Abstract
Introduction
Increasing rates of antimicrobial resistance in Neisseria gonorrhoeae cause problems for treating gonorrhoea.
Aim
This observational study aimed to describe isolates from all patients found infected with N. gonorrhoeae, in Barcelona, Spain, between 2013 and 2017, and with available antimicrobial susceptibility data.
Methods
Minimum inhibitory concentrations (MICs) of penicillin (PEN), cefixime (CFM), ceftriaxone (CRO), azithromycin (AZM), ciprofloxacin (CIP), spectinomycin (SPT), fosfomycin (FOF) and gentamicin (GEN) were determined by E-test. Susceptibility was assessed using clinical breakpoints from the European Committee on Antimicrobial Susceptibility Testing. Time trends for PEN, CFM, AZM and CIP were investigated using logistic regression.
Results
Of 1,979 patients with infection (2,036 isolates), 1,888 (95.4%) were men. Patient median age was 32 years. The proportions of isolates resistant to extended-spectrum cephalosporins were low, with 0.3% (5/1,982) resistant to CRO and 4.9% (98/1,985) to CFM. AZM resistance prevalence was 2.7% (52/1,981), including 16 isolates detected in 2016 and 2017, with high-level resistance. For CIP, 51.3% (1,018/1,986) of isolates were resistant, and for PEN, 20.1% (399/1,985). All isolates were susceptible to SPT. MIC50 and MIC90 values of GEN were 4 and 6 mg/L and of FOF 12 and 24 mg/L, respectively. Between 2013 and 2017, PEN and CFM resistance rates each decreased from 28.1% (92/327) to 12.2% (70/572) and from 8.3% (27/327) to 4.4% (25/572) (p ≤ 0.0073). In contrast, AZM resistance prevalence appeared to increase from 1.5% in 2014 (5/340) to 3.0% (17/572) in 2017. No trend was identified for CIP.
Conclusion
Antimicrobial susceptibility surveillance is important to timely detect new phenotypes and trends.
Keywords: Neisseria gonorrhoeae, antimicrobial resistance, extended-spectrum cephalosporins, azithromycin
Introduction
Gonorrhoea is the second most reported bacterial sexually transmitted infection (STI), after chlamydia. Untreated gonorrhoea can lead to pelvic inflammatory disease and infertility in women and epididymitis and orchitis in men. In the European Union/European Economic Area countries, a total of 89,239 confirmed cases of gonorrhoea were reported in 2017 with an overall rate of 22.2 cases per 100,000 population [1]. This represented a 17% increase over the previous year, which was particularly striking in certain groups, such as men who have sex with men (MSM), who represented almost half of the cases in 2017, and the 25–34-year-old population [1].
In Spain, 8,722 cases of gonococcal infection were reported in 2017 (rate: 18.74 per 100,000 inhabitants), with a very wide range of infection incidence among the different regions of the country, from 2.44 and 48.50 cases per 100,000 inhabitants. The highest rates were registered in Catalonia (48.50), Balearic Islands (41.79) and Madrid (28.48) [2]. Of the 3,622 cases reported in Catalonia in 2017, men accounted for 82% of the diagnoses. Data from 1,136 patients (31%) could be collected: 44% were MSM followed by heterosexual men (22%) and women (20%) [3].
Neisseria gonorrhoeae, the bacterial species responsible for gonorrhoea, has developed resistance to the different families of antibiotics used in the past, challenging future treatment. Currently, extended-spectrum cephalosporins (ESC) are the last-line treatment option for N. gonorrhoeae infection. Unfortunately, strains showing resistance to ESC have been reported worldwide [4-6]. In 2017, the European Centre for Disease Control and Prevention (ECDC) reported a resistance rate to cefixime of 1.9% within the European Union/European Economic Area countries and no isolates showing resistance to ceftriaxone [7]. The resistance rate to azithromycin was 7.5%; and high-level resistance (minimum inhibitory concentration (MIC) ≥ 256 mg/L) was detected in seven isolates [7].
This pathogen has moreover been able to acquire or develop nearly all known mechanisms of antimicrobial resistance: target modification, inactivation of the antimicrobial by enzymatic means, decreased influx of antimicrobials, and increased efflux of antimicrobials [8]. This situation highlights the importance of carrying out resistance monitoring programmes, in order to update therapeutic guidelines and to timely detect the emergence of multidrug-resistant strains.
Nowadays, most of the treatment guidelines recommend dual antimicrobial therapy (500 mg intramuscular ceftriaxone + 1 g or 2 g oral azithromycin) [9-12]. The rationale for gonococcal combination therapy using different antimicrobials with different mechanisms of action is to potentially mitigate the spread of antimicrobial resistance [13]. Combination therapy has been reported as possibly related to the decline in prevalence of N. gonorrhoeae isolates with decreased susceptibility to ceftriaxone (DSC), defined as a MIC > 0.032 mg/L [14,15].
The aim of this study is to describe antimicrobial susceptibility of N. gonorrhoeae in isolates collected between 2013 and 2017 in Barcelona, Spain.
Methods
Study population
The study population consisted of all patients who were diagnosed with a N. gonorrhoeae infection and with isolates for which antimicrobial susceptibility data were available. When patients tested positive for gonorrhoea on multiple sites and more than one culture was obtained, only the genital culture was included in the study.
Patients were attended in three different clinical settings: Drassanes-Vall d'Hebron Sexually Transmitted Infections unit, different medical departments of Vall d'Hebron University Hospital and primary healthcare units in Barcelona, Spain. Together these settings cover 1,200,000 of the 1,620,343 inhabitants of Barcelona (74.1%).
Culture
Urethral, rectal, vaginal, endocervical, and/or pharyngeal samples were cultured on selective Thayer–Martin medium and incubated at 35–37 °C in a 5% CO2 atmosphere for 24–48 hours. Probable N. gonorrhoeae strains were identified by oxidase reaction (oxidase-positive) and mass spectrometry (MALDI-TOF, Vitek MS system, Biomérieux, Marcy-I´Étoile, France). N. gonorrhoeae strains were subcultured in order to obtain fresh colonies for antimicrobial susceptibility testing (AST) and frozen at −80 °C in trypticase soy broth with 20% glycerol.
Antimicrobial susceptibility
AST was performed on fresh colonies (<24 hours). The MICs of penicillin, ceftriaxone, cefixime, ciprofloxacin, azithromycin, spectinomycin, gentamicin, and fosfomycin were determined by means of the E-test method (bioMérieux, France), as described by the Clinical and Laboratory Standard Institute (CLSI) [16]. Interpretation was performed using clinical breakpoints from the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [17], except for gentamicin and fosfomycin, since cut-off points for these antibiotics are not established by EUCAST (nor by CLSI). N. gonorrhoeae ATCC 49226 was used as a reference strain for antimicrobial susceptibility testing.
Descriptive and statistical analysis
Descriptive analyses of the study population were performed. Statistical analyses were conducted using Stata (StataCorp, College Station, Texas, US). Trends of penicillin, cefixime, azithromycin and ciprofloxacin resistance over the study period were calculated using logistic regression analyses.
Determinants for resistance for penicillin (MIC > 1 mg/L), cefixime (MIC > 0.125 mg/L), azithromycin (epidemiological cut-off (ECOFF) > 1 mg/L) and ciprofloxacin (MIC > 0.06 mg/L) were identified using logistic regression analyses. Univariable and multivariable analyses were performed. Differences with p < 0.05 were considered statistically significant. As there were very few strains that reached the 0.125 mg/L threshold of ceftriaxone resistance, we did not determine associations between potential factors for ceftriaxone resistance and resistance to this antibiotic.
Ethical statement
As this was a retrospective study, no ethical approval was needed. All data related to patients were coded to maintain confidentiality.
Results
Between 2013 and 2017, 2,054 strains were isolated from 1,979 patients. Eighteen strains were excluded because, in 16 patients, N. gonorrhoeae was recovered from two different body sites, and in one patient, gonococcus was recovered from three different sites. In the end, susceptibility testing was performed on 2,036 N. gonorrhoeae strains isolated from 1,979 patients, because 47 patients presented two different episodes and five patients presented three episodes during the study period.
Of the 1,979 patients included, 1,888 (95.4%) were from men. 1,292 (65.3%) were tended in the Drassanes-Vall d'Hebron Sexually Transmitted Infections Unit. Furthermore, 573 patients (29.0%) presented in primary healthcare units and 114 (5.8%) in other medical departments of Vall d'Hebron University Hospital. For 1,555 patients with data on age available, the median age was 32 years, and 1,173 (75.4%) were between 20 and 40 years old. A total of 1,598 (78.5%) of the 2,036 isolates studied were from urethral samples and 1,888 (95.4%) patients were men (Table 1).
Table 1. Characteristics of patients included in the study on antimicrobial susceptibility of Neisseria gonorrhoeae isolates, Barcelona, Spain, 2013−2017 (n = 1,979 patients).
| Characteristics | 2013 | 2014a | 2015b | 2016 | 2017 | TOTALc | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | Number | % | Number | % | Number | % | |
| Numbers of patients and isolatesd | ||||||||||||
| Isolates | 329 | 100 | 340 | 100 | 339 | 100 | 447 | 100 | 581 | 100 | 2,036 | 100 |
| Patients | 321 | 100 | 326 | 100 | 328 | 100 | 423 | 100 | 581 | 100 | 1,979 | 100 |
| Sex | ||||||||||||
| Men | 303 | 94.4 | 306 | 93.9 | 320 | 97.6 | 400 | 94.6 | 559 | 96.2 | 1,888 | 95.4 |
| Women | 18 | 5.6 | 18 | 5.5 | 8 | 2.4 | 23 | 5.4 | 22 | 3.8 | 89 | 4.5 |
| Unknown | 0 | 0.0 | 2 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 0.1 |
| Age in years | ||||||||||||
| < 20 | NA | NA | 14 | 6.1 | 9 | 2.8 | 12 | 2.8 | 9 | 1.5 | 44 | 2.8 |
| 20–29 | NA | NA | 73 | 31.7 | 118 | 36.8 | 153 | 36.2 | 236 | 40.6 | 580 | 37.3 |
| 30–39 | NA | NA | 91 | 39.6 | 121 | 37.7 | 167 | 39.5 | 214 | 36.8 | 593 | 38.1 |
| 40–49 | NA | NA | 39 | 17.0 | 52 | 16.2 | 68 | 16.1 | 84 | 14.5 | 243 | 15.6 |
| ≥ 50 | NA | NA | 13 | 5.7 | 21 | 6.5 | 23 | 5.4 | 38 | 6.5 | 95 | 6.1 |
| Median (range) | NA | NA | 32 (14–74) | NA | 32 (14–72) | NA | 32 (4–75) | NA | 31 (5–75) | NA | 32 (4–75) | NA |
| Clinical setting | ||||||||||||
| Drassanes STI unit | 214 | 66.7 | 232 | 71.2 | 209 | 63.7 | 275 | 65.0 | 362 | 62.3 | 1,292 | 65.3 |
| Primary healthcare units | 90 | 28.0 | 76 | 23.3 | 100 | 30.5 | 118 | 27.9 | 189 | 32.5 | 573 | 29 |
| Other HUVH departments | 17 | 5.3 | 18 | 5.5 | 19 | 5.8 | 30 | 7.1 | 30 | 5.2 | 114 | 5.8 |
| Specimen | ||||||||||||
| Urethral/balanoprepucial | 261 | 79.3 | 245 | 72.1 | 274 | 80.8 | 365 | 81.7 | 453 | 78.0 | 1,598 | 78.5 |
| Rectal | 38 | 11.6 | 53 | 15.6 | 48 | 14.2 | 56 | 12.5 | 88 | 15.1 | 283 | 13.9 |
| Endocervical/vaginal | 16 | 4.9 | 11 | 3.2 | 10 | 2.9 | 21 | 4.7 | 11 | 1.9 | 69 | 3.4 |
| Pharynx | 10 | 3.0 | 11 | 3.2 | 7 | 2.1 | 5 | 1.1 | 29 | 5.0 | 62 | 3.0 |
| Othere | 4 | 1.2 | 20 | 5.9 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 24 | 1.2 |
HUVH: Vall d'Hebron University Hospital; NA: not available; STI: sexually transmitted infection.
a Age data available from 230 patients (from April 2014 to December 2014).
b Age data available from 321 patients.
c Age data available from 1,555 patients.
d Number of isolates can be greater than number of patients because over the study period several patients had more than one episode of gonorrhoea.
e Other includes: synovial fluid, abdominal abscess, perianal exudates.
The denominators for the percentages concerning patients’ characteristics are the total numbers of patients with information available on the characteristic in question, for the year or period specified in the column header. Denominators for specimen percentages are total number of isolates.
Antimicrobial susceptibility data during the study period are shown in Table 2.
Table 2. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates collected in Barcelona, Spain, 2013−2017 (n = 2,036).
| Antimicrobial | MIC50 in mg/L |
MIC90 in mg/L |
MIC range in mg/L |
Susceptibility categorya | |||||
|---|---|---|---|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | |||||||
| Number | % | Number | % | Number | % | ||||
| PENb | 0.25 | 12 | 0.002 – > 32 | 135 | 6.8 | 1,451 | 73.1 | 399 | 20.1 |
| CROc | 0.016 | 0.047 | < 0.016 – 0.38 | 1,977 | 99.7 | 0 | 0.0 | 5 | 0.3 |
| CFMb | 0.016 | 0.094 | < 0.016 – 0.38 | 1,887 | 95.1 | 0 | 0.0 | 98 | 4.9 |
| AZMd | 0.125 | 0.25 | < 0.016 – > 256 | 1,929 | 97.4 | 0 | 0.0 | 52 | 2.6 |
| CIPe | 0.38 | > 32 | < 0.002 – > 32 | 965 | 48.6 | 3 | 0.2 | 1,018 | 51.3 |
| SPT | 8 | 12 | 1.5 – 64 | 2,036 | 100 | 0 | 0.0 | 0 | 0.0 |
| GENf | 4 | 6 | 0.25– 24 | NA | NA | NA | NA | NA | NA |
| FOFg | 12 | 24 | 0.064 – 128 | NA | NA | NA | NA | NA | NA |
AZM: azithromycin; CIP: ciprofloxacin; CFM: cefixime; CRO: ceftriaxone; ECOFF: epidemiological cut-off value; EUCAST: European Committee on Antimicrobial Susceptibility Testing; FOF: fosfomycin; GEN: gentamicin; MIC: minimum inhibitory concentration; NA: not applicable; PEN: penicillin; SPT: spectinomycin.
a According to EUCAST (2019) clinical breakpoints.
b Antimicrobial susceptibility testing for PEN and CFM was performed on 1,985 isolates.
c Antimicrobial susceptibility testing for CRO was performed on 1,982 isolates.
d Antimicrobial susceptibility testing for AZM was performed on 1,981 isolates.
e Antimicrobial susceptibility testing for CIP was performed on 1,986 isolates.
f GEN was determined in the period from 2013 to 2016.
g FOF was determined in 2015 and 2016.
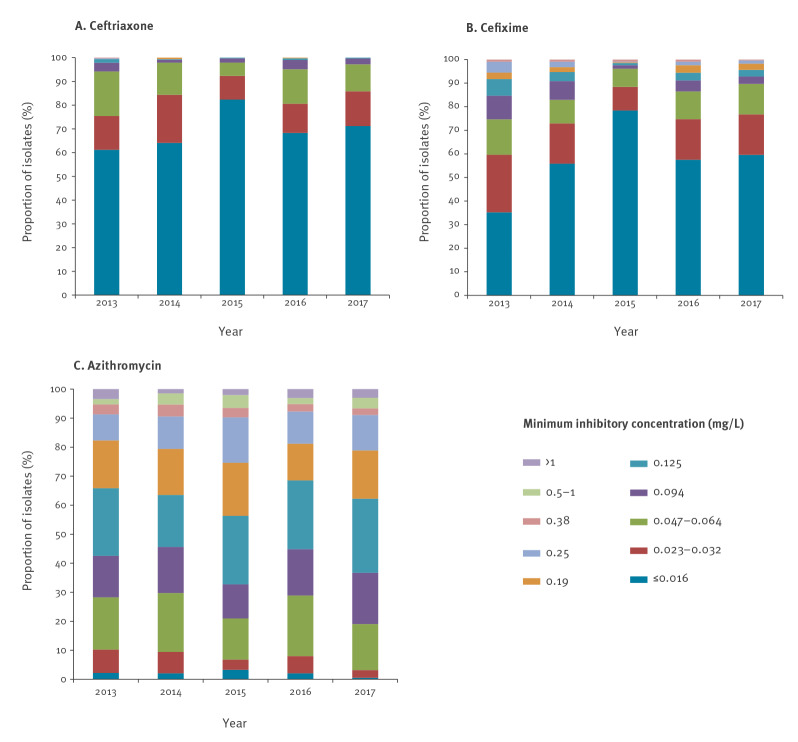
The distribution of MIC values by year for ceftriaxone, cefixime and azithromycin is shown in the Figure (panels A, B and C respectively). Most of the isolates (69.6%; 1,380/1,982) showed a ceftriaxone MIC ≤ 0.016 mg/L. The percentage of ceftriaxone resistant isolates (defined as MIC > 0.125 mg/L) appeared to decrease from 0.6% (2/324) in 2013 and 2014 to 0.2% (1/408) in 2016 (p = 0.4637). No isolates resistant to ceftriaxone were found either in 2015 or 2017. The average prevalence of decreased susceptibility to ceftriaxone (DSC; 0.032 < MIC < 0.125 mg/L) throughout the study period was 15.8% (314/1,982), seemingly declining from 2013 to 2015 (from 24.1% (78/324) to 7.4% (25/339)) but in 2016 and 2017 the proportions of isolates with DSC returned to higher levels, 19.1% (78/408) and 14.3% (82/572), respectively.
Figure.
Proportion of Neisseria gonorrhoeae isolates with different minimum inhibitory concentrations (mg/L) for (A) ceftriaxone (n = 1,982 isolates), (B) cefixime (n = 1,985 isolates) and (C) azithromycin (n = 1,981 isolates), by year, Barcelona, Spain, 2013−2017
EUCAST: European Committee on Antimicrobial Susceptibility Testing; MIC: minimum inhibitory concentration.
According to EUCAST, the MIC breakpoints for resistance to ceftriaxone and cefixime are identical and are MIC > 0.125 mg/L. The epidemiological cut-off for azithromycin is > 1mg/L.
The average percentage of isolates with cefixime resistance (MIC > 0.125 mg/L) at 4.9% was higher than the average percentage with ceftriaxone resistance at 0.3% (Table 2). Despite this, cefixime resistance rate decreased from 8.3% (27/327) in 2013 to 4.4% (25/572) in 2017 (p = 0.0073). For both ESC, the proportion of isolates with MIC ≤ 0.016 mg/L appeared to increase from 2013 to 2015, from 35.2% (115/327) to 78.5% (266/339) for cefixime and from 61.1% (198/324) to 82.3% (279/339) for ceftriaxone (Figure).
For azithromycin, most of isolates (54.6%; 1,082/1,981) showed MIC between 0.094 and 0.19 mg/L. Annual MIC50 and MIC90 values remain the same during the study period, 0.125 and 0.25 mg/L, respectively. Nevertheless, the proportion of resistant isolates, according to the EUCAST breakpoint (ECOFF > 1mg/L) seemed to increase slightly from 1.5% (5/340) in 2014 to 3.0% (17/572) in 2017 (p = 0.5295). A total of 16 strains showed high-level azithromycin-resistance (MIC ≥ 256mg/L): seven in 2016 and nine in 2017. These 16 strains accounted for almost a third (16/52) of all azithromycin resistant strains. No high-level azithromycin-resistant gonococcal isolates were found from 2013 to 2015.
However, the proportion of isolates showing both DSC and azithromycin resistance appeared to decrease from 1.5% (5/329) in 2013 to 0.3% (2/581) in 2017.
The prevalence of resistance to ciprofloxacin and penicillin was 51.3% and 20.1%, respectively (Table 2). For penicillin, the resistance rate presented a statistically significant decline over the study years (p = 0.0000), from 28.1% (92/327) in 2013 to 12.2% (70/572) in 2017. In addition, 14.7% (300/2,036) of isolates were penicillase-producing N. gonorrhoeae (PPNG). For ciprofloxacin, the resistance rate fluctuated between 48.8 and 56.8% during the study period, with no clear trend. All isolates were susceptible to spectinomycin (Table 2). The MIC50 and MIC90 values for gentamicin were 4 and 6 mg/L, while for fosfomycin these were 12 and 24 mg/L, respectively (Table 2).
Table 3 summarises the determinants of resistance to the different antibiotics tested during the study.
Table 3. Determinants, according to logistic regression analysis, of resistance to penicillin (MIC > 1 mg/L), cefixime (MIC > 0.125 mg/L), azithromycin (ECOFF > 1 mg/L) and ciprofloxacin (MIC > 0.06 mg/L) in Neisseria gonorrhoeae isolates from primary healthcare units and Drassanes-Vall d’Hebron sexually transmitted infection unit, Barcelona, Spain, 2013–2017 (n = 2,036).
| Variable | Number of resistant isolates | Total isolates tested | % | Univariable | Multivariable | Number of resistant isolates |
Total isolates tested | % | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | p value | OR (95%CI) | p value | OR (95%CI) | p value | OR (95%CI) | p value | |||||||
| Antibiotic | Penicillin | Cefimixime | ||||||||||||
| Year | ||||||||||||||
| 2013 | 92 | 327 | 28.1 | 1 (ref) | 0.0000 | 1 (ref) | 0.0000 | 27 | 327 | 8.3 | 1 (ref) | 0.0064 | 1 (ref) | 0.0073 |
| 2014 | 88 | 339 | 26.0 | 0.93 (0.66–1.33) | 0.97 (0.68–1.38) | 18 | 339 | 5.3 | 0.63 (0.33–1.20) | 0.67 (0.35–1.29) | ||||
| 2015 | 73 | 339 | 21.5 | 0.73 (0.51–1.05) | 0.70 (0.49–1.01) | 5 | 339 | 1.5 | 0.19 (0.07–0.50) | 0.20 (0.07–0.52) | ||||
| 2016 | 76 | 408 | 18.6 | 0.61 (0.42–0.86) | 0.59 (0.42–0.85) | 23 | 408 | 5.6 | 0.60 (0.32–1.12) | 0.61 (0.32–1.16) | ||||
| 2017 | 70 | 572 | 12.2 | 0.34 (0.24–0.49) | 0.33 (0.23–0.47) | 25 | 572 | 4.4 | 0.43 (0.23–0.80) | 0.43 (0.23–0.80) | ||||
| Total | 399 | 1,985 | NA | NA | NA | NA | NA | 98 | 1,985 | NA | NA | NA | NA | NA |
| Sex | ||||||||||||||
| Male | 388 | 1,895 | 20.5 | 1 (ref) | 0.1786 | 1 (ref) | 0.1067 | 88 | 1,896 | 4.6 | 1 (ref) | 0.0074 | 1 (ref) | 0.0303 |
| Female | 11 | 88 | 12.5 | 0.55 (0.23–1.31) | 0.49 (0.20–1.17) | 10 | 87 | 11.5 | 3.36 (1.38–8.16) | 2.72 (1.10–6.72) | ||||
| Total | 399 | 1,983a | NA | NA | NA | NA | NA | 98 | 1,983a | NA | NA | NA | NA | NA |
| Clinical setting | ||||||||||||||
| DVHSTI (n = 1,340b) | 242 | 1,303 | 18.6 | 1 (ref) | 0.0041 | 1 (ref) | 0.0006 | 46 | 1,303 | 3.5 | 1 (ref) | 0.0064 | 1 (ref) | 0.0050 |
| PH (n = 579b) | 138 | 566 | 24.4 | 1.41 (1.12–1.79) | 1.53 (1.20–1.95) | 36 | 565 | 6.4 | 1.86 (1.19–2.92) | 1.91 (1.22–3.01) | ||||
| Total | 380c | 1,869c | NA | NA | NA | NA | NA | 82c | 1,868c | NA | NA | NA | NA | NA |
| Antibiotic | Azithromycin | Ciprofloxacin | ||||||||||||
| Year | ||||||||||||||
| 2013 | 11 | 322 | 3.4 | 1 (ref) | 0.5295 | 1 (ref) | 0.5939 | 162 | 325 | 49.8 | 1 (ref) | 0.0771 | 1 (ref) | 0.0846 |
| 2014 | 5 | 340 | 1.5 | 0.42 (0.15–1.23) | 0.45 (0.15–1.30) | 167 | 340 | 49.1 | 1.01 (0.74–1.39) | 1.06 (0.77–1.45) | ||||
| 2015 | 7 | 339 | 2.1 | 0.60 (0.23–1.57) | 0.60 (0.23–1.57) | 192 | 338 | 56.8 | 1.45 (1.06–1.99) | 1.44 (1.05–1.98) | ||||
| 2016 | 12 | 408 | 2.9 | 0.81 (0.34–1.89) | 0.81 (0.35–1.90) | 213 | 407 | 52.3 | 1.11 (0.82–1.50) | 1.11 (0.82–1.50) | ||||
| 2017 | 17 | 572 | 3 | 0.87 (0.40–1.88) | 0.85 (0.39–1.84) | 284 | 576 | 49.3 | 1.002 (0.758–1.325) | 0.98 (0.74–1.30) | ||||
| Total | 52 | 1,981 | NA | NA | NA | NA | NA | 1,018 | 1,986 | 51.3 | NA | NA | NA | NA |
| Sex | ||||||||||||||
| Male | 50 | 1,891 | 2.6 | 1 (ref) | 0.5410 | 1 (ref) | 0.6184 | 965 | 1,896 | 50.9 | 1 (ref) | 0.9312 | 1 (ref) | 0.9832 |
| Female | 2 | 88 | 2.2 | 1.57 (0.37–6.65) | 1.45 (0.34–6.22) | 53 | 88 | 60.2 | 0.98 (0.55–1.73) | 0.99 (0.56–1.77) | ||||
| Total | 52 | 1,979a | NA | NA | NA | NA | 1,018 | 1,984a | NA | NA | NA | NA | NA | |
| Clinical setting | ||||||||||||||
| DVHSTI (n = 1,340b) | 28 | 1,302 | 2.2 | 1 (ref) | 0.0207 | 1 | 0.0272 | 612 | 1,303 | 47.0 | 1 | 0.0000 | 1 (ref) | 0.0000 |
| PH (n = 579b) | 23 | 563 | 4.1 | 1.94 (1.11–3.39) | 1.89 (1.07–3.31) | 332 | 567 | 58.6 | 1.63 (1.34–1.99) | 1.63 (1.33–1.99) | ||||
| Total | 51c | 1,865c | NA | NA | NA | NA | NA | 944c | 1,870c | NA | NA | NA | NA | NA |
CI: confidence interval; DVHSTI: Drassanes-Vall d’Hebron sexually transmitted infection unit; ECOFF: epidemiological cut-off value; MIC: minimum inhibitory concentration; NA: not applicable; OR: odds ratio; PH: primary healthcare unit.
a There are two patients for whom sex is not known.
b Number of isolates received by the setting.
c Isolates from Vall d’Hebron University Hospital are not included in the statistical analyses relating to clinical setting.
Antimicrobial resistance rates were compared between isolates from primary healthcare units and the Drassanes-Vall d’Hebron STI unit. Resistance rates of all antibiotics were higher in primary healthcare units than in Drassanes-Vall d‘Hebron STI unit, being statistically significant for penicillin (odds ratio (OR): 1.53; 95% confidence interval (CI): 1.20–1.95; p = 0.0006), cefixime (OR: 1.91; 95%CI: 1.22–3.01; p = 0.0050), azithromycin (OR: 1.89; 95%CI: 1.07–3.31; p = 0.0272) and ciprofloxacin (OR: 1.63; 95%CI: 1.33–1.99; p = 0.0000) (Table 3).
Discussion
This study describes antimicrobial surveillance data from 2,036 N. gonorrhoeae isolates in Barcelona during a 5-year period. To our knowledge, this is the first report in Spain describing antimicrobial resistance of such a considerable number of N. gonorrhoeae isolates. Other studies have been published previously [18,19], but the amount of strains included was much lower. Our findings show that the rate of ceftriaxone resistance remains low and stable. These results agree with data from the ECDC [7]. From 2013 to 2015 the number of isolates with MIC ≤ 0.016 mg/L of ceftriaxone and cefixime seemed to increase progressively. However, in 2016 and 2017 we observed an important decrease in the percentage of strains with MIC ≤ 0.016 mg/L and an increase in strains with higher MICs, which concur with a report that isolates with DSC have been emerging in Europe in recent years [15].
In our work, we were able to observe a decrease in the resistance rate of both ESC throughout the study. The reason that this decrease is statistically significant only for cefixime may be due to the small number of ceftriaxone resistant strains.
Since dual therapy (ceftriaxone 500 mg + azithromycin 1 g) was implemented in Barcelona in 2012, resistance to ceftriaxone has started to decrease: 2.8% in 2012 (data not shown) to 0.0% in 2017. This could be explained by the change in treatment regimen whereby, until 2011, gonorrhoea was treated with ceftriaxone 250 mg. On the other hand, the resistance rate of azithromycin has increased slightly from 1.5% in 2014 to 3.0% in 2017 and high-level resistant isolates (MIC ≥ 256 mg/L) were first detected in 2016 and 2017. An increase in azithromycin rate has been documented in several European countries [15].
Although the European and Spanish guidelines still recommend dual therapy [12,20], the increase in azithromycin resistance detected worldwide, not only in N. gonorrhoeae but also in other STIs such as Mycoplasma genitalium [21], brings into question the advisability of this therapeutic strategy. In fact, since 2019, the British Association for Sexual Health and HIV (BASHH) has recommended monotherapy with ceftriaxone 1 g [22]. However, some studies support that the selection/induction of azithromycin resistance of N. gonorrhoeae by the use of the current dual therapy is limited [23], and it may be associated with the general use of azithromycin for respiratory infections or the treatment of non-gonococcal urethritis [13].
The overall azithromycin resistance rate in our study was 2.6% based on the EUCAST breakpoint (ECOFF is 1 mg/L). This percentage is lower than that reported by other Spanish groups such as Cobo et al. [18], who showed a resistance percentage of 13.8% in Almería. On the other hand, Fuertes de Vega et al. reported a resistance rate of 5.2% in Barcelona [19], which is more similar to our results. We must bear in mind that the cut-off point used by these authors is that of prior EUCAST report versions (0.5 mg/L). If we analyse the azithromycin resistance rate of our study according to this cut-off, the percentage of resistance strains would be 4.1%.
The difference in the percentage of azithromycin resistance between different regions of Spain could be due to divergence in treatment patterns or the circulation of different genotypes with greater ability to develop antimicrobial resistance.
On other hand, the occurrence of extensively drug-resistant (XDR) strains of N. gonorrhoeae reported in countries such as the United Kingdom and Australia in 2018 is concerning [24]. These strains show a high level of azithromycin resistance and they are also resistant to ceftriaxone, resulting in resistance to the first line dual therapy for gonorrhoea (ceftriaxone intramuscularly and azithromycin orally) recommended by European, Australian and World Health Organization guidelines. The appearance and dissemination of these type of strains compromises the successful treatment of this infection. This highlights the need to keep the capability to culture N. gonorrhoeae, in order to monitor antibiotic susceptibility and rapidly detect the emergence of XDR strains.
Although the resistance rate to ciprofloxacin observed in this study is similar to that of neighbouring countries [25], it is higher than that reported in eastern European countries such as Ukraine [26].
The breakpoints of gentamicin are not yet established by EUCAST. In this study it appears that N. gonorrhoeae does not have high MIC values for gentamicin (MIC50 = 4mg/L and MIC90 = 6mg/L). These values are similar to those reported previously and suggest that gentamicin can be a low-cost and efficacious alternative therapy [27]. In addition, other studies support the in vitro activity of gentamycin, used in combination with cefixime or ertapenem, to control the spread of multidrug-resistant (MDR) and XDR N. gonorrhoeae strains [28].
Similar to gentamicin, no clinical breakpoints for fosfomycin exist. The review conducted by Tesh et al. suggests that fosfomycin can be an alternative treatment for gonococcal infection [29]. In this review, isolates with a MIC < 16 mg/L were considered as susceptible, while those with MICs between 32 and 64 mg/L were moderately susceptible. These MICs are similar to those found by our study, but it is necessary to establish susceptibility breakpoints in order to understand how to appropriately dose fosfomycin to treat N. gonorrhoeae infections [29].
It is surprising that the resistance rates of almost all antimicrobials tested were higher in isolates from primary healthcare than in those from the STI unit. One reason that might explain this is that 70% of the Drassanes-Vall d'Hebron STI Unit patients are MSM. Although we do not have access to this information, we hypothesise by deduction that the majority of patients who attend primary healthcare units are men who have sex with women (MSW) and if so, this might suggest that there are different N. gonorrhoeae populations circulating in the two populations.
One limitation of this study is that antimicrobial susceptibility tests of N. gonorrhoeae were performed mainly in symptomatic patients, since they are the ones from whom a sample is collected for culture. Asymptomatic patients are diagnosed by nucleic acid amplification tests (NAAT). Another limitation is that when N. gonorrhoeae was isolated in multiple sites in one patient, antimicrobial susceptibility was only performed on genital samples. This could lead to the loss of strains with higher MIC values in pharyngeal carriers and consequently to underestimate antimicrobial resistance. Moreover, due to the retrospective nature of the study, there is a lack of demographic and epidemiological information.
Although our results are only representative of Barcelona, the patients included in the current study represent 62.1% of those reported in Catalonia [30].
In conclusion, we analysed the susceptibility of 2,036 isolates in Barcelona from 2013 to 2017. We were able to observe that susceptibility to ceftriaxone remains high and resistance has decreased since dual therapy was implemented in 2012. However, azithromycin resistance increased during the study period and high-level azithromycin resistant strains were isolated for the first time in 2016.
Our study highlights the need to monitor antibiotic susceptibility and to perform molecular typing studies at a national level, which would allow identifying temporal and geographical changes, to detect the emergence and dissemination of new strains, and to maintain therapeutic guidelines updated.
Acknowledgements
We would like to thank all the Primary Health Centers included in the study, Drassanes-Vall d'Hebron STI Unit and laboratory of Vall d'Hebron University Hospital.
Conflict of interest: None declared.
Authors’ contributions: Salmerón P, Pumarola T, Larrosa N, Hoyos Y and Serra J contributed to the design and carrying out of the study.
Viñado B, El Ouazzani R and Hernández M were responsible for the susceptibility testing of the strains.
Jané M, Alberny M and Barbera MJ were responsible for the review of the data.
References
- 1.European Centre for Disease Prevention and Control (ECDC). Gonorrhoea. Annual epidemiological report for 2017. Stockholm: ECDC; 2019. [Google Scholar]
- 2.Unidad de vigilancia del VIH y conductas de riesgo. Vigilancia epidemiológica de las infecciones de transmisión sexual, 2017. [HIV and risk behaviors surveillance unit. Epidemiological surveillance of sexually transmitted infections, 2017]. Madrid: Centro Nacional de Epidemiología, Instituto de Salud Carlos III/Plan Nacional sobre el Sida, Dirección General de Salud Pública, Calidad e Innovación; 2019.
- 3.Centre d’Estudis Epidemiològics sobre les Infeccions de Transmissió Sexual i Sida de Catalunya (CEEISCAT). Vigilància epidemiològica de les infeccions de transmissió sexual a Catalunya. Informe anual 2017. [Center of Epidemiological Studies on Sexual Transmitted Infections and AIDS of Catalonia. Epidemiological surveillance of sexually transmitted infections in Catalonia. Annual report 2017] Badalona: CEEISCAT; 2018. [Google Scholar]
- 4.Cámara J, Serra J, Ayats J, Bastida T, Carnicer-Pont D, Andreu A, et al. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J Antimicrob Chemother. 2012;67(8):1858-60. 10.1093/jac/dks162 [DOI] [PubMed] [Google Scholar]
- 5.de Curraize C, Kumanski S, Micaëlo M, Fournet N, La Ruche G, Meunier F, et al. Ceftriaxone-resistant Neisseria gonorrhoeae isolates (2010 to 2014) in France characterized by using whole-genome sequencing. Antimicrob Agents Chemother. 2016;60(11):6962-4. 10.1128/AAC.01568-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lahra MM, Ryder N, Whiley DM. A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia. N Engl J Med. 2014;371(19):1850-1. 10.1056/NEJMc1408109 [DOI] [PubMed] [Google Scholar]
- 7.European Centre for Disease Prevention and Control (ECDC). Gonococcal antimicrobial susceptibility surveillance in Europe – Results summary 2017. Stockholm: ECDC; 2019. [Google Scholar]
- 8.Unemo M. Current and future antimicrobial treatment of gonorrhoea - the rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infect Dis. 2015;15(1):364. 10.1186/s12879-015-1029-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Workowski KA, Bolan GA, Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137. [PMC free article] [PubMed] [Google Scholar]
- 10.Public Health Agency of Canada (PHAC). Canadian Guidelines on Sexually Transmitted Infections. Ottawa: PHAC; 2013. Gonococcal Infections Chapter. [Google Scholar]
- 11.Australian Sexual Health Alliance (ASHA). Australian STI Management Guidelines for Use in Primary Care. Sydney: ASHA. [Accessed 12 Jun 2019]. Available from: http://www.sti.guidelines.org.au/sexually-transmissible-infections/gonorrhoea#management
- 12.Bignell C, Unemo M, Radcliffe K, Jensen JS, Babayan K, Barton S, et al. European STI Guidelines Editorial Board 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24(2):85-92. 10.1177/0956462412472837 [DOI] [PubMed] [Google Scholar]
- 13.Unemo M, Workowski K. Dual antimicrobial therapy for gonorrhoea: what is the role of azithromycin? Lancet Infect Dis. 2018;18(5):486-8. 10.1016/S1473-3099(18)30162-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofstraat SH, Götz HM, van Dam AP, van der Sande MA, van Benthem BH. Trends and determinants of antimicrobial susceptibility of Neisseria gonorrhoeae in the Netherlands, 2007 to 2015. Euro Surveill. 2018;23(36). 10.2807/1560-7917.ES.2018.23.36.1700565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Day MJ, Spiteri G, Jacobsson S, Woodford N, Amato-Gauci AJ, Cole MJ, et al. Euro-GASP network Stably high azithromycin resistance and decreasing ceftriaxone susceptibility in Neisseria gonorrhoeae in 25 European countries, 2016. BMC Infect Dis. 2018;18(1):609. 10.1186/s12879-018-3528-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 29th ed. CLSI supplement M100. Wayne, PA: CLSI; 2019. [Google Scholar]
- 17.The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoints tables for interpretation of MICs and zone diameters. Version 9.0. Växjö: EUCAST; 2019.
- 18.Cobo F, Cabezas-Fernández MT, Cabeza-Barrera MI. Antimicrobial susceptibility and typing of Neisseria gonorrhoeae strains from Southern Spain, 2012-2014. Enferm Infecc Microbiol Clin. 2016;34(1):3-7. 10.1016/j.eimc.2015.01.017 [DOI] [PubMed] [Google Scholar]
- 19.Fuertes de Vega I, Baliu-Piqué C, Bosch Mestres J, Vergara Gómez A, Vallés X, Alsina Gibert M. Risk factors for antimicrobial-resistant Neisseria gonorrhoeae and characteristics of patients infected with gonorrhea. Enferm Infecc Microbiol Clin. 2018;36(3):165-8. 10.1016/j.eimc.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 20.GESIDA. Documento de consenso sobre diagnóstico y tratamiento de las infecciones de transmisión sexual en adultos, niños y adolescents. 2017. [Consensus document on diagnostic and treatment of adult and teenage/adolescent sexually transmitted infections]. [Accessed 09 Jul 2019]. Available from: http://gesida-seimc.org/category/guias-clinicas/otras-guias-vigentes/
- 21.Barberá MJ, Fernández-Huerta M, Jensen JS, Caballero E, Andreu A. Mycoplasma genitalium Macrolide and Fluoroquinolone Resistance: Prevalence and Risk Factors Among a 2013-2014 Cohort of Patients in Barcelona, Spain. Sex Transm Dis. 2017;44(8):457-62. 10.1097/OLQ.0000000000000631 [DOI] [PubMed] [Google Scholar]
- 22.Fifer H, Saunders J, Soni S, Tariq Sadiq S, FitzGerald M. British Association for Sexual Health and HIV national guideline for the management of infection with Neisseria gonorrhoeae. 2019. [Accessed 04 Jul 2019]. Available from: https://www.bashh.org/guidelines [DOI] [PubMed]
- 23.Clifton S, Town K, Furegato M, Cole M, Mohammed H, Woodhall SC, et al. Is previous azithromycin treatment associated with azithromycin resistance in Neisseria gonorrhoeae? A cross-sectional study using national surveillance data in England. Sex Transm Infect. 2018;94(6):421-6. 10.1136/sextrans-2017-053461 [DOI] [PubMed] [Google Scholar]
- 24.Jennison AV, Whiley D, Lahra MM, Graham RM, Cole MJ, Hughes G, et al. Genetic relatedness of ceftriaxone-resistant and high-level azithromycin resistant Neisseria gonorrhoeae cases, United Kingdom and Australia, February to April 2018. Euro Surveill. 2019;24(8):1900118. 10.2807/1560-7917.ES.2019.24.8.1900118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris SR, Cole MJ, Spiteri G, Sánchez-Busó L, Golparian D, Jacobsson S, et al. Euro-GASP study group Public health surveillance of multidrug-resistant clones of Neisseria gonorrhoeae in Europe: a genomic survey. Lancet Infect Dis. 2018;18(7):758-68. 10.1016/S1473-3099(18)30225-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boiko I, Golparian D, Krynytska I, Bezkorovaina H, Frankenberg A, Onuchyna M, et al. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates and treatment of gonorrhoea patients in Ternopil and Dnipropetrovsk regions of Ukraine, 2013-2018. APMIS. 2019;127(7):503-9. 10.1111/apm.12948 [DOI] [PubMed] [Google Scholar]
- 27.Mann LM, Kirkcaldy RD, Papp JR, Torrone EA. Susceptibility of Neisseria gonorrhoeae to Gentamicin-Gonococcal Isolate Surveillance Project, 2015-2016. Sex Transm Dis. 2018;45(2):96-8. 10.1097/OLQ.0000000000000693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh V, Bala M, Bhargava A, Kakran M, Bhatnagar R. In Vitro Synergy Testing of Gentamicin, an Old Drug Suggested as Future Treatment Option for Gonorrhoea, in Combination With Six Other Antimicrobials Against Multidrug-Resistant Neisseria gonorrhoeae Strains. Sex Transm Dis. 2018;45(2):127-31. 10.1097/OLQ.0000000000000708 [DOI] [PubMed] [Google Scholar]
- 29.Tesh LD, Shaeer KM, Cho JC, Estrada SJ, Huang V, Bland CM, et al. Neisseria gonorrhoeae and fosfomycin: Past, present and future. Int J Antimicrob Agents. 2015;46(3):290-6. 10.1016/j.ijantimicag.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 30.Carmona G, Ruiz L, Fernández C, Jané M. Butlletí Epidemiològic de Catalunya. Març 2019. [Accessed 16 Jun 2019]. Available from: http://canalsalut.gencat.cat/web/.content/_Actualitat/Butlletins/Promocio_proteccio_salut/bec_butlleti_epidemiologic_de_catalunya/2019/bec-mar-2019.pdf