Abstract
Background
Polypharmacy and drug interactions are important issues for HIV-infected individuals. The number and nature of those interactions are continuously evolving with the use of new antiretroviral drugs and the aging of HIV-infected individuals. We aimed to analyze this evolution over time.
Methods
This retrospective cohort study was conducted in the University Hospital of Liège (Belgium). Treatments of HIV-infected outpatients attending Liège University Hospital were collected and analyzed in 2012 and 2016. The University of Liverpool HIV drug interactions database was used to determine drug interactions.
Results
We included 1038 patients in 2016, of whom 78% had 1 comedication. Polypharmacy was seen in 20% of the cohort. Four percent of the patients presented red flag interactions, and 38% had orange flag interactions. Nonantiretroviral (non-ARV) therapeutic classes involved in drug interactions were mostly cardiovascular and central nervous system drugs. They were followed by hormone drugs and dietary supplements for orange flag interactions. Two factors significantly contributed to both red and orange flag interactions: the number of non-ARV comedications and protease inhibitor–based ARV regimens. The proportion of patients with red or orange flag interactions remained stable from 2012 to 2016.
Conclusions
This study highlights the persistence of an alarming number of contraindicated drug interactions and a high prevalence of potential drug interactions over time. Identification, prevention, and management of drug interactions remain a key priority in HIV care.
Keywords: antiretroviral therapy, drug interactions, HIV
In an era of evolving in the use of antiretroviral drugs and polymedication, this retrospective cohort study highlights the persistence of an alarming number of contraindicated drug interactions and a high prevalence of potential drug interactions involving antiretroviral therapies in HIV-infected patients overtime.
Since the advent of combination antiretroviral therapy (ARV) in 1996, the lifespan of HIV-infected patients has been extended significantly. The disease has become a chronic condition requiring lifelong treatment.
Hence, the average age of HIV-infected patients is steadily increasing. In Belgium, 36% of all HIV-infected patients were aged 50 years or older in 2017, compared with 19% in 2006 [1]. This aging of the affected population leads to a paradigm shift for medical management. This shift must now be holistic, taking into account age-associated comorbidities (including, among others, cardiovascular diseases, kidney disease, liver disease, bone disorders, neurocognitive impairment, and non-AIDS-related cancers) [2]. It is all the more true that these comorbidities are highly prevalent in HIV-infected individuals compared with general population [3, 4]. Because of these comorbidities, many drugs are prescribed along with ARV therapy. It is well known that concomitant medication use is more prevalent in HIV-infected people than in the general population [5, 6].
As a result, these patients are more and more exposed to polypharmacy (generally defined as the use of 5 or more medications) and consequently to potential drug–drug interactions (PDDIs), which could lead to clinically significant events [7].
The incidence rate of PDDIs in HIV-infected patients may vary according to the ARV regimens used. For instance, ARVs can be substrates (eg, rilpivirine, maraviroc, bictegravir), inhibitors (eg, ritonavir, cobicistat), or inducers (eg, efavirenz, nevirapine) of cytochrome P450 3A enzymes (CYP450), which constitute the major mechanism of drug metabolism. Drug interactions with ARV can also occur through the alteration of other mechanisms including uridine 5’-diphospho-glucuronosyltransferase, drug transporters (eg, P-glycoprotein), nuclear receptor activation, pH-dependent drug absorption, and drug chelation [8].
PDDIs can lead to both reduction and increase in ARV drug concentrations, resulting in suboptimal disease or symptom management, development of ARV resistance, serious adverse reactions, and drug toxicity, which could lead to nonadherence to treatment [7].
Polypharmacy and PDDIs are thus persistent and evolving challenges faced by clinicians. However, little is known regarding the evolution of the number and the nature of PDDIs in the recent years, although important modifications could be predicted due to a major increase in the use of integrase inhibitors (INIs) and the aging of HIV-infected individuals.
The aim of this study was to retrospectively investigate the prevalence of drug interactions with ARV in 2016, to compare their evolution between 2012 and 2016, and finally to identify risk factors precipitating them.
METHODS
We performed a retrospective cohort study of individuals aged 18 years and older who were infected with HIV and were attending the University Hospital of Liège (Belgium) during 2012 and 2016 in an outpatient setting. The observation period for each year extended from January to December.
Demographic variables included age (categorized as <50 years, 50–64 years, and 65 years), gender, and ethnicity. Clinical variables included mode of transmission, comorbidities, coinfections, current CD4+ T-lymphocyte (CD4) count, HIV plasma viral load, ARV regimen, number of days on therapy, delayed diagnosis, duration of HIV infection, delayed initiation of treatment, and number of comedications.
Non-ARV medication data were collected at every visit to a specialist in infectious diseases and were categorized according to the Belgian Center for Pharmacotherapeutic Information classification (CBIP) [9]. Comedications were listed for each patient, and polypharmacy was defined as the use of ≥5 concomitant medications [6, 11, 14].
The University of Liverpool HIV drug interactions database [10] was used to determine interactions between ARV and non-ARV medications and classify them into red flag (contraindicated) and orange flag (potential dose adjustment and/or timing of administration and/or close monitoring required) interactions. The Liverpool Drug Interaction website provides reliable information that is regularly updated about drug interactions with ARV. To validate the occurrence of a red flag interaction, a medical report from an infectious diseases specialist mentioning the implicated medications had to be identified to ensure the concomitant use of the drugs.
Descriptive analyses are reported using means, standard deviations, medians, interquartile ranges (IQRs; 25th to 75th quartile), and extreme values. We compared patient characteristics between age groups using chi-square tests for categorical variables and analysis of variance or the Kruskal-Wallis test for continuous variables. We used McNemar’s test to compare drug interactions between 2012 and 2016. A multiple logistic regression analysis was performed to determine independent risk factors of drug interactions. Variables for which the significance level was <.1 were included in the model. The results are presented as P values, adjusted odds ratios (ORs), and 95% confidence intervals. Differences were considered statistically significant if the P value was <.05. All statistical analyses were performed with SAS Statistical Software, version 9.4 (SAS Institute Inc, Cary, NC, USA), graphs were built using R, version 3.6.1.
RESULTS
Patient Baseline Characteristics
A total of 1220 HIV-infected patients were enrolled in the study over 2 periods: 911 patients were followed in 2012 and 1038 patients in 2016; among these, 729 patients (60%) were followed during both years.
The baseline characteristics of the patients are presented in Supplementary Tables 1–3 according the year of follow-up (2016, 2012, and both).
In 2016, 1038 patients aged 18–81 years were under follow-up at our university hospital, of whom 62.6% were aged <50 years. Older HIV-infected individuals were more likely to be male and Caucasian (Supplementary Table 1). Conversely, 57.7% of younger patients were coming from Sub-Saharan Africa. Logically, older patients tended to have more comorbidities. The median CD4+ T-cell count (IQR) was 683 (495–915) cells/mm3, and 81% of the patients (838/1038) had a controlled HIV plasma viral load (≤200 copies/mL) on every blood sample collected during the year. The most prescribed ARV combination was an INI-based regimen, independent of age group. In particular, the association dolutegravir/abacavir/lamivudine was the most frequently reported ARV regimen. More than 90% of patients were on ARV treatment throughout the year (Supplementary Table 1).
In 2012, 911 patients aged 18–80 years were under follow-up at our hospital, of whom 71% were aged <50 years (Supplementary Table 2). The median CD4+ T-cell count (IQR) was 574 (420–780) cells/mm3, and 56.0% (505/911) had a controlled HIV plasma viral load on every blood sample. Importantly, the most frequently used ARV drug combination was a protease inhibitor (PI)–based regimen (Supplementary Table 2).
Among 729 patients followed in 2012 and 2016, 625 patients (85.7%) were taking at least 1 non-ARV comedication in 2016 compared with 565 patients (77.5%) in 2012 (P < .0001) with a median of 2 drugs for both years (Table 1). Polypharmacy was observed in 164 patients (22.5%) in 2016 compared with 129 patients (17.7%) in 2012. Older patients had a higher median number of comedications (IQR): 1 (0–3) for <50 years, 3 (1–6) for 50–64 years, 4 (3–7) for ≥65 years (P < .0001) in the population of patients followed in 2016 (n = 1038 patients) (Table 1).
Table 1.
Comedications and Drug Interactions
| Patients Followed in 2016 (n = 1038 Patients) | |||||
|---|---|---|---|---|---|
| Variables | All | Age <50 y | Age 50–64 y | Age ≥65 y | P Value |
| n = 1038 | n = 650 | n = 320 | n = 68 | ||
| No. (%) | No. (%) | No. (%) | No. (%) | ||
| Number of comedications | |||||
| None | 228 (22.0) | 188 (28.9) | 38 (11.9) | 2 (2.9) | <.0001a |
| ≥1 | 810 (78.0) | 462 (71.1) | 282 (88.1) | 66 (97.1) | |
| 1–4 | 601 | 382 | 184 | 35 | |
| ≥5 | 209 | 80 | 98 | 31 | |
| Total | 1038 (100.0) | 650 (100.0) | 320 (100.0) | 68 (100.0) | |
| Mean ± SD | 2.7 ± 2.9 | 2.0 ± 2.4 | 3.7 ± 3.2 | 4.8 ± 3.1 | <.0001b |
| Median (IQR) | 2 (1–4) | 1 (0–3) | 3 (1–6) | 4 (3–7) | |
| Extreme values | 0–19 | 0–19 | 0–16 | 0–12 | |
| Number of interactions | |||||
| Red flag | 85 | ||||
| Orange flag | 1337 | ||||
| Number of patients with at least 1 drug interaction | |||||
| Red flag | 45 (4.3) | ||||
| Orange flag | 396 (38.1) | ||||
| Evolution: patients followed both in 2012 and 2016 (n = 729 patients) | |||||
| Variables | 2012 | 2016 | P Value | ||
| Number of comedications | |||||
| None | 164 (22.5) | 104 (14.3) | <.0001c | ||
| ≥1 | 565 (77.5) | 625 (85.7) | |||
| 1–4 | 436 | 461 | |||
| ≥5 | 129 | 164 | |||
| Total | 729 (100.0) | 729 (100.0) | |||
| Mean ± SD | 2.4 ± 2.5 | 3.0 ± 2.9 | |||
| Median (IQR) | 2 (1–3) | 2 (1–4) | |||
| Extreme values | 0–15 | 0–19 | |||
| Number of interactions | |||||
| Red flag | 63 | 69 | |||
| Orange flag | 915 | 940 | |||
| Number of patients with at least 1 drug interaction | |||||
| Red flag | 34 (4.7) | 35 (4.8) | .88c | ||
| Orange flag | 300 (41.1) | 310 (42.5) | .50c |
Abbreviation: IQR, interquartile range.
aChi-square test.
bKruskal-Wallis test.
cMcNemar test for repeated measurements.
Drug Interactions in 2012
Based on the Liverpool HIV Drug Interactions website, 68 red flag interactions were identified in 37 patients, meaning that 4.1% (37/911) of patients had at least 1 red flag interaction. The most frequent non-ARV medications involved were cardiovascular drugs, followed by gastrointestinal (27.9%), respiratory (16.5%), otolaryngology (ENT) (8.8%), osteo-articular (2.9%), and central nervous system (CNS) agents (2.9%) (Table 2). The majority of ARV medications involved were PIs, except for 1 drug interaction with a non-nucleoside reverse transcriptase inhibitor (NNRTI; rilpivirine). Red flag interactions occurred mainly between atazanavir with proton pump inhibitor (omeprazole), ritonavir with antihypertensive calcium channel blocker (lercanidipine), and inhaled corticosteroids (budesonide). Coadministration of atazanavir or rilpivirine with proton pump inhibitor (PPI) may have decreased the plasma concentration of the ARV by reducing the solubility of the ARV, as intragastric pH increases with PPI.
Table 2.
Number of ARV and Non-ARV Treatments Affected by a Drug Interaction in 2016 and 2012
| 2016 | 2012 | |||
|---|---|---|---|---|
| Treatment | Red Flag Interactions (n = 85), No. (%) | Orange Flag Interactions (n = 1412), No. (%) | Red Flag Interactions (n = 68), No. (%) | Orange Flag Interactions (n = 1070), No. (%) |
| ARV | ||||
| Atazanavir | 9 (10.6) | 63 (5.5) | 25 (36.8) | 169 (15.8) |
| Darunavir | 17 (20.0) | 128 (11.3) | 3 (4.4) | 76 (7.1) |
| Ritonavir | 23 (27.1) | 177 (15.6) | 30 (44.1) | 314 (29.3) |
| Lopinavir | 5 (5.9) | 21 (1.9) | 9 (13.2) | 90 (8.4) |
| Fosamprenavir | - | 2 (0.2) | - | 22 (2.1) |
| Saquinarir | - | - | - | 1 (0.1) |
| Darunavir/cobicistat | 6 (7.1) | 45 (4.0) | - | - |
| Etravirine | 1 (1.2) | 42 (3.7) | - | 39 (3.6) |
| Efavirenz | - | 72 (6.3) | - | 93 (8.7) |
| Zidovudine | - | 1 (0.1) | - | 12 (1.2) |
| Rilpivirine | 9 (10.6) | 23 (2.0) | 1 (1.5) | - |
| Nevirapine | - | 111 (9.8) | - | 82 (7.7) |
| Abacavir | - | 11 (1.0) | - | 3 (0.3) |
| Didanosine | - | - | - | 1 (0.1) |
| Emtricitabine | - | 13 (1.1) | - | 44 (4.1) |
| Lamivudine | - | 33 (2.9) | - | 26 (2.4) |
| Tenofovir | - | 51 (4.5) | - | 58 (5.4) |
| Maraviroc | - | 9 (0.8) | - | 4 (0.4) |
| Dolutegravir | - | 140 (12.3) | - | - |
| Raltegravir | - | 31 (1.7) | - | 36 (3.4) |
| Emtricitabine/tenofovir alafenamide fumarate/elvitegravir/cobicistat | 6 (7.1) | 54 (4.8) | - | - |
| Emtricitabine/tenofovir disoproxil fumarate/elvitegravir/cobicistat | 9 (10.6) | 110 (9.7) | - | - |
| Non-ARV | ||||
| Ear, nose, and throat drugs | 5 (5.9) | 5 (0.4) | 6 (8.8) | 5 (0.5) |
| Osteoarticular drugs | 1 (1.2) | 57 (5.0) | 2 (2.9) | 48 (4.5) |
| Cardiovascular drugs | 35 (41.2) | 272 (23.9) | 21 (30.9) | 250 (23.4) |
| Gastrointestinal drugs | 17 (20.0) | 21 (1.9) | 19 (27.9) | 18 (1.7) |
| Respiratory drugs | 24 (28.2) | 21 (1.9) | 18 (16.5) | 33 (3.1) |
| Hemostasis drugs | 1 (1.2) | 46 (4.0) | - | 43 (4.0) |
| CNS agents | 2 (2.4) | 255 (22.4) | 2 (2.9) | 305 (28.5) |
| Analgesics | - | 34 (3.0) | - | 46 (4.3) |
| Obstetrics & gynecology | - | 27 (2.4) | - | 41 (3.8) |
| Immunity | - | 19 (1.7) | - | 10 (0.9) |
| Anti-infectives | - | 58 (5.1) | - | 82 (7.7) |
| Antineoplastic agents | - | 2 (0.2) | - | 1 (0.1) |
| Others drugs | - | 8 (0.7) | - | 5 (0.5) |
| Genitourinary drugs | - | 3 (0.3) | - | 12 (1.1) |
| Dietary supplements | - | 153 (13.5) | - | 32 (3.0) |
| Hormone drugs | - | 156 (13.7) | - | 139 (13.0) |
Abbreviations: ARV, antiretroviral; CNS, central nervous system.
A total of 1070 orange flag interactions were reported in 349 patients corresponding to 38.3% (349/911) of patients. Most of these interactions involved CNS agents (28.5%) with mainly anxiolytic drugs, followed by cardiovascular (23.4%), hormone (13%), and anti-infective (7.7%) agents (Table 2). The ARV medications involved were mainly PIs (62.8%), followed by NNRTIs (21.2%), NRTIs (6.9%), CCR5 receptor antagonists (5.4%), and INIs (3.8%). The most common individual orange flag interactions were ritonavir with levothyroxin, emtricitabine with trimethoprim/sulfamethoxazone, ritonavir with alprazolam, and ritonavir with rosuvastatin.
Drug Interactions in 2016
A total of 85 red flag interactions were found in 45 patients, meaning that 4.3% (45/1038) of patients had at least 1 red flag interaction, with a maximum of 6 contraindicated interactions per patient. The non-ARV medication classes involved included cardiovascular (41.2%), respiratory (28.2%), gastrointestinal (20%), ENT (5.9%), CNS (2.4%), osteo-articular (1.2%), and hemostasis (1.2%) drugs (Table 2). Regarding ARV medications, PIs were by far the most frequently involved ARV (70.7% of red flag interactions), followed by NNRTIs (11.8%) (Table 2). Overall, red flag interactions arose mainly between ritonavir with lercanidipine and budesonide.
Concerning orange flag interactions, 1137 interactions were identified in 396 patients in 2016. Thereby, 38.1% (396/1038) of patients had at least 1 orange flag interaction, which corresponds to 48.0% (396/810) of patients with at least 1 comedication. There were 1, 2, and 3 interactions in 38.6%, 21.7%, and 11.6% of cases, respectively, with a maximum of 34 interactions per patient (Table 1). The non-ARV medications involved included mainly cardiovascular agents (23.9%) and CNS agents (22.4%), followed by hormone drugs (13.7%) and dietary supplements (13.5%) (Table 2). Regarding ARV medications, these interactions most frequently involved PIs (38.5%), followed by NNRTIs (21.9%) and INIs (14%) (Table 2). Overall, individual orange flag interactions occurred mainly between dolutegravir and metformine followed by ritonavir and levothyroxine and dolutegravir and calcium. Calcium alone, with cholecalciferol or in multivitamin preparations, represented the most common drug of the dietary supplements therapeutic class.
Predictors of Drug Interactions in 2016
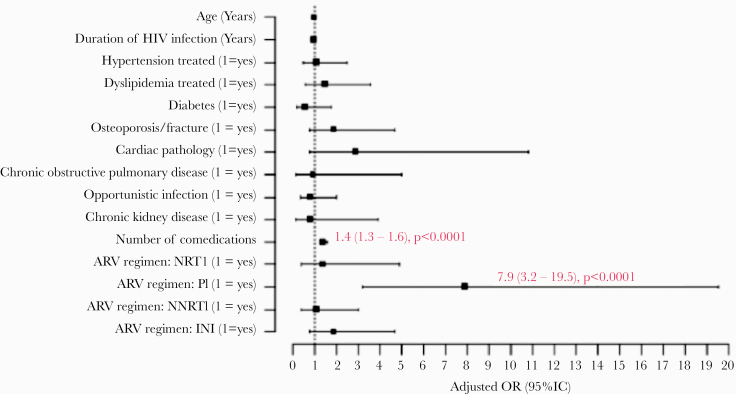
In 2016, multiple logistic regression analysis identified the use of PIs (OR, 7.5; 95% CI, 4.5–12.5), NNRTIs (OR, 2.4; 95% CI, 1.5–4.0) or INIs (OR, 1.6; 95% CI, 1.03–2.6), the duration of HIV infection (OR, 1.03; 95% CI, 1.001–1.05), the occurrence of osteoporosis or fracture (OR, 1.9; 95% CI, 1.03–3.6), and the number of non-ARV comedications (OR, 1.8; 95% CI, 1.6–2.0) as independent risk factors for orange flag interactions (Figure 1B). Among patients with red flag interactions, only the use of PIs (OR, 7.9; 95% CI, 3.2–19.5) and the number of non-ARV comedications (OR, 1.4; 95% CI, 1.3–1.6) were noted as independent risk factors (Figure 1A). The mode of HIV transmission had no impact on the risk of red flag (P = .58) or orange flag (P = .93) interactions.
Figure 1.
Independent risk factors for drug interactions in 2016. A, Red flag interactions. B, Orange flag interactions. Abbreviations: ARV, antiretroviral; INI, integrase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; OR, odds ratio; PI, protease inhibitor.
Analysis of Individuals Followed Both in 2012 and 2016
A total of 729 patients were followed during both 2012 and 2016 (Supplementary Table 3). In this population, 63 red flag and 915 orange flag interactions were reported in 2012 compared with 69 red flag and 940 orange flag interactions in 2016 (Table 1).
The rate of patients with at least 1 drug interaction was similar between 2012 and 2016: 4.7% vs 4.8% of patients for red flag interactions and 41.1% vs 42.5% for orange flag interactions (Table 1).
Among patients with red flag interactions, in 2012 the non-ARV medications involved were mainly gastrointestinal drugs, while the non-ARV medications involved were mostly cardiovascular drugs in 2016 (Table 4). About 30% of the patients with red flag interactions in both 2012 and 2016 presented at least 1 drug interaction, which could have reduced ARV plasma concentrations and thereby might have compromised the ARV’s efficacy. The implicated drug interactions were PPI with PI (atazanavir) or NNRTI (rilpivirine) and carbamazepine with NNRTI (etravirine). However, we found no correlation with the occurrence of a detectable viral load.
Table 4.
Comparison of the Proportion of Patients With at Least 1 Drug Interaction by Therapeutic Class Between 2012 and 2016 (n = 729), McNemar Test
| Non-ARV Treatment Class (CBIP) | 2012, No. (%) | 2016, No. (%) | P Value |
|---|---|---|---|
| Orange flag interactions | |||
| Ear, nose, and throat drugs | 5 (0.5) | 3 (0.4) | .71 |
| Osteoarticular drugs | 19 (2.6) | 17 (2.3) | .67 |
| Cardiovascular drugs | 109 (15) | 92 (12.6) | .081 |
| Gastrointestinal drugs | 6 (0.8) | 6 (0.8) | 1.00 |
| Respiratory drugs | 10 (3.2) | 6 (0.8) | .21 |
| Hemostasis drugs | 23 (3.2) | 25 (3.4) | .70 |
| CNS agents | 57 (7.8) | 47 (6.4) | .20 |
| Analgesics | 20 (2.7) | 20 (2.7) | 1.00 |
| Obstetrics & gynecology | 11 (1.5) | 13 (1.8) | .62 |
| Immunity | 7 (1.0) | 7 (1.0) | 1.00 |
| Anti-infectives | 40 (5.5) | 21 (2.9) | .0056 |
| Antineoplastic agents | 1 (0.1) | 2 (0.3) | .32 |
| Others drugs | 2 (0.3) | 4 (0.6) | .16 |
| Genitourinary drugs | 1 (0.1) | 1 (0.1) | 1.0 |
| Dietary supplements | 15 (2.1) | 49 (6.7) | <.0001 |
| Hormone drugs | 39 (5.4) | 46 (6.3) | .32 |
| Red flag interactions | |||
| Ear, nose, and throat drugs | 4 (0.5) | 2 (0.3) | .32 |
| Osteoarticular drugs | 2 (0.3) | 1 (0.1) | .32 |
| Cardiovascular drugs | 9 (1.2) | 16 (2.2) | .090 |
| Gastrointestinal drugs | 13 (1.8) | 11 (1.5) | .62 |
| Respiratory drugs | 8 (1.1) | 7 (1.0) | .71 |
| Hemostasis drugs | 0 (0.0) | 1 (0.1) | .32 |
| CNS agents | 0 (0.0) | 2 (0.3) | .16 |
Abbreviations: ARV, antiretroviral; CBIP, Belgian Center for Pharmacotherapeutic Information; CNS, central nervous system.
Orange flag interactions involving dietary supplements were found in 6.7% (49/729) of the patients in 2016, whereas only 2.1% (15/729) were found in 2012 (P < .0001) (Table 4). On the other hand, the rate of patients with orange flag interactions related to anti-infective agents showed a significant decrease (5.5% vs 2.9% of patients; P = .0056) (Table 3).
Table 3.
Comparison of the Proportion of Patients With at Least 1 Drug Interaction by Type of ARV Treatment Between 2012 and 2016 (n = 729), McNemar Test
| ARV treatment | 2012, No. (%) | 2016, No. (%) | P Value |
|---|---|---|---|
| Orange flag interactions | |||
| Atazanavir | 84 (11.5) | 33 (4.5) | <.0001 |
| Darunavir | 37 (5.1) | 57 (7.8) | .0055 |
| Ritonavir | 156 (21.4) | 86 (11.8) | <.0001 |
| Lopinavir | 46 (6.3) | 11 (1.5) | <.0001 |
| Fosamprenavir | 9 (1.2) | 1 (0.1) | .0047 |
| Darunavir/cobicistat | 0 (0.0) | 20 (2.7) | - |
| Saquinavir | 1 (0.1) | 0 (0.0) | - |
| Etravirine | 20 (2.7) | 13 (1.8) | .035 |
| Efavirenz | 49 (6.7) | 37 (5.1) | .077 |
| Rilpivirine | 0 (0.0) | 11 (1.5) | - |
| Nevirapine | 41 (5.6) | 47 (6.5) | .30 |
| Abacavir | 3 (0.4) | 7 (1.0) | .16 |
| Emtricitabine | 26 (3.6) | 7 (1.0) | .0001 |
| Lamivudine | 18 (2.5) | 13 (1.8) | .32 |
| Tenofovir | 44 (6.0) | 36 (4.9) | .24 |
| Zidovudine | 8 (1.1) | 1 (0.1) | .020 |
| Maraviroc | 4 (0.6) | 6 (0.8) | .16 |
| Dolutegravir | 0 (0.0) | 68 (9.3) | - |
| Raltegravir | 18 (2.5) | 9 (1.2) | .020 |
| Emtricitabine/tenofovir alafenamide fumarate/elvitegravir/cobicistat | 0 (0.0) | 24 (3.3) | - |
| Emtricitabine/tenofovir disoproxil fumarate/elvitegravir/cobicistat | 0 (0.0) | 41 (5.2) | - |
| Red flag interactions | |||
| Atazanavir | 19 (2.6) | 2 (0.3) | <.0001 |
| Darunavir | 3 (0.4) | 2 (0.3) | .66 |
| Ritonavir | 26 (3.6) | 2 (0.3) | <.0001 |
| Lopinavir | 8 (1.1) | 2 (0.3) | .058 |
| Darunavir/cobicistat | 0 (0.0) | 2 (0.3) | - |
| Etravirine | 0 (0.0) | 2 (0.3) | - |
| Rilpivirine | 1 (0.1) | 2 (0.3) | .56 |
| Emtricitabine/tenofovir alafenamide fumarate/elvitegravir/cobicistat | 0 (0.0) | 2 (0.3) | - |
Abbreviation: ARV, antiretroviral.
DISCUSSION
PDDIs between ARVs and non-ARVs in HIV-infected individuals were common in our study, both in 2012 and in 2016. The percentages of individuals receiving medications causing at least 1 red flag interaction were similar over time: 4.7% in 2012 compared with 4.8% in 2016. However, these alarmingly high rates are in the line with previous studies showing results ranging from 1% to 7% [7, 11–15]. The same evolution was observed with orange flag interactions, represented by 41.1% of patients with at least 1 drug interaction in 2012 and 42.5% in 2016.
All orange flag interactions are not equal, but they can usually be managed by dosage or timing administration adjustment or close monitoring. On the other hand, red flag interactions generally require a major shift in treatment [10].
Recognition of the risk factors for PPDI may help clinicians prevent it. Risk factors that were independently associated with red flag interactions included the number of non-ARV comedications, as expected, and PI intake. Logistic regression analysis showed that the use of PIs was an important independent risk factor for both orange flag and red flag interactions, in concordance with the literature [7, 11–15]. We observed a significant decrease of drug interactions involving PIs from 2012 to 2016. This is easily explained by the lower use of PIs in 2016. However, PIs are still drugs we must monitor because individuals on PIs were at higher risk (6.7 times) of having a red flag interaction in 2016. Ritonavir, especially, may be involved in interactions with numerous medications because of its potent inhibition of CYP3A4 and P-glycoprotein and potent induction of glucuronyl transferases, CYPP1A2, CYP2B6, CYP2C9, and CYP2C19 [16]. Coadministration of PIs with certain non-ARV drugs may increase the non-ARV concentration in plasma, resulting in adverse clinical outcomes. For example, PIs with inhaled corticosteroids, calcium channel blockers, and statins may lead to potential systemic corticosteroid effects including Cushing’s syndrome, arterial hypotension, and myopathy with rhabdomyolysis, respectively.
We also reported some red flag interactions that could lead to a decrease in ARV plasma concentrations. These interactions concerned some PIs or rilpivirine (NNRTI) with a PPI, which are frequently used treatments, and etravirine (NNRTI) with carbamazepine.
The non-ARV medications mainly involved in red flag interactions in 2012 were gastrointestinal drugs, while these were mostly cardiovascular drugs in 2016 [17–19]. This can be explained by a specific shift in the use of PIs over the time. In fact, between 2012 and 2016, we reported a significant decrease of orange flag interactions with atazanavir, which interacts in particular with PPIs, and a significant increase of orange flag interactions with darunavir, which had no interaction with PPIs.
In many studies, the use of an INI is associated with a decreased risk of drug interactions [14–16, 20, 21]. In the presented study, we reported a few red flag interactions involving INI (elvitegravir boosted) in 2016. This is explained by the fact that elvitegravir is metabolized predominantly by CYP450 enzymes with a minor pathway involving UGT1A1/3-glucuronidation and requires boosting with cobicistat (an inhibitor of the CYP3A isozyme family of proteins) to reach therapeutic concentrations [22]. As such, elvitegravir/cobicistat has a drug interaction profile similar to ritonavir-boosted PI and was thus the only INI reported to cause a red flag interaction in our study.
Our study showed a high rate of orange flag interactions from 2012 to 2016. These orange flag interactions involved disproportionally more cardiovascular drugs than other therapeutic classes, followed by CNS agents, as in the Swiss HIV Cohort Study [7]. The second most frequent therapeutic class associated with orange flag interactions was dietary supplements in 2016, which showed a significant increase compared with 2012. These interactions occurred mainly between INIs and calcium. Calcium and cholecalciferol supplements are commonly used in HIV-infected individuals because of the increased risk of osteoporosis directly linked to the disease and some ARVs (eg, tenofovir fumarate) [23]. Moreover, HIV-infected individuals face the significant challenge of coping with an incurable disease, leading to the frequent use of complementary and alternative medicine (CAM) as a way to manage their illness, with up to 60% of HIV-infected individuals using CAMs according to the literature [11, 24–27]. Indeed, divalent and trivalent metal cations (as calcium supplements) in co-administration with INI can lead to chelation-type drug interactions. Many oral multivitamin supplements also contain polyvalent cations. This drug interaction may result in reduced solubility and, consequently, a reduced oral absorption of INIs. Several studies have found clinically significant effects of concomitant administration of INIs with mineral supplements or antacids (that contain aluminium and magnesium) [28–31]. If known, these interactions could be managed easily by administering the INI 2 hours before or 6 hours after taking mineral supplements or with a meal [31].
Unlike elvitegravir, raltegravir and dolutegravir are options to consider in order to decrease the risk of red flag interactions with non-ARV medication because of their different metabolism. Raltegravir does not exhibit any effects on the CYP450 system, and dolutegravir is predominantly metabolized by UGTA1A-mediated glucuronidation with a minor pathway involving CYP450 enzymes [32]. However, our study underlines their potential for chelation-type drug interactions. Other studies have often underestimated the number of orange flag interactions with INI because dietary supplements were not or were incompletely reported [14]. In fact, physicians are often unaware of their patient’s use of CAM. The increase of interactions involving dietary supplements reported in 2016 compared with 2012 could be explained by an improvement of the listing of these drugs in medical reports and probably a greater attention given to dietary supplements in 2016.
Some limitations of our study should be acknowledged. First, drug interactions could be underestimated because we did not include interactions between ARV medications among themselves, and we probably did not report all over-the-counter drugs like herbal therapies, which are not always reported in the medical report. Furthermore, we have mentioned some drug interactions without considering if the dose or timing of administration adjustment had or had not already been carried out.
In response to these alarming rates of drug interactions, we developed a flag indicator in our medical program to inform the clinicians of potential drug interactions with an ARV. This interaction alert program might help clinicians prevent drug interactions and improve patient management in our hospital.
CONCLUSIONS
In conclusion, drug interactions are still common in the HIV-infected population. Indeed, although the type of interactions has changed overtime, with different ARV and comedications involved, the number of PDDIs remains an important concern. This is likely going to get worse as the HIV-infected population ages, implying an increase in use of comedications. Our study also highlights the importance of reporting and considering complementary and alternative medicine as a potential source of PDDIs, notably with INIs, which now often constitute the basis of therapy.
Following the alarming results of this study, we have implemented an informatics program to detect PDDIs. Together, thanks to an optimization of comedications report, the number of PPDIs will hopefully decrease in the coming years.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Jean-Baptiste Giot for his participation in discussions. We thank the Fonds Leon Fredericq.
Financial support. Gilles Darcis is postdoctoral clinical master specialist for the Belgian National Fund for Scientific Research (FNRS).
Potential conflicts of interest. G.D. and M.M. have served as a consultant, lecturer, or member of an advisory board for and have received research grants from Gilead, ViiV Healthcare, Janssen, and MSD. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. The design of the work has been approved by local ethical committees.
References
- 1. Sasse A, Deblonde J, Jamine D, et al. Épidémiologie du sida et de l’infection à VIH en Belgique. Sciensano 2017.
- 2. Gallant J, Hsue PY, Shreay S, et al. Comorbidities among US patients with prevalent HIV infection — a trend analysis. J Infect Dis 2017; 216:1525–33. [DOI] [PubMed] [Google Scholar]
- 3. Smit M, Brinkman K, Geerlings S, et al. ; ATHENA observational cohort Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gunter J, Callens S, De Wit S, et al. Prevalence of non-infectious comorbidities in the HIV-positive population in Belgium: a multicenter, retrospective study. Acta Clin Belg 2018; 73:50–3. [DOI] [PubMed] [Google Scholar]
- 5. Gimeno-Gracia M, Crusells-Canales MJ, Armesto-Gómez FJ, et al. Polypharmacy in older adults with human immunodeficiency virus infection compared with the general population. Clin Interv Aging 2016; 11:1149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Halloran MO, Boyle C, Kehoe B, et al. Polypharmacy and drug–drug interactions in older and younger people living with HIV: the POPPY study. Antivir Ther 2019; 24:193–201. [DOI] [PubMed] [Google Scholar]
- 7. Marzolini C, Elzi L, Gibbons S, et al. ; Swiss HIV Cohort Study Prevalence of comedications and effect of potential drug-drug interactions in the Swiss HIV Cohort Study. Antivir Ther 2010; 15:413–23. [DOI] [PubMed] [Google Scholar]
- 8. Alam C, Whyte-Allman SK, Omeragic A, Bendayan R. Role and modulation of drug transporters in HIV-1 therapy. Adv Drug Deliv Rev 2016; 103: 121–43. [DOI] [PubMed] [Google Scholar]
- 9. Thierry C. Centre Belge d’Information Pharmacothérapeutique (CBIP) Available at: https://www.cbip.be/fr/start. Accessed 1 January 2019.
- 10. Liverpool HIV Pharmacology Group. HIV drug interactions 2017 Available at: https://www.hiv-druginteractions.org. Accessed 1 January 2019.
- 11. Holtzman C, Armon C, Tedaldi E, et al. ; and the HOPS Investigators Polypharmacy and risk of antiretroviral drug interactions among the aging HIV-infected population. J Gen Intern Med 2013; 28:1302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller CD, El-Kholi R, Faragon JJ, Lodise TP. Prevalence and risk factors for clinically significant drug interactions with antiretroviral therapy. Pharmacotherapy 2007; 27:1379–86. [DOI] [PubMed] [Google Scholar]
- 13. Darque A, Enel P, Ravaux I, et al. Drug interactions in elderly individuals with the human immunodeficiency virus. J Am Geriatr Soc 2012; 60:382–4. [DOI] [PubMed] [Google Scholar]
- 14. Baecke C, Gyssens IC, Decoutere L, et al. Prevalence of drug-drug interactions in the era of HIV integrase inhibitors: a retrospective clinical study. Neth J Med 2017; 75:235–40. [PubMed] [Google Scholar]
- 15. Tseng A, Szadkowski L, Walmsley S, et al. Association of age with polypharmacy and risk of drug interactions with antiretroviral medications in HIV-positive patients. Ann Pharmacother 2013; 47:1429–39. [DOI] [PubMed] [Google Scholar]
- 16. Patel N, Abdelsayed S, Veve M, Miller CD. Predictors of clinically significant drug-drug interactions among patients treated with nonnucleoside reverse transcriptase inhibitor-, protease inhibitor-, and raltegravir-based antiretroviral regimens. Ann Pharmacother 2011; 45:317–24. [DOI] [PubMed] [Google Scholar]
- 17. Marzolini C, Back D, Weber R, et al. ; Swiss HIV Cohort Study Members Ageing with HIV: medication use and risk for potential drug-drug interactions. J Antimicrob Chemother 2011; 66:2107–11. [DOI] [PubMed] [Google Scholar]
- 18. Yiu P, Nguyen NN, Holodniy M. Clinically significant drug interactions in younger and older human immunodeficiency virus-positive patients receiving antiretroviral therapy. Pharmacotherapy 2011; 31:480–9. [DOI] [PubMed] [Google Scholar]
- 19. The Data Collection on Adverse Events of anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003; 349:1993–2003. [DOI] [PubMed] [Google Scholar]
- 20. Krikorian SA, Rudorf DC. Drug-drug interactions and HIV therapy: what should pharmacists know? J Pharm Pract 2005; 18:278–94. [Google Scholar]
- 21. Molas E, Luque S, Retamero A, et al. Frequency and severity of potential drug interactions in a cohort of HIV-infected patients identified through a multidisciplinary team. HIV Clin Trials 2018; 19:1–7. [DOI] [PubMed] [Google Scholar]
- 22. Unger NR, Worley MV, Kisgen JJ, et al. Elvitegravir for the treatment of HIV. Expert Opin Pharmacother 2016; 17:2359–70. [DOI] [PubMed] [Google Scholar]
- 23. Ahmad AN, Ahmad SN, Ahmad N. HIV infection and bone abnormalities. Open Orthop J 2017; 11:777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jalloh MA, Gregory PJ, Hein D, et al. Dietary supplement interactions with antiretrovirals: a systematic review. Int J STD AIDS 2017; 28:4–15. [DOI] [PubMed] [Google Scholar]
- 25. Colebunders R, Dreezen C, Florence E, et al. ; Eurosupport Study Group The use of complementary and alternative medicine by persons with HIV infection in Europe. Int J STD AIDS 2003; 14:672–4. [DOI] [PubMed] [Google Scholar]
- 26. Littlewood RA, Vanable PA. Complementary and alternative medicine use among HIV-positive people: research synthesis and implications for HIV care. AIDS Care 2008; 20:1002–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wootton JC, Sparber A. Surveys of complementary and alternative medicine: part III. Use of alternative and complementary therapies for HIV/AIDS. J Altern Complement Med 2001; 7:371–7. [DOI] [PubMed] [Google Scholar]
- 28. Ramanathan S, Shen G, Hinkle J, et al. Pharmacokinetic evaluation of drug interactions with ritonavir-boosted HIV integrase inhibitor GS-9137 (elvitegravir) and acid reducing agents [Abstract 69]. In: Abstracts ofthe Eighth International Workshop on Clinical Pharmacology of HIV Therapy, Budapest, Hungary Utrecht, the Netherlands: Virology Education; April 16–18, 2007. [Google Scholar]
- 29. Kiser JJ, Bumpass JB, Meditz AL, et al. Effect of antacids on the pharmacokinetics of raltegravir in human immunodeficiency virus-seronegative volunteers. Antimicrob Agents Chemother 2010; 54:4999–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grießinger JA, Hauptstein S, Laffleur F, et al. Evaluation of the impact of multivalent metal ions on the permeation behavior of dolutegravir sodium. Drug Dev Ind Pharm 2016; 42:1118–26. [DOI] [PubMed] [Google Scholar]
- 31. Song I, Borland J, Arya N, et al. Pharmacokinetics of dolutegravir when administered with mineral supplements in healthy adult subjects. J Clin Pharmacol 2015; 55:490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kassahun K, McIntosh I, Cui D, et al. Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the human immunodeficiency virus 1 integrase enzyme. Drug Metab Dispos 2007; 35:1657–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.