev technology in patients undergoing mechanical circulatory support therapy with HeartMate 3 (MOMENTUM 3) investigational device exemption clinical study protocol. J Heart Lung Transplant 2016;35:528–36. [DOI] [PubMed] [Google Scholar]
- 7.Bogaev RC, Pamboukian SV, Moore SA, et al. Comparison of outcomes in women versus men using a continuous-flow left ventricular assist device as a bridge to transplantation. J Heart Lung Transplant 2011;30:515–22. [DOI] [PubMed] [Google Scholar]
- 8.Saeed O, Shah A, Kargoli F, et al. Antiplatelet therapy and adverse hematologic events during Heart Mate II support. Circ Heart Fail 2016;9:e002296. [DOI] [PubMed] [Google Scholar]
- 9.Katz JN, Adamson RM, John R, et al. Safety of reduced anti-thrombotic strategies in HeartMate II patients: a one-year analysis of the US-TRACE Study. J Heart Lung Transplant 2015;34:1542–8. [DOI] [PubMed] [Google Scholar]
- 10.Netuka I, Litzler PY, Berchtold-Herz M, et al. Outcomes in HeartMate II patients with no antiplatelet therapy: 2-year results from the European TRACE Study. Ann Thorac Surg 2017;103:1262–8. [DOI] [PubMed] [Google Scholar]
- 11.Slaughter MS, Pagani FD, McGee EC, et al. HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2013;32:675–83. [DOI] [PubMed] [Google Scholar]
- 12.Consolo F, Raimondi Lucchetti M, Tramontin C, Lapenna E, Pappalardo F. Do we need aspirin in HeartMate 3 patients? Eur J Heart Fail 2019;21:815–7. [DOI] [PubMed] [Google Scholar]
- 13.Lim HS, Ranasinghe A, Mascaro J, Howell N. Discontinuation of aspirin in Heartmate 3 left ventricular assist device. ASAIO J 2019;65:631–3. [DOI] [PubMed] [Google Scholar]
- 14.Gustafsson F, Shaw S, Lavee J, et al. Six-month outcomes after treatment of advanced heart failure with a full magnetically levitated continuous flow left ventricular assist device: report from the ELEVATE registry. Eur Heart J 2018;39:3454–60. [DOI] [PubMed] [Google Scholar]
- 15.Saeed D, Garbade J, Gustafsson F, et al. Two-year outcomes in Real World patients treated with HeartMate 3TM left ventricular assist device for advanced heart failure: data from the ELEVATE Registry. J Heart Lung Transplant 2019;38(Suppl):S67. [Google Scholar]
- 16.Valerio L, Tran PL, Sheriff J, et al. Aspirin has limited ability to modulate shear-mediated platelet activation associated with elevated shear stress of ventricular assist devices. Thromb Res 2016;140:110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]