Abstract
Introduction
Approximately one million undiagnosed persons living with HIV in Southern and Eastern Africa need to test for HIV. Novel approaches are necessary to identify HIV testing options that match the heterogeneous testing preferences of high-risk populations. This pragmatic randomised controlled trial (PRCT) will evaluate the efficacy of a preference-informed, heterogeneity-focused HIV counselling and testing (HCT) offer, for improving rates of HIV testing in two high-risk populations.
Methods and analysis
The study will be conducted in Moshi, Tanzania. The PRCT will randomise 600 female barworkers and 600 male Kilimanjaro mountain porters across three study arms. All participants will receive an HIV testing offer comprised of four preference-informed testing options, including one ‘common’ option—comprising features that are commonly available in the area and, on average, most preferred among study participants—and three options that are specific to the study arm. Options will be identified using mixed logit and latent class analyses of data from a discrete choice experiment (DCE). Participants in Arm 1 will be offered the common option and three ‘targeted’ options that are predicted to be more preferred than the common option and combine features widely available in the study area. Participants in Arm 2 will be offered the common option and three ‘enhanced’ options, which also include HCT features that are not yet widely available in the study area. Participants in Arm 3, an active control arm, will be offered the common option and three predicted ‘less preferred’ options. The primary outcome will be uptake of HIV testing.
Ethics and dissemination
Ethical approval was obtained from the Duke University Health System IRB, the University of South Carolina IRB, the Ethics Review Committee at Kilimanjaro Christian Medical University College, Tanzania’s National Institute for Medical Research, and the Tanzania Food & Drugs Authority (now Tanzania Medicines & Medical Devices Authority). Findings will be published in peer-reviewed journals. The use of rigorous DCE methods for the preference-based design and tailoring of interventions could lead to novel policy options and implementation science approaches.
Trial registration number
Keywords: hiv & aids, public health, public health, statistics & research methods, health economics
Strengths and limitations of this study.
The pragmatic randomised controlled trial described in this protocol paper includes males and females at high risk of HIV infection; the implementation of the trial in collaboration with all HIV testing providers in the study area allows for the evaluation of testing uptake in a nearly closed system.
The study goes beyond the traditional approach of evaluating single-offer (‘one-size-fits-all’) interventions by identifying combinations of testing options that explicitly target preference heterogeneity in the target population.
The methods used to identify the intervention conditions evaluated in the trial, including the latent class analysis of data from the discrete choice experiment (DCE) used to elicit heterogeneous population preferences for HIV testing, may be applied to other contexts and may lead to the development of new implementation science approaches for systematically adapting effective interventions to local contexts.
The study design will allow for separate estimates of the effects of short messaging system (SMS) reminders, the issuance of HIV testing invitation cards, the heterogeneity-focused testing offer, and an incentive offer on HIV testing rates.
Potential limitations include loss to follow-up during the multiphase study, the finite range of HIV testing characteristics that can be included in a DCE, ordering effects and exogenous events during the study period that may influence rates of HIV testing across study arms, and limited generalisability of specific study findings to other populations and settings.
Background
In 2018, 37.9 million people were living with HIV worldwide, and 770 000 died of HIV-related illnesses.1 HIV counselling and testing (HCT) is a cost-effective intervention for increasing HIV serostatus awareness,2 3 a point of entry into HIV care and treatment, and an important means of primary and secondary HIV prevention.4 HIV Prevention Trials Network Protocol 052 conclusively demonstrated a marked reduction in HIV transmission among serodiscordant couples in which the HIV-infected partner was begun on antiretroviral therapy early in the course of infection.5 Subsequently, public health officials and policymakers, considering treatment as prevention, have called for dramatic increases in HIV testing—as frequently as annually in many populations and semiannually among individuals at high risk.6
In 2014, the Joint United Nations Programme on HIV/AIDS (UNAIDS) set for 2020 the ambitious 90-90-90 target: diagnosing 90% of all persons living with HIV (PLWH), initiating treatment for 90% of those diagnosed, and achieving viral suppression for 90% of those treated.7 While substantial progress has been made towards these targets since 2014, most countries remain short of at least one target, and the number of undiagnosed HIV infections in every region are considered a major hindrance to achieving the UNAIDS targets and ending the epidemic.8 Novel approaches are needed to increase testing uptake, especially among high-risk groups.
In order to establish the diagnosis of HIV in 90% of all PLWH in Eastern and Southern Africa, more than 1 million undiagnosed infected persons need to test, including 190 000 in Tanzania.4 6 9 Tanzania’s 2017–2022 Health Sector HIV and AIDS Strategic Plan (HSHSP-IV) lists as a key challenge that HIV testing services ‘need to be more efficient and ambitious to meet the 90-90-90 targets through more targeted testing approaches.’10 Evaluations of population preferences for testing have typically focused on the acceptability of specific testing options, such as home-based,11–13 provider-initiated,14–17 or workplace testing,18 19 usually without consideration or offer of other options. Results from these narrow assessments do not probe the potential diversity in testing preferences among target populations and cannot characterise which testing options will maximise uptake of testing.20–22
Discrete choice experiments (DCEs), grounded in the economic theory of utility maximisation, are specifically designed to provide information about individuals’ preferences for varying characteristics of multiattribute products. The DCE method is based on the assumption that a product or service such as HCT can be described in terms of its characteristics, namely attributes and levels within attributes. Participants are repeatedly asked to choose between two or more alternatives in choice scenarios simulating real choice decisions. Each alternative differs in the arrangement of attribute levels presented to the participant. The choice scenarios are systematically varied by means of an experimental design.23–26 Relative attribute importance, the utility that respondents derive from the diverse options, and trade-offs, that is, the willingness to trade between attribute levels, can be quantified analytically.27 DCEs are used increasingly to understand patient perspectives and to design patient-centred interventions. Although DCEs have been used in various contexts related to HIV, including testing,20 28–32 prevention,33–36 service delivery,37–39 and treatment,40–44 to our knowledge, DCEs have not yet been used to systematically design HCT interventions.
Below, we describe the study protocol for a pragmatic randomised controlled trial (PRCT) that evaluates the efficacy of a targeted, preference-informed HCT offer for improving rates of HIV testing in high-risk populations. The testing offer is developed using data from a DCE and designed to match the heterogeneous HIV testing preferences in the target population. To our knowledge, this is the first PRCT in which the study conditions are optimised using data from a DCE, and the first PRCT that evaluates an intervention explicitly targeting preference heterogeneity.
Methods and analysis
Study aim and hypothesis
The aim of this study is to evaluate the efficacy of a preference-informed, heterogeneity-focused HCT offer for improving rates of HIV testing among two high-risk populations. We hypothesise that an HCT offer matched to the specific preferences of the intended target population and explicitly accounting for preference heterogeneity within these populations will increase rates of testing relative to a control offer.
Study setting
The study is conducted in Moshi, Tanzania. Moshi is the commercial centre and administrative capital of the Kilimanjaro Region in Northern Tanzania and has an estimated population of about 200 000.45 Moshi has 25 HCT facilities, including 8 care and treatment centres (CTCs), which provide free HIV care to PLWH.46 The study is implemented with support from the Regional Medical Officer and the Regional AIDS Control Coordinator of the Kilimanjaro Region.
Study participants
The study population comprises women employed in bars, restaurants, and guesthouses serving alcohol to patrons (‘female barworkers’, FBW) and male mountain porters who are supporting climbers of nearby Mount Kilimanjaro (‘Kilimanjaro mountain porters’, KMP). The Regional AIDS Control Coordinator identified these groups as populations at high risk of HIV infection who could benefit from increased rates of testing; we subsequently showed that FBW and KMP engage in higher rates of HIV risk behaviours than randomly selected male and female community members in the same setting.20 For example, compared with randomly selected community members, FBW and KMP reported 2–3 times as many lifetime sexual partners, higher rates of sexually transmitted illnesses, and higher rates of having sex in exchange for money or gifts, but similar numbers of lifetime HIV tests.20 A census of bars and female barworkers, conducted by the study team between February and June of 2016, identified 612 venues within Moshi, with 2 059 age-eligible FBW. There are an estimated 10 000 KMP in the Kilimanjaro Region.47 48
Inclusion criteria
Eligible study participants are ages 18 or older, reside in Moshi, are able to read, and have no concrete plans to leave the study area during the 12–15-month period following study enrolment.
Outcome measure
The study outcome of interest is uptake of HIV testing. During the multiphase study (see below), the outcome is ascertained repeatedly by counsellors’ documentation of participants’ HIV tests, self-reports from study participants, or both. In the PRCT and one preceding study phase, coded HIV testing invitation cards will be distributed to participants and HIV tests will be tracked on the basis of cards returned to any HIV testing centre in the study area. Self-reports capture tests outside the study area and tests without cards. The primary outcome measure is counsellor-documented uptake of testing. A secondary outcome measure is counsellor-documented or self-reported uptake of testing.
Study design
The study is comprised of five sequential phases (figure 1). The target duration for each phase is 13 weeks (91 days).
Figure 1.
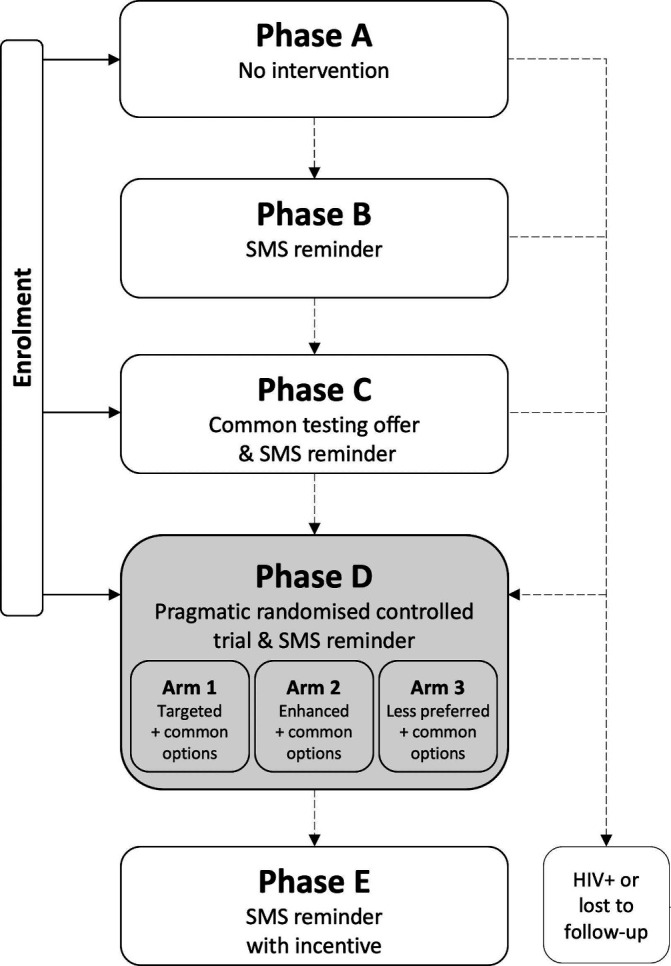
Study design. SMS, short messaging system.
Phase A: reference phase
Phase A includes no intervention. The purpose of this phase is to inform estimates of background rates of HIV testing among individuals participating in a research study focusing on HIV testing. A phone survey after 13 weeks (91 days) will ask participants about any HIV test during Phase A.
Phase B: SMS phase
In Phase B, a short messaging system (SMS) reminder message to test for HIV will be sent to participants 4 weeks (28 days) after the beginning of Phase B. The purpose of this phase is to inform estimates of the effect of an SMS reminder on rates of HIV testing. A phone survey after 13 weeks (91 days) will ask participants about any HIV test during Phase B.
Phase C: invitation phase
In Phase C, participants will be given a credit card-sized invitation card describing an HIV testing option that combines features commonly available in the study area, and that, on average, are most preferred among study participants (‘common option’). Four weeks (28 days) after the beginning of Phase C, participants will be sent an SMS reminder to test for HIV as shown on the invitation card given to them. The purpose of this study phase is to inform estimates of the effect of a testing invitation on rates of HIV testing. A phone survey after 13 weeks (91 days) will ask participants about any HIV test during Phase C.
Phase D: the pragmatic randomised controlled trial
Phase D is a PRCT that includes three parallel study arms (table 1). All participants will receive an HIV testing offer comprised of four invitation cards describing preference-informed HIV testing options. Participants will be asked to test for HIV using their individually most preferred of the four testing options given to them. Options will be identified using mixed logit and latent class analyses of data from a DCE with members of the target populations (see below). Arm 1 participants will be offered the common option and three ‘targeted’ options, predicted to be jointly more preferred than the common option and comprising testing features widely available in the study area. Arm 2 participants will be offered the common option and three ‘enhanced’ options, which are also predicted to be jointly more preferred than the common option but include additional features that are not yet widely available in the study area. Arm 3 participants will be offered the common option and three options that are jointly predicted to be ‘less preferred’ than the common option. In other words, for arm 3 participants, the common option is the predicted most preferred of the four options; the other three options, on average, provide no additional value. Arms 1 and 2 are intervention arms. Arm 3 represents an active control arm: study involvement in Arm 3 is the same as in Arms 1 and 2. Four weeks (28 days) after the beginning of Phase D, participants will be sent an SMS reminder to test for HIV using any of the testing options given to them. The purpose of this study phase is to obtain estimates of the effect of a heterogeneity-focused HIV testing offer on rates of HIV testing. A phone survey after 13 weeks (91 days) will ask participants about any HIV test during Phase D.
Table 1.
HIV testing options offered across the three study arms in the pragmatic randomised controlled trial
| Arm | Offers | Description |
| 1 | One common option | Combines the on average most preferred levels of each attribute included in the DCE, as described by the mean parameter estimates from a mixed logit model. |
| Three targeted options | Comprise features widely available in the study area. The targeted options are predicted to be jointly more-preferred than the common option by the largest possible share of participants. | |
| 2 | One common option | Combines the on average most preferred levels of each attribute included in the DCE, as described by the mean parameter estimates from a mixed logit model. |
| Three enhanced options | Includes additional features that are not yet widely available in the study area. The enhanced options are predicted to be jointly more-preferred than the common option by the largest possible share of participants. | |
| 3 | One common option | Combines the on average most preferred levels of each attribute included in the DCE, as described by the mean parameter estimates from a mixed logit model. |
| Three less preferred options | Includes options that are widely available in the study area and jointly predicted to be equally or less-preferred than the common option by the largest possible share of participants. |
DCE, discrete choice experiment.
Phase E: incentive phase
In phase E, participants will be offered an incentive to test for HIV using their choice of any of the testing options remaining to them from Phase D. An SMS reminder will be sent to participants 4 weeks (28 days) after the beginning of Phase E. The purpose of this phase is to inform estimates of the effect of a conditional financial transfer (CFT) offer on testing decisions and identify the most preferred testing option among those offered, among participants who did not test during Phase D.
The study design will allow for separate estimates of the effects on HIV testing rates of:
an SMS reminder message,
a testing invitation,
a heterogeneity-focused testing offer, and
a CFT offer.
Assignment to study arms
Participant IDs will be randomly assigned to study arms using a random number generator. The testing offer in Phase D will reflect the study arm assigned to the respective Participant ID. The random assignment is expected to result in approximately equal numbers of participants in each study arm.
Design of the intervention
Overview
A DCE will be used to elicit information on the distribution of preferences for feasible and modifiable characteristics of HIV testing options in the target population. DCE data will be analysed, and results of these analyses will be used to identify four types of testing options that will be offered to participants in the PRCT:
A ‘common’ option. This single testing option combines testing features that are widely available in the study area, and, on average, are most preferred among study participants. This option will be offered to all participants in Phases C and D.
Three ‘targeted’ options. This set of testing options, comprising features widely available in the study area, is predicted to be jointly more preferred than the common option by the largest possible share of participants.
Three ‘enhanced’ options. Enhanced testing options include additional features that are not yet widely available in the study area (eg, oral testing). The set of enhanced testing options is predicted to be jointly more preferred than the common option by the largest possible share of participants.
Three ‘less preferred’ options. This set of testing options includes options that are predicted to be equally or less preferred than the common option by the largest possible share of participants.
The design decision to offer three targeted, enhanced, and less preferred options was driven by practical considerations: (1) a choice from four alternatives (the common option plus three options specific to the study arm) is expected to be cognitively feasible for participants, (2) the implementation of 10 testing options (one common option, plus three targeted, three enhanced and three less preferred options) as part of this study is feasible from a logistical and budgetary perspective, and (3) the widespread implementation of three testing options that target preference heterogeneity is feasible in the study area. The statistical analysis of the DCE data (see below) will determine whether the testing offers differ between FBW and KMP.
Development and fielding of the DCE
A DCE with 300 FBW and 300 KMP recruited prior to the PRCT will characterise the patterns and variability in HIV testing preferences in the target population. The DCE development will follow guidelines and procedures established in our prior studies of HIV testing preferences.21 23 49 Focus group discussions with members of the target populations will be used to prioritise HIV testing features with respect to their expected influence on HIV testing decisions and to establish levels of features that represent plausible trade-offs in actual or hypothetical HIV testing interventions. Reconciling prior qualitative work22 with the objectives of the PRCT, the DCE is expected to include feasible attributes and levels across three domains: privacy and confidentiality (eg, testing venue, different types of counselling), accessibility and value (testing availability, additional services provided) and perceived quality and accuracy (eg, type of sample for the HIV test).
In the DCE survey, respondents will be introduced to each attribute and level and asked to complete 12–16 choice tasks. Each choice task will include three hypothetical testing options; participants will be asked to identify their preferred alternative. The combination of alternatives presented to respondents as part of the DCE will be varied according to a d-efficient statistical design,50 generated in Ngene software (ChoiceMetrics). Survey content and presentation will be tested in up to 40 guided individual pretest interviews. Pilot studies with at least 200 participants will yield statistical priors that inform the statistical design of the final DCE. DCE surveys will be administered in-person, in Kiswahili, using tablet devices, by trained research staff using the custom-built survey software, comet (Selway Labs).
Continuous recruitment and enrolment may result in overlap between DCE survey respondents and PRCT participants. All PRCT participants will complete the DCE survey to allow for comparisons of stated preferences (DCE survey responses) and revealed preferences (testing decisions).
Analysis of DCE data
The analysis of DCE data will follow established guidelines.23 49 To estimate mean (average) preferences in the study population, DCE data will be first analysed in Stata (StataCorp) using mixed, or random parameters, logit models,51 which are commonly used for analysing DCE data,52 but focus on average preferences. To model systematic variation in preferences across respondents, a random effects latent class logit (RELCL) model will be estimated in Latent Gold Choice V.5.0 (Statistical Innovations 2018). RELCL models allow for the joint modelling of systematic variation in preferences (latent classes) and random variation in preferences (random effects) across respondents.53 The Bayesian Information Criterion will be used to identify which model yields the best fit for the data.
To evaluate whether the distribution of preferences differs significantly between the two groups of participants (FBW versus KMP), participant type will be included in the model as a covariate. If the distributions of preferences differ significantly across groups, separate preference-informed testing options may need to be identified for FBW and KMP. On the other hand, if the preference distributions are broadly similar, the testing offer can be optimised for the joint preferences of both populations.
Selection of testing options for inclusion in the PRCT
Results from the mixed logit model will be used to identify the common option; results from the best-fitting latent class model will be used to identify the combinations of targeted, enhanced and less-preferred options to be in included in the PRCT.
Common option
The common option will combine the most preferred (on average) levels of each attribute included in the DCE, as described by the mean parameter estimates from the mixed logit model.
Targeted, enhanced and less-preferred options
The latent class analysis will identify statistical groupings of individuals with similar sets of preferences; these groupings are referred to as classes. Using parameter estimates from the latent class model, we will predict class-specific relative preferences for all feasible combinations of feature levels (ie, testing options), which, in turn, will be converted into predicted choice probabilities in a simulated choice between the respective testing option and the ‘common option’. Class-specific predicted choice probabilities will be aggregated across classes (taking into consideration the estimated class sizes) to calculate the share of the population predicted to prefer each testing option over the common option. These shares are used, as follows, to generate population-based rankings of all feasible combinations of three testing options. For targeted options, we will select from all options that combine features currently available in the study area the three options that jointly maximise the share of participants predicted to choose at least one of those three targeted options over the common option. Similarly, for enhanced options, we will select from all options that include additional features not yet widely available in the study area the three options that jointly maximise the share of participants predicted to choose at least one of those three enhanced options over the common option. For less preferred options, we will select the three options that jointly maximise the share of participants predicted to prefer the common option over all less preferred options.
Presentation of testing options to study participants
Testing options will be presented to participants on invitation cards. Each participant will be given four cards; each card will describe the characteristics of the testing option in a format similar to that presented in the DCE. The combination of cards given to a participant will be determined by the study arm assigned to the participant; references to specific testing venues may be varied according to participants’ location of residence or preferred testing venue. Cards will have unique codes that allow for the tracking of participants’ testing uptake across testing venues in the study area.
SMS delivery
SMS messages will be sent via a highly versatile, low-cost, mHealth system, called mobile phone based appointment reminder and incentive system (mParis), which can autonomously send large numbers of SMS messages according to prespecified algorithms and is based in the study area.54 55
Testing incentive
During Phase E, an incentive in the amount of TSH 5000 (~US$2.20) will be given in cash to participants presenting for testing with a coded testing invitation card at any of the testing venues in the study area. The amount is based on a willingness-to-accept study previously conducted in the same area.56
Sample size
The target sample size for the PRCT is 1200 participants, comprising equal numbers of FBW and KMP. Randomisation across study arms is expected to result in three groups with approximately 400 participants each.
Recruitment
Participants for formative work will be recruited using convenience and snowball sampling. For DCE surveys and the PRCT, the goal is to employ a systematic recruitment approach that minimises biases. Mountain porters will be recruited from the Mweka gate of Kilimanjaro National Park. The Mweka gate is selected because of its proximity to Moshi (~15 km); four of six popular climbing routes descend through this gate. Porters exiting the gate will be approached sequentially, and eligible porters will be handed an invitation card containing contact information and an invitation to the study’s research office for consent and enrolment. For the recruitment of female barworkers, bars will be randomised and visited in the order of randomisation. Eligible FBW will be consented at their place of work or given invitation cards containing contact information and an invitation to the study’s research office for consent and enrolment. Recruited participants may receive reminder phone calls or SMS messages to come to the study offices for more information and study enrolment.
Enrolment and informed consent
Eligible individuals contacted for participation in the study will be informed by trained study personnel of the study purpose, procedures, risks, and benefits during the informed consent process. Only consenting individuals will be included in the study. Study participants’ mobile phone numbers and the name and phone number of a contact person through whom they can be reached will be recorded to allow for phone-based follow-up.
Enrolment into the trial will be conducted in three sequential stages. Approximately half the participants will be enrolled into Phase A and one quarter each into Phases C and D. This approach ensures variation in the exposure to pre-PRCT intervention components across participants, thereby allowing for the estimation of potential ordering effects as participants move through the different study phases. The staggered enrolment also ensures a better alignment of study timelines for Phases D and E across participants.
Blinding
Participants will be blinded with respect to their assignment across the three study arms. While research staff are not blinded to participants’ study arm assignment, study procedures are the same for all arms except for the characteristics of the testing offer.
Study activities
Study activities and their schedule are shown in table 2.
Table 2.
Schedule of activities
| Phase | Time point | Target timing | Key activity | Key information collected |
| A | tA | Enrolment | Baseline survey | HIV testing preferences, history, HIV risk, sociodemographics |
| tAfu = tB* | tA + 91 days | Phone-based FU | HIV testing uptake since tA | |
| B | tBs | tB + 28 days | SMS reminder | |
| tBfu | tB + 91 days | Phone-based FU | HIV testing uptake since tB | |
| C† | tBx = tC | tBfu + <91 days | Testing invitation (‘common’ option) | |
| tCs | tC + 28 days | SMS reminder | ||
| tCfu | tC + 91 days | Card collection from testing sites, phone-based FU | HIV testing uptake since tC | |
| D† | tCx = tD | tCfu + <91 days | Four testing invitations, study arm specific | |
| tDs | tD + 28 days | SMS reminder | ||
| tDfu | tD + 91 days | Card collection from testing sites, phone-based FU | HIV testing uptake since tD | |
| E | tE | tDfu + <91 days | Phone call and SMS message with incentive offer | |
| tEs | tE + 28 days | SMS reminder | ||
| tEfu | tE + 91 days | Card collection from testing sites, phone-based FU | Choice among testing options offered, HIV testing uptake since tE |
*The phone-based follow-up at the end of Phase A constitutes the beginning of Phase B.
†To reduce variability across participants in the timing of Phase D, some participants will be enrolled directly into Phases C and D.
FU, follow-up; SMS, short messaging system.
Participants providing informed consent will be enrolled in the study. At the time of enrolment, a baseline survey will be conducted with all participants to assess sociodemographic characteristics, testing history, testing preferences, HIV serostatus, and HIV risk.
After enrolment, participants will progress through up to five study phases. Phase A represents a no-intervention phase. Phase B starts with the completion of the Phase A follow-up survey. Phase C starts with the distribution of an invitation card that describes the ‘common’ option. Phase D starts with the distribution of four invitation cards that describe the preference-informed HIV testing options, namely the ‘common’ option and three ‘targeted’, ‘enhanced’, or predicted ‘less preferred’ options, depending on the study arm. Phase E starts with a phone call or SMS message offering a financial incentive to test. SMS reminder messages will be sent 28 days after the beginning of Phases B, C, D and E. Phases A and B will end with a short phone-based survey with study participants. Phases C, D and E will end with a phone-based survey or the collection of a testing invitation card from testing sites, whichever occurs earlier. After the completion of Phases B and C, participants will be contacted by phone and SMS and invited to come to the local study office for follow-up.
HIV testing will be done in accordance with Tanzania’s National AIDS Control Program (NACP) guidelines.57 Since 2013, Tanzania’s National Comprehensive Guidelines for HIV Testing and Counselling describe specific retesting intervals ranging from 4 weeks to 6 months for most persons at elevated risk of HIV infection.58 Our own survey of HIV testing sites in the study area revealed that most counsellors continue to recommend retesting after 3 months for all clients testing negative for HIV, regardless of risk. As per NACP guidelines, participants testing positive for HIV will be linked to care at a local CTC. Participants who report having tested positive for HIV, or those for whom documentation of a positive HIV test is collected from testing sites, will discontinue participation in HIV testing related components of the study.
Study timeline
The schedule of activities implies a minimum time of 15 months for participants to progress through all five study phases. Delays in reaching participants by phone and delays in participants returning to study offices will extend the duration of follow-up. In order to minimise loss to follow-up prior to the PRCT and reduce variability in the timing of the PRCT across participants, all participants in Phases A, B or C who are 91 or more days late for a follow-up assessment will transition to Phase D during their next in-person visit. Additionally, participants may be directly enrolled into Phases C and D (figure 1). Study enrolment will continue until the target number of n=1200 participants in the PRCT (Phase D) has been reached. Follow-up will continue until 6 months after the last participant enters Phase D.
Participant retention
To maximise retention, study participants due for follow-up may receive multiple phone calls and SMS reminders to come to the study offices. Escalating incentives, that is, incentive amounts that increase across consecutive study phases, will be used. The effect of selective attrition on estimates will be evaluated in sensitivity analyses (see below).
Statistical analysis
The primary analysis involves the comparison of testing rates between study arms in Phase D. The effect of the intervention—a preference-informed, heterogeneity-focused, HIV testing offer—will be described by differences in testing uptake between those offered targeted or enhanced options, relative to those offered predicted less preferred options. Statistical significance will be evaluated in a bivariate analysis using a χ2 test. Logistic regression analysis will evaluate the statistical significance of differences in a multivariate framework. Uptake of HIV testing within 3 months of the beginning of Phase D will be the binary outcome variable; study arm will be the key explanatory variable. Systematic variation in the efficacy of the intervention, for example, by gender or with HIV risk, can be modelled using interactions between study arm and the respective covariates.
Survival models with up to five observations per participant (one each for Phases A, B, C, D and E) will be used to estimate the differential effects of study arm assignment, SMS reminders, invitations, and conditional financial incentives, on rates of HIV testing. The time until an HIV test following the beginning of the respective study phase constitutes the dependent variable. ‘Exposure’ to SMS reminders, invitations, and a financial incentive are hypothesised to increase the ‘hazard’ of testing relative to no intervention. To control for potential ordering effects, participants exposure to intervention components in prior study phases (Phase B and C SMS reminders, Phase C testing offer, recent testing uptake) will be included as covariates.
Statistical power
DCE
Statistical power in DCEs varies with sample size, the number of choice tasks, the number of alternatives per task and the number of attributes and levels, among other characteristics. An empirical power-test formula by Yang et al59 suggests that the DCE sample size (n=600) allows us to estimate the utility difference between the most and least-preferred testing options with a precision that is better than that of ‘the average’ DCE study. A sample size guidance by Orme60 suggests that the two study populations (n=300 each) are sufficiently large to derive independent estimates for each sub-cohort.
PRCT
The sample size for the three-arm trial (n=1200) was selected to ensure adequate statistical power to identify the statistical significance of policy-relevant differences in testing uptake between study arms. We expect testing rates in Arm 3 to range from 25% among porters (as in our preliminary data) to 40% among barworkers (lower than the 59% in our preliminary data where barworkers were enrolled at a health facility).20 Assuming an equal split between study arms, 400 participants per arm yield 65%–72% power to detect a difference of 10 percentage points, 94%–96% power for a difference of 15 points and >99% power for difference of 20 percentage points between the targeted, respectively enhanced, arms and the comparison arm (alpha=0.05, two-sided).
Reporting of results
Methods and results will be reported in accordance with the CONSORT reporting guidelines and its extensions for pragmatic randomised controlled trials (see online supplemental files 1–4).61
bmjopen-2020-039313supp001.pdf (48.7KB, pdf)
bmjopen-2020-039313supp002.pdf (21KB, pdf)
bmjopen-2020-039313supp003.pdf (64.5KB, pdf)
bmjopen-2020-039313supp004.pdf (175.4KB, pdf)
Sensitivity analyses
Extensive sensitivity analyses will describe the sensitivity of our estimates to the definition of the outcome variable, model specification and selective attrition. Estimates from the analysis of the secondary outcome measure (counsellor-documented or self-reported testing uptake) will be presented alongside the analysis of the primary outcome measure (counsellor-documented HIV testing). The DCE choice data will be analysed using a broad range of models in order to describe the sensitivity of the selected PRCT testing offers to model specification and assumptions. The effect of attrition will be estimated by modelling attrition as a function of observable characteristics at the time of enrolment and weighing individual-level predictions of the intervention effect by the inverse probability of attrition. Differences between the average intervention effect and the attrition-weighted average effect will characterise the effects of selective attrition on our estimates.
Data security and confidentiality
A research data security plan will ensure that data are kept in compliance with relevant privacy regulations, including HIPAA; access to identifying information will be strictly limited. Study personnel will be instructed to keep the identity of all research subjects confidential and will sign confidentiality agreements.
Monitoring and quality assurance
Adherence to intervention protocols and the completeness and quality of study data will be monitored by the principal investigators and a study monitor. Electronic data capture on tablet devices and daily uploads to secure servers allow for the continuous monitoring of study activities in near real time. All paper documents will be scanned. Rigorous quality assurance/quality control procedures will be established, including interviewer observation, validation and range checks during data entry, verification of entered data and the monitoring of time stamps for DCE choice tasks.
Patient and public involvement
Focus group discussions with members of the target populations will be used to prioritise HIV testing features with respect to their expected influence on HIV testing decisions and to establish levels of features that represent plausible trade-offs in actual or hypothetical HIV testing interventions. The results will inform the development of the DCE and the testing options in the PRCT.
Ethics and dissemination
The protocol was registered in ClinicalTrials.gov (Protocol NCT02714140) on 21 March 2016. The protocol was approved by the Institutional Review Boards at Duke University (Duke University Health System IRB, Protocol Pro00075996) and the University of South Carolina (University of South Carolina IRB, facilitated review, Pro00060760) in the USA as well as the Ethics Review Committee at Kilimanjaro Christian Medical University College (Protocol #901), the National Institute for Medical Research (NIMR/HQ/R.8a/Vol. IX/2603) and the Tanzania Food & Drugs Authority (now Tanzania Medicines and Medical Devices Authority, Authorization No. TZ18CT0017). Protocol amendments will be submitted to these entities as required. Findings will be published in peer-reviewed journals. The use of rigorous DCE methods for the preference-based design and tailoring of interventions could lead to novel policy options and implementation science approaches.
Discussion
This study will evaluate whether an HIV testing intervention, which is uniquely designed using data from a DCE and explicitly targets preference heterogeneity, will improve testing uptake. If testing rates differ between study arms, the results will support our hypothesis that DCE-derived preference data can be used to systematically design HIV testing interventions that target heterogeneous preferences among and within high-risk populations and that offering such interventions will increase testing uptake in target populations. With novel approaches to testing urgently needed to reach the 90-90-90 targets, the DCE and targeted methods used in this study may be broadly used to develop cost-effective testing offers that match the preferences of high-risk populations across diverse settings.
To our knowledge, this is the first PRCT in which the intervention conditions are designed using data from a DCE, and the first PRCT that evaluates an intervention explicitly targeting preference heterogeneity. If successful, the methods used to understand how different groups of users value key characteristics of a health intervention can readily be applied to other settings in which interventions are being developed or adapted to optimise their efficacy. This work may demonstrate the utility of DCEs as a tool in implementation research to replace the costly practice of iteratively evaluating narrowly focused interventions. Thus, even as we apply this approach to the specific area of HIV testing, the study has potential to significantly advance the fields of patient-oriented research and implementation science. The methods could be used to develop new approaches to adapt effective interventions to local contexts, by informing a priori which interventions should be rolled out, and with which modifications, in order to maximise uptake across different populations and subpopulations.
Our study design and implementation approach have several unique components. First, the implementation of the study, in collaboration with all HCT providers in the study area, allows for the evaluation of testing uptake in a nearly closed system. Second, the use of an automated mHealth system to send large numbers of SMS messages according to prespecified algorithms reduces both error potential and cost. Third, the similarity between hypothetical choice scenarios presented in the DCE and actual HIV testing options given to participants allows for explicit comparisons between stated and revealed preferences. Fourth, the study design allows for separate estimates of the effects of reminder SMS, the issuance of HIV testing invitation cards and an incentive offer, on HIV testing rates. Finally, the approach for identifying the targeted, enhanced and less-preferred options is not contingent on the use of RELCL analysis and proprietary software; instead, it can be approximated using open source alternatives for example, in R. In a sensitivity analysis, we will evaluate the effect of specific model assumptions on the selection of testing options for the PRCT.
The study is subject to several limitations. First, feasibility considerations limit the study area to include only HCT facilities in Moshi municipality. While coded invitation cards collected from all HCT providers offer definitive evidence of a completed HIV test, participants may test without invitation cards and may test outside the study area. Sensitivity analyses will characterise the effect of using only provider-documented testing uptake (primary outcome) versus provider-documented or self-reported testing uptake (secondary outcome) on estimates.
Second, the preference estimates from the DCE, preference informed testing options, and estimated effect sizes are not generalisable to other high-risk groups in Tanzania or other parts of Africa. However, if this study is successful, it will support the broader use of stated preference methods to systematically elicit the preferences of key populations and facilitate corresponding adaptations to HIV testing options. We acknowledge that study eligibility criteria include literacy, and study procedures involve phone-based and SMS-based contact with participants. While literacy in the region was 96% in 2012 and, in 2017, 93% of urban households had a mobile phone62 63 the exclusion of illiterate persons and limited mobile phone access may influence the results. It is also possible that individuals who are likely to move (and thus may not be enrolled or are lost to follow-up) may have different preferences and opportunities for HIV testing.
Third, participants’ progression through multiple study phases may influence testing uptake in the PRCT. Ideally, all tangential intervention components (SMS reminders, invitation cards, incentives) could be evaluated alongside the preference-informed HIV testing offer as part of a multiarm RCT; however, the sample size required for such a trial is not feasible. A multistage enrolment approach and the inclusion of variables describing participants’ exposure to SMS reminders, testing offers and HIV tests in prior study phases as covariates allow us to estimate the direction and magnitude of such ordering effects on uptake. In our study, we will not be able to estimate an unconditional effect of incentives on HIV testing uptake, as concurrent incentivised and non-incentivised testing offers were not considered viable among potentially closely knit community members (eg, barworkers in the same bar, porters climbing together).
Finally, DCE surveys contain a limited set of testing characteristics; the finite range of attributes and levels is a limitation of DCEs in general. Preference-relevant and choice-relevant testing characteristics may differ in other settings, and changes in the testing environment and available testing options may occur during the study period. While adaptations to the preference survey and analysis of DCE data may be necessary and require technical expertise, such costs are expected to be far smaller than costs associated with large-scale, iterative trials of potentially ineffective HCT testing interventions.
In conclusion, this study evaluates the critical link between preference-based intervention design and efficacy. If the PRCT indicates that a preference-informed, heterogeneity-focused HCT offer increases testing rates, the testing options evaluated in this study can be offered to high-risk populations in the study area, and the preference elicitation method and tools can be used to inform the design of testing options that better match the preferences of other high-risk populations, both locally and in other settings.
Supplementary Material
Acknowledgments
The authors are grateful to the study participants and to the study research assistants, Honoratha Israel, Beatrice Mandao, Elizabeth Mbuya, Yombya Madukwa, Leonia Rugalabamu, Suzan Kitomari, Stanny Komu, Blandina Zenze, Mohamed Mcharo, Upendo Nnko, Stephen Sikumbili, Edward Singo and Beldad Mmari, for input on study procedures and study implementation. The authors thank the staff of the Kilimanjaro Clinical Research Institute, especially Professor Blandina Mmbaga and Zuhura Lintu; the University of South Carolina’s Arnold School of Public Health, especially the Department of Health Services Policy & Management and the Center for Health Care Quality; the Duke Global Health Institute and Duke University’s Center for Health Policy and Inequalities Research, for administrative support and members of the Duke Center for AIDS Research and the study’s Scientific Advisory Board for feedback on study feasibility, design, analytic methods, and implementation. Finally, the authors acknowledge Dr Credianus Mgimba (Regional Medical Officer, Kilimanjaro Region), Dr Best Magoma (former Regional Medical Officer, Kilimanjaro Region), Dr Eligy Mosille (Regional AIDS Control Coordinator, Kilimanjaro Region), Ms Dafrosa Itemba (Director, Tanzania Women Research Foundation) and members of the Moshi District Council administration, for their support of the study’s development and implementation.
Footnotes
Twitter: @zwetsomics
Contributors: JO, NT and BN conceptualised the study. AH, ACM, BF, BN, DSB, JO and NT were involved in the development and submission of the funding application. All authors contributed to the development of the study protocol. JO and NT contributed equally to the development of this manuscript, wrote the first draft of the manuscript, and led subsequent revisions. MM developed the comet software for the collection of DCE data on iPads. AH, ACM, AS, BF, BN, DSB, JO, MLM, MM, MvZ, NT and TM read the manuscript and provided critical input. All authors read and approved the final manuscript.
Funding: This study is supported by a grant from the National Institute of Mental Health (R01MH106388).
Disclaimer: The funding body has no role in the design of the study, the collection, analysis, and interpretation of data or writing of the manuscript.
Competing interests: None declared.
Consent for publication: All authors of the manuscript have read and agreed to its content and are accountable for all aspects of the accuracy and integrity of the manuscript in accordance with ICMJE criteria.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data sharing is not yet applicable as datasets have not yet been generated and/or analysed for this study. Data from the proposed study will be stored in a data repository; data will be deidentified so that they cannot be linked back to individuals. Investigators wishing to use study data to answer new research questions may submit data analysis concept proposals for consideration by the Principal Investigators. The Principal Investigators will review the proposals in the context of relevant Data Transfer Agreements and will provide those submitting permissible, scientifically rigorous, and promising proposals access to the data repository to address their research questions.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.UNAIDS Unaids data 2019, 2019.. Available: https://www.unaids.org/en/resources/documents/2019/2019-UNAIDS-data
- 2.Sweat M, Gregorich S, Sangiwa G, et al. Cost-Effectiveness of voluntary HIV-1 counselling and testing in reducing sexual transmission of HIV-1 in Kenya and Tanzania. Lancet 2000;356:113–21. 10.1016/S0140-6736(00)02447-8 [DOI] [PubMed] [Google Scholar]
- 3.Thielman NM, Chu HY, Ostermann J, et al. Cost-Effectiveness of free HIV voluntary counseling and testing through a community-based AIDS service organization in northern Tanzania. Am J Public Health 2006;96:114–9. 10.2105/AJPH.2004.056796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dieffenbach CW, Fauci AS. Universal voluntary testing and treatment for prevention of HIV transmission. JAMA 2009;301:2380–2. 10.1001/jama.2009.828 [DOI] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fauci AS, Folkers GK, Dieffenbach CW. Hiv-Aids: much accomplished, much to do. Nat Immunol 2013;14:1104–7. 10.1038/ni.2735 [DOI] [PubMed] [Google Scholar]
- 7.UNAIDS 90-90-90. An ambitious treatment target to help end the AIDS epidemic, 2014. Available: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf
- 8.The Lancet Hiv Divergent paths to the end of AIDS. Lancet HIV 2017;4:e375. 10.1016/S2352-3018(17)30157-1 [DOI] [PubMed] [Google Scholar]
- 9.Granich RM, Gilks CF, Dye C, et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009;373:48–57. 10.1016/S0140-6736(08)61697-9 [DOI] [PubMed] [Google Scholar]
- 10.National AIDS Control Programme MoH Community development, gender, elderly and children. Health sector HIV and AIDS strategic plan, 2017: 2017–22. [Google Scholar]
- 11.Angotti N, Bula A, Gaydosh L, et al. Increasing the acceptability of HIV counseling and testing with three C’s: convenience, confidentiality and credibility. Soc Sci Med 2009;68:2263–70. 10.1016/j.socscimed.2009.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negin J, Wariero J, Mutuo P, et al. Feasibility, acceptability and cost of home-based HIV testing in rural Kenya. Trop Med Int Health 2009;14:849–55. 10.1111/j.1365-3156.2009.02304.x [DOI] [PubMed] [Google Scholar]
- 13.Sabapathy K, Van den Bergh R, Fidler S, et al. Uptake of home-based voluntary HIV testing in sub-Saharan Africa: a systematic review and meta-analysis. PLoS Med 2012;9:e1001351. 10.1371/journal.pmed.1001351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baggaley R, Hensen B, Ajose O, et al. From caution to urgency: the evolution of HIV testing and counselling in Africa. Bull World Health Organ 2012;90:652–8. 10.2471/BLT.11.100818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roura M, Watson-Jones D, Kahawita TM, et al. Provider-initiated testing and counselling programmes in sub-Saharan Africa: a systematic review of their operational implementation. AIDS 2013;27:617–26. 10.1097/QAD.0b013e32835b7048 [DOI] [PubMed] [Google Scholar]
- 16.Topp SM, Chipukuma JM, Chiko MM, et al. Opt-out provider-initiated HIV testing and counselling in primary care outpatient clinics in Zambia. Bull World Health Organ 2011;89:328–35. 10.2471/BLT.10.084442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanyenze R, Kamya M, Liechty CA, et al. Hiv counseling and testing practices at an urban hospital in Kampala, Uganda. AIDS Behav 2006;10:361–7. 10.1007/s10461-005-9035-9 [DOI] [PubMed] [Google Scholar]
- 18.Corbett EL, Marston B, Churchyard GJ, et al. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet 2006;367:926–37. 10.1016/S0140-6736(06)68383-9 [DOI] [PubMed] [Google Scholar]
- 19.Houdmont J, Munir F, Grey M. Acceptance of repeat worksite HIV voluntary counselling and testing in a rural South African factory. AIDS Care 2013;25:1199–202. 10.1080/09540121.2013.764388 [DOI] [PubMed] [Google Scholar]
- 20.Ostermann J, Njau B, Mtuy T, et al. One size does not fit all: HIV testing preferences differ among high-risk groups in northern Tanzania. AIDS Care 2015;27:595–603. 10.1080/09540121.2014.998612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostermann J, Njau B, Brown DS, et al. Heterogeneous HIV testing preferences in an urban setting in Tanzania: results from a discrete choice experiment. PLoS One 2014;9:e92100 10.1371/journal.pone.0092100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Njau B, Ostermann J, Brown D, et al. Hiv testing preferences in Tanzania: a qualitative exploration of the importance of confidentiality, accessibility, and quality of service. BMC Public Health 2014;14:838. 10.1186/1471-2458-14-838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mühlbacher A, Johnson FR. Choice experiments to quantify preferences for health and healthcare: state of the practice. Appl Health Econ Health Policy 2016;14:253–66. 10.1007/s40258-016-0232-7 [DOI] [PubMed] [Google Scholar]
- 24.Hensher DA, Rose JM, Greene WH. Applied choice analysis: a primer. Cambridge; New York: Cambridge University Press, 2005. [Google Scholar]
- 25.Louviere JJ, Hensher DA, Swait JD. Stated choice methods: analysis and applications. Cambridge, UK; New York, NY, USA: Cambridge University Press, 2000. [Google Scholar]
- 26.Ryan M, Gerard K, Amaya-Amaya M. Using discrete choice experiments to value health and health care. Dordrect, The Netherlands: Springer, 2008. [Google Scholar]
- 27.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ 2000;320:1530–3. 10.1136/bmj.320.7248.1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson FR, Ozdemir S, Phillips KA. Effects of simplifying choice tasks on estimates of taste heterogeneity in stated-choice surveys. Soc Sci Med 2010;70:183–90. 10.1016/j.socscimed.2009.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Indravudh PP, Sibanda EL, d'Elbée M, et al. 'I will choose when to test, where I want to test': investigating young people's preferences for HIV self-testing in Malawi and Zimbabwe. AIDS 2017;31 Suppl 3:S203–12. 10.1097/QAD.0000000000001516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips KA, Maddala T, Johnson FR. Measuring preferences for health care interventions using conjoint analysis: an application to HIV testing. Health Serv Res 2002;37:1681–705. 10.1111/1475-6773.01115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strauss M, George GL, Rhodes BD. Determining preferences related to HIV counselling and testing services among high school learners in KwaZulu-Natal: a discrete choice experiment. AIDS Behav 2018;22:64–76. 10.1007/s10461-016-1602-8 [DOI] [PubMed] [Google Scholar]
- 32.Ostermann J, Njau B, Brown DS, et al. Heterogeneous HIV testing preferences in an urban setting in Tanzania: results from a discrete choice experiment. PLoS One 2014;9:e92100. 10.1371/journal.pone.0092100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quaife M, Eakle R, Cabrera Escobar MA, et al. Divergent preferences for HIV prevention: a discrete choice experiment for multipurpose HIV prevention products in South Africa. Med Decis Making 2018;38:120–33. 10.1177/0272989X17729376 [DOI] [PubMed] [Google Scholar]
- 34.Cameron MP, Newman PA, Roungprakhon S, et al. The marginal willingness-to-pay for attributes of a hypothetical HIV vaccine. Vaccine 2013;31:3712–7. 10.1016/j.vaccine.2013.05.089 [DOI] [PubMed] [Google Scholar]
- 35.Newman PA, Cameron MP, Roungprakhon S, et al. Acceptability and preferences for hypothetical rectal microbicides among a community sample of young men who have sex with men and transgender women in Thailand: a discrete choice experiment. AIDS Behav 2016;20:2588–601. 10.1007/s10461-015-1258-9 [DOI] [PubMed] [Google Scholar]
- 36.Terris-Prestholt F, Hanson K, MacPhail C, et al. How much demand for new HIV prevention technologies can we really expect? results from a discrete choice experiment in South Africa. PLoS One 2013;8:e83193. 10.1371/journal.pone.0083193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zanolini A, Sikombe K, Sikazwe I, et al. Understanding preferences for HIV care and treatment in Zambia: evidence from a discrete choice experiment among patients who have been lost to follow-up. PLoS Med 2018;15:e1002636. 10.1371/journal.pmed.1002636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kruk ME, Riley PL, Palma AM, et al. How can the health system retain women in HIV treatment for a lifetime? a discrete choice experiment in Ethiopia and Mozambique. PLoS One 2016;11:e0160764. 10.1371/journal.pone.0160764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.d'Elbée M, Indravudh PP, Mwenge L, et al. Preferences for linkage to HIV care services following a reactive self-test: discrete choice experiments in Malawi and Zambia. AIDS 2018;32:2043–9. 10.1097/QAD.0000000000001918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beusterien KM, Dziekan K, Schrader S, et al. Patient preferences among third agent HIV medications: a US and German perspective. AIDS Care 2007;19:982–8. 10.1080/09540120701294278 [DOI] [PubMed] [Google Scholar]
- 41.Brégigeon-Ronot S, Cheret A, Cabié A, et al. Evaluating patient preference and satisfaction for human immunodeficiency virus therapy in France. Patient Prefer Adherence 2017;11:1159–69. 10.2147/PPA.S130276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hauber AB, Mohamed AF, Watson ME, et al. Benefits, risk, and uncertainty: preferences of antiretroviral-naïve African Americans for HIV treatments. AIDS Patient Care STDS 2009;23:29–34. 10.1089/apc.2008.0064 [DOI] [PubMed] [Google Scholar]
- 43.Mühlbacher AC, Stoll M, Mahlich J, et al. Patient preferences for HIV/AIDS therapy - a discrete choice experiment. Health Econ Rev 2013;3:14. 10.1186/2191-1991-3-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ostermann J, Mühlbacher A, Brown DS, et al. Heterogeneous patient preferences for modern antiretroviral therapy: results of a discrete choice experiment. Value Health 2020;23:851–61. 10.1016/j.jval.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.United Republic of Tanzania, National Bureau of Statistics 2012 Tanzania population and housing census, 2012. Available: https://www.nbs.go.tz/nbs/takwimu/census2012/Tanzania_Total_Population_by_District-Regions-2016_2017r.pdf
- 46.Ostermann J, Whetten K, Reddy E, et al. Treatment retention and care transitions during and after the scale-up of HIV care and treatment in northern Tanzania. AIDS Care 2014;26:1352–8. 10.1080/09540121.2014.882493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchell J, Keane J, Laidlaw J. Making success work for the poor: package tourism in northern Tanzania. Arusha, Tanzania: Overseas Development Institute, SNV Connecting People’s Capacities, 2009. [Google Scholar]
- 48.Peaty D. Kilimanjaro tourism and what it means for local porters and for the local environment. J Ritsumeikan Soc Sci and Humanities 2012;4:1–12. [Google Scholar]
- 49.Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user's guide. Pharmacoeconomics 2008;26:661–77. 10.2165/00019053-200826080-00004 [DOI] [PubMed] [Google Scholar]
- 50.Johnson F, Kanninen B, Bingham M. Experimental design for stated choice studies : BJ K, Valuing environmental amenities using stated choice studies. Dordrecht: Springer, 2007. [Google Scholar]
- 51.Hole AR. Fitting mixed logit models by using maximum simulated likelihood. Stata J 2007;7:388–401. 10.1177/1536867X0700700306 [DOI] [Google Scholar]
- 52.Hensher DA, Rose JM, Greene WH. Applied choice analysis. 2nd edition Cambridge: Cambridge University Press, 2015. [Google Scholar]
- 53.Zhou M, Bridges JFP. Explore preference heterogeneity for treatment among people with type 2 diabetes: a comparison of random-parameters and latent-class estimation techniques. J Choice Model 2019;30:38–49. 10.1016/j.jocm.2018.11.002 [DOI] [Google Scholar]
- 54.Ostermann J, Vasudevan L, Van Zwetselaar M. Mobile phone assisted reminder and incentive system (mParis). integrating mHealthreminders and conditional cash transfers to improve the timeliness of vaccinations inTanzania. poster presented at 2018 NIH mHealth technology showcase for health research. Washington, DC, 2018. [Google Scholar]
- 55.Ostermann J, Vasudevan L, Baumgartner JN, et al. Do mobile phone-based reminders and conditional financial transfers improve the timeliness of childhood vaccinations in Tanzania? study protocol for a quasi-randomized controlled trial. Trials 2019;20:397. 10.1186/s13063-019-3430-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ostermann J, Brown DS, Mühlbacher A, et al. Would you test for 5000 Shillings? HIV risk and willingness to accept HIV testing in Tanzania. Health Econ Rev 2015;5:60. 10.1186/s13561-015-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ministry of Health Community Development Gender Elderly and Children Ministry of health community development gender elderly and children. National AIDS control programme, 2017. Available: http://www.nacp.go.tz/site/news/HSHSPIV.pdf
- 58.Welfare MoHaS, Tanzania TURo, NACP . National comprehensive guidelines for HIV Testingand counseling. Dar es Salaam, 2013. http://library.tacaids.go.tz/handle/123456789/154 [Google Scholar]
- 59.Yang J-C, Johnson FR, Kilambi V, et al. Sample size and utility-difference precision in discrete-choice experiments: a meta-simulation approach. Journal of Choice Modelling 2015;16:50–7. 10.1016/j.jocm.2015.09.001 [DOI] [Google Scholar]
- 60.Orme B. Getting started with conjoint analysis: strategies for product design and pricing research. 2nd Madison, Wisconsin: Research Publishers LLC, 2010. [Google Scholar]
- 61.Zwarenstein M, Treweek S, Gagnier JJ, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337:a2390. 10.1136/bmj.a2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Bureau of Statistics, (OCGS) OotCGS National population projections, 2018. Available: https://www.nbs.go.tz/index.php/en/census-surveys/population-and-housing-census/174-2012-phc-literacy-and-education-monograph
- 63.Ministry of Health Community Development Gender Elderly and Children (MoHCDGEC) [Tanzania Mainland], Ministry of Health (MoH) [Zanzibar], National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS), ICF, TANZANIA Malaria Indicator Survey 2017, 2017. Available: https://www.dhsprogram.com/pubs/pdf/MIS31/MIS31.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-039313supp001.pdf (48.7KB, pdf)
bmjopen-2020-039313supp002.pdf (21KB, pdf)
bmjopen-2020-039313supp003.pdf (64.5KB, pdf)
bmjopen-2020-039313supp004.pdf (175.4KB, pdf)


