Abstract
Prostate cancer (PCa), an epithelial malignancy that occurs in the prostate, is the second leading cause of cancer death worldwide. MicroRNAs (miRs/miRNAs) are reported to have important applications in the field of cancer diagnosis and treatment. The present study aimed to investigate the function of miRNA-122 in the chemoresistance of PCa cells and the underlying mechanism. Significantly decreased miR-122 and increased pyruvate kinase (PKM2) levels were observed in docetaxel-resistant PCa cells, and PKM2 was negatively correlated with miR-122. MiR-122 mimic transfection in docetaxel-resistant LNCaP cells significantly inhibited cell proliferation, promoted apoptosis and decreased glucose uptake and lactate production, which was counteracted by PKM2 overexpression. Inhibition of miR-122 in LNCaP cells had an opposite effect to miR-122 mimic transfection. In addition, miR-122 mimic transfection significantly increased the sensitivity of docetaxel-resistant LNCaP cells to docetaxel, while inhibition of miR-122 significantly decreased the sensitivity of LNCaP cells to docetaxel. Luciferase reporter assays showed that miR-122 regulated PKM2 expression by binding to the 3'-untranslated region of PKM2. The results suggest that upregulation of miR-122 could enhance docetaxel sensitivity, inhibit cell proliferation and promote apoptosis in PCa cells,possibly through the downregulation of its target protein PKM2.
Keywords: prostate cancer, microRNA-122, pyruvate kinase, proliferation, apoptosis, glycolysis
Introduction
Prostate cancer (PCa) is an epithelial malignancy that occurs in the prostate (1). PCa mainly occurs in men over the age of 50 and is the second leading cause of cancer death worldwide (2,3). Currently, endocrine therapy, including surgical or drug castration and antiandrogen (bicalutamide or flutamide) therapy, is the main treatment for hormone-sensitive advanced PCa patients (4). However, the vast majority of patients are eventually treated with androgen-deprivation therapy and progress to metastatic castration-resistant PCa (CRPC), which is the leading cause of PCa-related mortality (5-7). Novel treatments, such as docetaxel and abiraterone, were shown to improve the survival of patients with metastatic CRPC; however, most patients develop drug resistance (8,9).
While healthy cells rely on carbohydrate molecules oxidized in mitochondria to acquire energy, most tumor cells obtain their energy supply through relatively low-yield glycolysis, which does not involve oxygen or mitochondria (10). Malignant, rapidly growing tumor cells typically have a 200-fold higher rate of glycolysis compared with normal tissues, even under oxygen-sufficient conditions (11). Therefore, it is speculated that this change in metabolism is the root cause of cancer (12). It was reported that glycolysis in cancer cells is characterized by high glucose consumption and lactate production (13). Cancer cells often take up high amounts of glucose and rely on glycolysis for ATP generation, more efficiently converting glucose into macromolecules that are needed for a variety of cellular processes (14-16). Pyruvate kinase (PKM2), a key rate-limiting enzyme that catalyzes the final step in glycolysis, was reported to be highly expressed in multiple cancers (17,18) and can promote glucose metabolism and cell growth (19). A number of studies showed that upregulation of PKM2 can promote malignancy and downregulation of PKM2 can inhibit cell growth, migration and invasion in various types of cancer (20-24).
MicroRNAs (miRs/miRNAs) are small noncoding, single-stranded RNAs which regulate gene expression by regulating the stability or translation of target mRNAs by binding to their 3'-untranslated regions (UTRs) (25,26). Studies showed that miRNAs have important applications in the field of cancer diagnosis and treatment (27-32). For example, exo-anti-miR-214 can reverse the resistance of gastric cancer cells to cisplatin (33). MiR-122, an abundant liver-specific miRNA, was shown to reverse doxorubicin resistance in liver cancer cells by inhibiting glycolysis in tumors via PKM2 inhibition (34,35). In colon cancer, overexpression of miR-122 can increase the sensitivity of fluorouracil (5-FU)-resistant colon cancer cells to 5-FU by PKM2 downregulation (36). Previous findings showed that miR-34a and miR-21 play a role in the chemoresistance of PCa cells (37-40). The present study aimed to investigate the function of miRNA-122 in the chemoresistance of PCa cells and the underlying mechanism.
Materials and methods
Cell culture
Prostate cancer docetaxel-resistant (LNCaP/Docetaxel) and docetaxel-sensitive (LNCaP) LNCaP cells were purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences. Cells were cultured with DMEM (cat. no. SH30243.01; HyClone; GE Healthcare Life Sciences) supplemented with 10% FBS (cat. no. 16000-044; Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin and streptomycin (100X; cat. no. P1400; Beijing Solarbio Science & Technology Co., Ltd.) in a 37˚C incubator (Forma 3111; Thermo Fisher Scientific, Inc.) with 5% CO2.
Isolation of primary PCa cells
PCa cells were isolated from 20 patients with PCa who were treated in Shaoxing People's Hospital, Shaoxing, China from February 2018 to February 2019 (age range, 60-80 years; mean age, 70.12±8.43 years). The inclusion criteria were as follows: i) patients did not receive any treatment and ii) clinical data of patients were complete. All cases were confirmed by a review by the Shaoxing People's Hospital Pathology Center. The exclusion criteria were cases without complete clinical data. Fresh prostate cancer tissues were washed three times with D-Hank's balanced salt solution containing 500 IU/ml penicillin and streptomycin. Once surrounding inactivated tissues (cloudy appearance, dull and loss of normal tissue elasticity) were removed with ophthalmic scissors, the tissues (normal color and elasticity) were cut into pieces (~300 times) in a sterile 5 ml syringe and incubated with 5 ml trypsin-EDTA (0.125% trypsin and 0.53 mol/l EDTA) for 5 min at 37˚C. A total of 10 ml RPMI-1640 medium (cat. no. 88365; Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) was added to terminate digestion in a 15 ml centrifuge tube. After centrifugation at 4˚C and 800 x g for 5 min, the pellet was resuspended in 5 ml RPMI 1640 containing 5 ng/ml epidermal growth factor (cat. no. PHG0311L; Gibco; Thermo Fisher Scientific, Inc.), 50 µg/ml bovine pituitary extract (cat. no. 13028014; Gibco; Thermo Fisher Scientific, Inc.) and 10% FBS. A total of 5 ml of the suspension was seeded in the T25 cell flasks for incubation in a 37˚C and 5% CO2 incubator. Fibroblasts can adherent growth in the T25 cell flasks after 4-6 days. Medium was replaced every two days, and the cells were passaged when they were reached 80% confluence. Cells that were sub-cultured 3 times were used for subsequent experiments.
Lentivirus construction
The coding sequence (CDS) of PKM2 (AY352517.1) was synthesized and validated by DNA sequencing. Following the insertion of the PKM2 CDS into the pLVX-Puro vector (Clontech Laboratories, Inc.) at the EcoRI-BamHI site, the pLVX-Puro-PKM2 plasmid was co-transfected into 293T cells (American Type Culture Collection) cultured with DMEM containing 10% FBS and 1% penicillin-streptomycin, with the viral packaging plasmids psPAX2 and pMD2.G (Addgene, Inc.) using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc). After 48 h of transfection, the supernatant was collected by centrifugation for 5 min at 1,000 x g and 4˚C.
Cell transfection
LNCaP and LNCaP/Docetaxel cells in the logarithmic growth phase were suspended to 1x106 cells/ml after trypsinization. Subsequently, 2 ml cell suspension was inoculated into six-well plates for overnight culture at 37˚C with 5% CO2. Once the cells grew to 60-70% confluence, LNCaP and LNCaP/Docetaxel cells were transfected with 5 µl negative control (NC)-miRNA (100 pmol; 5'-CAGUACUUUUGUGUAGUACAA-3'), 5 µl hsa-miR-122-5p inhibitor (100 pmol; 5'-CAAACACCATTGTCACACTCCA-3') or 5 µl of hsa-miR-122-5p mimic (100 pmol; 5'-UGGAGUGUGACAAUGGUGUUUG-3') using Lipofectamine™ 2000 (cat. no. 11668-019; Invitrogen; Thermo Fisher Scientific, Inc.). Following 24 h of transfection, serum-free transfer solution was replaced with complete medium to culture for a further 48 h.
Docetaxel treatment
LNCaP and LNCaP/Docetaxel cells were treated with gradient concentrations of docetaxel (0.25, 0.5, 1, 2, 4, 8, 16 and 32 µg/ml; cat. no. 114977-28-5; Shanghai Aladdin Biochemical Technology Co., Ltd.), followed by the detection of cell inhibition rate. After treatment with docetaxel, the apoptosis levels of primary PCa cells, treated LNCaP or treated LNCaP/Docetaxel cells were detected.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from PCa cells (LNCaP, LNCaP/Docetaxel or primary PCa cells) with or without miR-122 inhibitor or mimic, and/or combined with PKM2 overexpression (oePKM2) lentivirus using TRIzol® reagent (cat. no. 15996026; Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Following RNA quanrification and integrity confirmation, ~1 µg of RNA was reversed transcribed into cDNA using the RevertAid First Strand cDNA Synthesis kit (cat. no. K1622; Thermo Fisher Scientific, Inc.) using the following heat cycle: 37˚C for 5 min; 55˚C for 15 min and 85˚C for 5 min. qPCR was performed on a 7300 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with a miRNA RT-PCR Detection Kit (cat. no. AOMD-Q020; GeneCopoeia, Inc.) or a Maxima SYBR Green/ROX qPCR master mix (cat. no. K0223; Thermo Fisher Scientific, Inc.). The following thermocycling conditions were used for the qPCR: Initial denaturation at 95˚C for 10 min, 40 cycles of 95˚C for 15 sec and 60˚C for 45 sec and a final extension step at 95˚C for 15 sec. miR-122 expression were normalized to U6 levels and PKM2 expression were normalized to GAPDH levels using the 2-ΔΔCq method (41). The primer pairs used for the qPCR are listed in Table I.
Table I.
Primer sequences used for reverse transcription-quantitative PCR.
| Gene | Forward sequence | Reverse sequence |
|---|---|---|
| RT primer for miR-122 | 5'-GTCGTATCCAGTGCAGGGTCCGAGG | |
| TATTCGCACTGGATACGACGCCTAG-3' | ||
| miR-122 | 5'-CGCCATTATCACACTAAATAGCTACTG-3' | 5'-AGTGCAGGGTCCGAGGTATT-3' |
| PKM2 | 5'-TCCAGGTGAAGCAGAAAG-3' | 5'-CGGATGAATGACGCAAAC-3' |
| U6 | 5'-CTCGCTTCGGCAGCACA-3' | 5'-AACGCTTCACGAATTTGCGT-3' |
| GAPDH | 5'-AATCCCATCACCATCTTC-3' | 5'-AGGCTGTTGTCATACTTC-3' |
miR-122, microRNA-122; PKM2, pyruvate kinase; RT, reverse transcription.
Western blot analysis
Total protein was isolated from PCa cells (LNCaP, LNCaP/Docetaxel or primary PCa cells) with or without treatment of miR-122 inhibitor or mimic, and/or combined with oePKM2 lentivirus using RIPA buffer supplemented with protease and phosphatase inhibitors (cat. no. R0010; Beijing Solarbio Science & Technology Co., Ltd.). Following protein quantification using a BCA protein quantification kit (Thermo Fisher Scientific, Inc.), 25 µg of protein/lane was separated via 10% SDS PAGE followed by semi-dry transfer onto PVDF membranes (cat. no. HATF00010; EMD Millipore). After blocking with 5% skimmed milk for 1 h at room temperature, membranes were incubated overnight with primary antibodies against PKM2 (1:500; cat. no. ab137852; Abcam) and GAPDH (1:2,000; cat. no. 5174; Cell Signaling Technology, Inc.) at 4˚C with gentle agitationFollowing six washes with TBS-Tween-20, membranes were incubated for 2 h at room temperature with goat anti-rabbit horseradish peroxidase (HRP)-labeled secondary antibodies (1:1.000; cat. no. A0208; Beyotime Institute of Biotechnology). Protein bands were visualized using an Tanon 5200 chemiluminescent imaging system (Tanon Science and Technology Co., Ltd.) after 5 min of development with the Immobilon Western Chemiluminescent HRP substrate (cat. no. WBKLS0100; EMD Millipore) in the dark. PKM2 protein expression was quantified using ImageJ (version 1.47; National Institutes of Health) with GAPDH as the loading control.
Cell proliferation assay
PCa cells (LNCaP or LNCaP/Docetaxel) in the logarithmic growth phase were trypsinized and a 3x104 cells/ml suspension was prepared by counting the cells under an inverted microscope at x40 magnification (XDS-500C; Shanghai Caikon Optical Instrument Co., Ltd.). Subsequently, in a 96-well culture plate, 100 µl of the suspension was inoculated and cultured at 37˚C overnight. A total of 100 µl DMEM was used as the blank control. Cell Counting Kit-8 solution (CCK-8; cat. no. ab228554; Abcam) and serum-free DMEM were mixed at a volume ratio of 1:10. Following 0, 24, 48 and 72 h of treatment with miR-122 inhibitor or miR-122 mimic and oePKM2 lentivirus, 100 µl of the above CCK-8 mixture was added to the cells and incubated in a 5% CO2 incubator at 37˚C for 1 h. The absorbance was read at a wavelength of 450 nm using a microplate reader (cat. no. DNM-9602; Perlong Medical Equipent Co., Ltd.).
Cell apoptosis assay
Following 48 h of treatment, each group of cells (LNCaP, LNCaP/Docetaxel or primary PCa cells) was collected using 0.05% trypsin) to detect apoptosis using an Annexin V-FITC cell apoptosis detection kit (cat. no. C1062L; Beyotime Institute of Biotechnology). In brief, ~1x106 cells were centrifuged at 1,000 x g for 5 min at 4˚C. After discarding the supernatant, the cells were gently resuspended in 195 µl Annexin V-FITC binding solution, followed by a 15-min incubation with 5 µl Annexin V-FITC at 4˚C in the dark. Subsequently, 5 µl of propidium iodide (PI) staining solution was added and the cells were incubated for 5 min at 4˚C in the dark. A tube without Annexin V-FITC and PI was used as a negative control. Flow cytometry was performed and apoptosis percentages were assessed with BD Accuri C6 software (version 1.0.264.21; BD Biosciences).
Detection of glucose uptake and lactate production
PCa cells (LNCaP or LNCaP/Docetaxel) were seeded in 24-well plates and cultured overnight and treated with miR-122 inhibitor or miR-122 mimic and oePKM2 lentivirus. A 2-NBDG Glucose Uptake Assay kit (cat. no. K682-50; BioVision, Inc.) was used for glucose uptake detection. Following 72 h of treatment, the cells were incubated with 100 µM 2-NBDG for 1 h. After two washes with PBS, cells were trypsinized and resuspended in DMEM containing 10% FBS, followed by incubation with 5 µg/ml PI for staining. Subsequently, flow cytomtery was performed to measure the proportion of PI-negative and 2-NBDG-positive cells, and glucose uptake was calculated. Lactate production was measured using a lactate test kit (cat. no. A019-2; Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's instructions. The absorbance was measured at a wavelength of 530 nm using a spectrophotometer and lactate production was calculated.
Luciferase reporter assay
TargetScanHuman 7.2 (http://www.targetscan.org/vert_72/) was used to predict miR-122 target sites on PKM2. PCa cells (LNCaP or LNCaP/Docetaxel) in the logarithmic growth phasewere trypsinized and centrifuged at 800 x g for 5 min at room temperature. After discarding the supernatant, cells were gently resuspended in 1 ml DMEM and counted under an inverted microscope at x40 magnification (XDS-500C; Shanghai Caikon Optical Instrument Co., Ltd.). The cell suspension was inoculated into a six-well plate at a density of 5x105 cells/well and cultured in an incubator at 37˚C. Following 24 h of culture, the cells were co-transfected with 1.5 µg luciferase plasmid (pGL3-Promoter-PKM; Promega Corporation) and miR-122-5p inhibitor or mimic using Lipofectamine™ 2000 (cat. no. 11668-019; Invitrogen; Thermo Fisher Scientific, Inc.). Following transfection, the cells were washed with PBS, and then incubated in 500 µl PLB for 15 mins with gentle agitation at room temperature. The luciferase activity of the PKM2 reporter was determined using a Dual-Luciferase Reporter Assay system (cat. no. E1910; Promega Corporation) on a GloMax®-Multi+ Microplate Multimode reader (Promega Corporation). Firefly luciferase activity was detected following addition of 100 µl LAR II and 20 µl sample lysate in 96-well plates. Renilla luciferase activity was detected following addition of 100 µl Stop & Glo reagent.
Statistical analysis
GraphPad Prism 7.0 (GraphPad Software, Inc.) was used for statistical analysis. Data are presented as the mean ± SD from triplicate experiments. Unpaired Student's t-test was used to determine the significance between two groups, while multiple groups were compared by one-way ANOVA and Tukey's post hoc test. The Pearson correlation coefficient was used to analyze the correlation between miR-122 and PKM2. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of miR-122 significantly decreased in LNCaP/Docetaxel cells, and inhibition of miR-122 in LNCaP cells significantly promotes proliferation and glycolysis and inhibits apoptosis
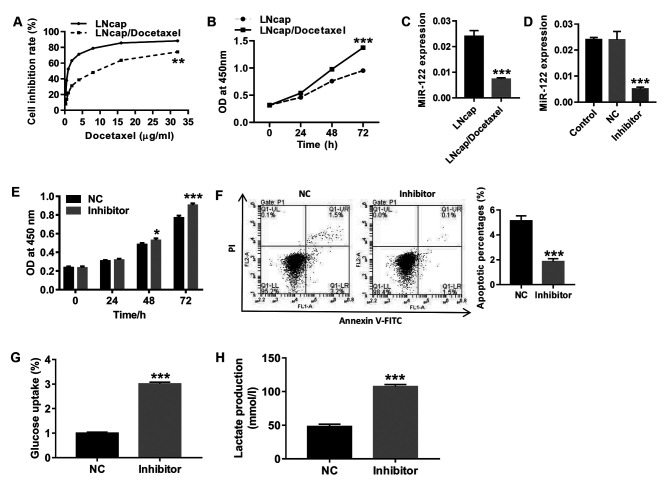
Following treatment with gradient concentrations of docetaxel, cell proliferation was detected to determine the drug resistance of LNCaP/Docetaxel cells to docetaxel. As shown in Fig. 1A, the half-maximal inhibitory concentration (IC50) in LNCaP/docetaxel cells was significantly higher compared with LNCaP cells, indicating that LNCaP/Docetaxel cells were docetaxel-resistant. Consistent with a previous report (42), 10 µg/ml of docetaxel was used for subsequent experiments. Baseline proliferation levels of LNCaP/Docetaxel cells were significantly higher compared with LNCaP cells (Fig. 1B). RT-qPCR was performed to detect the expression of miR-122 in human PCa docetaxel-resistant (LNCaP/Docetaxel) and -sensitive (LNCaP) cell strains. The results in Fig. 1C show that compared with LNCaP cells, miR-122 levels significantly decreased in LNCaP/Docetaxel cells. Furthermore, LNCaP cells were treated with miR-122 inhibitor (Fig. 1D). Following miR-122 inhibition, the proliferation of LNCaP cells significantly increased (Fig. 1E), and apoptosis was significantly decreased (Fig. 1F), which was accompanied by significantly increased glucose uptake (Fig. 1G) and lactate production (Fig. 1H) compared with the NC group. The findings suggested that miR-122 expression may be associated with docetaxel resistance in PCa.
Figure 1.
Expression of miR-122 significantly decreased in LNCaP/Docetaxel cells and inhibition of miR-122 in LNCaP cells significantly promotes proliferation and glycolysis and inhibits apoptosis. (A) Following treatment with gradient concentrations of docetaxel (0.25, 0.5, 1, 2, 4, 8, 16 and 32 µg/ml), cell proliferation was assessed to determine resistance to docetaxel in LNCaP and LNCaP/Docetaxel cells. **P<0.01. (B) Baseline proliferation levels of LNCaP and LNCaP/Docetaxel cells were detected. ***P<0.001. (C) Expression of miR-122 in LNCaP and LNCaP/Docetaxel cells was detected. ***P<0.001. (D) The expression of miR-122 in miR-122-treated LNCaP cells was detected. ***P<0.001 vs. NC. (E) Cell proliferation was detected at 0, 24, 48 and 72 h. *P<0.05 and ***P<0.001. (F) Cell apoptosis was detected at 48 h. The ordinate of the histogram is the sum of early and late apoptosis. ***P<0.001. (G) Glucose uptake and (H) lactate production were detected. ***P<0.001. miR-122, microRNA-122; OD, optical density; NC, negative control; PI, propidium iodide.
PKM2 may be a target gene of miR-122 in regulating PCa
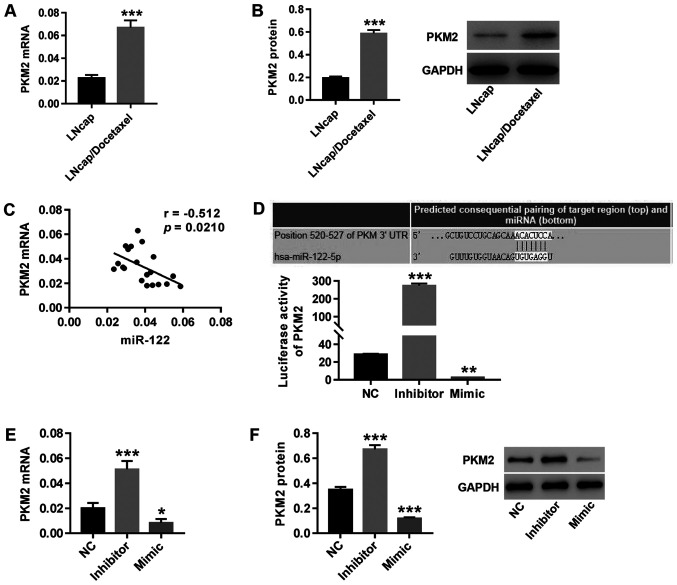
Both mRNA (Fig. 2A) and protein (Fig. 2B) expression of PKM2 significantly increased in LNCaP/Docetaxel cells compared with LNCaP cells. PKM2 expression was negatively correlated with miR-122 expression in primary PCa cells isolated from tumor tissues of 20 patients with PCa (Fig. 2C). Furthermore, TargetScan was used to predict the binding site between the PKM2 3'-UTR and miR-122. Compared with the NC group, theluciferase reporter assay showed that following inhibition of miR-122, the luciferase activity of the PKM2 reporter was significantly increased (Fig. 2D), accompanied by increased expression of PKM2 (Fig. 2E and F), whilemiR-122 mimic transfection had the opposite effect. The results indicated that PKM2 might be a target gene of miR-122 in regulating PCa.
Figure 2.
PKM2 may be a target gene for miR-122 in regulating PCa. The expression of PKM2 (A) mRNA and (B) protein in LNCaP and LNCaP/Docetaxel cells was detected. ***P<0.001. (C) The correlation between miR-122 and PKM2 in primary PCa cells was analyzed by Pearson's correlation coefficient analysis. (D) The binding site between the 3'-UTR of PKM2 and miR-122 was predicted by TargetScan. Following miR-122 inhibitor or mimic treatment, the luciferase activity of the PKM2 reporter was detected, and PKM2 (E) mRNA and (F) protein expression was detected. *P<0.05; **P<0.01 and ***P<0.001 vs. NC. miR-122, microRNA-122; NC, negative control; 3'-UTR, 3'-untranslated region.
miR-122 possibly regulates the docetaxel resistance of PCa cells via PKM2 regulation
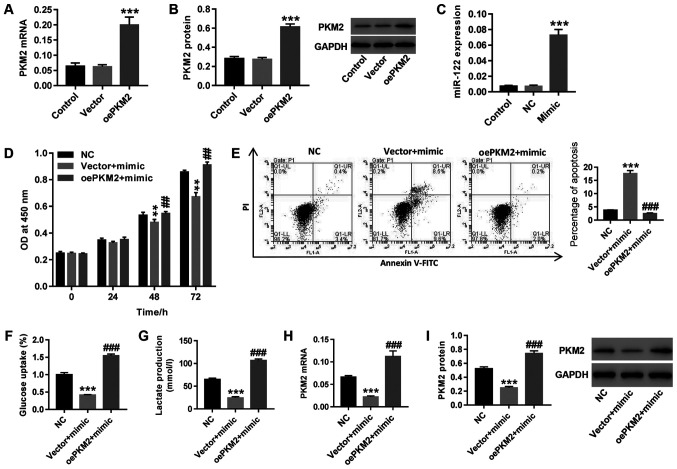
The human PCa docetaxel-resistant cell strain LNCaP/Docetaxel was treated with both miR-122 mimic and oePKM2 lentivirus. As shown in Fig. 3A-C, oePKM2 and miR-122 mimic treatment in LNCaP/Docetaxel cells significantly increased PKM2 and mir-122 expression compared with the vector and NC groups, respectively. Upregulation of miR-122 following miR-122 mimic transfection resulted in significantly increased cell proliferation (Fig. 3D), glucose uptake (Fig. 3F) and lactate production (Fig. 3G) in LNCaP/Docetaxel, cells, whereas cell apoptosis (Fig. 3E) increased, concurrent with a decrease inthe expression of PKM2 (Fig. 3H and I). Overexpression of PKM2 counteracted the effects of miR-122 mimic on LNCaP/Docetaxel cells. The results demonstrated that miR-122 regulated docetaxel resistance in PCa cells, possibly via regulating PKM2.
Figure 3.
miR-122 regulates docetaxel resistance of PCa cells possibly via PKM2 regulation. The upregulation efficiency of oePKM2 lentivirus was detected by (A) reverse transcription-quantitiative PCR and (B) western blotting. ***P<0.001 vs. vector. (C) The efficiency of miR-122 mimic transfection was detected. ***P<0.001 vs. NC. LNCaP/Docetaxel cells were treated with both oePKM2 lentivirus and miR-122 mimic. (D) Cell proliferation was detected at 0, 24, 48 and 72 h. **P<0.01 vs. NC and ##P<0.01 vs. vector + mimic. (E) Cell apoptosis was detected at 48 h. The ordinate of the histogram is the sum of early and late apoptosis. ***P<0.001 vs. NC and ###P<0.001 vs. vector + mimic. (F) Glucose uptake and (G) lactate production were detected. ***P<0.001 vs. NC and ###P<0.001 vs. vector + mimic. PKM2 (H) mRNA and (I) protein expression was detected. ***P<0.001 vs. NC and ###P<0.001 vs. vector + mimic. miR-122, microRNA-122; OD, optical density; NC, negative control; PI, propidium iodide.
Upregulation of miR-122 could reverse the resistance of LNCaP/Docetaxel cells to docetaxel
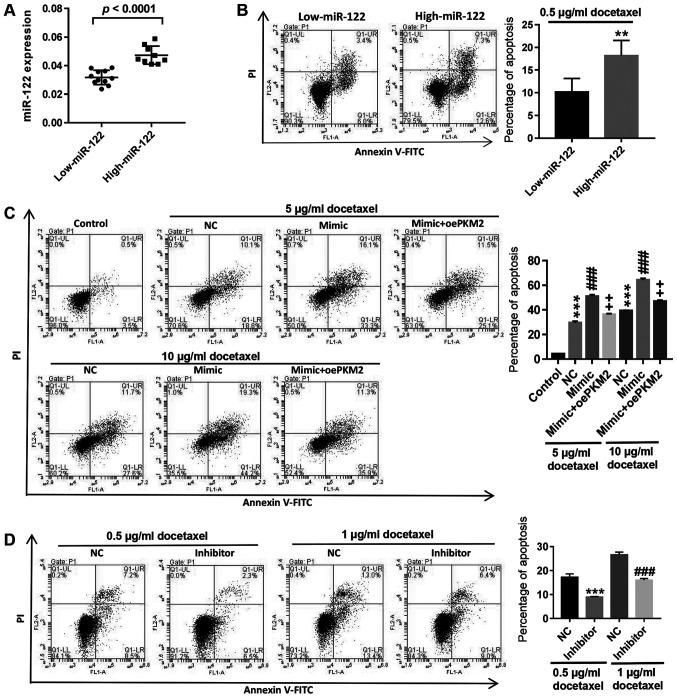
The primary cells isolated from 20 patients with PCa were divided into two groups: Low expression and high expression of miR-122 (Fig. 4A). Flow cytometry analysis showed that following treatment with 0.5 µg/ml docetaxel, apoptosis in primary PCa cells with high miR-122 expression significantly increased compared with cells with low miR-122 expression (Fig. 4B). In LNCaP/Docetaxel cells, upregulation of miR-122 expression by miR-122 mimic transfection significantly increased docetaxel-induced apoptosis, while overexpression of PKM2 counteracted the effect of miR-122 mimic transfection, and the effect of 10 µg/ml docetaxel was more significant compared with 5 µg/ml docetaxel (Fig. 4C). By contrast, inhibition of miR-122 in LNCaP cells significantly decreased docetaxel-induced apoptosis (Fig. 4D). The results demonstrated that high expression of miR-122 could promote docetaxel-induced apoptosis in PCa cells and upregulation of miR-122 could reverse the resistance of LNCaP/Docetaxel cells to docetaxel.
Figure 4.
Upregulation of miR-122 could reverse decetaxel resistance of LNCaP/Docetaxel cells.(A) Expression of miR-122 in primary PCa cells isolated from 20 patients with PCa was detected and divided into low-miR-122 and high-miR-122 groups. (B) Cell apoptosis of PCa cells with low miR-122 and high miR-122 expression was detected following 0.5 µg/ml docetaxel treatment. The ordinate of the histogram is the sum of early and late apoptosis. **P<0.01. (C) Following treatment with miR-122 mimic, oePKM2 or docetaxel (5 and 10 µg/ml), cell apoptosis in LNCaP/Docetaxel cells was detected. The ordinate of the histogram is the sum of early and late apoptosis. ***P<0.001 vs. control; ###P<0.001 vs. NC and ++P<0.01 vs. mimic. (D) Following treatment with miR-122 inhibitor and docetaxel (5 and 10 µg/ml), cell apoptosis in LNCaP cells was detected. The ordinate of the histogram is the sum of early and late apoptosis. ***P<0.001 vs. NC + 0.5 µg/ml docetaxel and ###P<0.001 vs. NC + 1 µg/ml docetaxel. miR-122, microRNA-122; NC, negative control; PI, propidium iodide.
Discussion
An increasing number of studies have reported that the sensitivity of tumor cells to anticancer drugs can be altered by miRNAs (43-45). miR-34a was reported to enhance the chemosensitivity of PC3 cells to paclitaxel and camptothecin and PCa cells treated with miR-143 showed higher chemosensitivity to docetaxel (37,46). Previous research also showed that miR-122 can reverse drug resistance in several cancers (34,36). In the present study, significantly decreased miR-122 levels were observed in human PCa LNCaP/Docetaxel cells compared with LNCaP cells. miR-122 mimic transfection in LNCaP/Docetaxel PCa cells significantly decreased cell proliferation, increased apoptosis and inhibited glycolysis, whilemiR-122 inhibitor transfection in LNCaP showed the opposite effect, suggesting that miR-122 expression may be associated with docetaxel resistance in PCa by regulating cell proliferation, apoptosis and glycolysis. In primary PCa cells, high expression of miR-122 could promote docetaxel-induced apoptosis in PCa cells, and miR-122 mimic transfection significantly increased docetaxel-induced apoptosis in LNCaP/Docetaxel cells, while inhibition of miR-122 showed the opposite effect. Thus, it was speculated that upregulation of miR-122 could reverse the resistance of LNCaP/Docetaxel cells to docetaxel, which may contribute to the treatment of PCa chemoresistance.
Furthermore, the underlying mechanism of miR-122 in regulating docetaxel resistance in PCa was investigated. In tumors, miRNAs primarily function via regulation of their target genes by targeting specific mRNAs for degradation or translation inhibition (47). A study reported that upregulation of miR-328 can enhance docetaxel sensitivity, decrease cell proliferation and increase apoptosis in PCa cells by directly targeting p21-activated protein kinase 6(48). Additionally, the ectopic expression of miR-21 can increase the resistance of PC3 cells to docetaxel by targeting the tumor suppressor programmed cell death protein 4(40). In addition, a previous study revealed that in human lung cancer xenografts in mice, inhibition of PKM2 could enhance the efficacy of docetaxel (49). Results of the present study showed that the expression of PKM2 in PCa cells negatively correlated with miR-122 expression, and the luciferase reporter assay showed that miR-122 regulated PKM2 expression by binding to the 3'-UTR of PKM2. In LNCaP/Docetaxel PCa cells, miR-122 mimic-induced cell proliferation decreased, apoptosis increased and glycolysis inhibition was counteracted by PKM2 overexpression. Consistent with previous reports on miR-122 in cancer chemoresistance (34-36), it can be inferred that the upregulation of miR-122 expression may reverse the resistance of PCa LNCaP/Docetaxel cells to docetaxel via downregulation of its target gene PKM2. However, the present study also had limitations, such as the lack of sequencing data and validation, as well as the lack of studies on other prostate cancer cell types. Mechanisms of miR-122 involved in docetaxel resistance and the function of miR-122 in other prostate cancer cell types can be investigated in future studies to further confirm the current results.
In conclusion, the results demonstrated that high expression of miR-122 could promote docetaxel-induced apoptosis in PCa cells and that the upregulation of miR-122 could reverse the resistance of LNCaP/Docetaxel cells to docetaxel, possibly via the regulation of its target protein PKM2 by binding to the 3'-UTR. These findings may provide a link between PCa chemoresistance and miRNAs, and targeting miRNA-122 may offer a novel therapy for the chemoresistance of PCa.
Acknowledgements
Not applicable.
Funding
This study was funded by the Shaoxing Municipal Bureau of Science and Technology in China (grant no. 2017B70032).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
ZZ and JY conceived and designed the study. ZZ, JY and GT performed the experiments. ZZ and JY wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experiments conducted in this study were approved by the Ethics Committee of Shaoxing People's Hospital. Written informed consent was obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1. doi: 10.1080/07391102.2019.1635913. Han W and Li J: Structure-activity relationship analysis of 3-phenylpyrazole derivatives as androgen receptor antagonists. J Biomol Struct Dyn: 1-10, Jul 5, 2019 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Fendler A, Jung M, Stephan C, Honey RJ, Stewart RJ, Pace KT, Erbersdobler A, Samaan S, Jung K, Yousef GM. miRNAs can predict prostate cancer biochemical relapse and are involved in tumor progression. Int J Oncol. 2011;39:1183–1192. doi: 10.3892/ijo.2011.1128. [DOI] [PubMed] [Google Scholar]
- 4.Pereira-Lourenço M, Vieira E, Brito D, Peralta JP, Godinho R, Conceiçao P, Reis M, Rabaça C, Sismeiro A. Influence of sociodemographic factors on treatment's choice for localized prostate cancer in Portugal. Arch Ital Urol Androl. 2020;92:45–49. doi: 10.4081/aiua.2020.1.45. [DOI] [PubMed] [Google Scholar]
- 5.van Brussel JP, Mickisch GH. Multidrug resistance in prostate cancer. Onkologie. 2003;26:175–181. doi: 10.1159/000071510. [DOI] [PubMed] [Google Scholar]
- 6.Sturge J, Caley MP, Waxman J. Bone metastasis in prostate cancer: Emerging therapeutic strategies. Nat Rev Clin Oncol. 2011;8:357–368. doi: 10.1038/nrclinonc.2011.67. [DOI] [PubMed] [Google Scholar]
- 7.Chi KN, Bjartell A, Dearnaley D, Saad F, Schröder FH, Sternberg C, Tombal B, Visakorpi T. Castration-resistant prostate cancer: From new pathophysiology to new treatment targets. Eur Urol. 2009;56:594–605. doi: 10.1016/j.eururo.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Cookson MS, Lowrance WT, Murad MH, Kibel AS. Castration-resistant prostate cancer: AUA guideline amendment. J Urol. 2015;193:491–499. doi: 10.1016/j.juro.2014.10.104. [DOI] [PubMed] [Google Scholar]
- 9.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 10.Bolten CJ, Heinzle E, Müller R, Wittmann C. Investigation of the central carbon metabolism of Sorangium cellulosum: Metabolic network reconstruction and quantification of pathway fluxes. J Microbiol Biotechnol. 2009;19:23–36. [PubMed] [Google Scholar]
- 11.Tyszka-Czochara M, Konieczny P, Majka M. Recent advances in the role of AMP-activated protein kinase in metabolic reprogramming of metastatic cancer cells: Targeting cellular bioenergetics and biosynthetic pathways for anti-tumor treatment. J Physiol Pharmacol. 2018;69 doi: 10.26402/jpp.2018.3.07. [DOI] [PubMed] [Google Scholar]
- 12.Liberti MV, Locasale JW. The warburg effect: How does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaneton B, Gottlieb E. Rocking cell metabolism: Revised functions of the key glycolytic regulator PKM2 in cancer. Trends Biochem Sci. 2012;37:309–316. doi: 10.1016/j.tibs.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Wang H, Liu A, Fang C, Hao J, Wang Z. Lactate dehydrogenase A negatively regulated by miRNAs promotes aerobic glycolysis and is increased in colorectal cancer. Oncotarget. 2015;6:19456–19468. doi: 10.18632/oncotarget.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ha TK, Her NG, Lee MG, Ryu BK, Lee JH, Han J, Jeong SI, Kang MJ, Kim NH, Kim HJ, Chi SG. Caveolin-1 increases aerobic glycolysis in colorectal cancers by stimulating HMGA1-mediated GLUT3 transcription. Cancer Res. 2012;72:4097–4109. doi: 10.1158/0008-5472.CAN-12-0448. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Li J, Sun X, Guo Y, Chu D, Wei L, Li X, Yang G, Liu X, Yao L, et al. Tumor suppressor NDRG2 inhibits glycolysis and glutaminolysis in colorectal cancer cells by repressing c-Myc expression. Oncotarget. 2015;6:26161–26176. doi: 10.18632/oncotarget.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong G, Mao Q, Xia W, Xu Y, Wang J, Xu L, Jiang F. PKM2 and cancer: The function of PKM2 beyond glycolysis. Oncol Lett. 2016;11:1980–1986. doi: 10.3892/ol.2016.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang J, Cao R, Zhang Y, Xia Y, Zheng Y, Li X, Wang L, Yang W, Lu Z. PKM2 dephosphorylation by Cdc25A promotes the Warburg effect and tumorigenesis. Nat Commun. 2016;7(12431) doi: 10.1038/ncomms12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luan W, Wang Y, Chen X, Shi Y, Wang J, Zhang J, Qian J, Li R, Tao T, Wei W, et al. PKM2 promotes glucose metabolism and cell growth in gliomas through a mechanism involving a let-7a/c-Myc/hnRNPA1 feedback loop. Oncotarget. 2015;6:13006–130018. doi: 10.18632/oncotarget.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu W, Cao Y, Zhang Y, Li S, Gao J, Wang XA, Mu J, Hu YP, Jiang L, Dong P, et al. Up-regulation of PKM2 promote malignancy and related to adverse prognostic risk factor in human gallbladder cancer. Sci Rep. 2016;6(26351) doi: 10.1038/srep26351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang HS, Zhang FJ, Li H, Liu Y, Du GY, Huang YH. Tanshinone ⅡA inhibits human esophageal cancer cell growth through miR-122-mediated PKM2 down-regulation. Arch Biochem Biophys. 2016;598:50–56. doi: 10.1016/j.abb.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 22.Xu Q, Zhang M, Tu J, Pang L, Cai W, Liu X. MicroRNA-122 affects cell aggressiveness and apoptosis by targeting PKM2 in human hepatocellular carcinoma. Oncol Rep. 2015;34:2054–2064. doi: 10.3892/or.2015.4175. [DOI] [PubMed] [Google Scholar]
- 23.Guo M, Zhao X, Yuan X, Jiang J, Li P. MiR-let-7a inhibits cell proliferation, migration, and invasion by down-regulating PKM2 in cervical cancer. Oncotarget. 2017;8:28226–28236. doi: 10.18632/oncotarget.15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taniguchi K, Sugito N, Kumazaki M, Shinohara H, Yamada N, Nakagawa Y, Ito Y, Otsuki Y, Uno B, Uchiyama K, Akao Y. MicroRNA-124 inhibits cancer cell growth through PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Lett. 2015;363:17–27. doi: 10.1016/j.canlet.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 26.Rigoutsos I. New tricks for animal microRNAS: Targeting of amino acid coding regions at conserved and nonconserved sites. Cancer Res. 2009;69:3245–3248. doi: 10.1158/0008-5472.CAN-09-0352. [DOI] [PubMed] [Google Scholar]
- 27.Gandellini P, Profumo V, Casamichele A, Fenderico N, Borrelli S, Petrovich G, Santilli G, Callari M, Colecchia M, Pozzi S, et al. miR-205 regulates basement membrane deposition in human prostate: Implications for cancer development. Cell Death Differ. 2012;19:1750–1760. doi: 10.1038/cdd.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudson RS, Yi M, Esposito D, Glynn SA, Starks AM, Yang Y, Schetter AJ, Watkins SK, Hurwitz AA, Dorsey TH, et al. MicroRNA-106b-25 cluster expression is associated with early disease recurrence and targets caspase-7 and focal adhesion in human prostate cancer. Oncogene. 2013;32:4139–4147. doi: 10.1038/onc.2012.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boll K, Reiche K, Kasack K, Mörbt N, Kretzschmar AK, Tomm JM, Verhaegh G, Schalken J, von Bergen M, Horn F, Hackermüller J. MiR-130a, miR-203 and miR-205 jointly repress key oncogenic pathways and are downregulated in prostate carcinoma. Oncogene. 2013;32:277–285. doi: 10.1038/onc.2012.55. [DOI] [PubMed] [Google Scholar]
- 30.Martens-Uzunova ES, Jalava SE, Dits NF, van Leenders GJ, Møller S, Trapman J, Bangma CH, Litman T, Visakorpi T, Jenster G. Diagnostic and prognostic signatures from the small non-coding RNA transcriptome in prostate cancer. Oncogene. 2011;31:978–991. doi: 10.1038/onc.2011.304. [DOI] [PubMed] [Google Scholar]
- 31.Musumeci M, Coppola V, Addario A, Patrizii M, Maugeri-Saccà M, Memeo L, Colarossi C, Francescangeli F, Biffoni M, Collura D, et al. Control of tumor and microenvironment cross-talk by miR-15a and miR-16 in prostate cancer. Oncogene. 2011;30:4231–4242. doi: 10.1038/onc.2011.140. [DOI] [PubMed] [Google Scholar]
- 32.Takayama K, Tsutsumi S, Katayama S, Okayama T, Horie-Inoue K, Ikeda K, Urano T, Kawazu C, Hasegawa A, Ikeo K, et al. Integration of cap analysis of gene expression and chromatin immunoprecipitation analysis on array reveals genome-wide androgen receptor signaling in prostate cancer cells. Oncogene. 2011;30:619–630. doi: 10.1038/onc.2010.436. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Zhang H, Bai M, Ning T, Ge S, Deng T, Liu R, Zhang L, Ying G, Ba Y. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol Ther. 2018;26:774–783. doi: 10.1016/j.ymthe.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan C, Wang X, Shi K, Zheng Y, Li J, Chen Y, Jin L, Pan Z. MiR-122 reverses the doxorubicin-resistance in hepatocellular carcinoma cells through regulating the tumor metabolism. PLoS One. 2016;11(e0152090) doi: 10.1371/journal.pone.0152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kishikawa T, Otsuka M, Tan PS Ohno M, Sun X, Yoshikawa T, Shibata C, Takata A, Kojima K, Takehana K, et al. Decreased miR122 in hepatocellular carcinoma leads to chemoresistance with increased arginine. Oncotarget. 2015;6:8339–8252. doi: 10.18632/oncotarget.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He J, Xie G, Tong J, Peng Y, Huang H, Li J, Wang N, Liang H. Overexpression of microRNA-122 re-sensitizes 5-FU-resistant colon cancer cells to 5-FU through the inhibition of PKM2 in vitro and in vivo. Cell Biochem Biophys. 2014;70:1343–1350. doi: 10.1007/s12013-014-0062-x. [DOI] [PubMed] [Google Scholar]
- 37.Fujita Y, Kojima K, Hamada N, Ohhashi R, Akao Y, Nozawa Y, Deguchi T, Ito M. Effects of miR-34a on cell growth and chemoresistance in prostate cancer PC3 cells. Biochem Biophys Res Commun. 2008;377:114–119. doi: 10.1016/j.bbrc.2008.09.086. [DOI] [PubMed] [Google Scholar]
- 38.Singh S, Chitkara D, Mehrazin R, Behrman SW, Wake RW, Mahato RI. Chemoresistance in prostate cancer cells is regulated by miRNAs and Hedgehog pathway. PLoS One. 2012;7(e40021) doi: 10.1371/journal.pone.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Yang X, Guan H, Mizokami A, Keller ET, Xu X, Liu X, Tan J, Hu L, Lu Y, Zhang J. Exosome-derived microRNAs contribute to prostate cancer chemoresistance. Int J Oncol. 2016;49:838–846. doi: 10.3892/ijo.2016.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi G, Ye D, Yao X, Zhang SL, Dai B, Zhang HL, Shen YJ, Zhu Y, Zhu YP, Xiao WJ, Ma CG. Involvement of microRNA-21 in mediating chemo-resistance to docetaxel in androgen-independent prostate cancer PC3 cells. Acta Pharmacol Sin. 2010;31:867–873. doi: 10.1038/aps.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 42.Egawa T, Kubota T, Suto A, Otani Y, Furukawa T, Watanabe M, Kumai K, Kitajima M. Docetaxel enhances the cytotoxicity of anthracyclines by increasing intracellular drug accumulation. Oncol Rep. 2002;9:777–781. [PubMed] [Google Scholar]
- 43. doi: 10.1002/2211-5463.12838. Lin W, Miao Y, Meng X, Huang Y, Zhao W and Ruan J: miRNA-765 mediates multidrug resistance via targeting BATF2 in gastric cancer cells. FEBS Open Bio: Mar 12, 2020 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong ST, Lin H, Wang CS, Chang CH, Lin AMY, Yang JCH, Lo YL. Improving the anticancer effect of afatinib and microRNA by using lipid polymeric nanoparticles conjugated with dual pH-responsive and targeting peptides. J Nanobiotechnology. 2019;17(89) doi: 10.1186/s12951-019-0519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hong BS, Ryu HS, Kim N, Kim J, Lee E, Moon H, Kim KH, Jin MS, Kwon NH, Kim S, et al. Tumor Suppressor miRNA-204-5p regulates growth, metastasis, and immune microenvironment remodeling in breast cancer. Cancer Res. 2019;79:1520–1534. doi: 10.1158/0008-5472.CAN-18-0891. [DOI] [PubMed] [Google Scholar]
- 46.Xu B, Niu X, Zhang X, Tao J, Wu D, Wang Z, Li P, Zhang W, Wu H, Feng N, et al. miR-143 decreases prostate cancer cells proliferation and migration and enhances their sensitivity to docetaxel through suppression of KRAS. Mol Cell Biochem. 2011;350:207–213. doi: 10.1007/s11010-010-0700-6. [DOI] [PubMed] [Google Scholar]
- 47.Bian Z, Li L, Tang R, Hou DX, Chen X, Zhang CY, Zen K. Identification of mouse liver mitochondria-associated miRNAs and their potential biological functions. Cell Res. 2010;20:1076–1078. doi: 10.1038/cr.2010.119. [DOI] [PubMed] [Google Scholar]
- 48.Liu C, Zhang L, Huang Y, Lu K, Tao T, Chen S, Zhang X, Guan H, Chen M, Xu B. MicroRNA-328 directly targets p21-activated protein kinase 6 inhibiting prostate cancer proliferation and enhancing docetaxel sensitivity. Mol Med Rep. 2015;12:7389–7395. doi: 10.3892/mmr.2015.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi HS, Li D, Zhang J, Wang YS, Yang L, Zhang HL, Wang XH, Mu B, Wang W, Ma Y, et al. Silencing of pkm2 increases the efficacy of docetaxel in human lung cancer xenografts in mice. Cancer Sci. 2010;101:1447–1453. doi: 10.1111/j.1349-7006.2010.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.