Abstract
Background
Lower Clostridium difficile spore counts in feces from C difficile infection (CDI) patients treated with fidaxomicin versus vancomycin have been observed. We aimed to determine whether environmental contamination is lower in patients treated with fidaxomicin compared with those treated with vancomycin/metronidazole.
Methods
The CDI cases were recruited at 4 UK hospitals (Leeds, Bradford, and London [2 centers]). Environmental samples (5 room sites) were taken pretreatment and at 2–3, 4–5, 6–8, and 9–12 days of treatment, end of treatment (EOT), and post-EOT. Fecal samples were collected at diagnosis and as often as produced thereafter. Swabs/feces were cultured for C difficile; percentage of C difficile-positive samples and C difficile bioburden were compared between different treatment arms at each time point.
Results
Pre-EOT (n = 244), there was a significant reduction in environmental contamination (≥1 site positive) around fidaxomicin versus vancomycin/metronidazole recipients at days 4–5 (30% vs 50% recipients, P = .04) and at days 9–12 (22% vs 49%, P = .005). This trend was consistently seen at all other timepoints, but it was not statistically significant. No differences were seen between treatment groups post-EOT (n = 76). Fidaxomicin-associated fecal positivity rates and colony counts were consistently lower than those for vancomycin/metronidazole from days 4 to 5 of treatment (including post-EOT); however, the only significant difference was in positivity rate at days 9–12 (15% vs 55%, P = .03).
Conclusions
There were significant reductions in C difficile recovery from both feces and the environment around fidaxomicin versus vancomycin/metronidazole recipients. Therefore, fidaxomicin treatment may lower the C difficile transmission risk by reducing excretion and environmental contamination.
Keywords: Clostridium difficile infection, environmental contamination, fidaxomicin
Environmental contamination of the area around patients with Clostridium difficile infection treated with fidaxomicin may be reduced compared with those treated with vancomycin/metronidazole. This may be related to a reduced bioburden in the feces of fidaxomicin-treated patients.
Clostridium (Clostridioides) difficile produces spores resistant to many disinfectants, which can survive for prolonged periods in the environment, particularly around infected patients [1–7]. Patients with C difficile infection (CDI) excrete between 1 × 104 and 1 × 107 of C difficile per gram of feces [2], and environmental contamination is likely due to direct or indirect fecal contact and aerosolization during diarrhea [6]. Contact with spores in the environment is the likely source for secondary cases of CDI in hospital settings [8]. Given the considerable time during which patients may shed spores, prompt isolation of symptomatic cases and adequate environmental decontamination are 2 central recommendations for preventing onward transmission [7, 9–11].
Fidaxomicin, a novel macrocyclic antibiotic, was approved to treat CDI in 2011/2012 12, 13, after 2 large trials showed it was noninferior to vancomycin for initial clinical cure [14, 15]. More importantly, recurrent CDI, a known complication, was significantly reduced with fidaxomicin (14% vs 26%) [14–16]. The mechanism by which fidaxomicin prevents recurrences of CDI is unclear. In vitro, fidaxomicin inhibits the outgrowth of C difficile spores, possibly due to its ability to adhere to the spore coat [17]. In an artificial gut model of CDI, fidaxomicin achieved intraluminal concentrations well above the minimum inhibitory concentration for C difficile; these were sustained for approximately 3 weeks after instillation, perhaps due to sequestration within biofilms [18]. In a Phase II trial, fidaxomicin-treated patients had significantly lower mean spore counts 11–18 days posttreatment, compared with vancomycin recipients (3.1 log10 colony-forming units [CFU]/g of feces versus 5.4 log10 CFU/g, respectively), and fidaxomicin was relatively sparing of other gut microflora [17].
Because fidaxomicin reduces spore counts in the feces of treated patients and inhibits spore outgrowth [17], we hypothesized that there may be less contamination of the patients’ skin and surrounding environment with C difficile, both during and immediately after treatment. In this case, fidaxomicin may help reduce onward transmission of C difficile to other patients, amplifying its benefit in reducing recurrences. Indeed, a single-center study comparing environmental contamination surrounding CDI patients on days 2–4 of treatment with fidaxomicin or conventional therapy (metronidazole/vancomycin [met/van]) found that those treated with fidaxomicin had significantly lower contamination rates [19]. However, the environment was tested at only 1 time point, so data on the period after treatment are lacking. We carried out a multicenter study to determine whether there is a difference in C difficile shedding and contamination of the skin and immediate environment between CDI patients treated with fidaxomicin versus vancomycin/metronidazole, both during and after treatment.
METHODS
Study
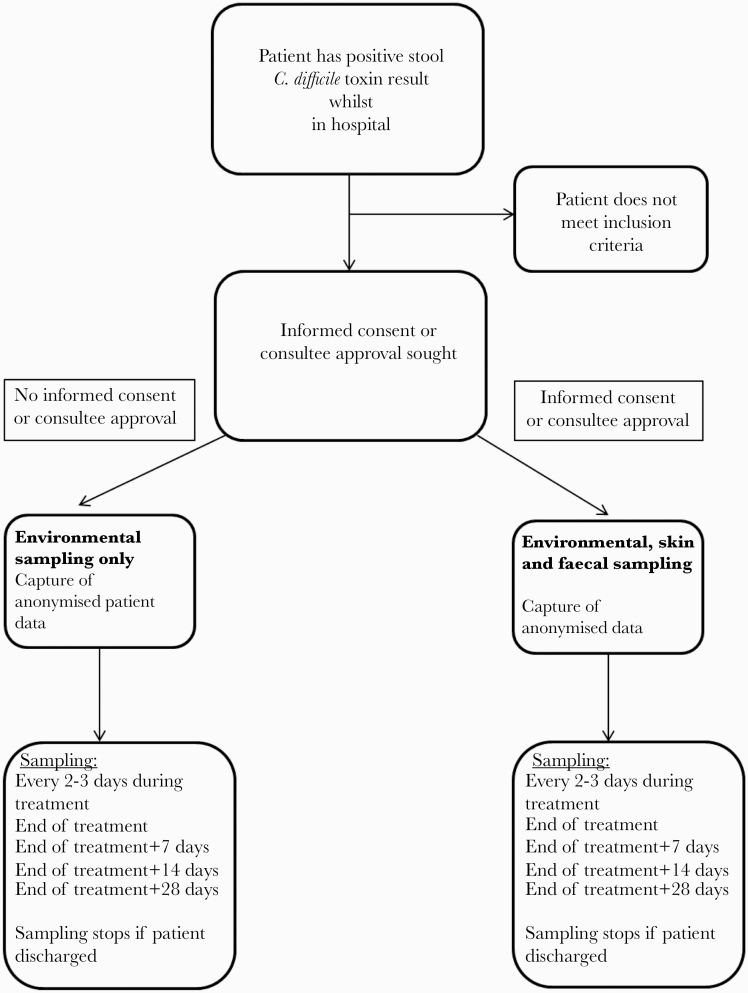
This was a prospective, observational study conducted in 4 UK hospitals (Leeds, Bradford, London [2 centers]). All hospital inpatients (aged ≥16 years) with CDI (defined by presence of C difficile toxin) between January 2015 and December 2016 were considered for inclusion. Local research staff assessed whether they met the following inclusion criteria: presence of diarrhea, defined as 3 or more episodes of unformed stools (Bristol stool type 5–7) in 24 hours within the last 7 days; and prescribed CDI-specific treatment (fidaxomicin, oral vancomycin, or metronidazole). All eligible participants had swabs of their environment taken from 5 room sites (call bell, commode/toilet, floor, bed rail, bedside table) at the following timepoints: after diagnosis, every 2–3 days during treatment, at the end of treatment (EOT), and on days 7, 14, and 28 post-EOT (Figure 1). Consent was not required for environmental screening; however, patients were approached after diagnosis for informed consent for skin swabbing and fecal sampling. The original diagnostic stool sample was also collected, where available. Skin swabbing took place at the same time as the environmental sampling; stool samples were collected as close as possible to these time points. All sampling ceased after patient discharge. Swabs and samples were stored at 5°C at each site, with shipments to the laboratory in Leeds once per week for processing.
Figure 1.
Flow chart of study, showing participant recruitment.
If a participant switched to fidaxomicin after receiving more than 24 hours of either metronidazole or vancomycin, they were withdrawn from the study, but data collected to that point was included. Participants who received any fidaxomicin before being switched to metronidazole/vancomycin were excluded. Participants who were switched between metronidazole and vancomycin, or simultaneously prescribed both agents, remained in the study.
Patient Consent Statement
The study was approved by the HRA North West Haydock research ethics committee (14/NW/1398). Patient’s written consent was obtained including recruitment of patients that lacked mental capacity for informed consent, via consultee approval.
Environmental Swabbing
All sites used chlorine-based cleaning products for the disinfection of hard surfaces and floors, and time (hours) since cleaning was recorded when samples were collected. Rooms at all sites were cleaned at least once daily. Sponge-sticks (3M, Saint Paul, Minnesota), moistened with sterile water, were used to sample a 5 × 20-cm area of flat surface, or the entire surface of the call bells, or a 13.3-cm length of bed rails (representing the same surface area). After transport to Leeds, swabs were placed in 50 mL neutralizing solution (0.1% [wt/vol] sodium thiosulfate, 3% [wt/vol] Tween80, 0.3% [wt/vol] lecithin in phosphate-buffered saline) and homogenized for 10 minutes before the solution was pulled through Microfil V 0.22-µM filters with 100-mL funnel onto the integral membrane using a gantry and pump assembly. Membranes were placed onto Brazier’s agar (Oxoid, UK) supplemented with 250 mg/L cycloserine and 8 mg/L cefoxitin (Oxoid) and 2% lysed horse blood (Oxoid) (CCEY agar). All plates were incubated anaerobically (A95 workstation; Don Whitley, Bradford, UK) for 48 hours before enumeration of typical colonies (gray-brown, irregular edge, typical odor). Atypical colonies were identified using matrix-assisted lazer desorption/ionization, time-of-flight mass spectrometry (MALDI-TOF; Bruker, Billerica. MA).
Skin Sampling
A 5 × 20-cm area of each participant’s skin sites (groin, abdomen, entire dominant hand) was sampled using flocked swabs (Sterilab Services, Harrogate, UK) moistened with sterile water. After transport to Leeds, swabs were broken off into 5 mL 50% (v/v) ethanol/water, mixed with a vortex for 15 seconds before the solution was pulled through Microfil filters as described above, and membranes were placed onto CCEY agar and incubated as described.
Fecal Clostridium difficile Enumeration
One gram of fecal sample was placed into 1 mL 50% (v/v) ethanol/water, mixed with a vortex for 15 seconds, and left at room temperature for an additonal hour before making a 10-fold dilution series in 4.5 mL peptone water (Sigma, Gillingham, UK) (from 10–1 to 10–7). Twenty milliliters of the original “alcohol shocked” sample and 20 µL of each of the dilutions were each inoculated onto one quarter of a CCEY plate in triplicate and incubated as described.
Polymerase Chain Reaction Ribotyping
All isolates of C difficile were typed by polymerase chain reaction (PCR) ribotyping at the Clostridium difficile Ribotyping Network of England and Northern Ireland (CDRN), as previously described [20]. One individual colony was picked from each plate for PCR ribotyping, as well as a sweep of growth from each plate, to check for multiple PCR ribotypes.
Clinical Data
Data on relevant demographic factors, past medical history, comorbidities, drug history, information about the CDI episode, and clinical markers of severe CDI were collected from medical records.
Analysis
For treatment comparisons, any patient who did not receive one of the antibiotics (fidaxomicin or vancomycin/metronidazole) for at least 48 hours was excluded. The following outcomes were compared between fidaxomicin and combined met/van treatment groups over time: percentage of environmental samples that were positive for C difficile, percentage of skin samples that were positive for C difficile, C difficile spore counts in fecal samples. Due to almost complete confounding (see results), comparisons between drugs could not be adjusted for site; therefore, comparisons were repeated within Leeds only, where all 3 drugs were used.
Analyses were conducted from diagnosis to EOT (+1 day to allow for visit windows), and then separately from EOT onwards for patients with EOT 7 days after diagnosis or later and with 1 or more post-EOT sample. All total spore counts were log10 transformed for normality. For each outcome, means (standard error of the mean) or percentages (95% confidence interval) as relevant were calculated in each group at each nominal time point (based on observed values) and to characterize the impact of time, including only the earliest sample per patient in each visit window (most conservative analysis). We used t tests (continuous) or exact tests (categorical) for comparisons.
RESULTS
Baseline Characteristics
There were 253 participants enrolled into the study: 202 of 253 (80%) were from Leeds. Fidaxomicin was used for 83 participants, 102 received vancomycin, and 70 received metronidazole. There was almost complete confounding between hospital site and treatment because Bradford almost never used fidaxomicin and St Georges almost never used met/van; Leeds used all 3 drugs.
There was no evidence of difference in the median time between cleaning and swabbing between fidaxomicin and met/van groups (6 vs 5 hours, P = .5). There were small differences (1 day) between fidaxomicin and met/van groups in median times from stool collection to positivity (P < .001) and from positivity to treatment (P = .04) but no evidence of any other imbalances in baseline characteristics between groups (P > .05) (Table 1). There was no evidence of difference in the median time to resolution of diarrhea for patients treated with fidaxomicin compared with met/van (6 vs 5 days, P = .4) or for patients treated with metronidazole compared with vancomycin (5 vs 6 days, P = .3). Resolution of diarrhea was defined as the first date of 2 consecutive days clear of diarrhea (defined as Bristol stool type 5–7).
Table 1.
Baseline Characteristics of Patients in the Studya
| Factor | Met/Van (N = 172) | Fidaxomicin (N = 81) | Total (N = 253) | P b |
|---|---|---|---|---|
| Site: Leeds | 141 (82%) | 61 (75%) | 202 (80%) | <.001 |
| Bradford | 26 (15%) | 1 (1%) | 27 (11%) | |
| St Georges | 3 (2%) | 15 (19%) | 18 (7%) | |
| Guys and St Thomas’s | 2 (1%) | 4 (5%) | 6 (2%) | |
| Age (years) | 75 (62–84) | 75 (61–82) | 75 (62–84) | .43 |
| Male | 91 (53%) | 35 (43%) | 126 (50%) | .18 |
| Temperature >38.5 | 23/168 (14%) | 12/78 (15%) | 35/246 (14%) | .70 |
| Clinical colitis | 62/172 (36%) | 34/80 (42%) | 96/252 (38%) | .33 |
| Creatinine rise >50% from baseline | 28/144 (19%) | 13/80 (16%) | 41/224 (18%) | .59 |
| Days from admission to first positive stool | 3 (1–12) | 6 (1–14) | 4 (1–13) | .33 |
| Days from stool collection to positivity | 2 (1–3) | 1 (1–2) | 2 (1–3) | <.001 |
| Days from stool collection to treatment | 2 (1–3) | 1 (1–2) | 2 (1–2) | .17 |
| Maximum total white cell count (×109/L) | 11.0 (8.2–14.9) | 11.5 (8.3–18.2) | 11.3 (8.2–15.9) | .34 |
| Serum creatinine (µmol/L) | 75 (57–117) | 82 (57–123) | 78 (57–123) | .82 |
| EOT (days) | 10 (7–14) | 10 (9–11) | 10 (8–13) | .89 |
| Any change in treatment, including dose | 25 (15%) | 13 (16%) | 38 (15%) | .85 |
| Experienced recurrence | 10 (6%) | 9 (11%) | 19 (8%) | .20 |
| Died within 30 days | 14 (8%) | 7 (9%) | 21 (8%) | 1.00 |
Abbreviations: EOT, end of treatment.
aMissing data shown by different denominators.
bExact test for categorical factors, rank-sum test for continuous factors.
Environmental Contamination
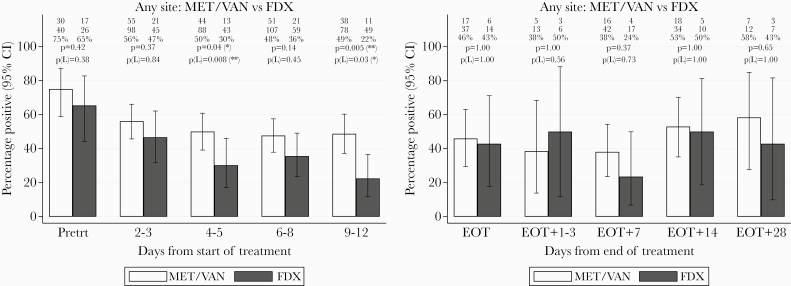
Between starting treatment and EOT, 3174 environmental samples were taken by approximately 244 patients (mean 13 samples/patient, range 3–30). Environmental contamination rates (at least 1 contaminated site) were generally lower in rooms housing patients treated with fidaxomicin compared with met/van, with 65% fidaxomicin versus 75% met/van rooms contaminated pretreatment (P = .42), 47% vs 56% on days 2–3 (P = .37), 30% vs 50% days 4–5 (P = .04), 36% vs 48% days 6–8 (P = .14), and 22% vs 49% days 9–12 (P = .005) (Figure 2A).
Figure 2.
(A) Percentage of environmental samples from at least 1 site positive for Clostridium difficile over time from treatment initiation. (B) Percentage of environmental samples from at least 1 site positive for C difficile after end of treatment (EOT). Note: Positive and total sample numbers at each time point are shown at the top of each panel, together with comparisons of metronidazole/vancomycin (met\van) versus fidaxomicin (FDX), overall, and within Leeds only (L). See Supplementary Figure 1 for results by individual site. CI, confidence interval; Pretrt, pretreatment.
Individual environmental sites showed similar downward trends in contamination rates over the course of treatment and generally lower rates with fidaxomicin versus met/van (Supplementary Figure 1). However, with lower rates at individual sites, almost all comparisons were nonsignificant except for bedrails after 6–8 days’ treatment (9% fidaxomicin vs 21% met/van, P = .05) and commodes after 2–3 days’ treatment (14% vs 39%, respectively, P = .003).
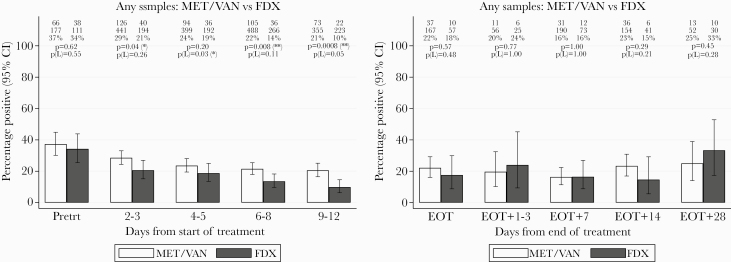
There was also a significant difference in environmental contamination when all samples were included (rather than considering any positive across all 5 sites) (Figure 3A). The environmental contamination rate was significantly lower for patients treated with fidaxomicin versus met/van on days 2–3 (21% vs 29%, respectively, P = .04), days 6–8 (14% vs 22%, respectively, P = .008) and days 9–12 (10% vs 21%, respectively, P < .001), with similar trends at days 4–5 (P = .20) and restricting to patients from Leeds.
Figure 3.
(A) Percentage of all environmental samples positive for Clostridium difficile over time from treatment initiation. (B) Percentage of all environmental samples positive for C difficile after end of treatment (EOT). Note: Positive and total sample numbers at each time point are shown at the top of each panel, together with comparisons of metronidazole/vancomycin (met\van) versus fidaxomicin (FDX), overall, and within Leeds only (L). CI, confidence interval; Pretrt, pretreatment.
After EOT, 905 environmental samples were taken from approximately 76 patients (mean 12/patient, range 4–25). Once treatment was stopped, the rate of environmental contamination appeared relatively constant, or if anything increased, regardless of drug, with no evidence of differences between fidaxomicin or met/van patients (Figures 2B, 3B; Supplementary Figure 1).
Skin Swabs
Between starting treatment and EOT, 705 skin swabs were obtained from 99 of 169 (59%) participants who consented. There was no evidence of difference in skin contamination rates between fidaxomicin and met/van patients overall (Supplementary Figure 3a) or for any individual sample type (Supplementary Figure 2), at any time point during treatment with 2 exceptions: lower contamination rates with fidaxomicin (27%) versus met/van (57%) on days 6–8 (P = .02) (Supplementary Figure 2e), and higher contamination rates on hands with fidaxomicin (29%) vs met/van (4%) at days 9–12 (P = .02) (Supplementary Figure 2g).
Fecal Samples
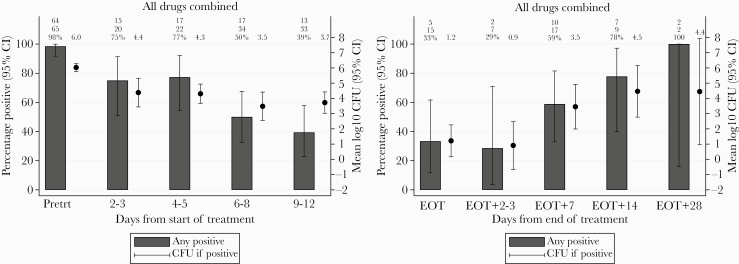
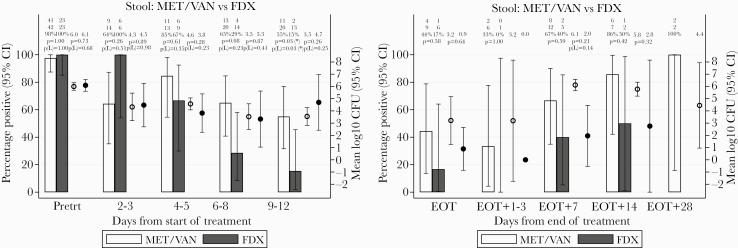
Between starting treatment and EOT, 300 stool samples were obtained from 129 of 169 (76%) of the consented participants. Immediately after initiation of treatment (fidaxomicin or met/van), there was a considerable impact on fecal spore counts with an abrupt decrease from a mean of 6.0 to 4.4 log10 cfu/mL but less than a 1.0 log10 cfu/mL further decrease over the following week (Figure 4A). There is a further decrease in fecal spore counts from a mean of 3.7 log10 cfu/mL on days 9–12 to a mean of 1.2 log10 cfu/mL at the EOT (Figure 4A and B). After EOT, 76 stool samples were obtained from 20 participants. It is interesting to note that there was a clear upward trend in both the percentage of samples positive for C difficile (by culture) and the spore counts within positive samples after completing treatment (Figure 4B).
Figure 4.
(A) Percentage of fecal samples positive for Clostridium difficile, and mean log10 colony-forming units (CFU)/mL C difficile within positive samples, after time from initiation of any treatment. (B) Percentage of fecal samples positive for C difficile, and mean log10 CFU/mL C difficile within positive samples, after end of treatment (EOT). CI, confidence interval; Pretrt, pretreatment.
Patients treated with fidaxomicin had lower fecal positivity rates from days 4 to 5 of treatment onwards, which reached statistical significance at days 9–12 (15% vs 55% in met/van, P = .03) (Figure 5A). However, there was no evidence of differences between colony counts in positive samples for the 2 treatment groups at any time point (Figure 5A). After EOT, colony counts in positive samples were consistently numerically lower by 2–4 log10 CFU/mL for patients treated with fidaxomicin compared with met/van, but there was no statistical evidence of difference, possibly due to low numbers (Figure 5B).
Figure 5.
(A) Percentage of fecal samples positive for Clostridium difficile, and mean log10 colony-forming units (CFU)/mL C difficile within positive samples, after time from initiation of metronidazole/vancomycin (met\van) versus fidaxomicin (FDX). (B) Percentage of fecal samples positive for C difficile, and mean log10 CFU/mL C difficile within positive samples, after end of treatment (EOT) with met/van versus fidaxomicin. CI, confidence interval; Pretrt, pretreatment.
Ribotyping
Overall, there were 559 pairs of ribotypes from a single colony and sweep from an environmental sample; 539 (96.4%) were identical (and one was a mixture on the sweep). Although there was no evidence that this varied by environmental site (P = .27) or treatment (P = .61), agreement was higher up to EOT (459 of 471, 97.5% identical) versus after EOT (80 of 88, 90.9%) (P = .007). Pooling all environmental ribotypes across sampling occasions, overall 365 of 400 (91.2%) sampling occasions had completely identical ribotypes, again greater up to EOT (304 of 328, 92.7%) versus after EOT (61 of 72, 84.7%) (P = .04). Likewise, 125 (91.9%) of 136 skin samples with paired ribotypes from a single colony and sweep were identical (plus 1 mixture on the sweep), as were 257 (95.5%) of 269 paired ribotypes from stool samples (plus 1 mixture on the sweep).
Comparing ribotypes from the first stool sample from 166 patients with skin/environmental samples (pretreatment in the vast majority, not available in all, 5 [3.0%] mixed ribotypes), all subsequent skin/environmental samples matched at least 1 baseline stool ribotype in 103 (62.0%) patients. Overall, 351 of 390 (90.0%) environmental sampling time points and 108 of 133 (81.2%) skin sampling time points matched at least 1 baseline stool ribotype, with again strong evidence that matching was lower after EOT for environmental time points (92.8% pre- vs 77.8% posttreatment [P = .001] compared with 82.1% pre- vs 77.8% posttreatment [P = .59] for skin time points). Considering individual samples, overall, 1244 of 1371 (90.7%) environmental samples, 298 of 350 (85.1%) skin samples, and 135 of 143 (94.4%) individual stool samples matched at least 1 baseline stool ribotype. Environmental and stool samples were significantly less likely to match the baseline ribotype after EOT (93.0% pre- vs 79.6% posttreatment [P < .001] and 98.0% pre- vs 86.7% posttreatment [P = .006], respectively), with a similar direction of effect in the smaller number of skin samples (86.1% pre vs 80.0% post, P = .24).
DISCUSSION
In this study, we found that C difficile environmental contamination of patient rooms was lower during fidaxomicin versus met/van treatment from approximately 4 days of treatment onwards, but it was similar between these agents after therapy was ended. There was a significant reduction in environmental contamination rates (at least 1 site positive) in rooms housing patients treated with fidaxomicin compared with those receiving met/van at multiple times after starting antibiotics. These results confirm those seen by Biswas et al [19], where there was a significant difference in the environmental contamination rates in rooms of patients treated with fidaxomicin versus met/van after 2–4 days of treatment (37% vs 58%, respectively, P = .02). Because we collected samples at several time points, we were able to show a downward trend in contamination rates over the duration of treatment for both met/van and fidaxomicin. One important limitation is that 1 study site predominantly used met/van, another predominantly used fidaxomicin, and a third recruited few patients. However, results were similar at the Leeds site, which used all 3 antibiotics and recruited 80% of patients. In addition, we also combined metronidazole and vancomycin treatment groups; time to resolution to diarrhea with these treatment options has been reported to be different [21, 22], but we found no evidence of a difference in our data (5 vs 6 days, respectively, P = .3).
The downward trend in environmental contamination rates during treatment was mirrored by the downward trend in fecal sample positivity, which again was lower in patients treated with fidaxomicin. However, it should be noted that although there was an initial large decrease in spore counts of 4–6 log10 cfu/mL after treatment initiation, the impact of treatment on further reductions in the bioload was smaller over the following week (<1.0 log10 cfu/mL). Because time to resolution of diarrhea was similar in both groups, increased environmental contamination in the met/van group is unlikely to be due to a prolonged period of diarrhea, indeed the median time to resolution was 5 days in this group compared with 6 days in the fidaxomicin-treated group (P = .4). This supports the hypothesis that fidaxomicin treatment reduces the microbial load within patients, and this in turn contributes to decreased spore shedding into the environment. Previous in vitro and in vivo studies have shown that fidaxomicin minimum inhibitory concentrations are sustained and that fecal colony counts are still lower than a comparator (vancomycin) 11–18 days after the EOT [14, 18]. However, there were no significant differences between spore counts in positive samples between 2 treatment groups at any time point, nor was there evidence of differences in skin contamination rates between groups. One important limitation is that the number of skin and stool samples collected in the study was much smaller than the number of environmental samples, and these comparisons may therefore be underpowered.
In addition, post-EOT colony counts were consistently lower for patients treated with fidaxomicin versus met/van, although the low number of samples meant that we were unable to exclude this being due to chance alone. It is possible that C difficile spores were present in samples, but spore outgrowth was prevented by fidaxomicin, as has been demonstrated in vitro [17], rather than there being a reduced number of spores. This reduced bioload did not translate to lower environmental contamination rates, however, because the rate of environmental and skin contamination appeared to increase again once treatment had been stopped, regardless of treatment, with no difference between fidaxomicin or met/van treated patients. More importantly, despite initial reduced bioload, patients continued to shed C difficile spores in their feces up to 28 days after treatment regardless of treatment choice; spore counts were initially lower in patients after EOT, for those treated with fidaxomicin compared with met/van, but counts in the fidaxomicin group increased from EOT+7 onwards. Again, the low number of samples collected towards the end of the study should caution strong conclusions here. All longitudinal studies have challenges with participants dropping out, as time progresses. An important limitation is that sampling after EOT was restricted to patients who remained in the hospital after finishing their initial treatment course, and therefore nonresponders are overpresented at later time points post-EOT. Thus, the increase in positivity post-EOT could partly reflect sampling bias towards these patients. In addition, results may not reflect the situation after EOT in patients well enough to be discharged home. However, even post-EOT, more than 80% of environmental and stool samples matched the patient’s baseline ribotype, suggesting that recovery of strains different to the index strain was making a relatively small contribution to the increases. In addition, fecal samples were difficult to obtain, again reducing power.
Our results have important implications for environmental cleaning of the surfaces around patients with CDI. All rooms in our study were cleaned (during and after the patient stay) with sporicidal cleaning agents, which have been shown to reduce the risk of environmental contamination with C difficile [23, 24], with similar times between cleaning and swabbing for both groups (6 vs 5 hours, P = .5). Despite this, environmental contamination continued in approximately one third to one half of all patients during and after treatment, suggesting that the environmental contamination seen here is due to continued shedding from patients and not residual contamination. This highlights the need for continued cleaning throughout a patient’s stay; indeed, recent focus on terminal room cleaning [23, 25] should be reviewed in light of this evidence. In addition, there is also evidence of environmental contamination from asymptomatic C difficile carriers, further emphasising the need for good, continued cleaning within hospital facilities, perhaps not only focused on those patients diagnosed with CDI [26].
Overall, we found that 5%–10% of samples from environmental sites, skin, and stool contained more than one ribotypes by comparing results from a single colony pick and a sweep, consistent with previously reported rates of mixed infection [27]. Given this, we did not attempt to restrict analyses of postbaseline contamination to identical ribotypes, because it is possible that ribotypes in the pretreatment stool could have been missed; moerover, baseline samples were not available for many patients. The fact that fewer samples post-EOT had identical ribotypes, both within the sampling time point and also compared with the baseline stool, supports ongoing contamination being a potential problem and a cause of onward transmission.
CONCLUSIONS
In summary, the results from our study suggests that environmental contamination from patients with CDI is likely to be reduced by treatment with fidaxomicin rather than met/van, although this effect may not persist after treatment has been completed. Our results underscore the need for continued optimal hygiene precautions even after diarrheal symptoms abate.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We like to thank the staff at the participating sites for their help with this study.
Author contributions. The study was conceived by M. H. W., D. M., and J. S. T. P., S. G., P. S., and D. M./K. D. acted as site lead for each of the 4 study sites. C. B. and D. M. wrote study paperwork, and C. B. cleaned data before analysis. Analysis was conducted by A. S. W. and K. D. The manuscript was prepared by K. D., A. S. W., and M. H. W. All authors reviewed the manuscript and commented before submission.
Disclaimer. The views expressed are those of the author(s) and not necessarily those of the NHS, the National Institute of Research Health (NIHR), or the Department of Health.
Financial support. This work was funded by an Investigator initiated grant from Astellas Pharma Europe. A. S. W. is supported by the Oxford Biomedical Research Centre and is an NIHR Senior Investigator.
Potential conflicts of interest. S. G. reports consultancy fees from Astellas, Enterobiotix, Menarini, MSD, Pfizer, Shionogi. D. M. has received travel grants from Astellas Pharma Europe. All have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Leggett MJ, McDonnell G, Denyer SP, et al. Bacterial spore structures and their protective role in biocide resistance. J Appl Microbiol 2012; 113:485–98. [DOI] [PubMed] [Google Scholar]
- 2. Mulligan ME, Rolfe RD, Finegold SM, Goerge WL. Contamination of a hospital environment by Clostridium difficile. Curr Microbiol 1979; 3:173–5. [Google Scholar]
- 3. McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med 1989; 320:204–10. [DOI] [PubMed] [Google Scholar]
- 4. Samore MH, Venkataraman L, DeGirolami PC, et al. Clinical and molecular epidemiology of sporadic and clustered cases of nosocomial Clostridium difficile diarrhea. Am J Med 1996; 100:32–40. [DOI] [PubMed] [Google Scholar]
- 5. Riggs MM, Sethi AK, Zabarsky TF, et al. Asymptomatic carriers are a potential source for transmission of epidemic and non-epidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis 2007; 45:992–8. [DOI] [PubMed] [Google Scholar]
- 6. Best EL, Fawley WN, Parnell P, Wilcox MH. The potential for airborne dispersal of Clostridium difficile from symptomatic patients. Clin Infect Dis 2010; 50:1450–7. [DOI] [PubMed] [Google Scholar]
- 7. Sethi AK, Al-Nassir WN, Nerandzic MM, et al. Persistence of skin contamination and environmental shedding of Clostridium difficile during and after treatment of C. difficile infection. Infect Control Hosp Epidemiol 2010; 31:21–7. [DOI] [PubMed] [Google Scholar]
- 8. Vonberg RP, Kuijper EJ, Wilcox MH, et al. ; European C difficile-Infection Control Group; European Centre for Disease Prevention and Control (ECDC) Infection control measures to limit the spread of Clostridium difficile. Clin Microbiol Infect 2008; 14(Suppl 5):2–20. [DOI] [PubMed] [Google Scholar]
- 9. Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments and outcomes. J Infect 2009; 58:403–10. [DOI] [PubMed] [Google Scholar]
- 10. Department of Health. Clostridium difficile infection: how to deal with the problem. Available at: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1232006607827. Accessed 27 May 2013.
- 11. Cohen SH, Gerding DN, Johnson S, et al. ; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–55. [DOI] [PubMed] [Google Scholar]
- 12. Optimer Pharmaceuticals. Optimer pharmaceuticals and astellas receive positive opinion from CHMP for European approval of DIFICID Available at: https://www.prnewswire.com/news-releases/optimer-pharmaceuticals-and-astellas-receive-positive-opinion-from-chmp-for-european-approval-of-dificid-130413033.html. Accessed 13 June 2013.
- 13. Federal Drug Administration. FDA approves treatment for Clostridium difficile infection. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/201699s000lbl.pdf. Accessed 13 June 2013.
- 14. Louie TJ, Miller MA, Mullane KM, et al. ; OPT-80-003 Clinical Study Group Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–31. [DOI] [PubMed] [Google Scholar]
- 15. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012; 12:281–9. [DOI] [PubMed] [Google Scholar]
- 16. Louie TJ, Emery J, Krulicki W, et al. OPT-80 eliminates Clostridium difficile and is sparing of bacteroides species during treatment of C. difficile infection. Antimicrob Agents Chemother 2009; 53:261–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allen CA, Babakhani F, Sears P, et al. Both fidaxomicin and vancomycin inhibit outgrowth of Clostridium difficile spores. Antimicrob Agents Chemother 2013; 57:664–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chilton CH, Crowther GS, Todhunter SL, et al. Fidaxomicin persistent in an in-vitro human gut model and on Clostridium difficile spores. ECCMID 2013. 23rd European Congress of Clinical Microbiology and Infectious Diseases (Berlin). April 27–30, 2013. [Google Scholar]
- 19. Biswas JS, Patel A, Otter JA, et al. Reduction in Clostridium difficile environmental contamination by hospitalised patients with fidaxomicin. J Hosp Infect 2015; 90:267–70. [DOI] [PubMed] [Google Scholar]
- 20. Stubbs SL, Brazier JS, O’Neill GL, Duerden BI. PCR targeted to the 16S-23S rRNA gene intergenic spacer region of Clostridium difficile and construction of a library consisting of 116 different PCR ribotypes. J Clin Microbiol 1999; 37:461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al-Nassir WN, Sethi AK, Nerandzic MM, et al. Comparison of clinical and microbiological response to treatment of Clostridium difficile-associated disease with metronidazole and vancomycin. Clin Infect Dis 2008; 47:56–62. [DOI] [PubMed] [Google Scholar]
- 22. Gerding DN, Meyer T, Lee C, et al. Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C. difficile infection: a randomized clinical trial. JAMA 2015; 313:1719–27. [DOI] [PubMed] [Google Scholar]
- 23. Wong T, Woznow T, Petrie M, et al. Postdischarge decontamination of MRSA, VRE, and Clostridium difficile isolation rooms using 2 commercially available automated ultraviolet-C-emitting devices. Am J Infect Control 2016; 44:416–20. [DOI] [PubMed] [Google Scholar]
- 24. Fawley WN, Underwood S, Freeman J, et al. Efficacy of hospital cleaning agents and germicides against epidemic Clostridium difficile strains. Infect Control Hosp Epidemiol 2007; 28:920–5. [DOI] [PubMed] [Google Scholar]
- 25. Barbut F. How to eradicate Clostridium difficile from the environment. J Hosp Infect 2015; 89:287–95. [DOI] [PubMed] [Google Scholar]
- 26. Gilboa M, Houri-Levi E, Cohen C, ShIC research group , et al. Environmental shedding of toxigenic Clostridioides difficile by asymptomatic carriers: a prospective observational study. Clin Microbiol Infect 2020; 6:1052–7. [DOI] [PubMed] [Google Scholar]
- 27. Dayananda P, Wilcox MH. A review of mixed strain Clostridium difficile colonization and infection. Front Microbiol 2019; 10:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.