Abstract
Background:
Neurovascular patterning is an emerging area of microvascular research. While overlapping molecular signals highlight links between angiogenesis and neurogenesis, advancing our understanding is limited by a lack of in vitro models containing both systems. One potential model is the rat mesentery culture model, which our laboratory has recently introduced as an ex vivo tool to investigate cellular dynamics during angiogenesis in a microvascular network scenario. The objective of this study was to demonstrate the use of the rat mesentery culture model as an ex vivo platform for maintaining spatiotemporal relationship with blood vessels and peripheral nerves during angiogenesis in adult microvascular networks.
Methods:
Adult male Wistar rat mesenteric tissue windows were harvested, rinsed in sterile DPBS and medium and then cultured per group: 1) MEM alone and 2) NBM with NGF and 20% FBS (nerve culture medium). On day 3 post culture tissues were labeled for endothelial (PECAM) and neural (class III β-tubulin, NG2, tyrosine hydroxylase, CGRP) markers.
Results:
In MEM alone tissues nerve segment degeneration was supported by discontinuous nerve or absence of nerve marker labeling. Nerve presence at the arteriole level and capillary level was maintained for nerve medium group compared to day 0, non-cultured control group (unstimulated).
Comparison with existing methods and conclusions:
The results support the use of specific medium types to maintain nerve presence across cultured microvascular networks and implicates the rat mesentery culture model as the first adult peripheral nerve ex vivo culture model and as a novel tool to investigate neurovascular patterning.
1. Introduction
A major challenge in biomimetic model development is the incorporation of multiple cell types and systems. This can be attributed to bottom-up approaches characterized by the addition of one to three cell types via co-culture methods. This challenge is exemplified by the lack of models to investigate the spatiotemporal coordination in neurovascular patterning, which represents an emerging area of research and is critical for tissue remodeling. The paracrine signaling between the neural and vascular systems is location specific and affects patterning during development, disease, and recovery [1–3] and thus is important for growing and therapeutically manipulating functional tissues. New ex vivo models that incorporate nerve and adult microvascular networks will enable real time investigation of neurovascular interactions not possible with chronic in vivo experiments.
The motivation to probe neurovascular interactions has motivated the coculture of neurons and endothelial cells. In one recent example, brain microvascular endothelial cells were shown to deter hypoxic injury to neurons through nitric oxide production [3]. Other models increase complexity by involving more cell types. Consider blood brain barrier models used to culture nonfenestrated endothelial cells with pericytes and astrocytes. These models are characterized by 2D cocultures of the multiple cell types or microfluidic devices to enable 3D architecture with fluid flow [4–9]. All together in vitro methods have been successful in integrating neural and vascular cells in spatially relevant patterns with and without flow, yet still models that “look” like a real tissue and include vessels and nerves are lacking. Alternative approaches to incorporate the complexity of a real tissue include ex vivo tissue culture models. One of the most common models is the brain slice that employs a vibratome to cut thin slices (100– 400 μm) of the rodent hippocampus for use as a CNS model with corresponding vasculature [10]. Variants include the spinal cord slice which when harvested from adult mice, provides a model of neurotrauma [11]. While brain slice studies have induced neurogenesis, an issue with culturing the adult brain slice is neurodegeneration [12]. The slicing method and the culture maintenance start an apoptotic cascade via intercellular connexin 43 gap junction channels [13,14]. The absence of adult peripheral neural culture models could be explained by Wallerian regression in peripheral nerve injury [15]. So, a need exists to develop tools that incorporate both intact microvascular networks and nerves – especially peripheral nerves.
The objective of this study was to demonstrate the use of the rat mesentery culture model as an ex vivo platform for maintaining spatiotemporal relationship with blood vessels and peripheral nerves during angiogenesis in adult microvascular networks. We have previously shown that rat mesenteric tissues can be cultured and used to investigate angiogenesis and lymphangiogenesis [16]. Importantly, smooth muscle cells, endothelial cells and vascular pericytes remain functional along the hierarchy of a microvascular network [17]. The tissues of adult rats are utilized because they are more vascularized compared to weanling or juvenile rats [18]. Based on immunolabeling of un-cultured, mesenteric tissues, peripheral nerves can be identified wrapping along arterioles and aligned along capillaries [19]. However, the ability to keep nerves viable in cultured mesenteric tissues has not been developed. Based on prevalent medium components used for the culture of postnatal and adult brain slices, we present a nerve culture medium that results in neural structure maintenance by 1) comparison of nerve presence near arterioles and capillaries over time course of 3 days, and 2) lectin identification of nerves did not colabel with dead marker. Subsets of the surviving axons coexpressed sensory and sympathetic nerve markers. To our knowledge this is the first ex vivo model to maintain peripheral nervous structure in culture within a microvascular network.
2. Materials and methods
2.1. Rat mesentery culture model
All animal experiments were approved by Tulane University’s or the University of Florida’s Institutional Animal and Care Use Committee and performed in accordance with the U.S. Animal Welfare Act, U.S. Public Health Service Policy on the Humane Care and Use of Laboratory Animals, and the NIH Guide for the Care and Use of Laboratory Animals. Rat mesenteric tissues were harvested and cultured according to a previously established protocol [16,20]. Adult male Wistar rats (350 – 400 g) were anesthetized with an intramuscular injection of ketamine (80 mg/kg body weight) and xylazine (8 mg/kg body weight). This weight range was selected based on previous work from our laboratory establishing that mesenteric tissues harvested from rats in this weight range contain branched microvascular networks [16, 17, 19, 20, 21]. The abdominal region was shaved then sterilized with 70% isopropyl alcohol before an incision was made along the linea alba. The mesentery was exteriorized onto a sterile plastic stage using cotton tip applicators to first remove the cecum and subsequently the ileum and jejunum. The rat was euthanized with an intracardiac injection of 0.2 ml Beuthanasia. Vascularized mesenteric tissues were excised and rinsed once in sterile saline with 0.9% NaCl (Baxter; Deerfield, IL) and immersed in minimum essential medium (MEM; Gibco; Grand Island, NY) containing 1% Penicillin-Streptomycin (PenStrep; Gibco; Grand Island, NY) at 37°C. After 1 hour, tissues were transferred randomly to petri dishes containing respective medium types either 1) MEM containing 1% PenStrep (Gibco; Grand Island, NY), or 2) Nerve culture medium. Tissues were cultured in standard incubation conditions (5% CO2, 37°C) for three days (Fig. 1A). A subset of tissues were fixed with methanol for 30 minutes at −20°C immediately after extraction to serve as a day 0 unstimulated control group. For this group, tissues were harvested and immunolabelled to represent the day 0 state at the start of tissue culture.
Figure 1.
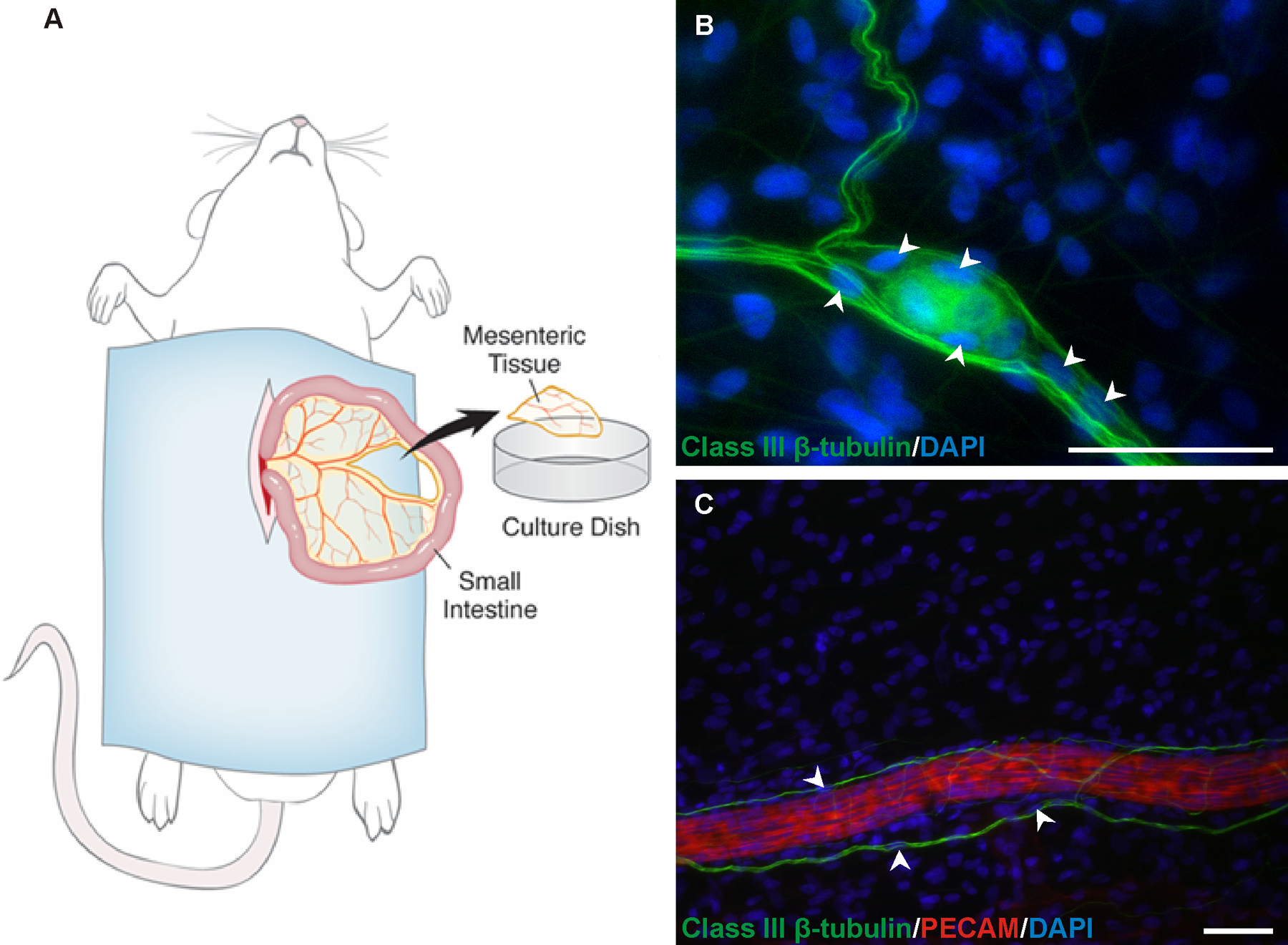
Figure 1. A) Schematic of the rat mesentery culture model. Mesenteric windows are surgically removed from the small intestine, rinsed in phosphate buffered saline, and placed in medium. B-C) Nuclei location along axons. B) DAPI (blue) positive nuclei (arrowheads) aligned with class III β-tubulin (green) nerve labeling. C) Class III β-tubulin (green) positive nerves associated with PECAM (red) positive arterioles characteristically align along the length of the vessels and display occasional DAPI (blue) positive nuclei (arrowheads), which are in line with the class III β-tubulin labeling and larger in diameter than the rest of the nerve structure. Scale bar = 50 µm.
2.2. Nerve culture medium composition
Nerve culture medium was composed of neurobasal medium (NBM; Gibco; Grand Island, NY), supplemented with 20 ng/ml nerve growth factor (NGF; Invitrogen; Carlsbad, CA), 20% FBS (Gibco; Grand Island, NY), 2% B27 supplement (Invitrogen; Carlsbad, CA), 2% Insulin-Transferrin-Selenium (ITS) supplement (Invitrogen; Carlsbad, CA), and 1% PenStrep.
2.3. Immunohistochemistry
After methanol fixation for thirty minutes at −20°C, tissues were labeled according to the following protocols. All antibodies were diluted in antibody buffer solution comprised of phosphate-buffered solution (PBS; Sigma-Aldrich) + 0.1% saponin + 2% bovine serum albumin (BSA; Jackson Immunoresearch Laboratories) + 5% normal goat serum. After each step, the tissues were submerged in PBS + 0.1% saponin for 10 minutes 3 times. PECAM/Class III β-tubulin/DAPI: 1) 1:100 mouse monoclonal anti-Class III β-tubulin antibody (2G10; Abcam, Cambridge, MA) for 1 hour at room temperature followed by 24 hours at 4°C; 2) 1:100 goat anti-mouse CY2-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 hours at room temperature; 3) 1:20 normal mouse serum (Jackson ImmunoResearch Laboratories) for 1 hour; 4) 1:200 mouse monoclonal biotinylated anti-PECAM antibody (CD31 antibody, BD Pharmigen; San Diego, CA) for 1 hour at room temperature; 5) 1:500 CY3-conjugated streptavidin antibody (Jackson ImmunoResearch Laboratories) for 1 hour at room temperature; 6) 1:3000 4’,6-diamidino-2-phenylindole (DAPI) Nucleic Acid Stain for 10 minutes at room temperature. PECAM/NG2: 1) 1:200 mouse monoclonal biotinylated anti-PECAM antibody with 1:100 rabbit polyclonal anti-NG2 antibody (Millipore/Chemicon, Billerica, MA) for 1 hour; 2) 1:500 CY3-conjugated Streptavidin secondary and 1:100 goat anti-rabbit CY2-conjugated antibody for 1 hour at room temperature. Class III β-tubulin /Tyrosine Hydroxylase: 1) 1:100 mouse monoclonal anti-class III β-tubulin antibody 1:100 rabbit polyclonal anti-tyrosine hydroxylase antibody (Millipore/Chemicon, Billerica, MA, USA) for 1 hour at room temperature followed by 24 hours at 4°C; 2) 1:100 goat anti-mouse CY2-conjugated secondary antibody and 1:100 goat anti-rabbit CY3-conjugated antibody for 2 hours at room temperature. Tyrosine Hydroxylase/CGRP: 1) 1:100 mouse monoclonal anti-CGRP (Abcam, Cambridge, MA) antibody with 1:100 rabbit polyclonal anti-tyrosine hydroxylase antibody (Millipore/Chemicon, Billerica, MA, USA) for 1 hour; 2) 1:100 goat anti-mouse CY3-conjugated secondary antibody and 1:100 goat anti-rabbit CY2-conjugated antibody for 1 hour at room temperature.
2.4. Quantification of nerve presence along arterioles and at the capillary level
To determine nerve maintenance along the microvasculature, tissues were harvested from adult male Wistar rats and divided into three experimental groups: 1) unstimulated, 2) MEM only culture, and 3) nerve culture medium. Following 3 days in culture, tissues were mounted on slides, fixed, and labeled for neural/endothelial cell markers. For the unstimulated group, tissues were immediately mounted on slides, fixed, and immunolabeled for neural/endothelial cell markers. The percentage of network feeding arterioles with corresponding nerve alignment and the length of nerves in capillary regions was measured per tissue (n = 8 tissues; 2 tissues per rat) according to two neural marker labels 1) class III β-tubulin and 2) NG2. PECAM labeling identified endothelial cells. For the percentage of network feeding arterioles with corresponding nerve alignment, images were taken of each network feeding arteriole at the periphery of the tissue. Network feeding arterioles were identified based on size (>20 μm diameter) and morphology. Nerve alignment was determined by direction and distance from arteriole (<10μm). For the analysis of nerve length at the capillary level, 4 4X images were taken per tissue in capillary regions. Nerve alignment was determined by direction and distance from capillaries (<10μm) in 4X images.
2.5. Image Acquisition
Images were acquired using an inverted, mercury burner illuminated microscope (Olympus IX70) combined with a Photometrics CoolSNAP EZ camera with 4x (dry, NA = 0.1), 10x (dry, NA = 0.3), 20x (oil, NA = 0.8), 40x (oil, NA = 1.0) and 60x (oil, NA = 1.4) objectives. Image analysis and quantification were done using ImageJ 2.0.0-rc-54 (U.S. National Institutes of Health, Bethesda, MD). Olympus fluorescent long pass filter cubes used for the imaging were U-MWU (excitation: 330–385 nm; dichroic mirror: 400 nm; emission: 420 nm), U-MNB2 (excitation: 470–490 nm; dichroic mirror: 500 nm; emission: 515 nm), and U-MWIY2 (excitation: 545–580 nm; dichroic mirror: 600 nm; emission: 610 nm).
2.6. Statistical Analysis
The percentages of network feeding arterioles with nerve alignment and nerve length along capillaries were compared among medium groups using one-way ANOVA followed by Student-Newman-Keuls pairwise comparison test using SigmaStat ver. 3.5 (Systat Software, San Jose, CA, USA). Statistical significance was accepted for p < 0.05. Values are presented as means ± SEM.
3. Results
Class III β-tubulin and PECAM labeling of tissues at day 0 identified peripheral nerves aligned with arterioles, venules, and capillaries. The neurovascular congruency was most pronounced at the periphery of the tissue along network feeding arterioles and draining venules. Nerve structures associated with arterioles and venules were characteristically patterned along the length of the vessels. Thinner extensions were also observed to intermittently cross the PECAM vessel labeling suggesting that the extensions were surrounding the vessel. These observations are consistent with previous characterization of neurovascular patterning in unstimulated tissues at day 0 [21]. The class III β-tubulin positive nerve structures were also associated with DAPI positive cell nuclei (Fig.1B–C). In addition, nerve labeling in rat mesenteric tissues can be observed in avascular areas suggesting that not all nerves are aligned with the microvasculature. Live lectin labeling also identified nerves and vessels that were cultured in nerve culture medium; the absence of EthD-1 labeling, which is indicative of cell death along the lectin positive nerve structures suggests that nervous structure remains viable during culture (data not shown). Importantly, the spatiotemporal patterning of nerves could be maintained during culture conditions. After three days in culture with MEM, class III β-tubulin and NG2 positive nerve presence along arterioles and at the capillary level was decreased compared to the unstimulated, day 0 control group (p < 0.001). Unstimulated tissues were harvested and immunolabelled without culturing. Hence, the unstimulated group represents a day 0 state at the start of tissue culture. In contrast, culturing with nerve culture medium maintained nervous structure along arterioles (Fig 2). Angiogenesis in nerve culture medium tissues was characterized by high vessel density regions and increased capillary sprouting consistent with our previous characterization of angiogenesis during culture conditions [21,22]. These angiogenic regions also maintained nervous structure presence (Fig. 3). The cultured nerves are heterogeneous and maintain components of multiple neurotransmitters. Class III β-tubulin labeling with neural markers at day 0 established the presence of both sympathetic and sensory neurotransmitters via tyrosine hydroxylase and CGRP respectively (data not shown). Labeling after 3 days in culture with nerve culture medium confirmed that tyrosine hydroxylase was maintained (Fig 4A–C). As tyrosine hydroxylase is a precursor for epinephrine and norepinephrine, this indicates that sympathetic nerves maintain the ability to produce neurotransmitters. CGRP was also still observable and the overlap of both these labels indicate that at least some axons possess multiple neurotransmitters (Fig 4D–F).
Figure 2.
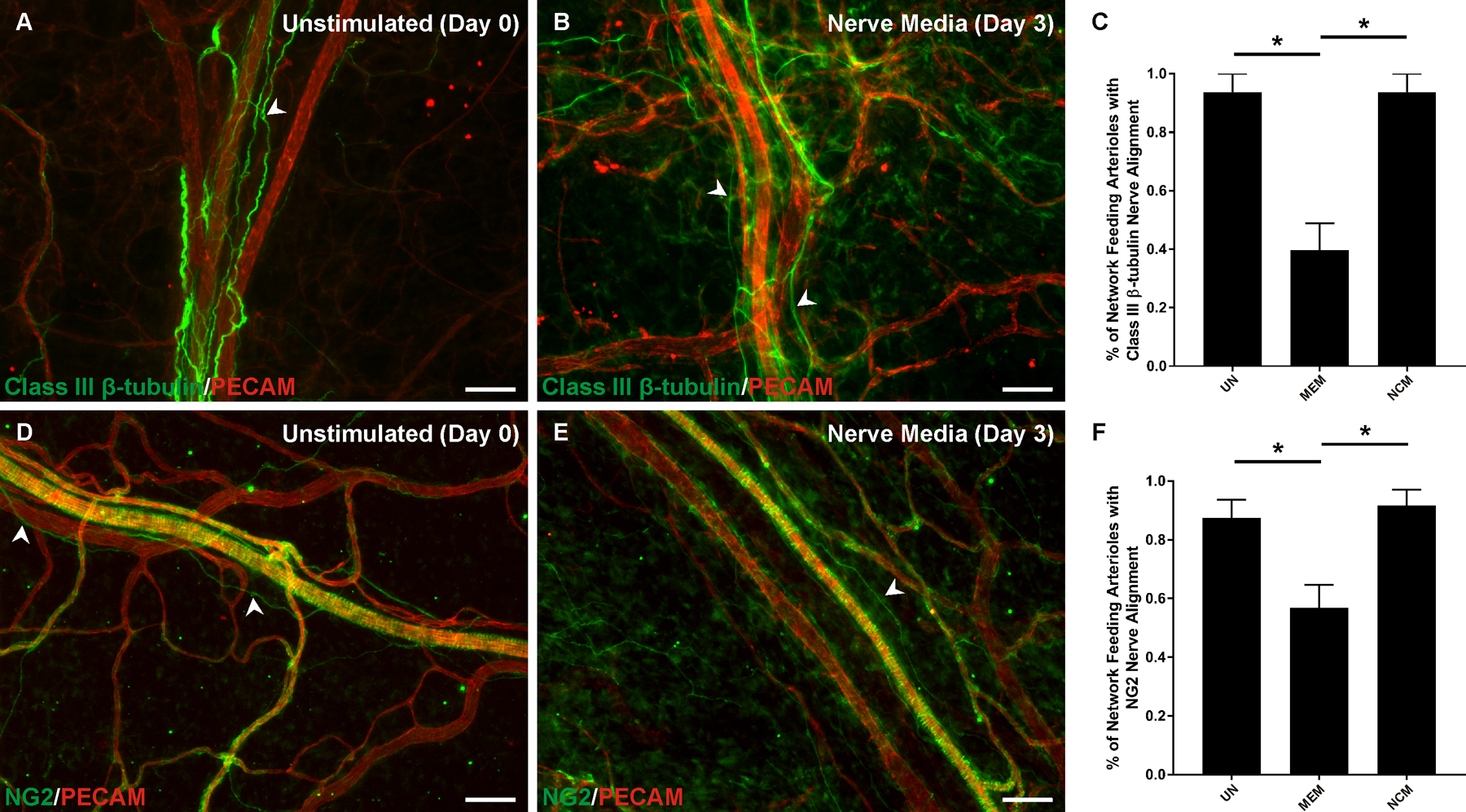
Neurovascular alignment along network feeding arterioles during culture. A-B) Comparison of class III β-tubulin nerve presence (arrowheads) at day 0 and day 3 at the arteriole level. C) Nerve culture medium maintained class III β-tubulin nerve presence compared to MEM alone. D-E) Comparison of NG2 nerve presence (arrowheads) at day 0 and day 3 at the arteriole level. NG2 labeling of vascular smooth muscle cells obscures observation of wrapping nerves. F) Nerve culture medium maintained NG2 nerve presence compared to MEM alone. The unstimulated group represents a day 0 reference as tissues were harvested and immediately immunolabelled without culturing. UN: unstimulated. MEM: minimum essential medium. NCM: nerve culture medium. P < 0.001. Scale bar = 100 µm.
Figure 3.
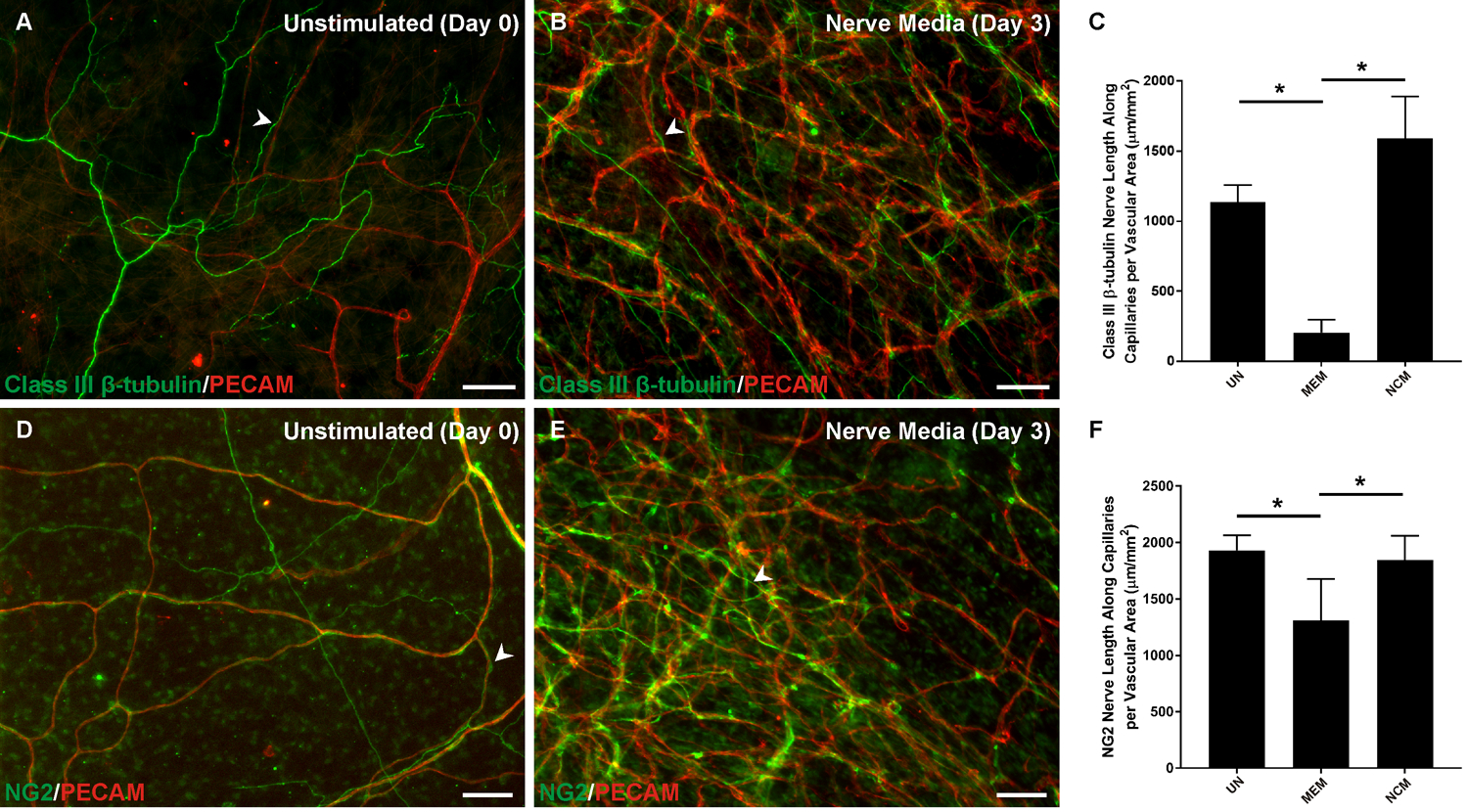
Neurovascular alignment at the capillary level in microvascular networks during culture. A-B) Comparison of class III β-tubulin nerve alignment (arrowheads) at day 0 and day 3 at the capillary level. C) Quantification of alignment of class III β-tubulin nerves found significantly more nerves in unstimulated and nerve culture medium groups compared to the MEM alone group. D-E) Comparison of NG2 nerve alignment (arrowheads) at day 0 and day 3 at the capillary level. NG2 labeling of vascular smooth muscle cells obscures observation of wrapping nerves. F) Quantification of alignment of NG2 found significantly more nerves in unstimulated and nerve culture medium groups compared to the MEM alone group. The unstimulated group represents a day 0 reference as tissues were harvested and immediately immunolabelled without culturing. UN: unstimulated. MEM: minimum essential medium. NCM: nerve culture medium.
P < 0.001; values are shown as mean ± SEM. Scale bar = 100 µm.
Figure 4.
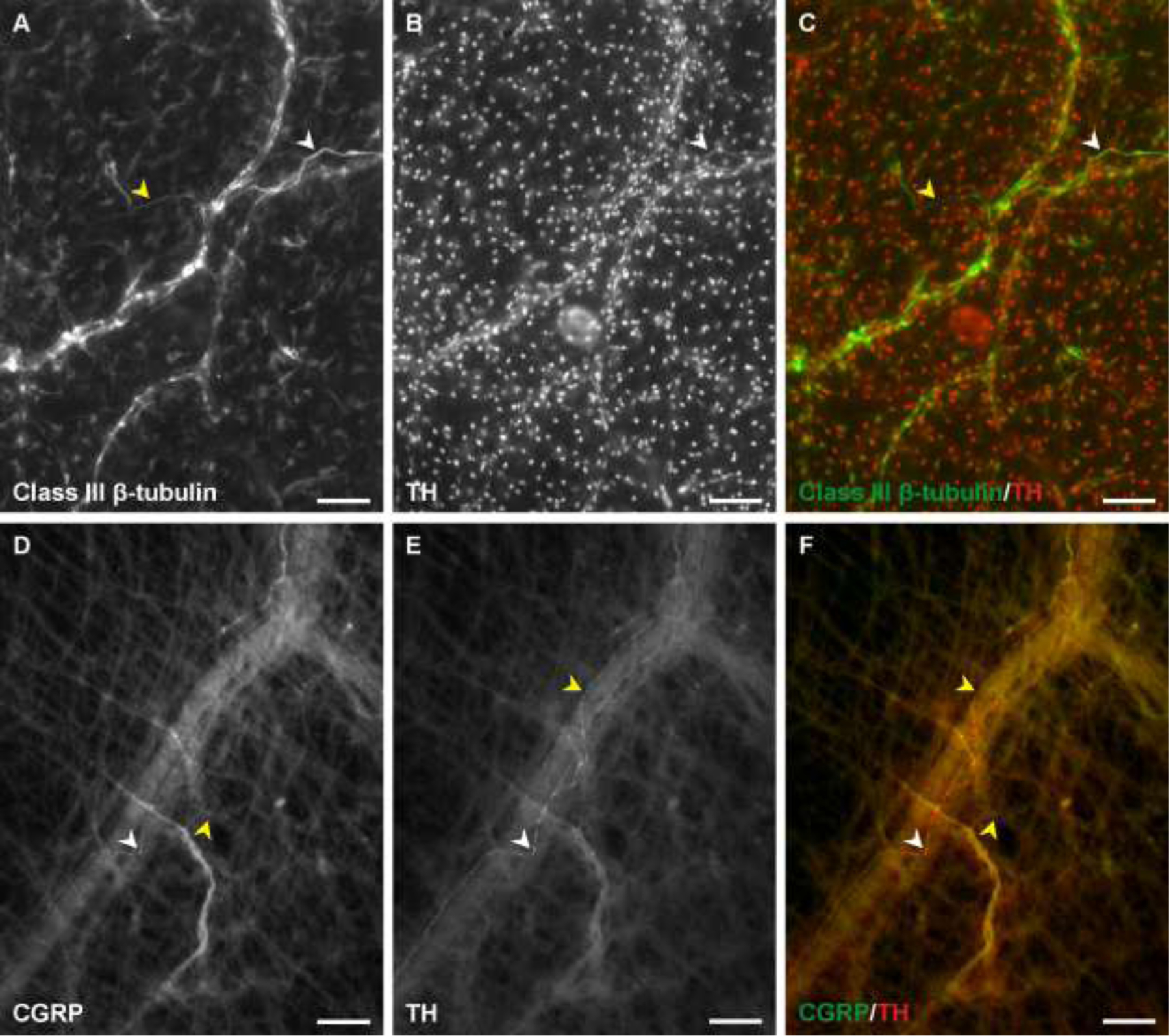
Sympathetic and sensory nerve labeling after three days. A-C) Tyrosine hydroxylase labels a subset of class III β-tubulin positive nerves. D-F) CGRP and tyrosine hydroxylase expression overlap at some locations within the nerve. White arrowheads indicate a segment co-labeled for both neural markers. Yellow arrowheads indicate segments that positively labelled for only one of the two markers. Scale bars = 100 µm.
4. Discussion
While neurovascular patterning remains an emerging area of research, there is a lack of adult peripheral culture models. Translating these systems into an experimental model comes with significant challenges. In vitro techniques like two dimensional cocultures of neurons and endothelial cells fail to recapitulate the region-specific growth patterns of the body. The use of microfluidic devices to increase the complexity and further mimic the physiological conditions has made promising advancements in blood brain barrier models [4,5]. Organotypic organ slice models such as the hippocampal brain and spinal cord slices are effective for investigating the dynamics in neonatal conditions [23–25]. The nature of the slicing method limits these models to ex vivo representations of neurodegeneration but may be culture medium dependent [11,12,26]. This is because slicing creates an apoptotic cascade via intercellular connexin 43 gap junction channels [13]. In the context of tissue engineering or biomimetic model development, the challenge remains how to recapitulate the spatial coordination between peripheral nerve and microvascular networks. Such a method could offer a novel experimental tool for probing neurovascular interactions in a new way. Our results support a method for maintaining peripheral nerve labeling ex vivo in intact microvascular networks using the rat mesentery culture model Nerve culture medium cultured mesenteric tissues displayed a similar presence of class III-β tubulin and NG2 positive nerve labeling at the arteriole and capillary level in microvascular networks compared to unstimulated tissues, which were harvested and immediately labelled and representative of a non-cultured, day 0 control group. In contrast, nerve labeling in the MEM alone group was significantly decreased compared to the control tissues.
Important issues for this methods development study are potential non-specific labeling of antibodies and false positives associated with bleed through of fluorophore detection due to CY2 and CY3 spectral overlap. While the long pass filters used for the imaging minimize the bleed through potential, some overlap is possible. However, it should be noted that we did not observe any overlap between the imaging channels (Supplemental Figures 1 – 2). In further support of neural primary antibody labeling, control IgG staining (Supplemental Figure 3) did not identify patterns expected by the neural markers. For example, class III β-tubulin and NG2 positively labeled nerve structures and perivascular cells. In contrast, this specific labeling of both cell types was not observable with IgG control labeling. In order to account for the potential for bleed through and false labeling, it is also paramount to consider specific cell morphologies and expected labeling patterning. For this study a big question is whether PECAM labeling identifies cells positive for a neural marker. PECAM labeling characteristically identifies the endothelial cell junctions along the vessels across a microvascular network. The specificity of endothelial cell labeling by PECAM in cultured and non-cultured rat mesenteric tissue is further supported by multiple previous studies [16, 17,19, 21, 22, 27, 28]. In addition, the decrease in observable nerve structures for the MEM control group suggests that false PECAM labeling cannot account for the positive marker identification of nerves.
Another potential labeling issues is that both PECAM and class III-β tubulin antibodies are derived from mouse. This resulted in a sequential labeling procedure and additional blocking step with mouse serum before the class III-β tubulin primary antibody incubation. The rationale is that mouse serum proteins would bind to free secondary sites prior to the class III-β tubulin primary labeling. However, there is still the potential for the binding of the second secondary antibody to both primary antibodies emphasizing more the impetus for paying attention to labeling patterns. Based on the sequence of labeling the CY2 secondary antibody could identify both PECAM and class III-β tubulin. While not ideal, nerve structure could still be identified in this scenario based on observation of nerve specific morphologies. Finally, it is important to note that the CY3 antibody is conjugated to strepdavidin and therefore should only label the biotinylated PECAM primary antibody, reducing the chance of false identification of nerves by PECAM labeling.
Recent work in our laboratory demonstrated the use of the tissue culture model for investigating angiogenesis, lymphangiogenesis, time-lapse, and drug testing studies [16,17,27]. The major contribution of this study is the identification and development of a culture protocol to maintain the presence of neural cell labeling during culture. Not surprisingly based on the literature, the maintenance of neural cells required a specific medium cocktail compared to other cell types in the tissue. To determine the components for the nerve culture medium, a literature search of an analogous CNS ex vivo model, the brain slice, was undertaken. A comprehensive list of every medium component and combination of components was compiled from 103 papers that attempted to culture adult or postnatal brain slices ex vivo (data not shown). After trial and error experiments of culturing, we selected the final nerve culture medium cocktail of NBM, 20 ng/ml NGF, 20% FBS, 2% B27, 2% ITS, and 1% PenStrep used for the current study.
A premise of this study is the ability to identify nerves based on class III β-tubulin and NG2 labeling. Our lab has previously shown that class III β-tubulin, most commonly used as neural cytoskeletal marker, can also label angiogenic perivascular cells [28,29] and NG2 is a pericyte and smooth muscle cell marker [29–31]. Importantly, class III β-tubulin and NG2 labeling of nerves is distinguishable from pericyte or smooth muscle cell labeling in rat mesenteric tissues based on morphology. Nerves are relatively thin, long and associated with an occasional DAPI positive nucleus that is larger in diameter compared to the rest of the labelled structure. Nerves associated with vessels commonly appear to be mostly aligned along the length of the vessel segments while pericytes and smooth muscle cells appear to be more tightly wrapped around vessels, indicative of endothelial cell coverage.
Along with sympathetic and sensory nerves, a subclass of nerves in the gut responsible for mechanical reflexes without CNS communication is the enteric nervous system. Enteric nerves are unique in their ability to respond to stimuli independent from the CNS and to express a multitude of neurotransmitters making nerve identity unique compared to other peripheral tissues [32]. This is important because the nerve bundles in the rat mesentery model may contain enteric nerves in addition to the sympathetic and sensory neurons. As enteric nerves may express both sympathetic and sensory markers, a challenge for this study was the selection of specific nerve type markers. In our experience markers suggested to be nerve type specific overlapped with other neural markers and can even identify other cell types. These labeling scenarios raise questions about their specificity in the rat mesentery. For example, PGP 9.5 was selected as a potential enteric nervous system label but overlapped completely with class III β-tubulin and marked interstitial cells [33,34] (data not shown). Tyrosine hydroxylase and calcitonin gene-related peptide (CGRP) labeling suggests that both sympathetic and sensory nerve types are indeed present in unstimulated and cultured conditions and that their neurotransmitters can be maintained. Tyrosine hydroxylase and CGRP labeling can also co-localize, suggesting these markers alone are not sufficient to identify nerve types. Furthermore, both tyrosine hydroxylase and CGRP do not label all of class III β-tubulin and NG2 positive vessels nerves, especially during culture. While future studies will be needed to evaluate the functional impact of reduced tyrosine hydroxylase or CGRP labelling of neural segments, the heterogeneous nerve labeling highlights issues with the identification on nerves in the mesentery. Complicating matters, co-labelling for S100 with class III β-tubulin suggests that nerve segments can be with and without Schwann cells (data not shown).
The 3 day culture time point was selected for this study based on our previous characterization of angiogenesis in the rat mesentery culture model [21,22]. Consistent with the previous work, angiogenesis in the nerve culture medium tissues for the current study is supported by the observation of high density capillary regions. The temporal characterization of neurovascular congruence in rat mesentery microvascular networks during chronic inflammation suggests that neurogenesis lags angiogenesis [19], motivating the need for future studies to confirm the potential for neurogenesis during culture. While examples of high density nerve plexuses at day 3 support the potential for such growth, the results in the current study only support the maintenance of nerves. In MEM only cultured tissues, nerve labeling was observed to be absent or characterized by discontinuous, punctate labeling consistent with Wallerian regression in peripheral nerve injury [15] motivating future studies at intermediate culture time points (i.e. day 1 and day 2) to characterize the potential degradation process. In contrast, the tissues cultured with the nerve culture medium displayed continuous labelling patterning consistent with nerve morphologies. Another limitation for our culture methods is the lack of blood flow, which could influence microvascular and consequently neural network structure. However, it should be noted that that this limitation is also present in most cell culture or ex vivo slice models. Additional studies will also be needed to confirm nerve functionality, which is supported by the presence of CGRP labeling and the presence of neurotransmitters.
5. Conclusions
In conclusion, a need exists for biomimetic models that bridge the gap between the lack of cellular complexity of in vitro constructs and the multi-system physiological environment of in vivo models. To meet this need, our lab has introduced the rat mesentery culture model as top down approach with intact microvascular networks. Previous development of the model has proven its time-lapse, angiogenic, and lymphangiogenic capabilities [16,21,22]. In this study we expand the rat mesentery culture model via the introduction of a nerve culture medium to maintain nerves and the spatiotemporal relationship between nerves and blood vessels in culture. To our knowledge, the results support the first successful attempt to maintain adult peripheral nerves in an ex vivo culture model.
Supplementary Material
Highlights.
A need exists for biomimetic models that bridge the gap between in vitro approaches and the in vivo multi-system physiological environment.
An ex vivo model with adult peripheral neural and microvascular networks does not exist.
This study presents a method for maintaining adult peripheral nerves along microvascular networks in cultured rat mesenteric tissues.
The rat mesentery culture model represents a new ex vivo tool for future investigation of neurovascular congruence for arterioles and capillaries within microvascular networks.
Acknowledgments
Research reported in this publication was supported by a National Institutes of Health grant R01AG049821.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interests
Declarations of interest: none.
References
- 1.Caillaud M, Richard L, Vallat J-M, Desmoulière A, Billet F. Peripheral nerve regeneration and intraneural revascularization. Neural Regen Res 2019;14: 24–33. doi: 10.4103/1673-5374.243699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer’s disease. Nat Rev Neurosci 2017;18: 419–434. doi: 10.1038/nrn.2017.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozberg M, Hillman E. Neurovascular coupling and energy metabolism in the developing brain. Prog Brain Res 2016;225: 213–242. doi: 10.1016/bs.pbr.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho H, Seo JH, Wong KHK, Terasaki Y, Park J, Bong K, et al. Three-Dimensional Blood-Brain Barrier Model for in vitro Studies of Neurovascular Pathology. Sci Rep 2015;5: 15222. doi: 10.1038/srep15222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naik P, Cucullo L. In vitro blood-brain barrier models: current and perspective technologies. J Pharm Sci 2012;101: 1337–1354. doi: 10.1002/jps.23022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor AM, Dieterich DC, Ito HT, Kim SA, Schuman EM. Microfluidic local perfusion chambers for the visualization and manipulation of synapses. Neuron 2010;66: 57–68. doi: 10.1016/j.neuron.2010.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan OF, Sefton MV. Endothelial cell behaviour within a microfluidic mimic of the flow channels of a modular tissue engineered construct. Biomed Microdevices. 2011;13: 69–87. doi: 10.1007/s10544-010-9472-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam SF, Shirure VS, Chu YE, Soetikno AG, George SC. Microfluidic device to attain high spatial and temporal control of oxygen. PloS One. 2018;13: e0209574. doi: 10.1371/journal.pone.0209574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato M, Sasaki N, Ato M, Hirakawa S, Sato K, Sato K. Microcirculation-on-a-Chip: A Microfluidic Platform for Assaying Blood- and Lymphatic-Vessel Permeability. PloS One. 2015;10: e0137301. doi: 10.1371/journal.pone.0137301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37: 173–182. [DOI] [PubMed] [Google Scholar]
- 11.Krassioukov AV, Ackery A, Schwartz G, Adamchik Y, Liu Y, Fehlings MG. An in vitro model of neurotrauma in organotypic spinal cord cultures from adult mice. Brain Res Brain Res Protoc 2002;10: 60–68. [DOI] [PubMed] [Google Scholar]
- 12.Legradi A, Varszegi S, Szigeti C, Gulya K. Adult rat hippocampal slices as in vitro models for neurodegeneration: Studies on cell viability and apoptotic processes. Brain Res Bull 2011;84: 39–44. doi: 10.1016/j.brainresbull.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 13.Yoon JJ, Nicholson LFB, Feng SX, Vis JC, Green CR. A novel method of organotypic brain slice culture using connexin-specific antisense oligodeoxynucleotides to improve neuronal survival. Brain Res 2010;1353: 194–203. doi: 10.1016/j.brainres.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 14.Frantseva MV, Kokarovtseva L, Naus CG, Carlen PL, MacFabe D, Velazquez JLP. Specific Gap Junctions Enhance the Neuronal Vulnerability to Brain Traumatic Injury. J Neurosci 2002;22: 644–653. doi: 10.1523/JNEUROSCI.22-03-00644.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: interactions at the axon level. Prog Neurobiol 2012;98: 16–37. doi: 10.1016/j.pneurobio.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 16.Sweat RS, Sloas DC, Murfee WL. VEGF-C induces lymphangiogenesis and angiogenesis in the rat mesentery culture model. Microcirc N Y N 1994. 2014;21: 532–540. doi: 10.1111/micc.12132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motherwell JM, Azimi MS, Spicer K, Alves NG, Hodges NA, Breslin JW, et al. Evaluation of Arteriolar Smooth Muscle Cell Function in an Ex Vivo Microvascular Network Model. Sci Rep 2017;7: 2195. doi: 10.1038/s41598-017-02272-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen-Smith FM, Joswiak GR, Baustert JL. Regional differences in spontaneously occurring angiogenesis in the adult rat mesentery. Microvasc Res 1994;47: 369–376. doi: 10.1006/mvre.1994.1029 [DOI] [PubMed] [Google Scholar]
- 19.Stapor PC, Murfee WL. Spatiotemporal distribution of neurovascular alignment in remodeling adult rat mesentery microvascular networks. J Vasc Res 2012;49: 299–308. doi: 10.1159/000336714 [DOI] [PubMed] [Google Scholar]
- 20.Azimi MS, Lacey M, Mondal D, Murfee WL. An Ex Vivo Tissue Culture Model for Anti-angiogenic Drug Testing. Methods Mol Biol Clifton NJ 2016;1464: 85–95. doi: 10.1007/978-1-4939-3999-2_8 [DOI] [PubMed] [Google Scholar]
- 21.Stapor PC, Azimi MS, Ahsan T, Murfee WL. An angiogenesis model for investigating multicellular interactions across intact microvascular networks. Am J Physiol Heart Circ Physiol 2013;304: H235–245. doi: 10.1152/ajpheart.00552.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azimi MS, Motherwell JM, Murfee WL. An Ex Vivo Method for Time-Lapse Imaging of Cultured Rat Mesenteric Microvascular Networks. J Vis Exp JoVE 2017. doi: 10.3791/55183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao D-A, Bi L-Y, Huang Q, Zhang F-M, Han Z-M. Isoflurane provides neuroprotection in neonatal hypoxic ischemic brain injury by suppressing apoptosis. Braz J Anesthesiol Elsevier. 2016;66: 613–621. doi: 10.1016/j.bjane.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 24.Sweda R, Phillips AW, Marx J, Johnston MV, Wilson MA, Fatemi A. Glial-Restricted Precursors Protect Neonatal Brain Slices from Hypoxic-Ischemic Cell Death Without Direct Tissue Contact. Stem Cells Dev 2016;25: 975–985. doi: 10.1089/scd.2015.0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witts EC, Nascimento F, Miles GB. Adenosine-mediated modulation of ventral horn interneurons and spinal motoneurons in neonatal mice. J Neurophysiol 2015;114: 2305–2315. doi: 10.1152/jn.00574.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H, Kim E, Park M, Lee E, Namkoong K. Organotypic hippocampal slice culture from the adult mouse brain: a versatile tool for translational neuropsychopharmacology. Prog Neuropsychopharmacol Biol Psychiatry. 2013;41: 36–43. doi: 10.1016/j.pnpbp.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 27.Azimi MS, Motherwell JM, Hodges NA, Rittenhouse GR, Majbour D, Porvasnik SL, et al. Lymphatic-to-blood vessel transition in adult microvascular networks: A discovery made possible by a top-down approach to biomimetic model development. Microcirc N Y N 1994. 2020;27: e12595. doi: 10.1111/micc.12595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stapor PC, Murfee WL. Identification of class III β-tubulin as a marker of angiogenic perivascular cells. Microvasc Res 2012;83: 257–262. doi: 10.1016/j.mvr.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 29.Stapor PC, Sweat RS, Dashti DC, Betancourt AM, Murfee WL. Pericyte dynamics during angiogenesis: new insights from new identities. J Vasc Res 2014; 51(3):163–74. doi: 10.1159/000362276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murfee WL, Skalak TC, Peirce SM. Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: identifying a venule-specific phenotype. Microcirculation. 2005; 12(2):151–60. doi: 10.1080/10739680590904955. [DOI] [PubMed] [Google Scholar]
- 31.Murfee WL, Rehorn MR, Peirce SM, Skalak TC. Perivascular cells along venules upregulate NG2 expression during microvascular remodeling. Microcirculation. 2006; 13(3):261–73. doi: 10.1080/10739680600559153. [DOI] [PubMed] [Google Scholar]
- 32.Nezami BG, Srinivasan S. Enteric Nervous System in the Small Intestine: Pathophysiology and Clinical Implications. Curr Gastroenterol Rep 2010;12: 358–365. doi: 10.1007/s11894-010-0129-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krammer HJ, Karahan ST, Sigge W, Kühnel W. Immunohistochemistry of markers of the enteric nervous system in whole-mount preparations of the human colon. Eur J Pediatr Surg Off J Austrian Assoc Pediatr Surg Al Z Kinderchir 1994;4: 274–278. doi: 10.1055/s-2008-1066117 [DOI] [PubMed] [Google Scholar]
- 34.Geramizadeh B, Akbarzadeh E, Izadi B, Foroutan H-R, Heidari T. Immunohistochemical study of enteric nervous system in Hirschsprung’s disease and intestinal neuronal dysplasia. Histol Histopathol 2013;28: 345–351. doi: 10.14670/HH-28.345 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


