INTRODUCTION
The novel coronavirus SARS-CoV-2 emerged in Wuhan, China, and is the seventh member of the coronavirus family to infect human beings.1 It is a causative factor of severe respiratory disease and pneumonia, leading to severe pulmonary manifestations requiring intensive care unit treatment.2 The typical clinical manifestations of COVID-19 include cough, fever, diarrhea, shortness of breath, and fatigue. SARS-CoV-2 has a significant propensity to infect the central nervous system along with the respiratory system.3 In patients with severe COVID-19 infection with respiratory system involvement, the incidence of neurological involvement is higher. Such manifestations range from impaired consciousness to acute cerebrovascular disease. Other potential neurological symptoms associated with COVID-19 include headache, dizziness, seizures, agitation, confusion, delirium, stupor, and coma. The patients with neurological manifestations are relatively sicker with more propensity to manifest disease complications and have a higher mortality rate.
In this case report, a 58-year-old patient with COVID-19 infection presented with a history of two to three days of worsening confusion. Magnetic resonance imaging (MRI) of his brain showed bilateral medial temporal edema.
CASE REPORT
A 58-year-old male with a past medical history of diabetes, hypertension, and coronary artery disease with morbid obesity presented with two to three days history of worsening confusion. He worked as a physician assistant. His confusion had been gradual in onset and progressively got worse. His history of presenting illness was limited at admission due to confusion and lack of attention. Two weeks before the onset of encephalopathy, the patient complained of left-sided facial numbness and ringing sensations in both ears, and he developed a rash on his legs that was progressive. The patient had a history of a recent tick bite/exposure a few weeks before the hospital admission. He also was treated recently for lower leg cellulitis by a primary care physician. On examination, there were no gross motor/sensory neurological deficits. He was febrile with a temperature up to 40°C, associated with thrombocytopenia/leukopenia with the white blood cell count of 3 × 109/L. His chest x-ray was normal. Specialists from infectious diseases and neurology were consulted at that time.
Upon admission, the patient underwent extensive workup for his encephalopathy. His symptoms were consistent initially with viral versus tick-borne illnesses. His first COVID-19 test was negative from an outpatient facility, and his second COVID-19 test, done at the time of hospital admission, was negative. However, his other COVID-19 specific labs, such as D-dimer, ferritin, lactate dehydrogenase (LDH), and fibrinogen were high. It raised the suspicion that the initial COVID-19 testing may be a false negative, as the test’s specificity is 95%. After two negative test results for COVID-19, his third test was positive on the fourth day of hospital admission. Viral panel and cerebrospinal fluid (CSF) cultures were negative. PCR testing for herpes simplex virus (HSV 1/2), cytomegalovirus (CMV) also were negative (Table 1).
Table 1.
Cerebrospinal fluid (CSF) report showing possible infectious etiology of symptoms.
| Cytology for malignancy | Negative |
| Appearance | Colorless |
| White Blood Cells | 56 cells/μL |
| Red Blood Cells | 43 cell/ μL |
| Total Cell Count | 59 cell/ μL |
| Segmented Neutrophils | 4 cell/ μL |
| Lymphocytes | 78 cell/ μL |
| Monocytes | 16 cell/ μL |
| Other cells | 2 cell/ μL |
| CSF Glucose | 119 mg/dl |
| CSF total proteins | 68 mg/dl |
| Herpes simplex virus PCR | Not detected |
| Cytomegalovirus PCR | Negative |
| Respiratory syncytial virus | Negative |
| Enterovirus | Not detected |
| Listeria Monocytogenes | Not detected |
| Neisseria Meningitis | Not detected |
| Strep. Pneumoniae | Not detected |
| E. coli | Not detected |
| Hemophilus Influenzae | Not detected |
| CSF Gram Stain Final Report | |
| No neutrophils | |
| No organisms seen | |
| CSF Culture Final | |
| No growth seen after 3 days | |
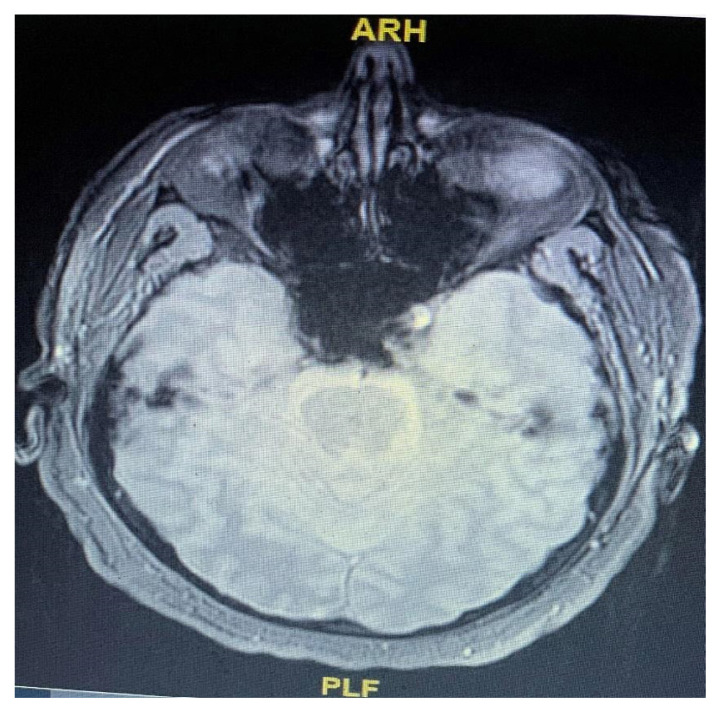
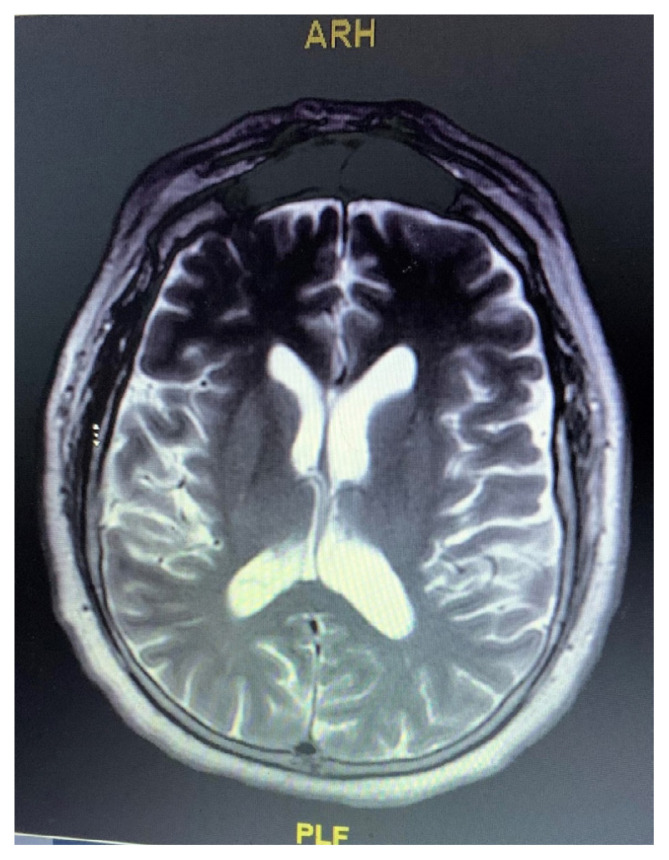
All tick-borne serologies were negative. COVID-19 testing was not performed on CSF due to the unavailability of the test at our facility. The patient’s brain MRI showed bilateral medial temporal T2 FLAIR bright signal consistent with edema with diffusion changes (Figures 1 and 2). Electroencephalography (EEG) showed background slowing and sharp wave discharges consistent with encephalopathy with no focal epileptiform activity.
Figure 1.
MRI (without contrast) of the brain showing bilateral medial temporal T2 FLAIR bright signal consistent with edema with diffusion changes.
Figure 2.
MRI (contrast enhanced view) of the brain showing bilateral medial temporal T2 FLAIR bright signal consistent with edema with diffusion changes.
In the presence of constant confusion and absence of an alternative explanation for altered mental status, the patient was started empirically on doxycycline for presumed anaplasmosis. He also was treated with acyclovir for possible herpes encephalitis. He initially was placed on levetiracetam, but was switched to divalproex sodium due to emotional lability. His acyclovir was stopped after the negative test results for herpes encephalitis. His carotid ultrasound did not show any acute pathology. He had leukopenia, anemia, and thrombocytopenia on admission that improved over time. He had mild acute kidney injury as well as transaminitis and hyponatremia that improved as well. After one week of hospital admission, the patient’s condition improved, and he was discharged to rehabilitation in a stable condition.
DISCUSSION
The current body of evidence has suggested that COVID-19 can involve the central nervous system (CNS), peripheral nervous system (PNS), and skeletal muscles.3 The common neurological manifestations include consciousness impairment, acute stroke, and skeletal muscle injury. Furthermore, patients with more severe COVID-19 disease are at a higher risk of developing neurological manifestations such as conscious disturbance, confusion, cerebrovascular disease, and skeletal muscle injury. Many of these neurological manifestations appeared early in the disease course. Some patients have been admitted to the hospital with only neurological manifestations as their presenting symptoms, without the typical symptoms (e.g., cough, fever, diarrhea, anorexia) of COVID-19.
Our case highlighted the significance of recognizing encephalopathy as the presenting symptom of COVID-19. The patient presented with confusion and was tested positive by the COVID-19 nasal swab test. He did not have any prior significant neurological history. His MRI highlighted the bilateral medial temporal lobe edematous changes consistent with T2 FLAIR hyperintensity. Helms et al.4 reported confusion in 26 of 40 COVID-19 patients admitted to the hospital with neurological symptoms. They performed MRI on 13 patients and found a leptomeningeal enhancement in eight patients. They also noted frontotemporal hypoperfusion in 11 patients who underwent perfusion imaging. However, this case underscored the presence of bilateral temporal lobe edematous changes with T2 FLAIR hyperintensity, a possible COVID-19 related brain pathology.
Several case studies have reported negative tests for viral RNA detection in CSF; however, others have detected SARS-CoV-2 nucleic acid in cerebrospinal fluid of COVID-19 patients who presented with neurological symptoms.5–9 Andrius et al.10 identified the antibodies against protein S1, protein S2, and SARS-CoV-2 nucleoprotein in the CSF of encephalopathy patients. CSF testing for CMV, herpes, and respiratory syncytial virus were negative in our case. However, the PCR test for coronavirus on CSF could not be run at our facility because of its unavailability. Our hospital also lacked the facility to test for CSF antibodies against SARS-CoV-2 protein. Lastly, our patient’s EEG findings showed background slowing and sharp wave discharges with no focal epileptiform activity. These findings were consistent with the small case series which reported sharp frontal waves in COVID-19 patients presented with encephalopathy.11 They reported sporadic epileptiform discharges in the EEG of acutely ill COVID-19 patients presenting with encephalopathy, and these epileptiform discharges appeared as frontal sharp waves.
It has been postulated that SARS-CoV-2 acts through the angiotensin-converting enzyme 2 receptors (ACE2).12 These receptors are present in multiple body organs, including the lungs, central nervous system, and skeletal muscles.13 SARS-CoV-2 can cause neurological signs through its direct or indirect actions on these ACE2 receptors. Other coronaviruses such as SARS-CoV and MERS-CoV are known to cause neuronal injury, and their nucleic acids have been detected in the CSF.14 Autopsy reports of some COVID-19 patients’ brain tissues have shown some hyperemic, edematous, and degenerated neurons.15
Mao et al.3 proposed the possible pathological mechanism of CNS invasion of SARS-CoV-2. Like the other coronaviruses, it may enter the CNS through the hematogenous or retrograde neurological route. This retrograde neurological pathway can be supported by the fact that many patients with COVID-19 have been reported with smell impairment. Moreover, Liu et al.16 reported eosinopenia (eosinophils < 0.02 × 109cells/L) at the time of hospital admission in a small cohort of COVID-19 patients. They proposed eosinopenia as the possible cause of immunosuppression in these patients. Notably, their eosinophilic count improved with lopinavir treatment at the time of discharge, suggesting eosinopenia may be a potential indicator for improving clinical status in COVID-19 patients.
CONCLUSION
This case report highlighted the importance of recognizing neurological symptoms in COVID-19 patients. These patients can present with encephalopathy without the usual COVID-19 presentation picture in acute settings or hospitals. Health care workers should be aware of it to avoid rapid clinical deterioration or worsen the patient’s condition, contributing to the high mortality rate. Further research studies are needed to determine the detrimental effect of SARSCoV-2 on the brain.
REFERENCES
- 1.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Saiegh F, Ghosh R, Leibold A, et al. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J Neurol Neurosurg Psychiatry. 2020;91(8):846–848. doi: 10.1136/jnnp-2020-323522. [DOI] [PubMed] [Google Scholar]
- 6.Caamaño DSJ, Beato RA. Facial diplegia, a possible atypical variant of Guillain-Barré Syndrome as a rare neurological complication of SARSCoV-2. J Clin Neurosci. 2020;77:230–232. doi: 10.1016/j.jocn.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perchetti GA, Nalla AK, Huang M-L, et al. Validation of SARS-CoV-2 detection across multiple specimen types. J Clin Virol. 2020;128:104438. doi: 10.1016/j.jcv.2020.104438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou L, Zhang M, Wang J, Gao J. Sars-Cov-2: Underestimated damage to nervous system. Travel Med Infect Dis. 2020;36:101642. doi: 10.1016/j.tmaid.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andriuta D, Roger P-A, Thibault W, et al. COVID-19 encephalopathy: Detection of antibodies against SARS-CoV-2 in CSF. J Neurol. 2020;267(10):2810–2811. doi: 10.1007/s00415-020-09975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galanopoulou AS, Ferastraoaru V, Correa DJ, et al. EEG findings in acutely ill patients investigated for SARS-CoV-2/COVID-19: A small case series preliminary report. Epilepsia Open. 2020;5(2):314–324. doi: 10.1002/epi4.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-n Cov. Jan 26, 2020. [Accessed August 12, 2020]. https://www.biorxiv.org/content/10.1101/2020.01.26.919985v1.
- 13.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desforges M, Favreau DJ, Brison E, et al. Human Coronavirus: Respiratory Pathogens Revisited as Infectious Neuroinvasive, Neurotropic, and Neurovirulent Agents. In: Singh SK, Ruzek D, editors. Neuroviral Infections: RNA Viruses and Retroviruses. Boca Raton, FL: CRC Press; 2013. pp. 93–122. [Google Scholar]
- 15.National Health Commission of the People’s Republic of China. Diagnosis and treatment of the novel coronavirus pneumonia (Trial version 7) Mar 29, 2020. [Accessed August 12, 2020]. http://en.nhc.gov.cn/2020-03/29/c_78469.htm.
- 16.Liu F, Xu A, Zhang Y, Xuan W, Yan T, Pan K. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]