Abstract
Objective
The Alchemilla genus, which belongs to the Rosaceae family, is known as Lady’s mantle and is commonly used in traditional medicine. This study was designed to investigate the major metabolites isolation and gastroprotective effects of Alchemilla caucasica.
Materials and Methods
Phytochemical studies were carried out using column chromatography on Alchemilla caucasica. The gastroprotective effect of ethanol extract of this plant was tested on indomethacin-induced gastric ulcer model in rats. In addition, superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione (GSH) parameters in the stomach tissue were examined.
Results
Quercetin-3-O-glucuronide, apigenin, and catechin were isolated from aerial parts of Alchemilla caucasica. When macroscopic ulcer index and histopathological results were analyzed, the extract at 200 mg/kg dose was found to be most effective. All doses of extract reduced MDA level and enhanced SOD activity and GSH level.
Conclusion
The results of this study showed that Alchemilla caucasica has significant antiulcer activity. This effect was thought to be caused by antioxidant properties of flavonoids.
Keywords: Antioxidants, medicinal plants, ulcer, phythochemicals
Introduction
Peptic ulcer is a gastrointestinal system disease characterized by mucosal damage secondary to pepsin and gastric acid secretion. Helicobacter pylori infection, nonsteroidal anti-inflammatory drugs (NSAIDs), critical illness, surgery, smoking, and stress are the causes of peptic ulcer disease [1]. Reactive oxygen species (ROS) cause a significant increase in the formation of gastric ulcer. Indomethacin (IND)-induced inflammation in the stomach tissue causes ROS formation [2]. Antioxidants such as superoxide dismutase (SOD) and glutathione (GSH) have protective effects against stomach damage [3].
The Alchemilla genus (family: Rosaceae) contains nearly 80 species in Turkey [4, 5]. This genus is known as Lady’s mantle and is commonly used in traditional medicine. Some of the species have been used as a diuretic, hypocholesterolemic, sedative, and memory booster [6] and have been used for diarrhea, dysmenorrhea, menopausal complaints, eczema, skin rashes, ulcers [7], liver disorder, menstruation problem, and skin diseases [8]. Species belonging to Alchemilla genus contain mainly flavonoids [4, 9]. They also include triterpenes [10, 11] and tannins [12, 13]. It is known that flavonoids have antibacterial, hepatoprotective, anti-inflammatory, anticancer, antiviral, and strong antioxidant effects [14].
Alchemilla caucasica is a species belonging to this genus. This study was designed 1) to isolate secondary metabolites of A. caucasica, 2) to evaluate the activity of its ethanol extract in IND-induced gastric ulcer model, and 3) to define its effects on antioxidant parameters in rat stomach tissue.
Materials and Methods
Animals
In the experiments, 36 male albino Wistar rats weighing 220–280 g were used. The studies were conducted in the Atatürk University’s Experimental Animal Laboratory at the Medicinal and Experimental Application and Research Centre in accordance with the national guidelines for animal use in experiments. An ethics permit was obtained from the local animal care committee of Atatürk University (93722986-000-E.1800120587).
Plant Material
The plant samples were collected from Konakli Mountain (Erzurum Province, Turkey). A sample of the species is stored in the Herbarium of the Faculty of Pharmacy (Atatürk University, AUEF1023).
Extraction and Isolation Studies
The aerial parts of the plant were dried and powdered. The powdered parts (430 g) were extracted with methanol at 40 °C (3×2 L). The methanol extract (72.77 g) was evaporated in the rotary evaporator. The dried extract was dissolved in water and subjected to liquid-liquid extraction with dichloromethane and ethyl acetate. Later, the solvents were evaporated in a rotary evaporator. Ethyl acetate, dichloromethane, and water extracts were obtained. Compound 1 was isolated from water extract using silica gel (Merck, Darmstadt, Germany) column (solvent systems: CH2Cl2:MeOH:H2O [90:10:1→50:50:5]), Sephadex LH-20 (Sigma, St. Louis, MO, USA) column (solvent system: MeOH), and then silica gel column (solvent systems: CH2Cl2:MeOH:H2O [70:30:3→65:35:3.5]). Compound 2 was isolated from ethyl acetate (EtOAc) extract using silica gel (solvent systems: CH2Cl2:MeOH:H2O [90:10:1→50:50:5]), reverse phase silica gel column (solvent systems: H2O:MeOH [90:10→0:100]), and Sephadex LH-20 column (solvent system: MeOH). Compound 3 was isolated from EtOAc extract using silica gel column (solvent systems: CH2Cl2:MeOH:H2O [90:10:1→50:50:5]), Sephadex LH-20 column (solvent system: MeOH), reverse phase silica gel column (solvent systems: H2O:MeOH [90:10→0:100]), and silica gel column (solvent systems: CH2Cl2:MeOH:H2O [80:20:2, 70:30:3, 60:40:4]). The structures of the compounds were determined by 1H nuclear magnetic resonance (NMR), 13C NMR, 2D NMR, and quadrupole time-of-flight (Q-TOF) mass spectrometer.
Ulcer Model
The IND-induced ulcer model procedure was used in this experiment [15, 16]. NSAIDs are frequently referred to in the ulcerative formation model [17]. In the experiment, rats were not fed for 24 hours with free access to water. A total of 1 ml of saline was administered to healthy and IND control groups by oral gavage as vehicle. Then, 40 mg/kg dose of famotidine (FAM) (Famodin 40 mg, Sandoz Drug Company, İstanbul, Turkey) (positive control) [15] and 50, 100, and 200 mg/kg concentration of plant extracts (ALC) were administered to the corresponding rat groups by oral gavage. After five minutes, 25 mg/kg dose of IND (Endol 25 mg, DEVA Holding A.S., İstanbul, Turkey) was administered to all groups except for healthy group by oral gavage. After 6 hours, thiopental (Thiopental sodium, IE Ulagay A.S., Istanbul, Turkey) at a dose of 50 mg/kg was applied intraperitoneally to all groups, and euthanasia was performed. The ulcerated areas on the stomach surfaces of rats were evaluated macroscopically and were measured by mm2 paper.
Experimental Groups of Indomethacin-Induced Ulcer Model
Each experiment group consisted of 6 rats, which were classified as follows:
Healthy;
25 mg/kg IND;
25 mg/kg IND+40 mg/kg FAM (IND+FAM);
25 mg/kg IND+50 mg/kg ALC (IND+ALC50);
25 mg/kg IND+100 mg/kg ALC (IND+ALC100); and
25 mg/kg IND+200 mg/kg ALC (IND+ALC200).
Histopathological Evaluation
The tissues were fixed in a 10% buffered formalin solution for 24 hours for histopathological evaluation. After fixation, tissue samples were immersed in paraffin. Sections that were 5-μm thick were cut from the paraffin-immersed tissue samples and placed on positively charged slides. Then, the samples underwent deparaffinization and rehydration and were spotted with Mayer’s hematoxylin and eosin. The sections were investigated under the microscope for histopathological changes using a light photomicroscope (Nikon Eclipse E600; USA).
Biochemical Studies
The tissues were stored at −80 ° C for biochemical experiments. The samples were ground with liquid nitrogen by a TissueLyser II grinding jar set. The milled tissues (50 mg) were centrifuged after mixing with 1 ml PBS buffer. SOD activity [18] and malondialdehyde (MDA) [19] and GSH levels [20] were measured at room temperature using an enzyme-linked immunosorbent assay reader [21]. A standard curve was formed by calculating the medial absorbance. Linear SOD activity and GSH and MDA concentrations were calculated according to the equation obtained from the standard absorbance.
Statistical Analysis
For the statistical analysis, IBM Statistics 20 version of Statistical Package for the Social Sciences (IBM SPSS Corp.; Armonk, NY, USA) software was used. One-way analysis of variance and Duncan’s Multiple Comparison Test were performed. The mean values in the same column by the same letter are not significantly different from the test of Duncan. The level with the p-value <0.05 was considered significant. Results are expressed as mean ± standard deviation.
Results
Isolation and Structure Identification
Quercetin-3-O-glucuronide (Miquelianin) (Compound1): C21H18O13, m/z 479.0820 [M+H]+, 1H-NMR (400 MHz, CD3OD) δ: 3.47–3.63 (ov, H-2″-H-5″), 5.32 (d, J 7.2 Hz, H-1″), 6.19 (d, J 2.1 Hz, H-6), 6.38 (d, J 2.1 Hz, H-8), 6.86 (d, J 8.5 Hz, H-5′), 7.49 (dd, J 8.5; 2.2 Hz, H-6′), 7.94 (d, J 2.1 Hz, H-2′). 13C-NMR (100 MHz, CD3OD) δ: 157.1 (C-2), 134.4 (C-3), 178.0 (C-4), 161.5 (C-5), 98.5 (C-6), 164.7 (C-7), 93.3 (C-8), 157.8 (C-9), 104.3 (C-10), 121.4 (C-1′), 114.7 (C-2′), 144.5 (C-3′), 148.5 (C-4′), 116.6 (C-5′), 121.3 (C-6′), 103.1 (C-1″′), 74.1 (C-2″), 76.7 (C-3″), 71.9 (C-4″), 76.2 (C-5″), 175.0 (C-6″).
Apigenin (Compound 2): C15H10O5, 1H-NMR (400 MHz, DMSO-d6) δ: 6.19 (d, J 2.0 Hz, H-6), 6.48 (d, J 2.0 Hz, H-8), 6.79 (s, H-3), 6.92 (d, J 8.0 Hz, H-3′, 5′), 7.93 (d, J 8.0 Hz, H-2′,6′), 12.97 (s, 5-OH). 13C-NMR (100 MHz, DMSO-d6) δ: 164.7 (C-2), 103.3 (C-3), 182.2 (C-4), 161.9 (C-5), 99.3 (C-6), 164.2 (C-7), 94.4 (C-8), 157.8 (C-9), 104.1 (C-10), 121.6 (C-1′), 128.9 (C-2′), 116.4 (C-3′), 161.6 (C-4′), 116.4 (C-5′), 128.9 (C-6′).
Catechin (Compound 3): C15H14O6, m/z 291.0853 [M+H]+, 1H-NMR (400 MHz, CD3OD) δ: 2.52 (dd, J 16.1; 8.1 Hz, H-4a), −2.87 (dd, J 16.1; 5.4 Hz, H-4b), −3.99 (m, H-3), −4.58 (d, J 7.5 Hz, H-2), 5.87 (d, J 2.3 Hz, H-8), −5.95 (d, J 2.3 Hz, H-6), 6.73 (dd, J 8.2; 1.8 Hz, H-6′), 6.78 (d, J 8.0 Hz, H-5′), 6.86 (d, J 1.8 Hz, H-2′). 13C-NMR (100 MHz, CD3OD) δ: 81.42 (C-2), 67.42 (C-3), 27.11 (C-4), 156.18 (C-5), 94.91 (C-6), 156.45 (C-7), 94.12 (C-8), 155.52 (C-9), 99.43 (C-10), 130.84 (C-1′),113.87 (C-2′), 144.83 (C-3′), 144.85 (C-4′), 114.68 (C-5′), 118.63 (C-6′).
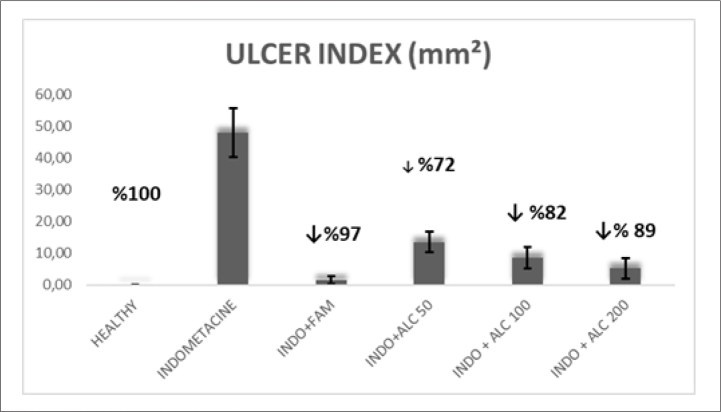
Macroscopic Ulcer Index
When macroscopic ulcer indexes were examined, the extract at 200 mg/kg concentration showed a strong antiulcer activity (89%, p<0.05), which is compatible to the standard drug FAM (97%, p<0.05). The ulcer index area of extract (50, 100, and 200 mg/kg doses), FAM (40 mg/kg dose), and control groups are shown in Figure 1.
Figure 1.
A Ulcer index of all groups
Histopathological Evaluation
The tissue sections were evaluated for histopathological changes under a microscope. In the healthy group, it was observed that the gastric pits were normal, and the parietal and surface mucous cells had a healthy appearance. In the IND group, epithelial losses and irregular gastric pits were observed in the mucosa; the necrotic appearance of the surface mucous cells and the increase of lymphatic cells in the lamina propria were remarkable. There was also an increase in eosinophilic staining properties of some parietal cells. The IND+ALC200 group had a similar appearance to a healthy group. Nevertheless, some epithelial cells were cast. Histopathological ulcerated area scoring results are shown in Table 1. Macroscopic photographs and the histopathological results are shown in Figure 2.
Table 1.
Histopathological ulcerated area scoring results
| Lymphatic cell increase | Hemoragy | Epithelial cell loss | |
|---|---|---|---|
| ALC50 | ++ | ++ | ++ |
| ALC100 | + | − | −/+ |
| ALC200 | − | − | − |
| IND | +++ | + | +++ |
| IND+FAM | − | − | − |
| Healthy | − | − | − |
Histopathological damage: − (none), + (little damage), ++ (moderate damage), +++ (severe damage)
ALC: Alchemilla caucasica extract (50,100, and 200 mg/kg concentrations); IND: Indomethacin; FAM: Famotidine
Figure 2.
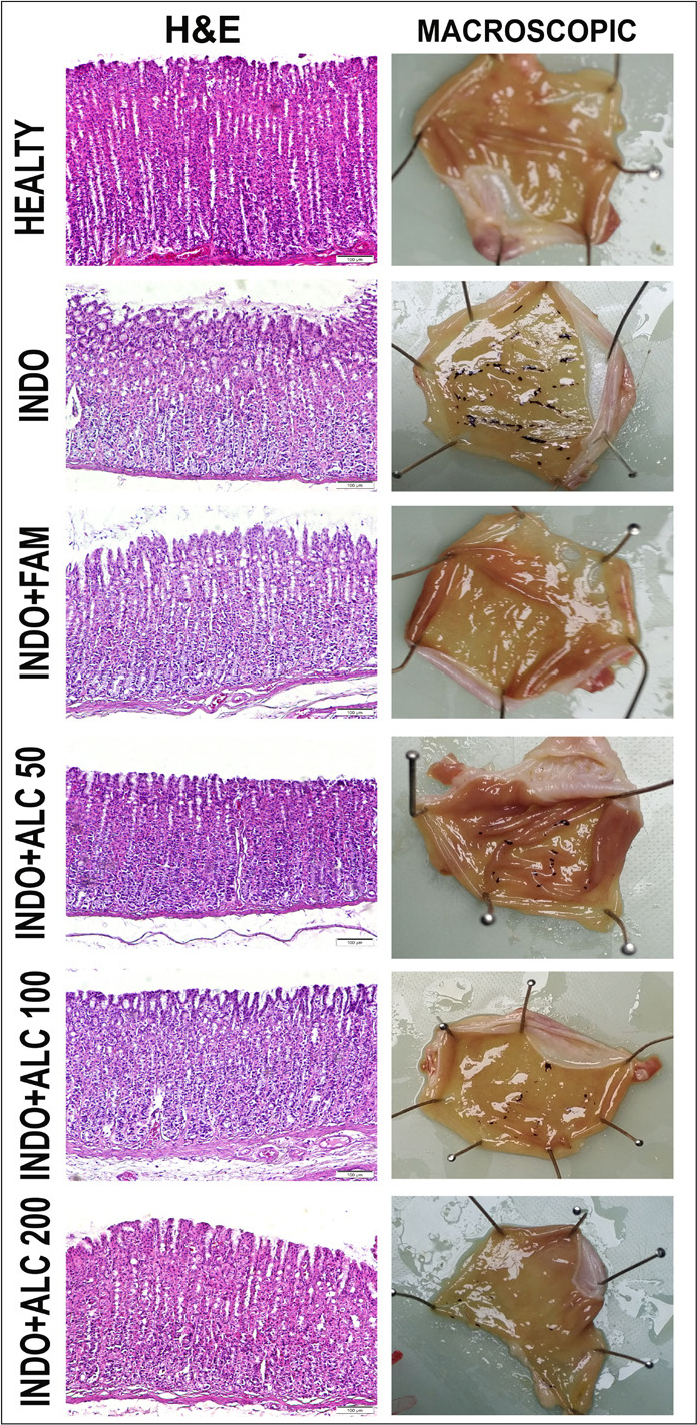
Macroscopic and histopathological evaluation
Biochemical Evaluation
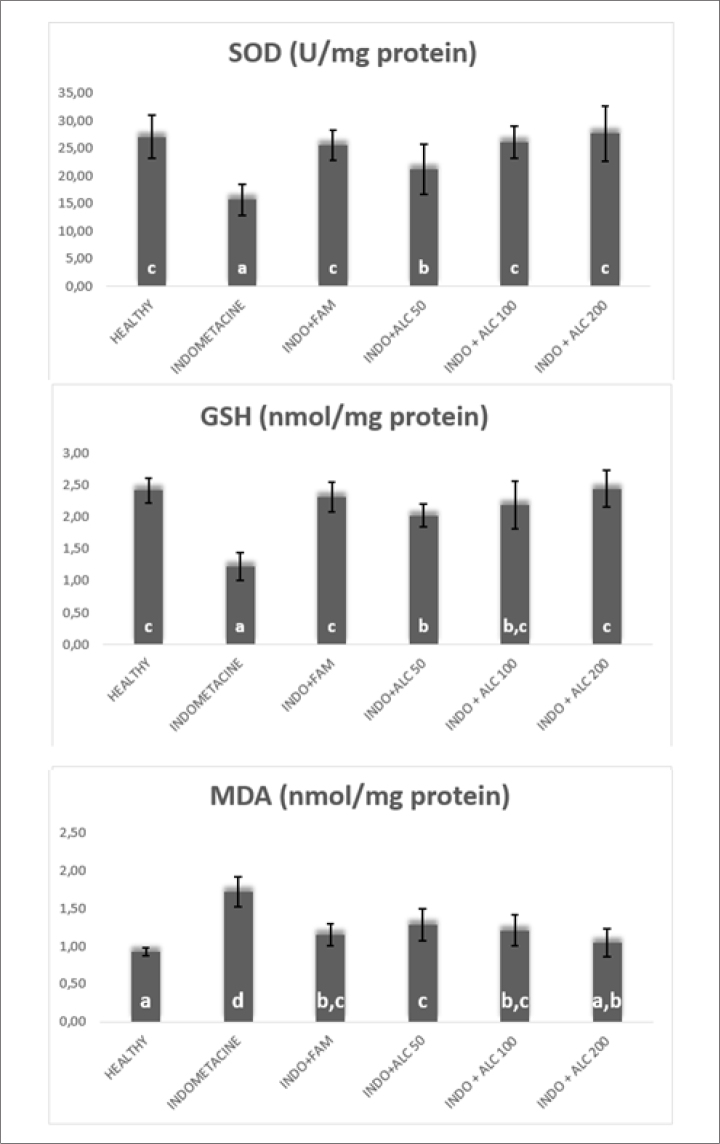
When biochemical parameters were analyzed, it was observed that the extract at 200 mg/kg concentration increased the SOD activity (p<0.05) and GSH level (p<0.05) at the highest rate compared with the standard drug FAM. In addition, the extract at 200 mg/kg concentration decreased the MDA level at highest degree (p<0.05). The results of the SOD activity and GSH and MDA levels are shown in Figure 3.
Figure 3.
SOD activity and GSH and MDA levels. SOD: superoxide dismutase; GSH: glutathione; MDA: malondialdehyde
Discussion
The genus Alchemilla belongs to the Rosaceae family. This genus is rich in flavonoids and has traditionally various medicinal usage. Flavonoids are good antioxidant substances [22]. They are also thought to have antiulcer activities [23]. One of the most important hypotheses in the formation of ulcers caused by NSAIDs is increased oxidative stress and disruption of antioxidant system. When we looked at the literature, we did not encounter any phytochemical activity and antiulcer activity studies on A. caucasica. Therefore, secondary metabolites isolation was carried out by column chromatography on the aerial parts of A. caucasica in this study. The activity of the ethanol extract of A. caucasica in IND-induced gastric ulcer model was evaluated, and the effects on antioxidant parameters in rat stomach tissue were defined.
Three compounds were isolated from the aerial parts of A. caucasica using column chromatography. The results of NMR of these compounds were found to be compatible with the literature. The first component was quercetin-3-O-glucuronide (miquelianin) [24, 25], the second component was apigenin [26], and the third component was catechin [27]. The genus Alchemilla contains mainly different flavonoids [4, 9]. The result of our study supported this information.
Gastroprotective effect of A. caucasica ethanol extract on IND-induced gastric ulcers in rats was investigated. In addition, the effect of the extract on antioxidant parameters in rat stomach tissues was evaluated biochemically. It was observed that all concentrations of plant extracts significantly reduced ulcerated index areas (Figure 1). According to the histopathological examination, drop-out of epithelial cells, irregular pit observation, and necrotic appearance are directly related to ulcers. When ulcerated area index and histopathological results were evaluated, it was observed that IND+ALC200 group was similar to the healthy group. However, some spilled epithelial cells have been seen. Macroscopic and histopathological results are shown in Figure 2. It is reported that some species belonging to the genus Alchemilla are used traditionally in the ulcer treatment [7]. The results of this study have been found compatible with this literature.
SOD prevents the formation of more toxic products such as hydroxyl radical by preventing superoxide radicals from reacting with potential substrates [28]. GSH defends cells from the toxicity of reactive oxygen compounds [29]. As a result of biochemical evaluation, all doses of extract increased SOD activity and GSH level. It was observed that IND+ALC200 group, especially, increased SOD activity and GSH level similar to the standard drug FAM. MDA is a product of lipid peroxidation and prostaglandin biosynthesis. It has mutagenic and carcinogenic effects [30]. All doses of extract decreased MDA level. IND+ALC200 group reduced MDA level similar to FAM. SOD activity and GSH and MDA levels are shown in Figure 3.
One of the most important hypotheses in the formation of ulcers caused by NSAIDs is increased oxidative stress and disruption of antioxidant system. Flavonoids have high antioxidant effects and are thought to have antiulcer effect. In this study, it was determined that A. caucasica contains some flavonoids. As a result of the study, it was observed that all tested doses had antiulcer effects. We think that significant effects of this plant on IND-induced ulcer is due to the single or synergistic effects of its antioxidant flavonoids or other undetectable compounds.
Main Points.
Quercetin-3-O-glucuronide, apigenin and catechin were isolated from aerial parts of Alchemilla caucasica.
All doses of extract increased SOD activity and GSH level and decreased MDA level.
According to the histopathological examination, IND+ALC200 group had similar characteristics with the healthy group.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Ataturk University (93722986-000-E.1800120587).
Informed Consent: N/A
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - E.S.K.; Design - E.S.K., Y.B., A.A., E.T.; Supervision - E.S.K., Y.B., A.A.; Resources - E.S.K., Y.B., C.K.; Materials - E.S.K., Y.B., A.A., E.T.; Data Collection and/or Processing - E.S.K., U.O., A.K.; Analysis and/or Interpretation - E.S.K., Y.B., A.A., E.T., C.K., A.K.; Literature Search - E.S.K., U.O.; Writing Manuscript E.S.K.; - Critical Review - E.Ç.
Conflict of Interest: Authors have no conflicts of interest to declare.
Financial Disclosure: This research was supported by Scientific Research Projects Comission of Ataturk University with project number THD-2018-6798.
References
- 1.Ramakrishnan K, Salinas RC. Peptic ulcer disease. Am Fam Physician. 2007;76:1005–12. [PubMed] [Google Scholar]
- 2.Das D, Bandyopadhyay D, Bhattacharjee M, Banerjee RK. Hydroxyl radical is the major causative factor in stress-induced gastric ulceration. Free Radic Biol Med. 1997;23:8–18. doi: 10.1016/S0891-5849(96)00547-3. [DOI] [PubMed] [Google Scholar]
- 3.Mates JM, Perez-Gomez C, Nunez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595–603. doi: 10.1016/S0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 4.Kaya B, Menemen Y, Saltan FZ. Flavonoids in the Endemic Species of Alchemilla L., (Section Alchemilla L. Subsection Calycanthum Rothm. Ser. Elatae Rothm.) from North-East Black Sea Region in Turkey. Pak J Bot. 2012;44:595–7. [Google Scholar]
- 5.Hayirlioglu-Ayaz S, Inceer H. Three New Alchemilla L. (Rosaceae) Records from Turkey. Pak J Bot. 2009;41:2093–6. [Google Scholar]
- 6.Vitalini S, Iriti M, Puricelli C, Ciuchi D, Segale A, Fico G. Traditional knowledge on medicinal and food plants used in Val San Giacomo (Sondrio, Italy)--an alpine ethnobotanical study. J Ethnopharmacol. 2013;145:517–29. doi: 10.1016/j.jep.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Menkovic N, Savikin K, Tasic S, et al. Ethnobotanical study on traditional uses of wild medicinal plants in Prokletije Mountains (Montenegro) J Ethnopharmacol. 2011;133:97–107. doi: 10.1016/j.jep.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Redzic SS. The ecological aspect of ethnobotany and ethnopharmacology of population in Bosnia and Herzegovina. Coll Antropol. 2007;31:869–90. [PubMed] [Google Scholar]
- 9.Trendafilova A, Todorova M, Gavrilova A, Vitkova A. Flavonoid glycosides from Bulgarian endemic Alchemilla achtarowii Pawl. Biochem Syst Ecol. 2012;43:156–8. doi: 10.1016/j.bse.2012.03.013. [DOI] [Google Scholar]
- 10.Olafsdottir ES, Omarsdottir S, Jaroszewski JW. Constituents of three Icelandic Alchemilla species. Biochem Syst Ecol. 2001;29:959–62. doi: 10.1016/S0305-1978(01)00038-2. [DOI] [PubMed] [Google Scholar]
- 11.Sokolowska-Wozniak A, Krzaczek T. Sterols and triterpene acids from the herb Alchemilla pastoralis Bus. Herba Polonica. 1993;39:173–8. [Google Scholar]
- 12.Geiger C, Scholz E, Rimpler H. Ellagitannins from Alchemilla-Xanthochlora and Potentilla-Erecta. Planta Med. 1994;60:384–5. doi: 10.1055/s-2006-959510. [DOI] [PubMed] [Google Scholar]
- 13.Duckstein SM, Lotter EM, Meyer U, Lindequist U, Stintzing FC. Phenolic Constituents from Alchemilla vulgaris L. and Alchemilla mollis (Buser) Rothm. at Different Dates of Harvest (vol 67c, pg 529, 2012) Z Naturforsch C. 2013;68:76. doi: 10.5560/ZNC.2012.67c0529. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. 2013;2013 doi: 10.1155/2013/162750. 162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halici Z, Polat B, Cadirci E, et al. Inhibiting renin angiotensin system in rate limiting step by aliskiren as a new approach for preventing indomethacin induced gastric ulcers. Chem-Biol Interact. 2016;258:266–75. doi: 10.1016/j.cbi.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Atalay F, Odabasoglu F, Halici M, et al. Gastroprotective and Antioxidant Effects of Lobaria pulmonaria and Its Metabolite Rhizonyl Alcohol on Indomethacin-Induced Gastric Ulcer. Chem Biodivers. 2015;12:1756–67. doi: 10.1002/cbdv.201400432. [DOI] [PubMed] [Google Scholar]
- 17.Suleyman H, Albayrak A, Bilici M, Cadirci E, Halici Z. Different Mechanisms in Formation and Prevention of Indomethacin-induced Gastric Ulcers. Inflammation. 2010;33:224–34. doi: 10.1007/s10753-009-9176-5. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Oberley LW, Li Y. A Simple Method for Clinical Assay of Superoxide-Dismutase. Clin Chem. 1988;34:497–500. doi: 10.1093/clinchem/34.3.497. [DOI] [PubMed] [Google Scholar]
- 19.Ohkawa H, Ohishi N, Yagi K. Assay for Lipid Peroxides in Animal-Tissues by Thiobarbituric Acid Reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 20.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 21.Bayir Y, Karagoz Y, Karakus E, et al. Nigella sativa reduces tissue damage in rat ovaries subjected to torsion and detorsion: oxidative stress, proinflammatory response and histopathological evaluation. Gynecol Obstet Invest. 2012;74:41–9. doi: 10.1159/000336295. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez I, Alegre L, Van Breusegem F, Munne-Bosch S. How relevant are flavonoids as antioxidants in plants? Trends Plant Sci. 2009;14:125–32. doi: 10.1016/j.tplants.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Parmar NS, Parmar S. Anti-ulcer potential of flavonoids. Indian J Physiol Pharmacol. 1998;42:343–51. [PubMed] [Google Scholar]
- 24.Smolarz HD. Flavonoids from Polygonum lapathifolium ssp tomentosum. Pharm Biol. 2002;40:390–4. doi: 10.1076/phbi.40.5.390.8455. [DOI] [Google Scholar]
- 25.Pacifico S, D’Abrosca B, Scognamiglio M, Galasso S, Monaco P, Fiorentino A. Antioxidant Polyphenolic Constituents of Vitis × Labruscana cv. ‘Isabella’ Leaves. Open Nat Prod J. 2013;6:5–11. doi: 10.2174/1874848101306010005. [DOI] [Google Scholar]
- 26.Hu J, Ma W, Li N, Wang KJ. Antioxidant and Anti-Inflammatory Flavonoids from the Flowers of Chuju, a Medical Cultivar of Chrysanthemum Morifolim Ramat. J Mex Chem Soc. 2017;61:282–9. doi: 10.29356/jmcs.v61i4.458. [DOI] [Google Scholar]
- 27.Watanabe M. Catechins as antioxidants from buckwheat (Fagopyrum esculentum Moench) groats. J Agr Food Chem. 1998;46:839–45. doi: 10.1021/jf9707546. [DOI] [Google Scholar]
- 28.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 29.Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–8. [PubMed] [Google Scholar]
- 30.Marnett LJ. Lipid peroxidation-DNA damage by malondialdehyde. Mutat Res. 1999;424:83–95. doi: 10.1016/S0027-5107(99)00010-X. [DOI] [PubMed] [Google Scholar]