Abstract
Objective
Chronic obstructive pulmonary disease (COPD) is one of the most prevalent respiratory diseases in the world. There is an impressive relationship between periodontal status and airflow limitation in patients with COPD. Therefore, in this study, we aimed to investigate the periodontal status, its treatment needs, and its relationship with the severity of airway obstruction and quality of life in patients with COPD.
Materials and Methods
In this case-control study, 36 healthy men (control group) and 35 men afflicted with COPD (case group) were investigated. On the basis of spirometry results and Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria, patients with COPD were further divided into 4 groups. The participants’ quality of life was evaluated using COPD Assessment Test (CAT) questionnaire. Thereafter, both groups of participants were referred to a dentistry clinic so that the related specialist could investigate their periodontal health status. The relationship between the periodontal indices and the variables under study including GOLD stage, CAT score, Forced Expiratory Volume in first second and Forced Vital Capacity (FEV1/FVC) ratio, Forced Expiratory Volume in first second (FEV1), and the exacerbation rate were statistically analyzed using independent t-test, one-way analysis of variance, Tukey’s test, and Pearson correlation coefficient.
Results
The results revealed that probing pocket depth (PDD), bleeding on probing (BOP), and loss of attachment (LOA) are negatively correlated with FEV1% (r=−0.53, p=0.001), (r=−0.62, p=0.001), and (r=−0.72, p=0.001) as well as FEV1/FVC ratio (r=−0.45, p=0.007), (r=−0.47, p=0.004), and (r=−0.61, p=0.001), respectively. The results showed that PDD, BOP, and LOA are positively correlated with CAT score (r=0.51, p=0.002), (r=0.47, p=0.004), and (r=0.71, p=0.001), respectively.
Conclusion
Periodontal problems are positively associated with COPD severity as determined by GOLD criteria and negatively associated with quality of life of patients with COPD.
Keywords: Periodontal parameters, COPD, FEV1, periodontal disease, COPD assessment test
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most prevalent respiratory diseases and the fourth leading cause of death in the world, which causes substantial economic and social burden on people and societies all over the world [1, 2]. This disease has a progressive nature and causes pulmonary as well as extrapulmonary complications [3]. It is characterized by irreversible airflow limitation in the afflicted patients [4]. Each year, more than 3 million people in the world die of COPD [5]. Despite its increasing prevalence, few methods have been proposed to prevent and control it [6]. Smoking has been shown to be the most important risk factor for COPD [7]. However, various types of bacteria, especially those residing in the oral cavity, can also play a very important role in the incidence and progression of this disease and cause exacerbations as the result of the aspiration of oropharyngeal bacteria into the lower airways [8].
Recently, the relationship between periodontal status and some chronic systemic diseases has attracted researchers’ attention [9]. Systemic diseases are often reported to be affected by oral hygiene [10]. Therefore, many studies have been conducted to determine the relationship between oral hygiene and various systemic diseases such as diabetes, cardiovascular diseases, and pulmonary diseases [11, 12]. Therefore, it is quite possible that oral health problems, especially periodontal diseases, which are considered as important indicators of personal hygiene, affect and exacerbate chronic respiratory diseases such as COPD [13]. Periodontal diseases are inflammatory conditions that affect tissues surrounding and supporting teeth [14]. Some studies have shown that poor oral hygiene increases the incidence risk of pulmonary diseases [15] and decreases the quality of life [16]. Therefore, therapeutic interventions aimed at improving oral health can decrease the incidence rate and severity of pulmonary diseases in susceptible individuals [17]. Recent studies have shown that oral cavity can be one of the determining factors affecting the incidence of pulmonary diseases including COPD because it hosts a large number of bacterial pathogens [18]. COPD is more prevalent among people with unhealthy teeth than those with healthy teeth [19]. Furthermore, periodontal infections are likely to deteriorate the health status of patients suffering from pulmonary diseases, raise the risk of severe attacks of these diseases, and increase the occurrence rate of exacerbations [20].
The ultimate goal in the treatment of COPD is not only to control and cure the disease, but also to improve the patients’ quality of life [21]. Hence, our aim, in this study, is to investigate the periodontal status, its treatment needs, and its relationship with the severity of airway obstruction in patients with COPD as determined based on GOLD criteria and their quality of life as determined by the COPD Assessment Test (CAT) questionnaire [22, 23].
Materials and Methods
In this case-control study, 35 smokers with stable COPD (case group) and 36 healthy subjects were selected from the same hospital from among cases that had normal spirometry and did not have pulmonary symptoms. These subjects were matched in terms of age and dental number with the patients in the case group. The patients included in the study were already diagnosed to suffer from this disease based on the criteria proposed by the American Thoracic Society, that is coughing, sputum disposal, experiencing chronic dyspnea, having Forced Expiratory Volume in first second and Forced Vital Capacity (FEV1/FVC) ratio lower than 70%, and having at least 8 teeth. The sampling technique employed for the selection of participants was simple random sampling. The control group was matched with the case group in terms of age and gender. Written consent form was obtained from each of the participants before beginning the study. The exclusion criteria for this study were the presence of malignancies, cardiovascular diseases, diabetes, or HIV infection, having periodontal surgery in the last 6 months, and unwillingness to participate in the study. The spirometry indices of FEV1/FVC ratio, Forced Expiratory Volume in first second (FEV1), and FVC were recorded in the predesigned questionnaires for all participants; and based on the results obtained and the GOLD criteria, patients with COPD were further divided into 4 groups with moderate, mild, severe, and very severe airflow limitation. The participants’ quality of life was also evaluated using CAT questionnaire, and the estimated CAT scores were recorded. Both groups of participants were then referred to a dentistry clinic so that the related specialist could investigate their periodontal health status. The information obtained from the participants’ oral examination such as their probing pocket depth (PPD), bleeding on probing (BOP), and loss of attachment (LOA) were also recorded in the questionnaires for the subsequent analysis of the indices and the treatment needs of the participants. In addition, to assess the oral hygiene status of the participants, their teeth cleaning practices as well as their history of referring to dentist were recorded. The relationship between the periodontal indices and the variables under study including GOLD stage, CAT score, FEV1/FVC ratio, FEV1, and the exacerbation rate were statistically analyzed. This study was approved by the Research Ethics Committee of Ardabil University of Medical Sciences with the following code: ‘IR.ARUMS.REC.1393.44’.
Statistical Analysis
The data obtained in this study were analyzed by SPSS Software, Version 16.0 (SPSS Inc.; Chicago, IL, USA) available from Ardabil University. The relationship between different variables of the study were analyzed using independent t-test, one-way analysis of variance (ANOVA), Tukey’s test, and Pearson correlation coefficient with the significance level set at 0.05 for all of them.
Results
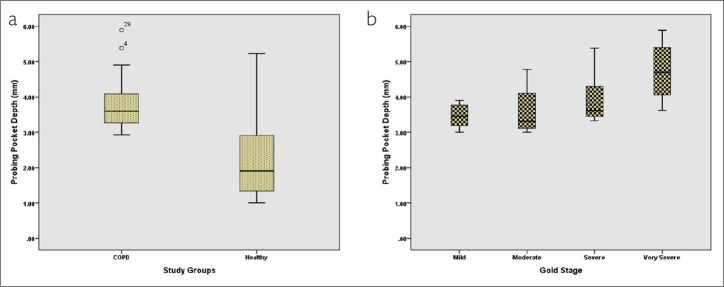
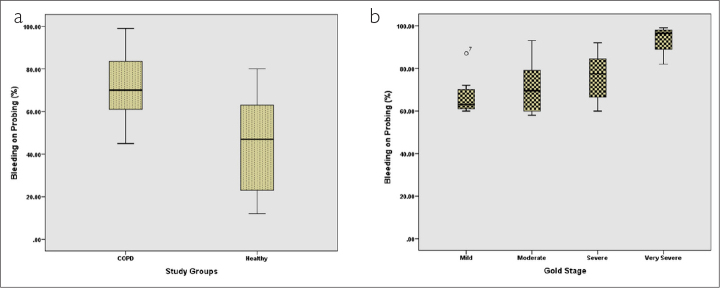
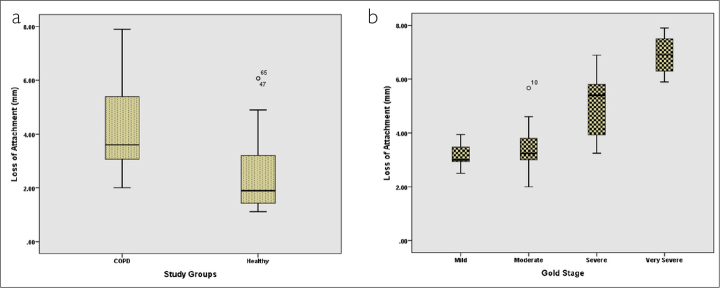
From among the total participants, 35 participants (49.3%) were in the COPD group (case group) and 36 participants belonged to the group without any respiratory disease (control group). The mean age of the participants in the COPD group was 57.88 years and that of the participants in the control group was 56.58 years. The 2 groups were not significantly different with respect to their mean age (p=0.59). The mean PPD, mean percentage of BOP, and mean LOA for both groups are presented in Table 1. In this study, the 2 groups of participants were compared in terms of mean PDD, mean percentage of BOP, and mean LOA using independent t-test. On the basis of the results obtained, the 2 groups were significantly different with respect to all of these 3 variables (p=0.001) (Figures 1a, 2a, and 3a).
Table 1.
Mean and SD of periodontal parameter
| Variable | Patient group Mean±SD | Control group Mean±SD | p |
|---|---|---|---|
| Age, year | 57.6±11.04 | 56.58±10.83 | 0.59 |
| Probing pocket depth, mm | 3.77±0.73 | 2.23±1.07 | 0.001* |
| Bleeding on probing, % | 73.31±13.71 | 46.11±23.44 | 0.001* |
| Loss of attachment, mm | 4.15±1.51 | 2.44±1.35 | 0.001* |
SD: standard deviation
Significant difference between groups based on independent t-test
Figure 1.
Comparison of mean probing pocket depth in (a) 2 groups and (b) patients based on different GOLD stages
Figure 2.
Comparison of mean bleeding on probing in (a) 2 groups and (b) patients based on different GOLD stages
Figure 3.
Comparison of mean loss of attachment in (a) 2 groups and (b) patients based on different GOLD stages
On the basis of the severity of airflow limitation and the GOLD criteria, patients in the case group were further divided into 4 different groups (Table 2). To compare the mean PPD of these 4 groups, one-way ANOVA was employed. The results indicated that the 4 groups of patients with COPD were significantly different based on their mean PDD (p=0.023) (Figure 1b). The mean PDD in patients with mild airflow limitation was 3.46 mm and in patients with very severe airflow limitation was 4.73 mm. Post hoc analysis with Tukey’s test demonstrated that the mean PDD of patients with very severe airflow limitation was significantly different from that of the patients with mild (p=0.024) and moderate (p=0.029) airflow limitation. However, the differences between other groups in this regard were not found to be significant (p≥0.05) (Table 2).
Table 2.
Post hoc comparison of mean PPD based on GOLD stages
| Obstruction Severity | Numbers | Mean±SD | PPD(mm) Mild | Moderate | Severe | Very Severe |
|---|---|---|---|---|---|---|
| Mild | 7 | 3.46±0.68 | * | |||
| Moderate | 14 | 3.62±0.72 | * | |||
| Severe | 8 | 3.93±0.69 | ||||
| Very Severe | 4 | 3.73±0.78 | * | * |
SD: standard deviation; PPD: probing pocket depth
Significant difference between groups based on analysis of variance
The mean BOP percentages of the 4 groups of patients with COPD were compared using one-way ANOVA (Table 3). The results revealed that these 4 groups were significantly different regarding their mean BOP percentage (p=0.005). The mean BOP percentage in the patients with mild airflow limitation was 67.43% and that of the patients with very severe airflow limitation was 93.50%. To find out that which of the 4 groups differ significantly, post hoc analysis was conducted using Tukey’s test. The results showed that the mean BOP of patients having very severe airflow limitation was significantly different from that of the patients with mild (p=0.003) and moderate (p=0.008) airflow limitation (Figure 2b). The differences between the BOPs of other groups were not found to be statistically significant (p≥0.05).
Table 3.
Post hoc comparison of mean BOP based on GOLD stages
| Obstruction Severity | BOP (%) Mean±SD | Mild | Moderate | Severe | Very Severe |
|---|---|---|---|---|---|
| Mild | 67.43±13.65 | * | |||
| Moderate | 71.86±13.51 | * | |||
| Severe | 76.12±13.86 | ||||
| Very Severe | 93.50±13.97 | * | * |
SD: standard deviation; BOP: bleeding on probing
Significant difference between groups based on analysis of variance
One-way ANOVA was also used to compare the mean LOAs of the 4 groups of patients with COPD (Table 4). The results indicated that these 4 groups were significantly different in this regard (p=0.001). The mean LOA of the patients with mild airflow limitation was 3.18 mm and that of the patients with very severe airflow limitation was 6.90 mm. To determine the significance of differences between specific pairs of groups, post hoc analysis was conducted using Tukey’s test. The results demonstrated that the LOA of the patients with mild airflow limitation was not significantly different from that of the patients with moderate airflow limitation (p≥0.05), while the differences between other groups in this regard were all found to be statistically significant (p≤0.05) (Figure 3b).
Table 4.
Post hoc comparison of mean LOA based on GOLD stages
| Obstruction Severity | LOA (mm) Mean±SD | Mild | Moderate | Severe | Very Severe |
|---|---|---|---|---|---|
| Mild | 67.43±13.65 | * | * | ||
| Moderate | 71.86±13.51 | * | * | ||
| Severe | 76.12±13.86 | * | * | * | |
| Very Severe | 93.50±13.97 | * | * | * |
SD: Standard deviation; LOA: loss of attachment
Significant difference between groups based on analysis of variance
To determine the relationship between periodontal variables and spirometry indices in patients with COPD, Pearson correlation coefficient was used. The results revealed that PDD was negatively correlated with FEV1% (r=−0.53, p=0.001) and FEV1/FVC ratio (r=−0.45, p=0.007). BOP was also found to be negatively correlated with FEV1% (r=−0.62, p=0.001) and FEV1/FVC ratio (r=−0.47, p=0.004). LOA, too, was found to be negatively correlated with FEV1% (r=−0.72, p=0.001) and FEV1/FVC ratio (r=−0.61, p=0.001) (Table 5).
Table 5.
Correlation coefficient of periodontal status with FEV1% and FEV1/FVC
| Variables | Mean | SD | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|---|---|
| 1 | PPD (mm) | 3.77 | 0.73 | ||||
| 2 | BOP (%) | 73.31 | 13.73 | 0.40* | |||
| 3 | LOA (mm) | 4.15 | 1.51 | 0.59** | |||
| 4 | FEV1%Predict | 61.91 | 25.24 | −0.53** | −0.72** | ||
| 5 | FEV1/FVC | 59.26 | 12.02 | −0.47** | −0.61** | −0.78** |
SD: standard deviation; PPD: probing pocket depth; BOP: bleeding on probing; LOA: loss of attachment; FEV1: Forced Expiratory Volume in first second; FEV1/FVC: Forced Expiratory Volume in first second and Forced Vital Capacity ratio
p≤0.05
p≤0.01
The analysis of the mean ages of patients in different GOLD stages with ANOVA did not show any statistically significant difference (p=0.95). Also, the analysis of the mean smoking history in patients at different GOLD stages did not show a statistically significant difference among them (p=0.43). Furthermore, Pearson correlation did not show any statistically significant correlation between periodontal variables (PPD, BOP, and LOA) and age (p=0.78, p=0.95, and p=0.88, respectively).
To determine the relationship between periodontal variables and health status of patients with COPD as determined by CAT score, again Pearson correlation coefficient was used. The results showed that PDD was positively correlated with CAT score (r=0.51, p=0.002). Positive correlation was also observed between BOP and CAT score (r=0.47, p=0.004). LOA was also found to be positively correlated with CAT score (r=0.71, p=0.001) (Table 6).
Table 6.
Correlation coefficient of periodontal status with CAT score
| Variables | Mean | SD | 1 | 2 | 3 | |
|---|---|---|---|---|---|---|
| 1 | PPD (mm) | 3.77 | 0.73 | |||
| 2 | BOP (%) | 73.31 | 13.71 | 0.40* | ||
| 3 | LOA (mm) | 4.15 | 1.51 | 0.59** | 0.43** | |
| 4 | CAT Score | 18.31 | 8.12 | 0.51** | 0.47** | −0.71** |
SD: standard deviation; PPD: probing pocket depth; BOP: bleeding on probing; LOA: loss of attachment
p≤0.05
p≤0.01
Discussion
The results of the present study indicated that the periodontal status of patients with COPD was worse than that of the normal people. More specifically, the periodontal variables of PPD, BOP, and LOA were found to be significantly related to the severity of airflow limitation, quality of life as determined through CAT score, and severity of dyspnea as determined through MMRC scale in patients with COPD.
CAT is an easily available and precise tool to evaluate the quality of life of patients with COPD and gain an understanding of the effect of this disease on various aspects of their life. As the airflow limitation decreases, CAT score improves [24]. The results of the present study demonstrated that there is a significantly positive correlation between periodontal variables (PPD, BOP, and LOA) and CAT score in patients with COPD (p<0.01 in all cases).
Few studies have investigated the relationship between periodontal variables and CAT score. In one of them, Zhou et al. reported that there is a direct correlation between poor oral health and lower quality of life in patients with COPD; that is, their quality of life decreases as their oral health deteriorates while oral care treatments improve the quality of life [25]. Our findings in this study are in line with those of Zhou et al. with the only difference being that they have used St George’s Respiratory Questionnaire to evaluate the quality of life for patients with COPD whereas we have employed the CAT questionnaire for this purpose.
In the present research, the severity of dyspnea was also investigated as one of the criteria related to the quality of life of patients with COPD, and the MMRC scale was used for this purpose. The results indicated a significant relationship between poor periodontal status and the severity of dyspnea in patients with COPD. Barrionuevo et al. showed in their study that poor oral hygiene is accompanied by increased airway obstruction in relatively young and healthy individuals and that enhancing oral hygiene can improve the general status of respiratory system [26]. Another study in Netherlands demonstrated that poor oral hygiene specifically results in airflow limitation and decreases lung capacity [27]. In another study, Zhou Xuan et al. investigated the effect of the treatment of chronic periodontitis on the health status of patients with COPD and reported that its treatment can improve pulmonary function and decrease the rate of exacerbations [28]. The results obtained in the current study indicated that poor oral health can increase airflow limitation in patients with COPD. They also revealed that a decrease in spirometry indices can cause a decrease in the patients’ quality of life and an increase in the severity of their dyspnea.
According to the results of the present research, the mean PPD in patients with COPD was 3.77 mm, which was significantly lower than that of the participants without any respiratory disease (p=0.001). This finding is consistent with the findings reported by Scannapieco et al. [29], Garcia et al. [30], and Peter et al. [31].
In this study, the mean BOP percentage of patients with COPD was found to be significantly higher than that of the participants in the control group (74.41% vs. 46.11%) (p=0.001). Similar findings have been reported by Scannapieco et al. [17] and Peter et al. [31].
According to the results of this study, the mean LOA of patients with COPD was found to be 4.15 mm while that of the participants in the control group was 2.44 mm. The difference between these 2 groups in this regard was statistically significant (p=0.001). This finding was consistent with the findings reported in the study by Scannapieco et al. [17]. In another study, Peter et al. [31] also reported that LOA was significantly higher in patients with severe COPD.
In this study, patients with COPD were divided into 4 groups based on GOLD criteria and were found to be significantly different with respect to their mean PPD and LOA. Furthermore, mean PPD and LOA of the patients were observed to increase with the intensification of airflow obstruction. Moreover, the 4 groups of patients with COPD were found to be significantly different with respect to their mean BOP (p=0.005). The findings of the present study indicated that with the increase in the severity of COPD, the severity of periodontal issues also increases. This finding is in line with the findings reported by Zhou et al. [25] and Mojon et al. [32].
The significant differences between the case and control groups regarding the oral hygiene indices investigated in this study might stem from the proliferation of pulmonary pathogens in the dental plaque or gingival cleft of the patients with COPD. In both periodontal disease and COPD, the inflammatory response of the host increases as the result of exposure to a harmful agent, which are either dental plaque bacteria in the case of periodontal disease or factors such as tobacco consumption in the case of COPD. This condition causes neutrophils to enter the respiratory system and release oxidative enzymes, which can directly cause tissue destruction.
Contrary to our findings in this study, Baldomero et al. did not report any relationship between oral hygiene and the exacerbation rate of COPD [33]. In another study, Liu et al. reported that the number of the remaining teeth in patients with COPD negatively correlates with the exacerbation rate of the disease; however, they did not observe any specific relationship between oral hygiene indices and the exacerbation rate of COPD in the groups of patients with frequent and infrequent exacerbation [34]. The differences between these results stem from the difference in the types of studies.
One of the potential limitations of this study was the limited number of patients meeting the criteria to be included in the study, which was removed because the researchers had access to the patients referred to the hospital and to the lung clinic. Another limitation was the inclusion of only male participants in the study, the reason for which was to minimize the interventional effects of female hormones.
In conclusion, the findings of the present study indicated that periodontal problems were positively associated with COPD severity as determined by GOLD criteria and negatively associated with the quality of life of patients with COPD. Therefore, dentists, especially periodontists, can play a key role in controlling COPD and improving the quality of life of the patients suffering from this disease via preventing periodontal diseases or stopping their progression.
Main Points.
Patients with COPD should be closely investigated for their periodontal health status.
Periodontal status in patients with COPD is associated with COPD severity.
Periodontal problems in patients with COPD negatively impact their quality of life.
Periodontal health status evaluation of patients with COPD may be beneficial in their quality of life.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Ardabil University of Medical Sciences (IR.ARUMS.REC.1393.44).
Informed Consent: Informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - H.G., A.A., A.B.; Design - H.G., N.J., A.B., A.A.; Supervision - H.G., A.B.; Resources - H.G., N.J., A.B., A.A.; Materials - H.G., N.J., A.B., A.A.; Data Collection and/or Processing - H.G., N.J., A.B., A.A.; Analysis and/or Interpretation - A.N.B., H.G., S.M.; Literature Search - H.G., N.J., A.N.B., S.M.; Writing Manuscript - S.M., N.J., H.G.; Critical Review - H.G., S.M.
Conflict of Interest: Authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Alrajeh AM, Aldabayan YS, Aldhair AM, et al. Global use, utility, and methods of tele-health in COPD: a health care provider survey. Int J Chron Obstruct Pulmon Dis. 2019;14:1713–9. doi: 10.2147/COPD.S202640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharifi H, Ghanei M, Jamaati H, et al. Burden of obstructive lung disease study in Iran: First report of the prevalence and risk factors of copd in five provinces. Lung India. 2019;36:14–9. doi: 10.4103/lungindia.lungindia_129_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sin DD, Man SP. Systemic inflammation and mortality in chronic obstructive pulmonary disease. Can J Physiol Pharmacol. 2007;85:141–7. doi: 10.1139/y06-093. [DOI] [PubMed] [Google Scholar]
- 4.Bajpai J, Kant S, Bajaj DK, Pradhan A, Srivastava K, Pandey AK. Clinical, demographic and radiological profile of smoker COPD versus nonsmoker COPD patients at a tertiary care center in North India. J Family Med Prim Care. 2019;8:2364–8. doi: 10.4103/jfmpc.jfmpc_347_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yorgancıoğlu A. Implementing Precision Medicine in Best Practices of Chronic Airway Diseases. Elsevier; 2019. The Global Burden of Chronic Airway Diseases; pp. 33–7. [DOI] [Google Scholar]
- 6.Agustí A, Hogg JC. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med. 2019;381:1248–56. doi: 10.1056/NEJMra1900475. [DOI] [PubMed] [Google Scholar]
- 7.Santoro A, Tomino C, Prinzi G, et al. Tobacco Smoking: Risk to Develop Addiction, Chronic Obstructive Pulmonary Disease, and Lung Cancer. Recent Pat Anticancer Drug Discov. 2019;14:39–52. doi: 10.2174/1574892814666190102122848. [DOI] [PubMed] [Google Scholar]
- 8.Si Y, Fan H, Song Y, Zhou X, Zhang J, Wang Z. Association between periodontitis and chronic obstructive pulmonary disease in a Chinese population. J Periodontol. 2012;83:1288–96. doi: 10.1902/jop.2012.110472. [DOI] [PubMed] [Google Scholar]
- 9.Magnus MC, Henderson J, Tilling K, Howe LD, Fraser A. Independent and combined associations of maternal and own smoking with adult lung function and COPD. Int J Epidemiol. 2018;47:1855–64. doi: 10.1093/ije/dyy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peres MA, Macpherson LM, Weyant RJ, et al. Oral diseases: a global public health challenge. Lancet. 2019;394:249–60. doi: 10.1016/S0140-6736(19)31146-8. [DOI] [PubMed] [Google Scholar]
- 11.Seymour G, Ford P, Cullinan M, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13(Suppl 4):3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 12.Falcao A, Bullón P. A review of the influence of periodontal treatment in systemic diseases. Periodontol 2000. 2019;79:117–28. doi: 10.1111/prd.12249. [DOI] [PubMed] [Google Scholar]
- 13.Pinto EH, Longo PL, de Camargo CCB, et al. Assessment of the quantity of microorganisms associated with bronchiectasis in saliva, sputum and nasal lavage after periodontal treatment: a study protocol of a randomised controlled trial. BMJ Open. 2016;6:e010564. doi: 10.1136/bmjopen-2015-010564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moghadam SA, Shirazaiy M, Risbaf S. The associations between periodontitis and respiratory disease. J Nepal Health Res Counc. 2017;15:1–6. doi: 10.3126/jnhrc.v15i1.18023. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Zhou X, Zhang J, et al. Periodontal health, oral health behaviours, and chronic obstructive pulmonary disease. J Clin Periodontol. 2009;36:750–5. doi: 10.1111/j.1600-051X.2009.01448.x. [DOI] [PubMed] [Google Scholar]
- 16.Deo V, Bhongade ML, Ansari S, Chavan RS. Periodontitis as a potential risk factor for chronic obstructive pulmonary disease: a retrospective study. Indian J Dent Res. 2009;20:466–70. doi: 10.4103/0970-9290.59456. [DOI] [PubMed] [Google Scholar]
- 17.Scannapieco FA, Cantos A. Oral inflammation and infection, and chronic medical diseases: implications for the elderly. Periodontol 2000. 2016;72:153–75. doi: 10.1111/prd.12129. [DOI] [PubMed] [Google Scholar]
- 18.Bozejac BV, Stojšin I, Đuric M, et al. Impact of inhalation therapy on the incidence of carious lesions in patients with asthma and COPD. J Appl Oral Sci. 2017;25:506–14. doi: 10.1590/1678-7757-2016-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhavsar NV, Dave BD, Brahmbhatt NA, Parekh R. Periodontal status and oral health behavior in hospitalized patients with chronic obstructive pulmonary disease. J Nat Sci Biol Med. 2015;6(Suppl 1):S93–7. doi: 10.4103/0976-9668.166097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–65. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 21.Bender BG, Depew A, Emmett A, et al. A patient-centered walking program for COPD. Chronic Obstr Pulm Dis. 2016;3:769–77. doi: 10.15326/jcopdf.3.4.2016.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khyalappa R, Thanekar S. Use of CAT score & it’s correlation with spirometry in stable COPD patients. Journal of Advanced Medical and Dental Sciences Research. 2019;7:72–5. [Google Scholar]
- 23.Amani M, Ghadimi N, Aslani MR, Ghobadi H. Correlation of serum vascular adhesion protein-1 with airflow limitation and quality of life in stable chronic obstructive pulmonary disease. RRespir Med. 2017;132:149–53. doi: 10.1016/j.rmed.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Ghobadi H, Ahari SS, Kameli A, Lari SM. The relationship between COPD assessment test (CAT) scores and severity of airflow obstruction in stable COPD patients. Tanaffos. 2012;11:22–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou X, Wang Z, Song Y, Zhang J, Wang C. Periodontal health and quality of life in patients with chronic obstructive pulmonary disease. Respir Med. 2011;105:67–73. doi: 10.1016/j.rmed.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Barrionuevo AMP, Real FG, Igland J, et al. Periodontal health status and lung function in two Norwegian cohorts. PloS One. 2018;13:e0191410. doi: 10.1371/journal.pone.0191410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holtfreter B, Richter S, Kocher T, et al. Periodontitis is related to lung volumes and airflow limitation: a cross-sectional study. Eur Respir J. 2013;42:1524–35. doi: 10.1183/09031936.00109112. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Han J, Liu Z, Song Y, Wang Z, Sun Z. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: A 2-year pilot randomized controlled trial. J Clin Periodontol. 2014;41:564–72. doi: 10.1111/jcpe.12247. [DOI] [PubMed] [Google Scholar]
- 29.Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review. Ann Periodontol. 2003;8:38–53. doi: 10.1902/annals.2003.8.1.38. [DOI] [PubMed] [Google Scholar]
- 30.Garcia RI, Nunn ME, Vokonas PS. Epidemiologic associations between periodontal disease and chronic obstructive pulmonary disease. Ann Periodontol. 2001;6:71–7. doi: 10.1902/annals.2001.6.1.71. [DOI] [PubMed] [Google Scholar]
- 31.Peter KP, Mute BR, Doiphode SS, Bardapurkar SJ, Borkar MS, Raje DV. Association between periodontal disease and chronic obstructive pulmonary disease: a reality or just a dogma? J Periodontol. 2013;84:1717–23. doi: 10.1902/jop.2013.120347. [DOI] [PubMed] [Google Scholar]
- 32.Mojon P. Oral health and respiratory infection. J Can Dent Assoc. 2002;68:340–5. [PubMed] [Google Scholar]
- 33.Baldomero AK, Siddiqui M, Lo C-Y, et al. The relationship between oral health and COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2019;14:881–92. doi: 10.2147/COPD.S194991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z, Zhang W, Zhang J, et al. Oral hygiene, periodontal health and chronic obstructive pulmonary disease exacerbations. J Clin Periodontol. 2012;39:45–52. doi: 10.1111/j.1600-051X.2011.01808.x. [DOI] [PubMed] [Google Scholar]