Abstract
We report a case of chronic hepatosplenic aspergillosis following immune reconstitution complicating colic aspergillosis in an AIDS patient with multicentric Castleman disease. Symptoms mimicked the clinical presentation of chronic disseminated candidiasis and responded to corticosteroid. This emerging entity enlarges the spectrum of fungal immune reconstitution inflammatory syndrome in the HIV setting.
Keywords: aspergillosis, hepatosplenic, immune reconstitution inflammatory syndrome
Immune reconstitution inflammatory syndrome (IRIS) is defined as the clinical worsening or appearance of an infectious disease after reversal of immune deficiency. In the setting of HIV, it has been commonly described during the first months of antiretroviral therapy (ART) and associated with a wide range of opportunistic agents. Among fungal IRIS, cryptococcosis has been best described in HIV-infected patients and solid organ transplant recipients, whereas reports of IRIS associated with other fungi remain anecdotal [1]. We report here a new HIV-associated fungal IRIS, which we propose to name “chronic disseminated aspergillosis” given its similarities with chronic disseminated candidiasis [2, 3].
CASE REPORT
A 39-year-old Caucasian man was admitted to the intensive care unit for multi-organ failure. He was diagnosed with HIV infection and HHV-8-associated multicentric Castleman disease with hemophagocytic syndrome confirmed by an excisional biopsy of an axillary lymphadenopathy and a bone marrow biopsy, associated with a Kaposi sarcoma of the foot. His CD4+ T-cell count was 13/mm3 (2%), and his HIV viral load was 28 979 copies/mL. He received intravenous etoposide (100 mg/m2/d for 2 days) and rituximab (375 mg/m2/wk for 2 weeks). His condition rapidly improved with resolution of hemophagocytosis.
Five days after etoposide infusion, he developed febrile neutropenia (day 0) (Supplementary Appendix 1) with septic shock requiring vasopressors and antibiotic therapy with meropenem and aminoglycoside. Caspofungin was added on day 3 for persistent fever. Bone marrow recovery occurred on day 4, and antiretroviral therapy was started with emtricitabine, dolutegravir, and enfuvirtide. On day 6, serum galactomannan antigen returned strongly positive (index value 9), and a culture of bronchial aspirate was positive for Aspergillus fumigatus without any suggestive feature of invasive pulmonary aspergillosis on thoracic computed tomography (CT). Caspofungin was switched to voriconazole to cover a possible invasive mold infection.
On day 8, a new septic shock with multi-organ failure occurred. An abdominal CT scan showed pneumoperitoneum. Laparotomy revealed fecal peritonitis and transmural colic necrosis requiring peritoneal toilet, subtotal colectomy with double-end ileostomy, sigmoidostomy, and cholecystectomy. Examination of the colectomy sample evidenced proven invasive aspergillosis with ulcerative, necrotizing, and hemorrhagic colitis containing fungal hyphae invading the digestive wall blood vessels (homogenous size, absence of pigmentation, acute angle branching and septation) with a positive labeling after anti-Aspergillus spp. immunohistochemistry (Supplementary Appendices 2 and 3). Caspofungin therapy was thus initiated on day 13, in combination with voriconazole because of the severity of illness. There was no radiological evidence of pulmonary, cerebral, or sinus aspergillosis. The patient’s condition gradually improved, apyrexia was obtained, and serum galactomannan antigen was negative on day 34. Combination therapy was switched to voriconazole alone after 13 days.
The patient presented an early immune reconstitution, with an increase in CD4+ T-cell count reaching 141/mm3 (13%) on day 16 (after 12 days of antiretroviral therapy) and an undetectable HIV viral load.
On day 25, he developed isolated fever without localizing signs or symptoms, elevated C-reactive protein serum levels, or eosinophilia (4, 600/mm3), and liver function tests were normal. An abdominal CT scan revealed multiple infracentimetric liver abscesses, and treatment with ceftriaxone and metronidazole was initiated. After 3 weeks of this empirical antibiotic therapy driven by what were considered pyogenic hepatic abscesses, abdominal magnetic resonance imaging showed no improvement, with multiple hepatosplenic lesions and a more localized lesion that appeared to be a peritoneal abscess in the left upper quadrant. A biopsy of this lesion was performed; microscopic examination revealed acute septate hyphae, fungal cultures were negative, and in-house real-time polymerase chain reaction (PCR) for A. fumigatus was positive (Supplementary Appendix 2). Histologic examination showed granulomas, necrosis, and acute septate hyphae (Supplementary Appendix 4). Serum galactomannan antigen remained negative. Diagnosis of hepatosplenic aspergillosis with HIV-associated IRIS triggered by antiretroviral therapy was retained. Antiretroviral therapy and voriconazole were continued, and the patient’s condition spontaneously improved with apyrexia, normal inflammatory blood parameters, and normal eosinophilic count on day 58.
After 4 months of voriconazole therapy, asthenia with weight loss appeared, with elevated C-reactive protein serum levels and anicteric cholestasis. The alkaline phosphatase serum level was 462 U/L (reference range, <120 U/L), and the gamma glutamyltransferase serum level was 920 U/L (reference range, <55 U/L). Galactomannan antigen in serum was negative. The patient’s HIV viral load had remained undetectable, and his CD4+ T-cell count was 204/mm3 (10%). 18-FDG positron emission tomography (PET)/CT showed increased hepatosplenic metabolism (Figure 1). There was no hemophagocytosis and no hypermetabolic lymph nodes. Histologic examination of a liver biopsy showed fibrosis, granulomas, and necrosis, without hyphae (Supplementary Appendix 4). Microscopic examination as well as bacterial, fungal, and mycobacterial cultures were negative. PCR for A. fumigatus was also negative. We concluded worsening of hepatosplenic aspergillosis related to an IRIS. Corticosteroid therapy was initiated at 0.5 mg/kg daily, and antifungal treatment was continued. Corticosteroid therapy was tapered down gradually over 2 months. Inflammatory blood parameters and liver function tests rapidly normalized. 18-FDG PET/CT showed improvement with partial metabolic response (Figure 1).
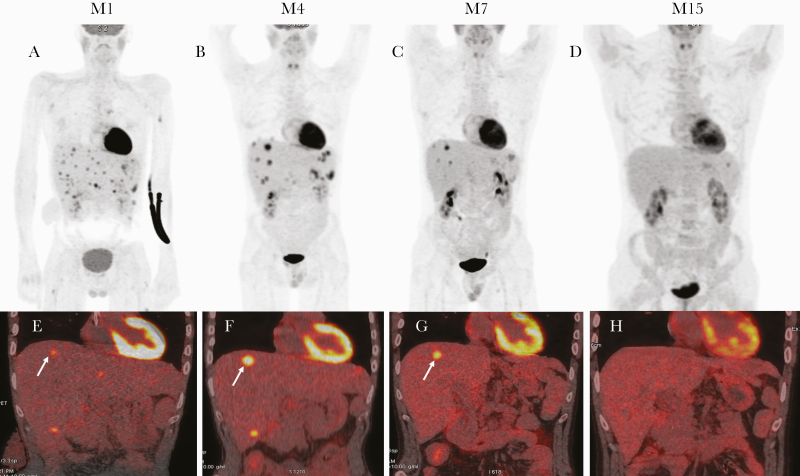
Figure 1.
18-FDG positron emission tomography/computed tomography (PET/CT) during disease course. 18-FDG PET/CT scan maximum intensity projection (MIP) showed a metabolic modification with progression of several liver nodules despite the regression in numbers between month 1 (M1) (A) and month 4 (M4) (B), then decreased FDG uptake in the liver abscess and regression in uptake of numbers of lesions at month 7 (M7) (C) and complete regression at month 15 (M15) (D). Fused coronal PET and CT images show several abscesses with a target on the liver dome. In (E) (M1), the arrow indicates a liver node (SUV max, 6; metabolic tumor volume, 2.3 cm3). In (F), the arrow indicates an increase in FDG uptake in liver target (SUV max, 11.6; metabolic tumor volume, 4.5 cm3) at M4. G, Decreased metabolic activity (SUV max, 9.8; metabolic tumor volume, 2.5 cm3) at M7. H, No metabolic activity at M15.
Seven months after relapse, the patient still presented immunovirologic response: undetectable HIV viral load and CD4+ T-cell count of 414/mm3 (13%). Liver function tests were normal, as well as inflammatory blood parameters and galactomannan antigen. At 15 months, 18-FDG PET/CT showed complete metabolic response (Figure 1).
DISCUSSION
Here, we report the first case, to our knowledge, of what we propose to name “chronic disseminated aspergillosis” as an IRIS fungal syndrome occurring during HIV infection.
The patient originally presented with proven primary colic invasive aspergillosis, without evidence of pulmonary disease, as described by Eggimann et al., for whom the digestive tract is considered a potential portal of entry for Aspergillus spp. in immunocompromised patients [4]. Colic invasive aspergillosis was in our case characterized by transmural tissue necrosis with tissue invasion by septate hyphae and angioinvasion associated with elevated galactomannan antigen and positive immunohistochemistry with Aspergillus spp. antibody, fulfilling the 2008 definition criteria [5].
Our patient developed during immune reconstitution (neutrophil recovery on day 4 and CD4+ T-cell recovery >140/mm3 on day 16), symptoms strongly mimicking those presented by patients with chronic disseminated candidiasis: fever that fails to respond to antibiotics, increased alkaline phosphatase and C-reactive protein levels, hepatosplenic micro-abscesses on imaging, granulomatous lesions associated with fungal hyphae, but negative fungal cultures. Initially, clinical course was spontaneously favorable without any change in antifungal treatment. A relapse of IRIS occurred 4 months later, requiring corticosteroid therapy, with subsequent favorable outcomes.
Since the availability of highly active antiretroviral therapy (HAART), IRIS has been described as the paradoxical worsening of treated opportunistic infections or the unmasking of previously untreated infections, occurring classically when CD4+ T-cell counts rise and function improves. HIV-associated IRIS has been described for several fungal infections, such as cryptococcosis, Pneumocystis jiroveci pneumonia, histoplasmosis, candidiasis, and talaromycosis [1, 6–12]. Sambatakou et al. also reported a case of IRIS with invasive pulmonary aspergillosis in an end-stage HIV-infected patient [13].
Miceli et al. described pulmonary IRIS among patients with hematological malignancies and invasive pulmonary aspergillosis [14]. This entity is characterized by worsening of pulmonary symptoms and radiological lesions during neutrophil recovery, associated with a persistent microbiological response (eg, decrease in galactomannan index titers).
In hematological patients with profound and prolonged neutropenia, chronic disseminated candidiasis (CDC), also known as hepatosplenic candidiasis, is regarded as being due to an IRIS upon neutrophil recovery, favoring antigen-driven response [3]. Indeed, we recently evidenced an expansion of Candida-specific interferon-γ-producing T cells together with features of T-cell activation and systemic inflammation during CDC [2].
Invasive hepatosplenic aspergillosis without IRIS has been described in few cases in immunocompromised patients [15–17], but it has not been described in HIV-infected patients in historical papers [18], nor in a recent nation-based study [19] or an immunocompetent patient [20]. In our patient, we assume that initial colonization of the digestive tract with Aspergillus and subsequent colic invasive aspergillosis with high inoculum and transmural colic necrosis favored peritoneal dissemination, translocation to the bloodstream, and hematogenous spread via portal venous circulation, leading to occult hepatosplenic aspergillosis. Furthermore, hepatosplenic aspergillosis occurred when neutrophil and CD4+ T-cells started to rise and continued as immune reconstitution was going on. The patient developed peritoneal and hepatosplenic aspergillosis lesions almost 3 weeks after beginning treatment with voriconazole, with normal plasma concentrations and a microbial load control characterized by serum galactomannan antigen decrease. In addition, there was no evidence of new lesions of aspergillosis or other infectious processes in other organs. Fungal cultures remained negative, and subsequent improvement of the patient’s condition without treatment modifications or corticosteroid therapy supports the immune-related nature of the symptoms.
To conclude, this case supports the idea that independent of fungal species, colonization and primary invasive fungal infection of the digestive tract could be responsible for hepatosplenic IRIS in immunocompromised patients, including neutropenic AIDS patients with early immune restoration. Given the similarities with CDC, we propose to name this new entity “chronic disseminated aspergillosis” (CDA).
Supplementary Material
Acknowledgments
Financial support. None.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dellière S, Guery R, Candon S, et al. Understanding pathogenesis and care challenges of immune reconstitution inflammatory syndrome in fungal infections. J Fungi (Basel) 2018; 4:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Candon S, Rammaert B, Foray AP, et al. Chronic disseminated candidiasis during hematological malignancies: an immune reconstitution inflammatory syndrome with expansion of pathogen-specific T helper type 1 cells. J Infect Dis 2020; 221:1907–16. [DOI] [PubMed] [Google Scholar]
- 3. Rammaert B, Desjardins A, Lortholary O. New insights into hepatosplenic candidosis, a manifestation of chronic disseminated candidosis. Mycoses 2012; 55:e74–84. [DOI] [PubMed] [Google Scholar]
- 4. Eggimann P, Chevrolet JC, Starobinski M, et al. Primary invasive aspergillosis of the digestive tract: report of two cases and review of the literature. Infection 2006; 34:333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lortholary O, Fontanet A, Mémain N, et al. ; French Cryptococcosis Study Group Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. AIDS 2005; 19:1043–9. [DOI] [PubMed] [Google Scholar]
- 7. Wislez M, Bergot E, Antoine M, et al. Acute respiratory failure following HAART introduction in patients treated for Pneumocystis carinii pneumonia. Am J Respir Crit Care Med 2001; 164:847–51. [DOI] [PubMed] [Google Scholar]
- 8. Breton G, Adle-Biassette H, Therby A, et al. Immune reconstitution inflammatory syndrome in HIV-infected patients with disseminated histoplasmosis. AIDS 2006; 20:119–21. [DOI] [PubMed] [Google Scholar]
- 9. Peigne V, Dromer F, Elie C, et al. ; French Mycosis Study Group Imported acquired immunodeficiency syndrome-related histoplasmosis in metropolitan France: a comparison of pre-highly active anti-retroviral therapy and highly active anti-retroviral therapy eras. Am J Trop Med Hyg 2011; 85:934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berkeley JL, Nath A, Pardo CA. Fatal immune reconstitution inflammatory syndrome with human immunodeficiency virus infection and Candida meningitis: case report and review of the literature. J Neurovirol 2008; 14:267–76. [DOI] [PubMed] [Google Scholar]
- 11. Thanh NT, Vinh LD, Liem NT, et al. Clinical features of three patients with paradoxical immune reconstitution inflammatory syndrome associated with Talaromyces marneffei infection. Med Mycol Case Rep 2018; 19:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sambatakou H, Denning DW. Invasive pulmonary aspergillosis transformed into fatal mucous impaction by immune reconstitution in an AIDS patient. Eur J Clin Microbiol Infect Dis 2005; 24:628–33. [DOI] [PubMed] [Google Scholar]
- 14. Miceli MH, Maertens J, Buvé K, et al. Immune reconstitution inflammatory syndrome in cancer patients with pulmonary aspergillosis recovering from neutropenia: proof of principle, description, and clinical and research implications. Cancer 2007; 110:112–20. [DOI] [PubMed] [Google Scholar]
- 15. Chasan R, Patel G, Malone A, et al. Primary hepatic aspergillosis following induction chemotherapy for acute leukemia. Transpl Infect Dis 2013; 15:E201–5. [DOI] [PubMed] [Google Scholar]
- 16. Gupta KL, Rajaram KG, Joshi K, Sakhuja V. Progression of hepatic aspergillosis following second renal transplantation in a patient with recurrent glomerulonephritis. Indian J Pathol Microbiol 2012; 55:580–2. [DOI] [PubMed] [Google Scholar]
- 17. van der Velden WJ, Blijlevens NM, Klont RR, et al. Primary hepatic invasive aspergillosis with progression after rituximab therapy for a post transplantation lymphoproliferative disorder. Ann Hematol 2006; 85:621–3. [DOI] [PubMed] [Google Scholar]
- 18. Lortholary O, Meyohas MC, Dupont B, et al. Invasive aspergillosis in patients with acquired immunodeficiency syndrome: report of 33 cases. French Cooperative Study Group on Aspergillosis in AIDS. Am J Med 1993; 95:177–87. [DOI] [PubMed] [Google Scholar]
- 19. Denis B, Guiguet M, de Castro N, et al. ; French Hospital Database on HIV National Agency for Research on AIDS and Viral Hepatitis, France CO4 Relevance of EORTC criteria for the diagnosis of invasive aspergillosis in HIV-infected patients, and survival trends over a 20-year period in France. Clin Infect Dis 2015; 61:1273–80. [DOI] [PubMed] [Google Scholar]
- 20. Chen L, Liu Y, Wang W, Liu K. Adrenal and hepatic aspergillosis in an immunocompetent patient. Infect Dis (Lond) 2015; 47:428–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.