Abstract
Background
Antimicrobial resistance (AMR) is a serious threat to humanity. This paper describes the French efforts made since 2001 and presents data on antimicrobial consumption (AC) and AMR.
Methods
We gathered all data on AC and AMR recorded since 2001 from different national agencies, transferred on a regular basis to standardized European data on AC and resistance in both humans and animals.
Results
After a large information campaign implemented in France from 2001 to 2005 in humans, AC in the community decreased significantly (18% to 34% according to the calculation method used). It remained at the same level from 2005 to 2010 and increased again from 2010 to 2018 (8%). Contrasting results were observed for AMR. The resistance of Staphylococcus aureus decreased significantly. For gram-negative bacilli, the results were variable according to the microorganism. The resistance of Enterobacteriaceae to third-generation cephalosporins increased, remaining moderate for Escherichia coli (12% in 2017) but reaching 35% in the same year for Klebsiella pneumoniae. Resistance to carbapenems in those 2 microorganisms remained below 1%. Both global AC and resistance to most antibiotics decreased significantly in animals.
Conclusions
Antibiotic consumption decreased significantly in France after a large public campaign from 2001 to 2005, but this positive effect was temporary. The effect on AMR varied according to the specific microorganism: The effect was very impressive for gram-positive cocci, variable for gram-negative bacilli, and moderate for E. coli, but that for K. pneumoniae was of concern. The consumption of and resistance to antibiotics decreased significantly in animals.
Keywords: antibiotics, antibiotic consumption, antibiotic resistance, bacterial transmission, gram-negative bacilli, gram-positive cocci
Antimicrobial resistance (AMR) has sharply increased in the past 15 years and has become a major concern worldwide [1–4]. In France, 12% of Escherichia coli strains isolated from blood cultures are now resistant to third-generation cephalosporins, while in several countries, the resistance rate for this microorganism may reach 50% to 80% globally [2]. Not surprisingly, carbapenem-resistant Enterobacteriaceae have emerged over the past 10 years as a growing threat worldwide, reaching ≥50% for gram-negative bacteria in several countries [2, 5]. Treatment of infections due to those highly resistant bacteria requires, in particular when carbapenemases are present, complex combinations of old and sometimes toxic drugs, such as colistin. Unfortunately, resistance to colistin has recently emerged [6], and bacteria resistant to all available classes of antibiotics are now encountered in some countries. This has ignited the fear of returning to a “pre-antibiotic era.”
In France, antimicrobial consumption (AC) has been high for many decades in both hospitals and the community. Consumption within the community is 30% higher than the mean European rate and 2–3 times higher than the rates in countries with very low consumption and resistance levels, such as Scandinavian countries [7].
There are very few European publications that provide in-depth descriptions of long-term national programs aimed at reducing AMR and the results of those actions for both AC and AMR over a long period of time (almost 20 years) in humans and animals [8–10]. A strong national program addressing AC and resistance as well as the cross-transmission of multidrug-resistant bacteria in humans has been implemented in France within the last 25 years, and a similar program for animals was initiated in 2012. This article describes the French anti-AMR program from 2000 to 2017 and analyzes its results regarding AC and AMR in hospitals and the community in humans and in animals.
METHODS
We gathered all the data on AC and AMR in France since 2001. The data on AC were collected by the French Agence Nationale de Sécurité Sanitaire for all French structures. They are transferred on a yearly basis to the European AC Network (ESAC-Net) coordinated by the European Centre for Disease Control and Prevention (ECDC). The data on antibiotic resistance were collected through a large French network, the National Observatory of the Epidemiology of the Bacterial Resistance to Antibiotics (ONERBA), gathering data on resistance to antibiotics throughout the whole country, provided by various networks in both hospitals and the community. They were then transferred to Santé Publique France and ultimately to the European Antimicrobial Resistance Surveillance Network (EARS-Net) at the ECDC [2]. The methodology to assess and treat all data used in this study were checked and harmonized by the different agencies. All the information from French public agencies and from ECDC networks is publicly available. More details on the methodology are available at the sites of these entities [2, 7].
The data from animals were collected through the large national “Résapath” network, which is part of the French Agence Nationale de Sécurité Sanitaire de l’Alimentation. The bacteria in this network were sampled in sick animals. The French data were included in the European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) network.
RESULTS
French Plans for Addressing Antimicrobial Resistance Over the Years
AMR and AC surveillance programs have been in place in France for a long time [11]. A comprehensive set of plans was implemented for humans beginning in 2001 and for animal medicine beginning in 2012.
A. The first national plans for tackling antibiotic resistance in human medicine
Three national plans for human medicine were successively launched from 2001 to 2005 (“Antibiotics Are Not Automatic”), 2007 to 2010, and 2011 to 2016 by the Ministry of Health together with the French National Health Insurance System (Figure 1). Measures were directed toward the public, general practitioners (GPs), and hospitals. Actions included television campaigns targeting unnecessary antibiotic prescriptions for viral respiratory tract infections. Rapid strep tests were also promoted to avoid unnecessary use of antibiotics for viral tonsillitis/pharyngitis. Other interventions targeted antibiotic use in hospitals, such as the designation of a local antibiotic committee in each hospital and of a dedicated physician for antibiotic stewardship.
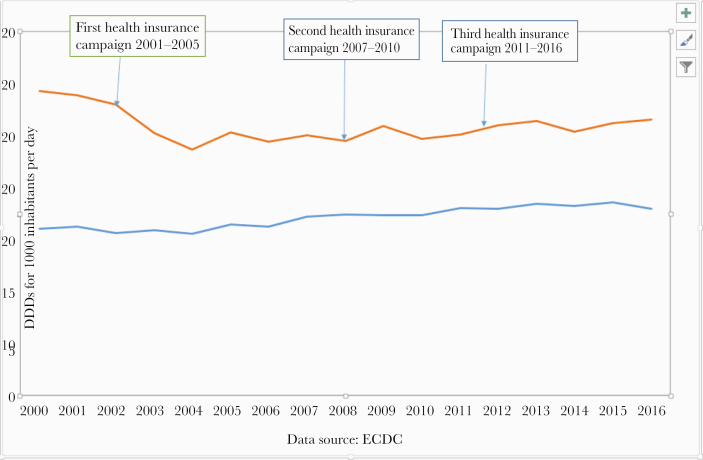
Figure 1.
Comparison over time of the French and European mean antibiotic consumption rates in the community, presented in defined daily doses per 1000 patient-days. The arrows show the start of the 3 French programs. Abbreviations: DDD, defined daily dose; ECDC, European Centre for Disease Control and Prevention.
Mandatory public reporting of annual quality indicators for infection prevention was implemented in France in 2004. Specific indicators devoted to infection prevention (eg, volume of hand-rub alcoholic solutions used) and antibiotic stewardship as well as prevention of multidrug-resistant (MDR) transmission became mandatory for every hospital and were made publicly available in 2006. In 2012, the French National Health Insurance system introduced a financial incentive for performance process, called Pay for Performance (PFP) for antibiotic usage in general practice.
B. The national action plan to reduce antibiotic resistance in veterinary medicine
In line with the “One World, One Health” concept and the policy defined by the European parliament, the European Commission, the World Health Organization (WHO), the Food and Agriculture Organization, and the World Organization for Animal Health, a 5-year national plan for the veterinary sector (ECOANTIBIO) was launched in 2012 under the auspices of the Ministry of Agriculture [12]. This plan operates in close cooperation with several other ministries and agencies, such as the risk assessment agency, and all stakeholders (particularly breeders and veterinarians).
The strategic domains of the ECOANTIBIO plan include reducing antibiotic use in veterinary medicine, particularly use that plays a critical role in humans, and preserving therapeutic options on a sustainable basis, given that the prospects for development of new antibiotics are limited in veterinary medicine. The 4 key objectives of this plan are as follows: (a) raising awareness in all stakeholders, (b) improving livestock farming practices (ie, hygiene, farm building maintenance, animal health monitoring), (c) reinforcing partnerships between prescribers and animal owners, and (d) reducing global antibiotic use by 25% over a period of 5 years (2012–2017).
Moving Forward After the Initial Plans
Despite some successes obtained over the period covered by the above plans, the continued increase in multidrug-resistant Enterobacteriaceae remained worrisome. More intensive actions had to be taken, combining infection control measures and antibiotic stewardship. Two major steps were then taken in 2015.
A. Renewing the commitment to tackle AMR: the Task Force for Antibiotic Preservation (2015)
Deeply concerned by the antibiotic resistance problem and the paucity of new antibiotics, the Minister of Health (Mme Marisol Touraine) implemented a task force in January 2015 whose mandate was to propose a limited number of well-focused innovative but pragmatic recommendations to fight against antibiotic resistance in order to decrease antibiotic consumption by 25% within 5 years and to facilitate research and innovation. Five topics were assigned to distinct working groups: morbidity and mortality attributable to antibiotic resistance, antibiotic stewardship, communication and educational issues, research and innovation, and finally antibiotic resistance in the environment. Many propositions were made to the minister. Most of them were implemented.
B. The Inter-ministerial Committee on Health; the “One Health” approach in motion
In 2014, the Prime Minister set up the Inter-ministerial Health Committee (IHC) with the aim of fostering dialogue between relevant ministries and agencies on health-related issues [13]. The IHC had to formulate a roadmap for the fight against antimicrobial resistance by September 2016, which addresses the issue from an interministerial viewpoint. A steering committee was established to prepare this roadmap covering the following domains: surveillance and indicators, research and innovation, appropriate use, tools and guidelines, training, communication, and raising awareness [14]. This steering committee was coordinated by a ministerial delegate for AMR. The objectives of this national plan are available on the websites of the Ministries of Health and Agriculture [12, 13].
Results of the French Programs Addressing AMR
A. Results of the human plan
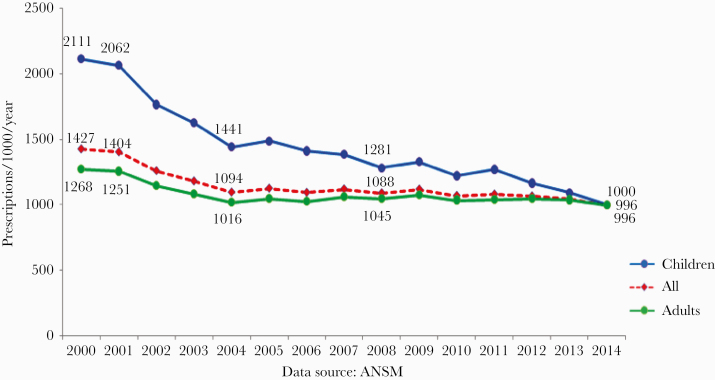
As seen in Figure 1, the initial notable decrease in antibiotic consumption in the community from 2001 to 2005 in the community (18% to 35% according to the method used) was followed by a plateau between 2005 and 2010 and an 8% increase between 2010 and 2015 [15]. However, consumption in 2016 remained 12.6% lower than that in 2000. Of note, the decrease in AC was far higher in children than in adults, in whom the effect was rather limited (Figure 2).
Figure 2.
Antibiotic consumption during and after the first and second French national plans, in both adults and children. Abbreviation: ANSM, Agence Nationale de Sécurité Sanitaire.
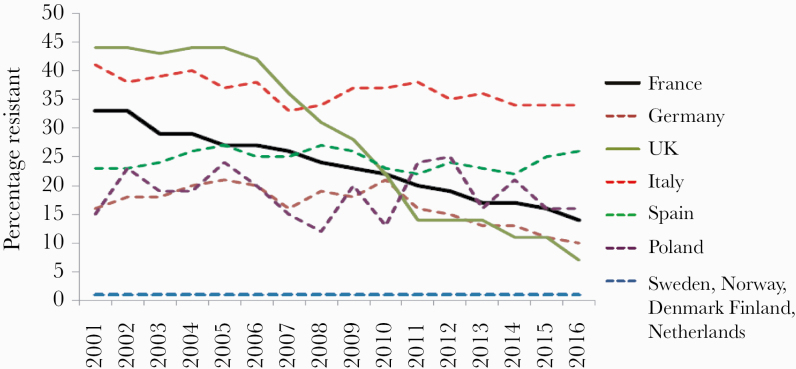
In the hospital sector, the situation has been somewhat different. During the past 10 years, the AC appears to have leveled off. AC in France after the campaigns remains far higher than the mean European consumption rate (Figure 1). Considering the rates of resistance to antibiotics, the results vary according to the microorganisms (Figures 3 and 4). For gram-positive cocci, the results were the following: (a) a 58% decrease between 2000 and 2016 in the proportion of MRSA among Staphylococcus aureus isolated from blood cultures (33% to 14%) [2, 16] and an incidence density decrease from 0.68 to 0.26 cases for 1000 hospital days. Even if the prevalence of MRSA remains high in France compared with Northern European countries, the decrease recorded in France, as well as the huge decrease achieved in the United Kingdom, contrasts with the evolution in other European countries with large populations (Figure 3). (b) The rate of resistance to vancomycin in Enterococcus faecium has remained very low since 2002 (≤1%), as in the Netherlands and Sweden, but contrasting with the trends in other countries. (c) The rate of resistance to penicillin in pneumococci decreased by 46% since 2000. However, this rate remains extremely high compared with other European countries.
Figure 3.
Evolution over time of the percentage of resistance to methicillin in Staphylococcus aureus bacteremia in France, Germany, the United Kingdom, Italy, Spain, Poland, Sweden, Norway, Denmark, Finland, and the Netherlands (European Antimicrobial Resistance Surveillance Network from the European Centre for Disease Control and Prevention).
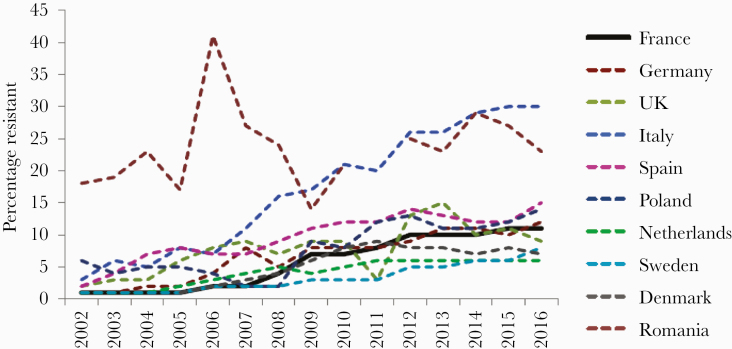
Figure 4.
Evolution over time of the resistance to third-generation cephalosporins in Escherichia coli bacteremia in France, Germany, the United Kingdom, Italy, Spain, Poland, the Netherlands, Sweden, Denmark, and Romania (European Antimicrobial Resistance Surveillance Network from the European Centre for Disease Control and Prevention).
For gram-negative bacilli, the results are contrasting [2]. Rates of resistance to imipenem in Pseudomonas aeruginosa remained stable at around 20%, while resistance to ceftazidime decreased slightly (from 18% to 12%). In both cases, the French resistance rates were very close to the population-weighted mean rates of the European Union (EU-PWRR). Resistance to carbapenems in Acinetobacter baumannii remained low (around 6%) and far lower than that of EU-PWRR (35%). Resistance of E. coli to third-generation cephalosporins, mainly through ESBL, increased (from 2% to 12%) from 2001 to 2013 (Figure 4) [2] and has remained stable since then.
These percentages are equal to those of EU-PWRR and far lower than the rates observed in several EU countries (Figure 4). In contrast, resistance of K. pneumoniae to third-generation cephalosporins is a serious issue in France, as this rate increased from 5% to 30% from 2005 to 2013. Although the resistance level to this microorganism seems to have stabilized since 2013, it is slightly higher than that of EU-PWRR (26%). Incidence densities for E. coli increased from 0.02 in 2002 to 0.38 in 2015 and from 0.31 in 2012 to 0.41 in 2017. Incidence densities for K. pneumoniae increased from 0.02 in 2002 to 0.17 in 2015, and then from 0.11 in 2012 to 0.18 in 2017. Finally, the resistance of both E. coli and K. pneumoniae to carbapenems remains below 1%. These results, in particular the low rate of resistance to vancomycin in Enterococci and of Enterobacteriaceae to carbapenems, are largely due to strong recommendations set up in 2006 and recalled in 2013 by the French health authorities to specifically limit the spread of “extensively resistant bacteria.” The measures included a screening of multidrug-resistant bacteria in intensive care units and drastic isolation procedures, often with a cohorting of infected or colonized patients. These encouraging results have been pinpointed in the recent rapid risk assessment “Carbapenem-Resistant Enterobacteriaceae” published by the ECDC [17].
C. Results of the first animal plan (ECOANTIBIO 2012–2016)
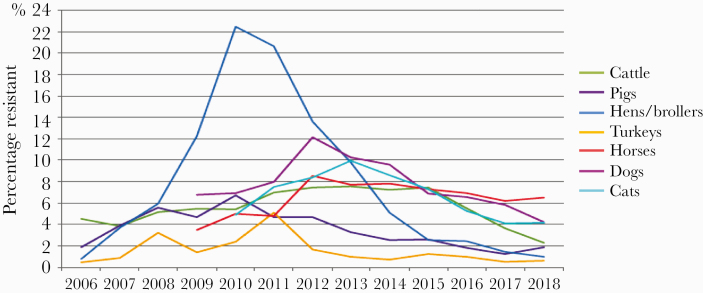
From 2011 to 2016, overall AC of antibiotics in animals decreased by 36.6% (Figure 5). The decline in exposure to antimicrobials was observed for all species compared with results recorded in 2011 (cattle: –24.3%; pigs: –41.5%; poultry: –42.8%; rabbits: 37.6%; cats and dogs: –19.4%). Exposure to new-generation cephalosporins and fluoroquinolones decreased by 81.3% in 2016 and 74.9% in 2013, respectively (all species combined). Thus, the initial quantitative and qualitative objectives have been achieved and even exceeded. The decline in exposure to antimicrobials has been accompanied by a decline or stabilization in resistance to the vast majority of antimicrobials tested (Figure 5). Resistance to broad-spectrum cephalosporins is mainly observed for E. coli. In 2016, the highest rate of resistance to ceftiofur in clinical E. coli isolates of animal origin in France was ~5%–7%, and resistance to ceftiofur was found in veal calves, cats, dogs, and horses.
Figure 5.
Evolution of the percentage of Escherichia coli isolates resistant (I + R) to ceftiofur in cattle, pigs, poultry, turkeys, horses, cats, and dogs (2006–2018).
Ceftiofur resistance in E. coli in other animal species (poultry, pigs, adult cattle, turkeys) was <3%. Resistance to fluoroquinolones, with the highest rates of enrofloxacin or marbofloxacin resistance in E. coli of sick animal origin, was recorded in cattle (16.5% in 2016). Overall, a continuous downward trend in fluoroquinolone resistance has been observed over the last 6 years in almost all animal species. Nevertheless, there is a need for these achievements to be consolidated into a “One Health” perspective. A second ECOANTIBIO plan, which is part of the interministerial roadmap, was implemented in 2018 to further increase communication with all people responsible for the care of animals and to assess their motivation.
DISCUSSION
There are very few papers in the literature with in-depth descriptions of long-term national programs that provide data on both AC and AMR in both humans (adults and children) and animals with a One Health philosophy. Scandinavian countries and the Netherlands began providing data on AC and AMR, using very efficient networks, a long time ago. Most recent articles have come from non-European countries and look at very limited outcomes in a rather short period of time. Comparisons between European countries, presented in part in this paper, are available on the ECDC website but have not been extensively published in peer-reviewed journals.
The French AC has been very high for many years, making French authorities and health care professionals somewhat guilty, in particular when the European levels of MRSA were publicized. Since that time, French agencies have been very active in fighting against antibiotic resistance over the years in both animals and humans and in both hospitals and the community. These information campaigns have been repeated in humans [18]. The results within the community have been initially positive in humans [19], in particular in children (Figure 2), but were not sustained in adults. The effect in children is interesting and is probably due to the motivation of general practitioners, and even more so pediatricians, to reduce antibiotic therapy for upper airway infections. The results are weaker in adults, probably explained in part by the increase in underlying comorbidities in the oldest patients. Antimicrobial consumption has slowly increased since 2010, although it has remained 12.7% lower than at the very beginning of the programs. There was a slight decrease in 2017, which has been confirmed since that time. Such contrasting results are not limited to France [20]. The global European mean AC has increased the over years (Figure 1). Out of the 21 European countries participating in the ESAC-net whose data are available for both 2000 and 2016 [7], AC increased in 10 countries, decreased in only 8, and remained stable in 3. France remains a very high antibiotic consumer in the community, ranking as the fourth highest consumer in the ESAC network (30.3 defined daily doses [DDDs] for 1000 inhabitants per day), and going from 10.4 to 36.3 DDDs for 1000 inhabitants per day in Europe [21, 22]. The reasons for the long-term relative failure of the French successive plans in the community in AC, as well as the European trends, are not fully understood. The argument given by GPs is that patients’ ages and comorbidities have increased significantly in recent decades. In France, general practitioners are not often challenged by very resistant strains in their daily practice, and therefore are not convinced that AMR is a dramatic public health issue. French doctors are primarily clinicians, and some of them are convinced that they can differentiate bacterial from viral infections more accurately than the rapid strep test, C-reactive protein, or procalcitonin. Only 40% of the GPs use the rapid strep test in their daily practice, although the test is completely free of charge. In hospitals, antibiotic consumption is close to the mean European rate. The contrast between hospital and community AC is obvious and could be due, in part, to the implementation of an accreditation program in every French hospital, which contains several important quality indicators, including AC and AMR.
The program for animals (ECOANTIBIO) was implemented later than the program for humans (2012 vs 2002). This program has been very effective, with a 37% decrease in antibiotic consumption from 2012 to 2017. Therefore, the French position in the ESVAC network [21, 22] is now competitive, with an exposure to antibiotics in France of 70.2 mg of antibiotics/population correlation unit (PCU; kg of animal) against the average of 30 European countries of 135.5 mg/PCU [22].
Antibiotic resistance has decreased in France for some pairs of microorganisms/antibiotics. Those very positive results are likely to be due to the strength of the program, fully supported by the Ministry of Agriculture, and the quality of the consensus between numerous partners in the veterinary field. This trend in animals toward a better antibiotic usage in France has also been noted in many European countries, with a mean decrease of AC of 26% in Europe. France, which was the highest consumer of antibiotics in animals along with the Netherlands 10 years ago, is now in the middle range of consumption among European countries.
The level of antibiotic resistance in humans may be considered moderate in France for most microorganisms, contrasting with a very high AC. The programs for detecting MDR and for limiting their spread have been very active and sustained in France for many years and have led to very encouraging results for some bacteria, including methicillin-resistant staphylococci [23], penicillin-resistant pneumococci, vancomycin-resistant enterococci (VRE), as well as carbapenem-resistant Enterobacteriaceae. These measures have included the detection of resistant microorganisms at entry into intensive care units and sometimes in the rest of the hospital, strict isolation, and often cohorting. The results for pneumococci are likely to be due, in part, to the vaccination program for this microorganism. Very strict measures, in particular cohorting, are extremely time- and money-consuming, usually making decision-makers very reluctant to implement them. Obviously, the differences in infection control measures might explain the differences in the European prevalence of VRE.
It appears that in France, we have reached a plateau since 2013 in the prevalence of resistant E. coli and K. pneumoniae to third-generation cephalosporins via extended beta-lactamases [2]. It is not a simple matter to know if this is due to the measures that were implemented or to the spontaneous evolution of the epidemic with those 2 microorganisms.
The direct relationship between AC and resistance rates, which is widely accepted worldwide, in particular after the study of Albrich et al. [24], is not easy to study and needs a specific methodology, which was not used in our study. This relationship is not obvious when looking at French and European data [2, 7]. Some countries, such as Belgium and France, have high AC levels but moderate resistance levels. Other ones, in particular Eastern European countries, have moderate AC levels but very high AMR levels, probably due to suboptimal infection control programs. Some articles, such as that of McDonnell et al., show that there is some relationship between AC and AMR in 29 European countries, but with very impressive disparities [25]. Controlling MDR bacteria in hospitals, the community, animal settings, and the environment will require far more ambitious and multifaceted programs that should include increased hygiene among the general population (sanitation), strong environment policies (ie, processing in sewage treatment plants and food control), and strong organization in farming to terminate the intricate chains of transmission. A key objective would be to work on the appropriateness of antibiotic usage (antibiotic stewardship) in each patient, which is the overall goal, not only looking at the consumption of those drugs. Although several papers, often positive in nature, have been published on the results of antibiotic stewardship [26], very few have been published on the “appropriate” usage of antibiotics in individual patients [27]. The aims of the French program in the next few years, apart from continuing the present program, will also include the use of indicators to assess the quality of antibiotic therapy, prevention of resistant bacteria coming from the environment, and reinforcement of antibiotic stewardship multidisciplinary units in hospitals.
The topic of antibiotic resistance is becoming fashionable. This is good news, but it is not certain that people, including decision-makers, realize the very high degrees of morbidity and mortality and the high cost of AMR [28–30]. This probably explains the difficulty involved in convincing French doctors, in particular GPs, that AMR is a very urgent matter.
The main strengths of this program, and of this article, are the high quality of data for both antibiotic consumption and resistance, the long duration of the program, and the strong cooperation between human and veterinary medicine (One Health). Very few countries, with the exception of Sweden, Finland, Denmark, and the Netherlands, have been able to set up national networks providing long-term data for both AC and AMR in humans and animals. In Scandinavian countries, very precise and high-quality data are available on the countries’ websites and through the EARSS-net, but relatively few of these data have been published in peer-reviewed journals. There are also several important weaknesses of our study. The main weakness is that the study, although it shows important data on this issue, is not able to provide a final demonstration of the relationship between AC and AMR, mostly because the study, which was epidemiologic, was not designed for that purpose. However, the European data are not in favor of a strong and systematic correlation. A more careful analysis of European and international data, using an appropriate methodology, would help to bring precise answers to this very important question.
CONCLUSIONS
In France, after a significant initial decrease in humans, likely due to a very active campaign using a persuasive advertisement technique, AC remains high within the community, in particular among elderly patients, despite strong programs that have been implemented since 2000. Conversely, the results of the first animal plan are very significant. However, these efforts need to be followed to ensure that these positive results are sustainable. The program ECOANTIBIO 2 will be able to answer this question. Antibiotic resistance levels vary according to the microorganisms involved. The main issue in France, as well as in many other countries, is resistance to third-generation cephalosporins in Enterobacteriaceae. However, it appears that in France, we have maintained a plateau since 2013 in the prevalence of both E. coli and K. pneumoniae. Resistance for several antibiotics is currently decreasing in animals for most bacteria. Continuous and multidisciplinary efforts are still essential and must gather people from many different ministries and from various horizons. Additional information, especially information from in-depth analysis of the European data from ECDC networks and other international data, is necessary to elucidate the mechanisms of AMR, particularly the possible but still controversial relationship between AC and AMR.
Acknowledgments
We are indebted to the National Agency for Drug Safety (ANSM), Santé Publique France (SPF), and ECDC for the data on AC and antibiotic resistance. We are also indebted to Mr. Philippe Cavalié, who worked at ANSM until recently and provided excellent comments on the AC data. We want to thank Antoine Andremont and Gilles Pipien for their efforts to provide data on AMR in the environment. We are grateful to Dominique Monnet, from the ECDC, and Marc Sprenger, from the WHO, for their encouragement.
Names and missions of French agencies and networks (chronological appearance in the text). Santé Publique France (SPF): Gathers all infection information related to epidemiologic data in France. Agence Nationale de Sécurité Sanitaire (ANSM): Many missions on safety, in particular on antibiotic side effects and consumption. Observatoire National de l’ Epidémiologie de la Résistance Bactérienne aux Antibiotiques (ONERBA): Gathers all yearly national data from French networks on antibiotic resistance in both the hospitals and the community. Agence Nationale de Sécurité Sanitaire de l’Alimentation (ANSES): Gathers yearly data on antibiotic consumption and resistance in animals. Réseau d’Épidémio-Surveillance de l’Antibiorésistance Chez les Animaux (RESAPATH): Gathers all yearly data on antibiotic resistance in all animals. French National Health Insurance Network (URSSAF): Takes care of health reimbursements for all citizens. ECOANTIBIO: National program for antibiotic resistance in animals (2012/2017, and 2017/2021). Inter-ministerial Health Committee (IHC): Interministerial cooperation for public health issues, presently antibiotic resistance.
Financial support. There was no funding for this study.
Potential conflicts of interest. Jean Carlet participated in 3 boards with Beckton-Dickinson and gave a talk for Biomerieux and a talk for Mylan. Pierre Tattevin has had several activities associated with Astellas, AstraZeneca, Biomerieux, Cerrevio, Gilead Sciences, MSD, Mylan, and Pfizer. All the other authors have no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. J.C. proposed the idea to publish the French program against AMR and wrote the first draft of the document. V.J. made important improvements to the paper and provided and commented on the ECDC data on antibiotic resistance in France and Europe. J.A. made interesting comments. O.D. wrote the sections on the programs for animals. B.G., P.D., P.L.C., G.L., C.R., J.S., F.R., B.S., and B.V. looked carefully at the paper and provided useful comments. Y.P. provided the data of the ONERBA network and made comments on the paper.
Patient consent. The informed consent of the patients was not necessary for this study, which was purely epidemiological. Access to the names of the patients or other personal information was not possible at any step of the process.
References
- 1. Carlet J, Collignon P, Goldmann D, et al. Society’s failure to protect a precious resource: antibiotics. Lancet 2011; 378:369–71. [DOI] [PubMed] [Google Scholar]
- 2. European Centre for Disease Prevention and Control (ECDC). European Antimicrobial Resistance Surveillance Network (EARS-Net) Available at: https://www.google.fr/search?q=earss-net&oq=earssnet&aqs=chrome..69i57j0l5.6322j0j4&sourceid=chrome&ie=UTF-8. Accessed 10 September 2019.
- 3. Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance—the need for global solutions. Lancet Infect Dis 2013; 13:1057–98. [DOI] [PubMed] [Google Scholar]
- 4. Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev 2005; 18:657–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nordmann P, Poirel L. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin Microbiol Infect 2014; 20:821–30. [DOI] [PubMed] [Google Scholar]
- 6. Liu YY, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 2016; 16:161–8. [DOI] [PubMed] [Google Scholar]
- 7. European Centre for Disease Prevention and Control (ECDC). European Antimicrobial Resistance Surveillance Network: antimicrobial consumption data base (ESAC-Net) Available at: https://ecdc.europa.eu/en/antimicrobial-consumption/surveillance-and-disease-data/database. Accessed 5 September 2019.
- 8. Beović B, Pulcini C, Dumartin C, et al. ; LEASH Study Group on behalf of ESCMID Study Group for Antimicrobial StewardshiP (ESGAP). Legal framework of Antimicrobial Stewardship in Hospitals (LEASH): a European Society of Clinical Microbiology and Infectious Diseases (ESCMID) cross-sectional international survey. Int J Antimicrob Agents 2018; 52:616–21. [DOI] [PubMed] [Google Scholar]
- 9. Joshi MP, Chintu C, Mpundu M, et al. Multidisciplinary and multisectoral coalitions as catalysts for action against antimicrobial resistance: implementation experiences at national and regional levels. Glob Public Health 2018; 13:1781–95. [DOI] [PubMed] [Google Scholar]
- 10. Xiao Y, Li L. China’s national plan to combat antimicrobial resistance. Lancet Infect Dis 2016; 16:1216–8. [DOI] [PubMed] [Google Scholar]
- 11. Carlet J, Astagneau P, Brun-Buisson C, et al. French national program for prevention of healthcare-associated infections and antimicrobial resistance, 1992–2008: positive trends, but perseverance needed. Infect Control Hosp Epidemiol 2009; 30:737–45. [DOI] [PubMed] [Google Scholar]
- 12. Ministry of Agriculture and Food. Écoantibio 2: plan national de réduction des risques d’antibiorésistance en médecine vétérinaire (2017 - 2021) 2018. Available at: http://agriculture.gouv.fr/le-plan-ecoantibio-2-2017-2021. Accessed 30 August 2019.
- 13. Carlet J, Le Coz P; Propositions du Groupe de Travail Spécial pour la Préservation des Antibiotiques. Tous ensemble, sauvons les antibiotiques Ministère des Solidarités et de la Santé et des Droits des Femmes. 2017. Available at: https://solidarites-sante.gouv.fr/IMG/pdf/rapport_antibiotiques.pdf. Accessed 10 September 2019.
- 14. Touraine M. Tackling antimicrobial resistance in France. Lancet 2016; 387:2177–9. [DOI] [PubMed] [Google Scholar]
- 15. Cavalie P. Evolution 2000–2010 de la consommation d’antibiotiques en ville. Bul Epidemiol Hebd 2012; 42:480–4. [Google Scholar]
- 16. Carbonne A, Arnaud I, Maugat S, et al. ; MDRB Surveillance National Steering Group (BMR-Raisin) National multidrug-resistant bacteria (MDRB) surveillance in France through the RAISIN network: a 9 year experience. J Antimicrob Chemother 2013; 68:954–9. [DOI] [PubMed] [Google Scholar]
- 17. European Centre for Disease Prevention and Control (ECDC). Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: guidance from the European Centre for Disease Prevention and Control 2017. Available at: https://ecdc.europa.eu/en/publications-data/directory-guidance-prevention-and-control/prevention-and-control-infections-1. Accessed 10 June 2019.
- 18. Chahwakilian P, Huttner B, Schlemmer B, Harbarth S. Impact of the French campaign to reduce inappropriate ambulatory antibiotic use on the prescription and consultation rates for respiratory tract infections. J Antimicrob Chemother 2011; 66:2872–9. [DOI] [PubMed] [Google Scholar]
- 19. Sabuncu E, David J, Bernède-Bauduin C, et al. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002–2007. PLoS Med 2009; 6:e1000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huttner B, Goossens H, Verheij T, Harbarth S; CHAMP Consortium Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect Dis 2010; 10:17–31. [DOI] [PubMed] [Google Scholar]
- 21. European Surveillance of Veterinary Antimicrobial Consumption (ESVAC). Antimicrobial resistance in veterinary medicine Available at: https://www.ema.europa.eu/en/veterinary-regulatory/overview/antimicrobial-resistance/european-surveillance-veterinary-antimicrobial-consumption-esvac. Accessed 10 June 2019.
- 22. Grave K, Greko C, Kvaale MK, et al. ; ESVAC Group Sales of veterinary antibacterial agents in nine European countries during 2005-09: trends and patterns. J Antimicrob Chemother 2012; 67:3001–8. [DOI] [PubMed] [Google Scholar]
- 23. Jarlier V, Trystram D, Brun-Buisson C, et al. ; Collégiale de Bactériologie-Virologie-Hygiène des Hôpitaux Universitaires de l’Ile de France Curbing methicillin-resistant Staphylococcus aureus in 38 French hospitals through a 15-year institutional control program. Arch Intern Med 2010; 170:552–9. [DOI] [PubMed] [Google Scholar]
- 24. Albrich WC, Monnet DL, Harbarth S. Antibiotic selection pressure and resistance in Streptococcus pneumoniae and Streptococcus pyogenes. Emerg Infect Dis 2004; 10:514–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McDonnell L, Armstrong D, Ashworth M, et al. National disparities in the relationship between antimicrobial resistance and antimicrobial consumption in Europe: an observational study in 29 countries. J Antimicrob Chemother 2017; 72:3199–204. [DOI] [PubMed] [Google Scholar]
- 26. Dellit TH, Owens RC, McGowan JE Jr, et al. ; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44:159–77. [DOI] [PubMed] [Google Scholar]
- 27. Le Marechal M, Tebano G, Monnier AA, et al. Quality indicators assessing antibiotic use in the outpatient setting: a systematic review followed by an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73(Suppl_6):vi40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cassini A, Hogberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 2019; 19:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Colomb-Cotinat M, Lacoste J, Brun-Buisson C, et al. Estimating the morbidity and mortality associated with infections due to multidrug-resistant bacteria (MDRB), France, 2012. Antimicrob Resist Infect Control 2016; 5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Organization for Economic Cooperation and Development (OECD). Stemming the Superbug Tide: Just a Few Dollars More. Paris: Editions OECD; 2018. [Google Scholar]