Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of a current pandemic worldwide. This virus can reach all organs and disturbs the immune system, leading to a cytokine storm in severe forms. We aimed to report cutaneous features among coronavirus disease 2019 (COVID-19) hospitalized patients.
Methods
We performed a cross-sectional study on 1 given day among all patients hospitalized in acute care for COVID-19 and included all patients with cutaneous features. Follow-up 48 hours later was obtained.
Results
Among 59 adult patients hospitalized on the day of the study in an infectious diseases ward for SARS-CoV-2 infection who were confirmed by molecular assay and/or radiological findings (computed tomography scan), 40 were included. Several cutaneous manifestations were found: macular exanthema (80%), face edema (32%), livedo (13%), urticarial rash (8%), purpura (5%), oral lichenoid lesions (33%), and conjunctivitis (18%). Cutaneous biopsy was performed in 17 patients. Histological findings showed mast cell hyperplasia (100%), superficial perivascular infiltrate of lymphocytes (94%), and superficial edema (47%) consistent with capillary leak.
Conclusions
Various dermatological signs can be encountered during COVID-19. A macular rash was the most frequent. All cutaneous features could be related to a vascular leak process.
Keywords: COVID-19, dermatology, exanthema, pandemics, pneumonia
This cross-sectional study showed that cutaneous features are various among hospitalized patients with COVID-19. Macular rash was the most frequent and could be predictive of worsening. They seem related to an immune system disturbance, leading to a vascular leak process.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus that was first detected in a cluster of cases on December 31, 2019, in Wuhan, Hubei Province, China. It causes coronavirus disease 2019 (COVID-19) [1]. Since the initial detection of the virus, >3 450 000 cases of COVID-19 have been confirmed worldwide [2].
Most common signs at onset of illness are fever, cough, myalgia, or asthenia. Less common symptoms are sputum production, headache, and diarrhea. Dyspnea usually appears secondarily. COVID-19 is mostly associated with moderate symptoms; nevertheless, some patients require hospitalization mostly due to respiratory failure.
The critical period of illness, with possible worsening, seems to occur from 7 to 12 days [3]. The median delay from onset of symptoms to hospital admission is 7.0 days, and 9.0 days to mechanical ventilation [4]. Few reports of cutaneous involvement during the hospitalization period have been published in the literature [5–8]. An early study, performed by Italian colleagues, reported 18 patients with cutaneous manifestations. They found erythematous rash (78%) mainly located on the trunk in which itching was reported as low or absent, widespread urticaria (16%), and chickenpox-like vesicles (5%) [5].
Despite these preliminary data, cutaneous manifestations of SARS-CoV-2 infection remain poorly known, especially specific skin characteristics. Their detailed clinical pictures and histological analyses have not yet been reported.
We aim to describe extensively clinical cutaneous features of patients hospitalized for COVID-19 pneumonia.
METHODS
Study Design and Patients
We performed a cross-sectional study on a given day (March 31, 2020) in a French university hospital (Raymond-Poincaré University Hospital, Garches) among all adult patients hospitalized for COVID-19 in an infectious diseases (ID) department with follow-up at 48 hours after inclusion. Were included all hospitalized patients with a COVID-19 diagnosis, confirmed by molecular and/or radiological evidence, and presenting with cutaneous manifestations.
Epidemiological, clinical, biological, and histological data were collected through a standardized questionnaire.
The local institutional review board approved the study protocol. Patient consent was obtained for all included patients for compilating data gathering, picturing, and skin biopsy. The study was done in accordance with the ethical principles of the Declaration of Helsinki and the Guidelines for Good Clinical Practice. The clinical trial number is NCT04364698.
Data Collection/Definitions
We registered the following information from the patients’ medical charts:
-
-
Time interval between first signs or symptoms and the cutaneous examination (inclusion)
-
-
Clinical signs and symptoms, especially mucocutaneous
-
-
Vital parameters at inclusion: blood pressure, heart rate, oxygen saturation, respiratory rate, temperature
-
-
Molecular confirmation of SARS-CoV-2 infection
-
-
Biological parameters at inclusion: C-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin and procalcitonin (PCT) levels
-
-
Radiographic evidence of pulmonary lesions (computed tomography [CT] scan): extent of pulmonary injury
-
-
Histologic analysis of skin biopsies (if performed in routine care) at inclusion
-
-
Anti-infective agent prescription at inclusion
-
-
Outcome at day 2:
-
◦
Failure: death, transfer to intensive care unit (ICU)
-
◦
Stable: patient still hospitalized in ID ward
-
◦
Cure: patient discharged from the hospital
-
◦
Molecular Detection
First, upper respiratory samples were obtained with nasopharyngeal swabs (Sigma Virocult, Medical Wire Instrument, Corsha, UK); if negative, a second nasopharyngeal swab was performed; if also negative, a tracheal aspiration was performed.
Imaging
Chest CT scan was performed in all but 1 patient (for whom the diagnosis was done on chest x-ray).
Skin Evaluation
All skin and mucosal evaluation was performed by a physician with both specialties: ID and immuno-dermatology (H.M.). Pictures were reviewed by an expert dermatologist (I.B.V.).
Histopathology
Formalin-fixed, paraffin-embedded (FFPE) skin biopsies were analyzed on 3-µm-thick sections after hematoxylin, eosin, and saffron (HES) and periodic acid-Schiff staining (B.B.).
Statistical Analysis
Statistical analysis was performed with the Statistical Package for Social Science for Windows, version 17.0 (SPSS, Chicago, IL, USA).
Quantitative variables are presented as median and interquartile range (IQR), and qualitative variables are presented as number of occurrences and relative frequencies.
RESULTS
Study Population
Among the 59 patients hospitalized for COVID-19 in our ID department, 40 (67%) had confirmed COVID-19-related pneumonia with skin or mucosal manifestations and were included in our study. Table 1 summarizes the study population’s characteristics.
Table 1.
Main Characteristics of the Study Population and COVID-19 Presentation (n = 40)
| n = 40 | |
|---|---|
| Age, median (IQR), y | 57.6 (49.4–69.1) |
| Sex ratio (M/F) | 3.7 |
| Body mass index, median (IQR), kg/m2 | 26.20 (23.2–29.5) |
| Time between onset and skin evaluation, median (IQR), d | 14.5 (9.0–16.3) |
| Temperature ≥38°C | 3 (7.5) |
| Oxygen requirements | 32 (80.0) |
| O2 ≥1 L/min | 32 (80.0) |
| O2 ≥2 L/min | 24 (60.0) |
| O2 ≥3 L/min | 14 (35.0) |
| O2 ≥4 L/min | 6 (15) |
| O2 ≥5 L/min | 4 (10) |
| O2 ≥6 L/min | 2 (5) |
| WHO progression scale of COVID-19 | |
| 4 = hospitalized; no oxygen therapy | 8 (20) |
| 5 = hospitalized; oxygen by mask or nasal prongs | 31 (77.5) |
| 6 = hospitalized; oxygen by noninvasive ventilation or high flow | 1 (2.5) |
| Cutaneous signs | |
| Macular exanthema | 2 (5) |
| Head and neck macular exanthema | 31 (77.5) |
| Maculopapular exanthema | 3 (7.5) |
| Urticarial rashes | 3 (7.5) |
| Face edema | 13 (32.5) |
| Livedo reticularis | 5 (12.5) |
| Purpura | 2 (5.0) |
| Exacerbation of atopic dermatitis | 1 (2.5) |
| Oral lichenoid reaction | 13 (32.5) |
| Herpes | 1 (2.5) |
| Oral enanthema | 11 (27.5) |
| Macroglossia | 10 (25.0) |
| Cheilitis | 5 (12.5) |
| Conjunctivitis | 7 (17.5) |
| Molecular detection of SARS-CoV-2 infection by PCR | 37 (92.5) |
| Extend of pulmonary injury | 39 (97.5) |
| <10% | 5 (12.5) |
| 10%–25% | 14 (35.0) |
| 25%–50% | 13 (32.5) |
| 50%–75% | 7 (17.5) |
| Chest x-ray, No. (%) | 1 (2.5) |
| Biological parameters, median (IQR) | |
| CRP, mg/L | 57 (17.4–93.2) |
| LDH, UI/L | 311.5 (250.8–351.5) |
| Ferritin, µg/L | 1411 (875–1863) |
| PCT, µg/L | 0.125 (0.1–0.2) |
| Treatments taken at time of skin evaluation | |
| No anti-infective treatment | 17 (42.5) |
| Hydroxychloroquine | 15 (37.5) |
| Macrolides | 13 (32.5) |
| Beta-lactams | 8 (20.0) |
| Outcome | |
| Failure | 3 (7.5) |
| Stable | 17 (42.5) |
| Cure | 19 (47.5) |
Abbreviations: COVID-19, coronavirus disease 2019; CRP, C-reactive protein; IQR, interquartile range; LDH, lactate dehydrogenase; PCR, polymerase chain reaction; PCT, procalcitonin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization.
The sex ratio was 3.7 (M/F), and the median age (IQR) was 57.6 (49.4–69.1) years.
All patients, except 3, were confirmed infected with SARS-CoV-2 by reverse transcription polymerase chain reaction.
Patients who had negative molecular assays for SARS-CoV-2 had clinical symptoms, biological features, and typical chest CT scan images compatible with COVID-19. Thirty-nine patients had typical chest CT scans according to the literature [9]. Most patients presented with mild pulmonary infiltrates involving 10%–25% of lung volume (n = 14). One patient had a severe interstitial bilateral pneumonia on chest x-ray and did not have a CT scan when skin evaluation was performed.
Finally, 32 patients required oxygen with a flow varying from 1 L/min to 15 L/min. Among them: 6 (19%) required >4 L/min of oxygen. All patients, except 3, were afebrile. Based on the WHO Progression Scale of COVID-19 (https://clinicaltrials.gov/ct2/show/NCT04324073), 1 patient had a severity score evaluated at 6, 31 patients at 5, and 8 patients at 4.
Skin Manifestations
Among the 40 patients with skin manifestations, the median delay between symptom onset and skin evaluation (IQR) was 14.5 (9.0–16.3) days. Characteristics of all patients are summarized in Supplementary Table 1. Several cutaneous patterns were observed and are shown in Table 2.
Table 2.
Main Skin, Eyes, and Mouth Manifestations Observed in the Study Patients
| No. of Cases | Dermatological Semiology and Clinical Pictures of our Patients Presenting Each Skin Manifestation Encountered | |
|---|---|---|
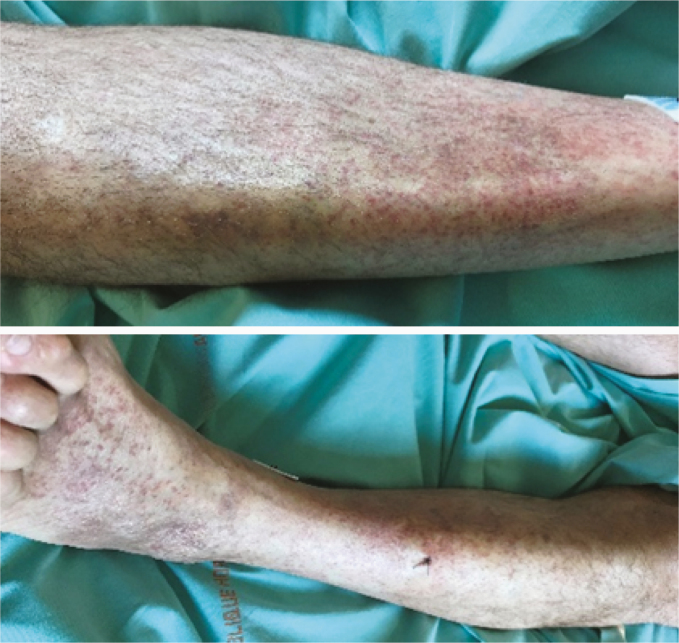
| Skin examination: Macular exanthema (2.A) |
32 |
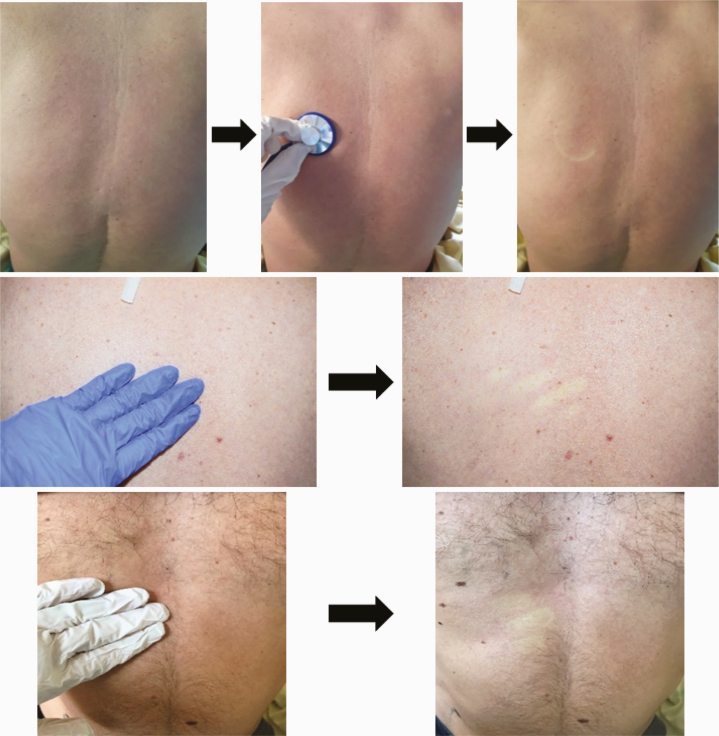
|
| Skin examination: Head and neckline macular exanthema (2.B) |
31 |
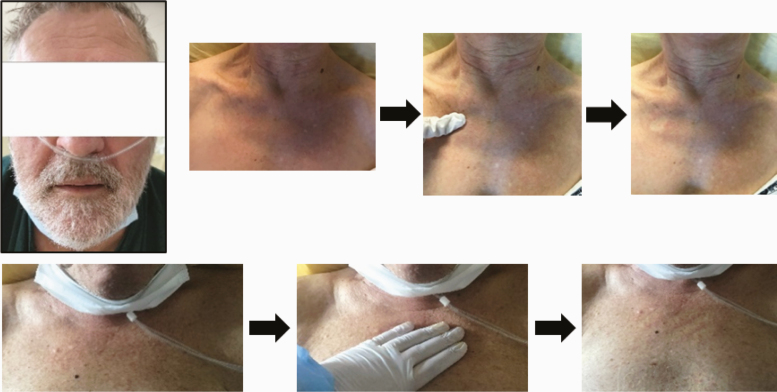
|
| Skin examination: Maculo-papular exanthema (2.C) |
3 |
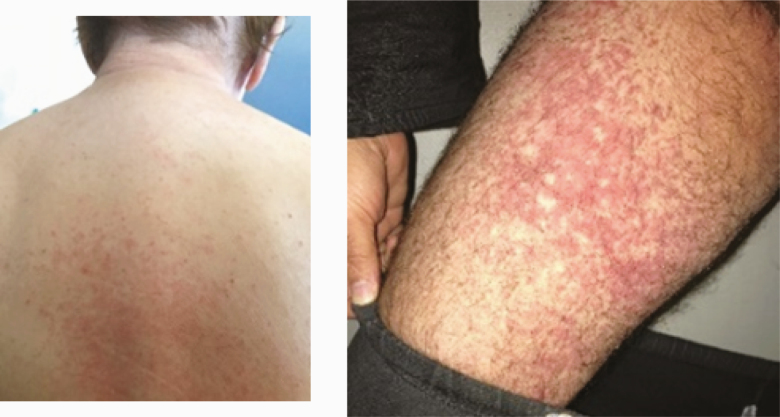
|
| Skin examination: Urticarial rashes (2.D) |
3 |
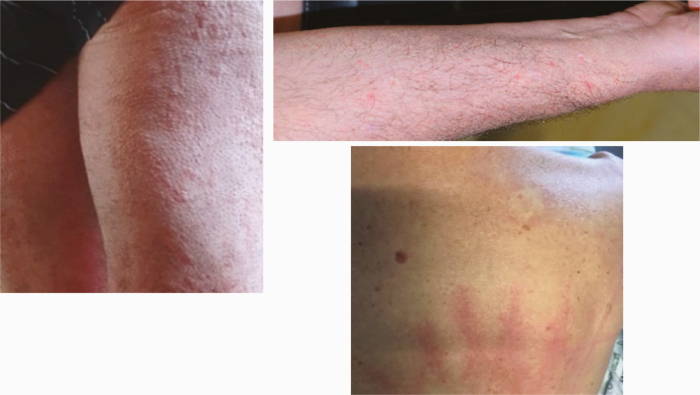
|
| Skin examination: Face edema (2.E) |
13 |
|
| Skin examination: Livedo reticularis (2.F) |
5 |
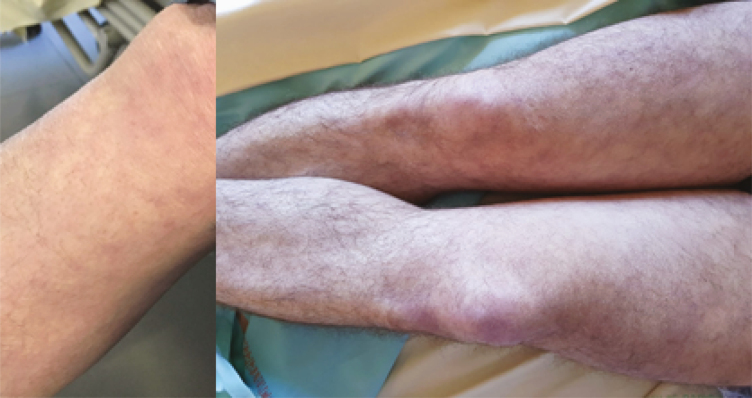
|
| Skin examination: Purpura (2.G) |
2 |
|
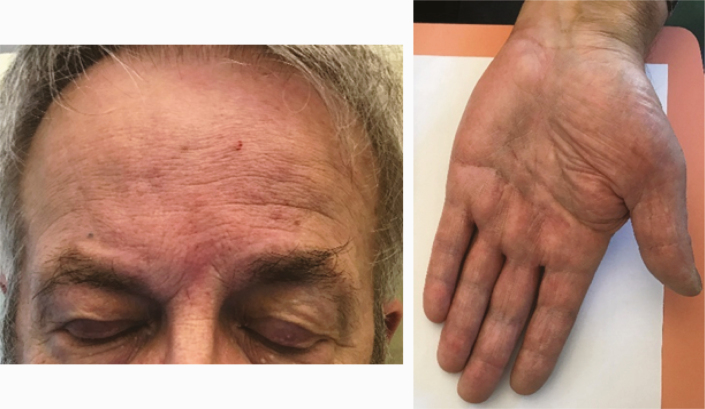
| Skin examination: Atopic dermatitis (2.H) |
1 |
|
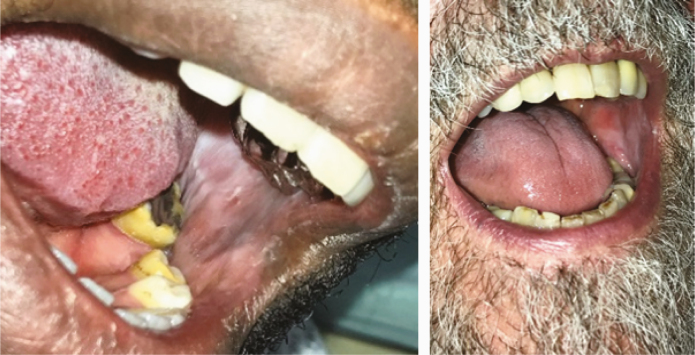
| Oral cavity examination: Oral lichenoid reaction (2.I) |
13 |
|
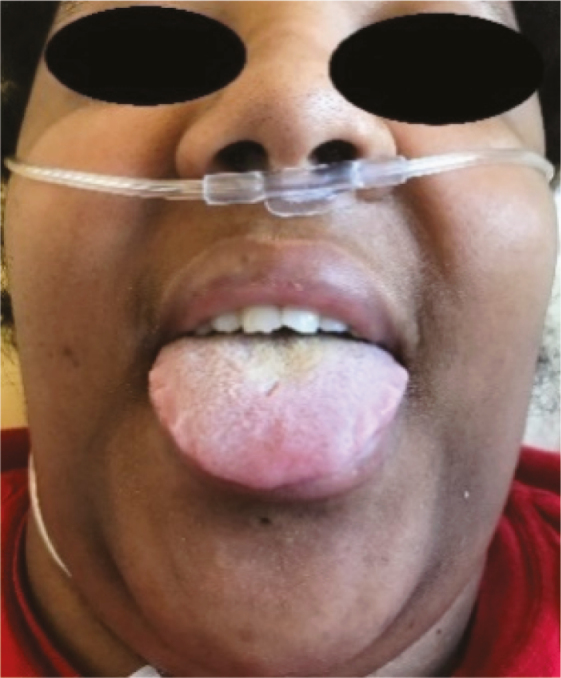
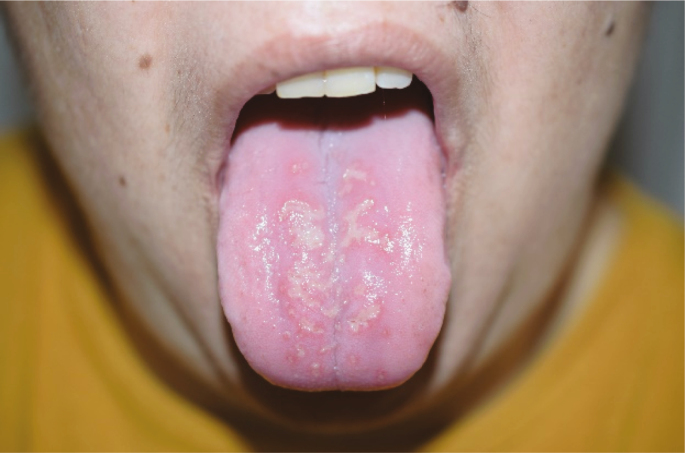
| Oral cavity examination: Macroglossia (2.J) |
10 |
|
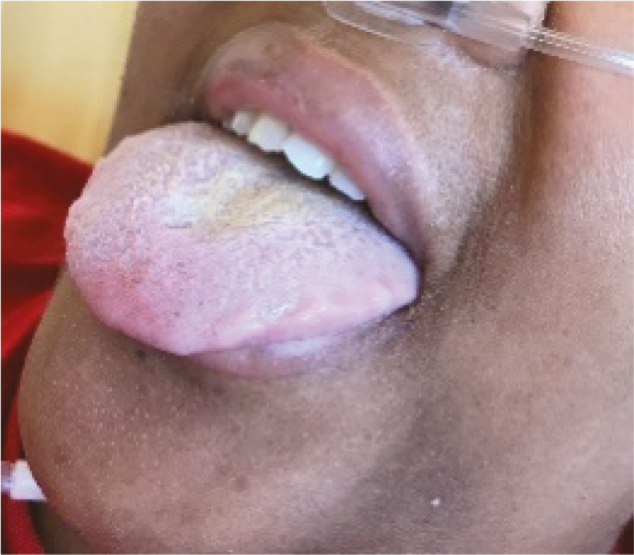
| Oral cavity examination: Herpes (2.K) |
1 |
|
| Oral cavity examination: Enanthema (2.L) |
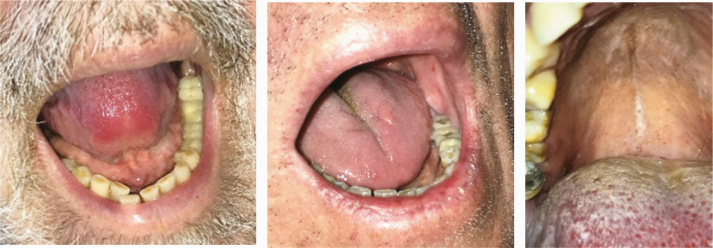
11 |
|
| Oral cavity examination: Cheilitis (2.M) |
5 |
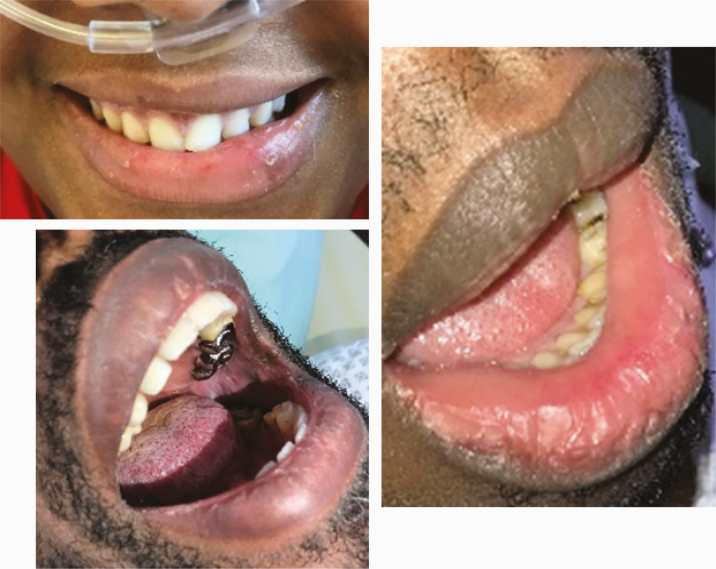
|
| Eye examination: Conjunctivitis (2.N) |
7 |
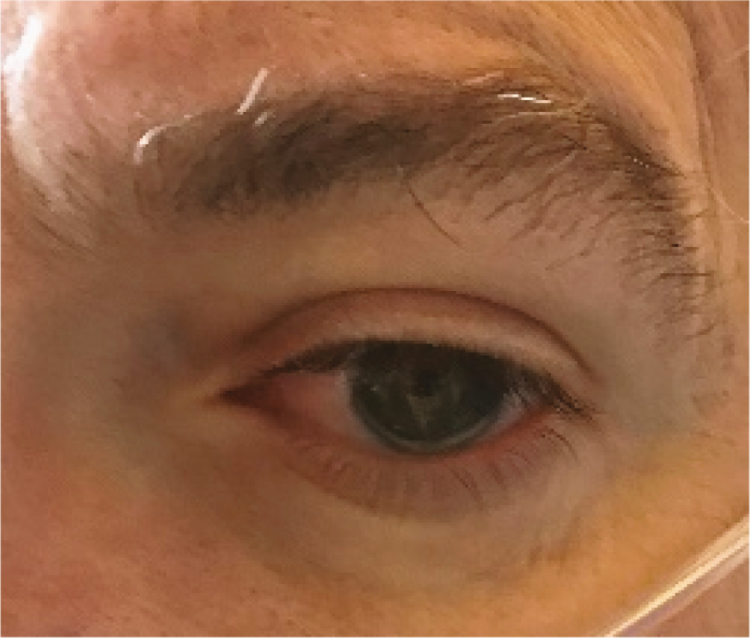
|
Macular exanthema was observed in 32 patients (80%) (Table 2A). Trunk and head & neck were the areas preferentially involved in 30 and 31 patients, respectively (Table 2B). Hands and feet were spared. Only 3 patients had fever. None of them had pruritis. Furthermore, 12/32 patients (37.5%) were not taking any drug at the time of skin evaluation.
We noticed 3 maculopapular exanthemas (patients 18, 19, 35). Two of them had an important concomitant face edema (patients 19, 35) (Table 2C).
One patient (patient 36) had a generalized de novo papular urticarial rash at day 9 after the onset of COVID-19 signs and symptoms, with a spontaneous resolution (Table 2D). He was not taking any medication. The second and third patients (patients 22, 40) had acute urticaria at days 21 and 15, respectively, after the onset of COVID-19 signs and symptoms.
All the patients presented with extremely itchy lesions.
Face edema was found in 13 patients, without associated urticarial papules. None of them reported previous angioedema (Table 2).
Five patients had persistent bilateral livedo reticularis of the lower limbs (Table 2F). None had livedo racemosa.
Two patients presented with petechial purpura of the leg extremities (Table 2G).
One patient had an exacerbation of his atopic dermatitis (AD) of the face and limbs and palm dyshidrosis (Table 2H).
Mucosal Involvement
Several lesions were observed on the lips, oral mucosa, and ocular mucosa. A typical lichenoid network was observed on the inner cheeks of 13 patients (Table 2I). Ten patients had macroglossia (Table 2J). Interestingly, we noticed that 9 of them complained of anosmia and ageusia. Herpes simplex virus (HSV)–1 was found in extensive ulcerations of the tongue in the 37th patient (Table 2K). Eleven patients had oral enanthema (Table 2L). Five patients had cheilitis (Table 2M). Seven patients had conjunctivitis (Table 2N).
None of the patients reported any prior cutaneous or mucosal disorder, except the patient who presented with atopic dermatitis.
Treatment
At the time of the skin evaluation, 23 patients (57.5%) had received an anti-infective treatment. Table 3 shows skin manifestations under each type of anti-infective treatment combination.
Table 3.
Anti-infective Treatment Durations of Study Patients Before Skin Evaluation According to Skin Manifestations
| Type of Cutaneous Manifestation | No. of Patients With at Least 1 Anti-infective Treatment (n = 23) | Treatment Duration Before Skin Evaluation, d | |||||
|---|---|---|---|---|---|---|---|
| HCQ (n = 6) | Beta-lactam (AMX or CRO) (n = 4) |
AZM (n = 2) | HCQ + AZM (n = 7) | SP + CRO (n = 2) | HCQ + AZM + Beta-lactam (n = 2) | ||
| Macular exanthema (n = 32) | 19 | n = 4 | n = 4 | n = 2 | n = 5 | n = 2 | n = 2 |
| •P2: 7 •P5: 6 •P7: 6 •P11: 7 |
•P4: 13 •P8: 1 •P12: 3 •P24: 1 |
•P21: 3 •P32: 3 |
•P3: 5 •P13: 5 •P17: HCQ (6) + AZM (5) •P22: 8 •P27: 1 |
•P25: 1 •P34: 1 |
•P1: HCQ (5) + AZM (5) + CRO (4) •P14: HCQ (5) + AZM (5) + AMX (2) |
||
| Maculo-papular exanthema (n = 3) | 1 | - | - | - | n = 1 | - | - |
| P19: 5 | |||||||
| Urticarial rashes (n = 3) |
1 | - | - | - | n = 1 | - | - |
| P22: 8 | |||||||
| Face edema (n = 13) | 8 | n = 1 | n = 2 | n = 1 | n = 2 | - | n = 2 |
| P23: 6 | •P8: 1 •P24: 1 |
P21: 3 | •P13: 5 •P19: 5 |
•P1: 5 •P14: 5 |
|||
| Livedo reticularis (n = 5) | 3 | - | n = 1 | - | n = 2 | - | - |
| P24: 1 | •P17: HCQ 6 + AZM 5 •P22: 8 |
||||||
| Purpura (n = 2) | 1 | - | - | - | - | - | n = 1 |
| P14: 5 | |||||||
| Exacerbation of atopic dermatitis (n = 1) | 1 | n = 1 | - | - | - | - | - |
| P23: 6 | |||||||
| Oral lichenod reaction (n = 13) | 9 | n = 2 | n = 2 | - | n = 5 | - | - |
| •P5: 6 •P28: 4 |
•P12: 3 •P24: 1 |
•P3: 5 •P17: HCQ 6 + AZM 5 •P19: 5 •P22: 8 •P27: 1 |
|||||
| Macroglossia (n = 10) | 8 | - | n = 1 | n = 1 | n = 5 | - | n = 1 |
| P12: 3 | P21: 3 | •P3: 5 •P13: 5 •P16: HCQ 3 + AZM 4 •P19: 5 •P22: 8 |
P1: 5 | ||||
| Herpes (n = 1) | 0 | - | - | - | - | - | - |
| Enanthema (n = 11) | 9 | n = 1 | n = 3 | n = 1 | n = 3 | - | n = 1 |
| P5: 6 | •P8: 1 •P12: 3 •P24: 1 |
P21: 3 | •P19: 5 •P22: 8 •P27: 1 |
P1: 5 | |||
| Cheilitis (n = 6) | 2 | - | - | - | n = 2 | - | - |
| •P19: 5 •P27: 1 |
|||||||
| Conjonctivitis (n = 7) | 4 | - | n = 1 | - | n = 2 | - | n = 1 |
| P24: 1 | •P19: 5 •P22: 8 |
P1: 5 |
Abbreviations: AMX, amoxicillin; AZM, azithromycin; CRO, ceftriaxone; HCQ, hydroxychloroquin; P, patient; SP, spiramycin.
Twelve patients received an anti-infective molecule monotherapy: beta-lactams (4 patients; mean duration, 4.5 days), azithromycin (2 patients; mean, 3 days), and hydroxychloroquine (6 patients; mean, 6 days). Nine patients received a combination of 2 anti-infective molecules: hydroxychloroquine + azithromycin (7 patients; mean, 4.4 days), and spiramycin + ceftriaxone (2 patients; mean, 1 day). Two patients received a combination of hydroxychloroquine + azithromycin + beta-lactam for a mean duration of 3 days.
The median treatment duration before the evaluation (IQR) was 5 (5–6) days for hydroxychloroquine, 5 (3–5) days for macrolides, and 1.5 days (1–3.25) days for beta-lactam.
Histology
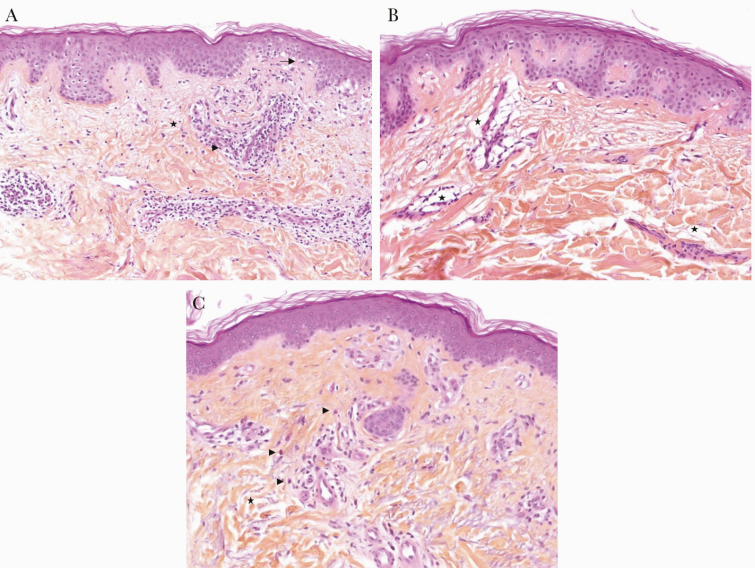
Cutaneous biopsy was performed in 17 patients. Histological findings showed a superficial perivascular infiltrate of lymphocytes (16/17; 94.1%), sometimes with mild interface changes (4/17; 23.5%). The second most frequent finding was superficial edema (8/17; 47.1%). Edema was either part of urticaria (2/17; 11.8%), predominantly in perivascular distribution without eosinophilic infiltrate (3/17; 17.5%), or with purpura (1/17; 5.9%). All biopsies had mast cell (MC) hyperplasia (100%). Malassezia folliculitis was frequently observed (7/17; 41.2%). Figure 1 illustrates a superficial perivascular infiltrate of lymphocytes (1.A) and vascular leak (1.B) in 2 patients presenting with macular exanthema of the trunk, as well as urticaria in 1 patient (1.C).
Figure 1.
Mean histopathological aspects of rash associated with COVID-19 (haematoxylin, eosin, and saffron, ×100). A, Superficial perivascular infiltrate of lymphocytes (→) with few mast cells, eosinophils, and edema (★) associated with light interface changes (basal cell hydropic degeneration): viral exanthemata aspect (patient 34). B, Important superficial edema especially in pericapillary forming perivascular retraction artifact (★) with a light infiltrate of lymphocytes: capillary leak syndrome aspect (patient 33). C, Inconspicuous edema (collagen fibers appear separated: ★), perivascular and interstitial infiltrate with few sparse eosinophils (→): urticaria aspect (patient 36).
Outcome
At 48 hours of follow-up, no patient had passed away, 3/40 were hospitalized in the ICU (failure), and 18/40 were still in the ID department (stable), while 19/40 were discharged from the hospital (cure).
DISCUSSION
We observed 40 patients, among 59 patients, with cutaneous manifestations, hospitalized in an ID ward in a cross-sectional study on 1 given day. All of them were hospitalized for moderate to severe COVID-19, with various cutaneous and mucosal manifestations.
The dermatological features found in our COVID-19 patients were sometimes similar to cutaneous manifestations encountered in other well-documented infectious diseases. Thus, hypotheses on the pathophysiology behind those mucocutaneous manifestations of COVID-19 could be suggested, especially on the potential role of the immune system. Table 4 describes the type of cutaneous findings in our COVID-19 patients with their presumed pathogenic mechanisms based on histological findings and similarities with other well-known viral infectious diseases and inflammatory diseases.
Table 4.
Skin Manifestations Observed in our Study Patients and Their Presupposed Pathogenic Mechanisms
| Presupposed Mechanism | Dermatological Features | Other Infectious Diseases and/or Inflammatory Diseases With Similar Mucocutaneous Features | Pathophysiological Hypotheses |
|---|---|---|---|
| Vascular leak | Macular exanthema |
Dengue:
Endothelial swellings of small blood vessels, perivascular edema, and infiltration with mononuclear cells consistent with vascular leak (histology) [21] |
“Inflammatory phase” of COVID-19 (7th to 12th days of symptom onset): Due to a “cytokine storm” involving in particular IL-2, IL-6, IL-1, IL-10, and TNFα [22]. Among them, IL-2 and TNFα are thought to be involved in the pathogenesis of the vascular leak syndrome [23, 24]. |
| Clarkson disease: Capillary leak syndrome [25] | |||
| Angio-edema | None of our patients took converting enzyme inhibitor drugs or angiotensin II receptor blockers. | ||
| EBV: Drug-induced eruption [19] | Synergy between SARS-CoV-2 and drugs, as observed with EBV infection and beta-lactam rash [19]. | ||
| Face oedema | Clarkson disease: Capillary leak syndrome [25] | “Inflammatory phase” of COVID-19 pneumonia, leading to “cytokine storm” and vascular leak process (IL-2 and TNFα). | |
| Angio-edema | No drug and no urticarial lesion associated with face edema in our study. | ||
| Urticarial rashes | - Viral hepatitis - Parvovirus B19 - HHV-6 - Zika virus - Dengue |
Mast cells directly infected by viruses [11, 12], leading to MC degranulation, endothelial activation (IL2 and TNFα), and vascular leak process. | |
| Acute hypersensitivity, type 1 | No drug given in the 6 hours before the eruption. | ||
| Urticarial vasculitis | No vasculitis on histological section. Resolution of urticaria within 24 h. | ||
| Vasculopathy | Livedo reticularis |
Intracellular bacteria: Coxiella burnetti
+ Anti-phospholipid Antibodies |
Hypothesis 1:
Increased thromboembolic events have been described in COVID-19 [26]. Antiphospholipid antibodies and associated thromboembolic events have been described in COVID-19 [27]. |
|
Hypothesis 2:
Vasculitis or local vasculopathy [28]. |
|||
| Disseminated intravascular coagulation | In our study, none of our patients had DIC at the time of skin evaluation. | ||
| Purpura |
- Parvovirus B19
- Dengue |
Pauci-inflammatory thrombogenic vasculopathy, with deposition of C5b-9 and C4d co-localized with SARS-CoV-2 spike glycoprotein [29]. | |
| T-cell lymphocytes | Oral lichenoid reaction |
- HCV
- HPV-16 |
Lymphopenia, with absolute number of T lymphocytes, CD4+ T cells, and CD8+ T cells decreased in blood of COVID-19 patients and low expression of IFN-gamma by CD4+ T cells, probably linked to a redistribution of Tc1 CD8+ T-cell lymphocytes in the organs [15]. Keratinocytes infected by SARS-CoV-2 could be the target of Tc1 CD8+ T-cell lymphocytes. |
| Oral erosion | HSV-1 | Viral reactivation: Related to the decrease of CD4+ and CD8+ T-cell activity against HSV-1 antigen. | |
| Direct excretion of SARS-CoV-2 | Conjonctivitis | SARS-CoV-2 was isolated from the conjunctival swabs of 12 patients with ocular manifestations among 38 COVID-19 patients [16]. |
Abbreviations: COVID-19, coronavirus disease 2019; DIC, disseminated intravascular coagulation; EBV, Epstein-Barr virus; HCV, hepatitis C virus; HHV, human herpesvirus; HPV, human papillomavirus; HSV, herpes simplex virus; IFN, interferon; IL, interleukin; MC, mast cells; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF, tumor necrosis factor.
We mainly encountered extensive macular exanthemas, which were present in 32 patients (80%). The trunk, head, and neck areas were preferentially involved. Histological examinations were compatible with viral reaction. For instance, the histological section in Figure 1A shows superficial dermal edema with mild lymphocytic infiltrates consistent with a vascular leak.
Additionally, face edema was observed in 13 patients, which could also be compatible with a capillary leak syndrome.
In our study, all patients, except 1, were hospitalized within 7 days following the onset of symptoms, with a median time between onset of COVID-19 and skin evaluation (IQR) of 14.5 (9.0–16.3) days. Nevertheless, it is of note that 3 of our patients developed a sudden flare-up of their macular rash, while experiencing clinical worsening and requiring mechanical ventilation in the ICU.
We observed 3 cases of acute urticaria (on the ninth, 15th, and 21st days after onset of COVID-19), due to MC degranulation without vasculitis, and without any causative drug identified. In the literature, urticaria and urticaria vasculitis have recently been reported during COVID-19 [6, 10]. Also, in our study, MC hyperplasia was observed in all of the performed biopsies, and the sudden onset of extremely pruritic urticarial rash was observed in the late phase of the disease. However, many studies focused on the ability of MCs to become infected with viruses [11, 12]. Therefore, this skin manifestation could be correlated to viral replication in the MCs infected with SARS-CoV-2, leading to vascular leakage.
Livedo reticularis of the lower limbs was observed among 5 patients. Two cases of transient livedo reticularis [13] and 7 cases of acro-ischemia were recently reported in an ICU [14]. Two cases of purpura were also found. Among them, 1 patient had a skin biopsy that showed an inflammatory perivascular lymphocytic infiltrate with extravasated erythrocytes in the dermis. No evidence of vasculitis was noted.
Chen et al. demonstrated that the absolute number of T lymphocytes, CD4+ T cells, and CD8+ T cells decreased in the blood of COVID-19 patients with a low expression of interferon-gamma by CD4+ T cells [15]. This lymphopenia is probably linked to a redistribution of Tc1 CD8+ T-cell lymphocytes in the organs, such as in the oral cavity of our 13 patients who had oral lichenoid de novo reactions. Conversely, an extensive HSV-1 infection was observed on the tongue and palate of a patient. This viral reactivation could be related to the decreased activity of CD4+ and CD8+ T cells against HSV-1 antigen.
In our study, 7 patients had conjunctivitis. In a recent series of 12 patients, ocular manifestations were consistent with conjunctivitis, including conjunctival hyperemia, chemosis, epiphora, or increased secretions. SARS-CoV-2 was isolated from conjunctival swabs [16].
Finally, Zheng et al. reported worsening of previous skin chronic disorders (AD, eczema, rosacea, neurodermatitis, and facial acne) linked to an induction of systemic inflammatory responses [6]. In our study, exacerbation of AD and dyshidrosis of the palms were observed in 1 patient.
Overall, in the literature, Recalcati et al. reported that 20.4% of patients with COVID-19 presented with cutaneous manifestations (14 erythematous rash, 3 widespread urticaria, and 1 chickenpox-like vesicles) [5]. In his cohort, the trunk was preferentially involved, with low or absent itch, which is concordant with our findings. The author did not report any correlation between those skin symptoms and disease severity. Interestingly, 3 of our patients developed a sudden flare-up of their macular rash while experiencing clinical worsening and requiring mechanical ventilation in the ICU; however, the predictive value of this clinical sign cannot be assured because of the small number of patients. Chilblains seem to be a potential marker of the nonsevere form of COVID-19 [10, 17, 18]. In our study, we did not observe any acrosyndrome or chilblain-like injury. These lesions could be related to the recovery phase.
Our study has several limitations, including the small sample size and short follow-up.
Also, many cutaneous lesions could be explained by drug-related reactions. However, drug reaction was formally excluded in 17 patients who did not take any prior drugs. We did not retain the drug imputability for other patients, but a synergy between SARS-CoV-2 and drugs cannot be formally excluded, as observed with EBV infection and beta-lactam rash [19].
Nevertheless, this is the largest study of cutaneous lesions with histological evaluation during severe SARS-CoV-2 infection. Estébanez et al. have recently reported that the rate of COVID-19 patients with skin manifestations is probably underestimated [20]. In our cross-sectional study, 67% of patients hospitalized for COVID-19 had skin manifestations, which should be systematically looked for.
CONCLUSIONS
Our study presents several cutaneous signs occurring during SARS-CoV-2 infection that are common and helpful in practice for the diagnosis and prognosis of COVID-19. The main cutaneous manifestations (exanthema, face edema) are consistent with a vascular leak process.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. None.
Potential conflicts of interest. The authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. H.M., I.B.V., and C.P. were involved in the conception and design of the work. H.M., I.B.V., C.P., B.B., A.D., F.A., V.P., and C.D. were involved in the analysis or interpretation of data for the work. B.B. and J.F.E. were involved in the histological analyses. F.M., M.A.R.W., E.G., A.L.R., V.S.T., E.S., F.E.S., J.L.G. were involved in the molecular detection of SARS-CoV-2. F.B. was involved in the drug data analyses. R.C. were involved in the radiological data acquisition. H.M., A.D., F.A., V.P., T.L., M.M., A.L.G., J.N.V.T., A.L., L.J., N.K., S.S., S.B., P.D.T., S.L., and B.D. were involved in the clinical data acquisition. H.M., I.B.V., C.P., B.B., A.D., and C.D. wrote the first draft of the manuscript. All authors were involved in drafting the work and revising it critically. All authors gave final approval of the version to be published.
Patient consent. The local institutional review board approved the study protocol. Patient consents were obtained for all included patients for compilating data gathering, picturing, and skin biopsy. The study was done in accordance with the ethical principles of the Declaration of Helsinki and the Guidelines for Good Clinical Practice.
Trial registration. The clinical trial number is NCT04364698.
References
- 1. World Health Organization. Novel coronavirus – China Available at: http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/. Accessed 9 April 2020.
- 2. Johns Hopkins Coronavirus Resource Center COVID-19 map. Available at: https://coronavirus.jhu.edu/map.html. Accessed 18 April 2020.
- 3. Lescure FX, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis 2020; 20:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol 2020; 34:e212–3. [DOI] [PubMed] [Google Scholar]
- 6. Zheng Y, Lai W. Dermatology staff participate in fight against Covid-19 in China. J Eur Acad Dermatol Venereol 2020; 34:e210–1. [DOI] [PubMed] [Google Scholar]
- 7. Tammaro A, Adebanjo GAR, Parisella FR, et al. Cutaneous manifestations in COVID-19: the experiences of Barcelona and Rome. J Eur Acad Dermatol Venereol 2020; 34:e306–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol 2020; 83:280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020; 20:425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bouaziz JD, Duong T, Jachiet M, et al. Vascular skin symptoms in COVID-19: a french observational study. J Eur Acad Dermatol Venereol 2020. doi: 10.1111/jdv.16544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marshall JS, King CA, McCurdy JD. Mast cell cytokine and chemokine responses to bacterial and viral infection. Curr Pharm Des 2003; 9:11–24. [DOI] [PubMed] [Google Scholar]
- 12. Syenina A, Jagaraj CJ, Aman SAB, et al. Dengue vascular leakage is augmented by mast cell degranulation mediated by immunoglobulin Fcγ receptors. eLife 2015; 4:e05291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manalo IF, Smith MK, Cheeley J, et al. A dermatologic manifestation of COVID-19: Transient livedo reticularis. J Am Acad Dermatol 2020; 83:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi 2020; 41:E006. [DOI] [PubMed] [Google Scholar]
- 15. Chen G, Wu D, Guo W, et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130:2620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol 2020; 138:575–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: A case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol 2020; 83:e61–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the Time of COVID-19. J Eur Acad Dermatol Venereol 2020; 34:e346–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gruchalla RS, Pirmohamed M. Clinical practice. Antibiotic allergy. N Engl J Med 2006; 354:601–9. [DOI] [PubMed] [Google Scholar]
- 20. Estébanez A, Pérez-Santiago L, Silva E, et al. Cutaneous manifestations in COVID-19: a new contribution. J Eur Acad Dermatol Venereol. 2020; 34:e250–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas EA, John M, Kanish B. Mucocutaneous manifestations of dengue fever. Indian J Dermatol 2010; 55:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mehta P, McAuley DF, Brown M, et al. ; HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sivakumar PV, Garcia R, Waggie KS, et al. Comparison of vascular leak syndrome in mice treated with IL21 or IL2. Comp Med 2013; 63:13–21. [PMC free article] [PubMed] [Google Scholar]
- 24. Park KY, Kim SJ, Oh E, Heo TH. Induction of vascular leak syndrome by tumor necrosis factor-alpha alone. Biomed Pharmacother 2015; 70:213–6. [DOI] [PubMed] [Google Scholar]
- 25. Druey KM, Greipp PR. Narrative review: the systemic capillary leak syndrome. Ann Intern Med 2010; 153:90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation 2020; 142:68–78. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 2020; 382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chasset F, Francès C. Cutaneous manifestations of medium- and large-vessel vasculitis. Clin Rev Allergy Immunol 2017; 53:452–68. [DOI] [PubMed] [Google Scholar]
- 29. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020; 220:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.