Abstract
Objectives: Telehealth is promoted as a strategy to support self-management of long-term conditions. The aim of this systematic review is to identify which information and communication technology features implemented in mobile apps to support asthma self-management are associated with adoption, adherence to usage, and clinical effectiveness.
Methods: We systematically searched 9 databases, scanned reference lists, and undertook manual searches (January 2000 to April 2016). We include randomized controlled trials (RCTs) and quasiexperimental studies with adults. All eligible papers were assessed for quality, and we extracted data on the features included, health-related outcomes (asthma control, exacerbation rate), process/intermediate outcomes (adherence to monitoring or treatment, self-efficacy), and level of adoption of and adherence to use of technology. Meta-analysis and narrative synthesis were used.
Results: We included 12 RCTs employing a range of technologies. A meta-analysis (n = 3) showed improved asthma control (mean difference −0.25 [95% CI, −0.37 to −0.12]). Included studies incorporated 10 features grouped into 7 categories (education, monitoring/electronic diary, action plans, medication reminders/prompts, facilitating professional support, raising patient awareness of asthma control, and decision support for professionals). The most successful interventions included multiple features, but effects on health-related outcomes were inconsistent. No studies explicitly reported adoption of and adherence to the technology system.
Conclusion: Meta-analysis of data from 3 trials showed improved asthma control, though overall the clinical effectiveness of apps, typically incorporating multiple features, varied. Further studies are needed to identify the features that are associated with adoption of and adherence to use of the mobile app and those that improve health outcomes.
Keywords: asthma, self-management, telemedicine, mobile application, medical informatics
INTRODUCTION
Asthma is common and associated with significant morbidity. The World Health Organization reports that 235 million people worldwide currently suffer from asthma.1 Supported self-management, including a personalized asthma action plan (PAAP), reduces morbidity.2–5 However, implementation is challenging. Practical, conceptual, and organizational barriers hinder the use of written PAAPs. Practical barriers include lack of time and resources (eg, no immediately available paper-based PAAPs).6 Conceptual barriers include a mismatch between advice given by professionals and advice patients want on how to live with their asthma.7 Organizational barriers include a lack of flexible systems for effective communication between professionals and patients.4,8
A mobile application (app) has the potential to support self-management, though it needs to engage patients and encourage adherence. This year, it is predicted that 500 million people around the world will use a health care app, and 71% of all UK citizens have a smartphone.9 Apps have penetrated into people’s daily lives and are increasingly accepted as a tool to monitor health. However, many people stop using a health care app shortly after downloading it.10 To realize the benefits of self-management, apps need to not only attract potential users, but sustain awareness of and adherence to ongoing use of the system.
Previous research has been focused on clinical outcomes rather than on informing the development of system features that are attractive and adherent, such that patients continue to use the app in routine self-management. We therefore aimed to systematically review the literature to (1) assess clinical effectiveness, (2) characterize the features of the interventions and their association with outcomes, and (3) assess adoption and adherence to usage.
METHODS
The systematic review is registered with, and the protocol is available from, the PROSPERO database, registration number CRD42015016414. We followed the procedures described in the Cochrane Handbook for Systematic Reviews of Interventions.11
Search strategy
The search strategy, inclusion criteria, exclusion criteria, and analysis plan were specified in advance and are documented in the protocol. Table 1 summarizes the PICOS (population, intervention, comparison, outcome, and setting) strategy. We searched 9 databases and 2 trial registries, and undertook manual searches of key relevant journals. Search terms were asthma AND technology terms (3 categories: smartphone/tablet app, information and communication technology [ICT] services, devices and platforms) limited to RCTs and quasiexperimental studies with a date limit of 2000 (because this was the year of approval of the global technical specifications for third-generation [3G] cellular systems under the brand IMT-2000 by the International Telecommunication Union, which enable faster ICT application and services, including voice, fax, and Internet).12 The detailed search strategy for MEDLINE and EMBASE are provided in Supplementary Appendix A.
Table 1.
Search strategy
| Inclusion and exclusion criteria, data range, and sources of searches | ||
|---|---|---|
| PICOS search strategy | ||
| Population | Adults and teenagers with asthma. We excluded young children because (i) the format of effective self-management in preschool children is unclear, and (ii) the dynamics of ICT use are likely to be different if the parent is taking responsibility. We did not set an absolute age threshold, but included any intervention in which the primary target is the person with asthma (as opposed to a parent); we anticipated this would include teenagers 12 years and over. Studies of multiple conditions were included if data specifically about people with asthma could be extracted. | |
| Intervention | Any ICT intervention with any currently available device, such as smartphone, tablet, smart TV, or computer, to support self-management of asthma. We did not include interventions where the only ICT component was a telephone as an alternative mode of delivery of a consultation or to impart information (eg, with an educational video), unless there was ongoing facilitation of self-management. | |
| Comparator | Patients who were not provided with or did not have access to the ICT system to support their asthma self-management. | |
| Outcomes |
|
|
| Settings | Any health care setting. | |
| Study design | Studies were included if they were randomized controlled trials (RCTs) and quasiexperimental studies. | |
| PICOS search strategy | ||
| Other exclusion criteria | We excluded papers not published in English. | |
| Date range | The date range for all searches was January 1, 2000, to January 1, 2015, with an updated search in April 2016. | |
| Databases | MEDLINE, EMBASE, CINAHL, PsychINFO, AMED, BNI, Cochrane Library (Database of Abstracts of Reviews of Effects, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials), Web of Science Core Collection, and ISI Proceedings (SCI-EXPANDE, SSC, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH), ScienceDirect. | |
| Manual searching | Journal of Medical Internet Research (2010–2015), Journal of Asthma (2010–2015), Journal of Telemedicine and e-Health (2010–2015). | |
| Forward citations | A forward citation search was performed on all included papers using International Statistical Institute Proceedings (Web of Science). The bibliographies of all eligible studies were scrutinized to identify additional possible studies. | |
| Unpublished and in-progress studies | UK Clinical Research Network Study Portfolio (www.clinicaltrials.gov) and Meta Register of Controlled Trials (www.controlled-trials.com). | |
| Definition |
|
Screening and Data Extraction
Titles and abstracts were screened by 1 reviewer (CyH), with 100 random titles checked by a second reviewer (HP) for training and quality control (with 100% agreement). The full text of all potentially eligible studies was retrieved and assessed against the inclusion criteria (see Table 1 PICOS description) by 1 reviewer (CyH), with a random sample of 20 papers reviewed by a second reviewer (TJ) initially with 75% agreement. The disagreement was due to different interpretations of the ICT interventions that would be included in the review. This was clarified in discussion with a third reviewer (HP), and we subsequently achieved 100% agreement.
Two reviewers (CyH and HP) extracted data using a piloted data extraction sheet under the heading characteristics of the included studies (study method, demographics of participants, asthma severity, sample size, intervention duration, intervention, and control setting); features of the ICT; clinical outcomes (control and exacerbations); and adherence. Disagreements were resolved by discussion.
Risk of bias
Two reviewers (CyH and HP) assessed and documented the methodological quality of included studies using the methods detailed in section 8 of the Cochrane Handbook for Systematic Reviews of Interventions,11 and used Review Manager 5.3 to record and generate a risk of bias graph. The overarching risk of bias was summarized based on the Cochrane risk of bias tool.11
Data synthesis and analysis
Meta-analysis
Heterogeneity of the included studies, such as measures used, intervention setting, and duration, was assessed to judge the appropriateness of performing meta-analysis. For groups of trials where meta-analysis was judged appropriate, mean difference was estimated using a fixed-effect model by R software,13 and a pooled estimate with 95% confidence intervals reported. We used a fixed-effects method due to the small number of studies and so that the weightings could be dependent on within-study variability and study size rather than influenced by estimates of heterogeneity. If long-term and short-term measures were presented, the long-term measures were taken to determine the treatment effect of the intervention.
Narrative synthesis
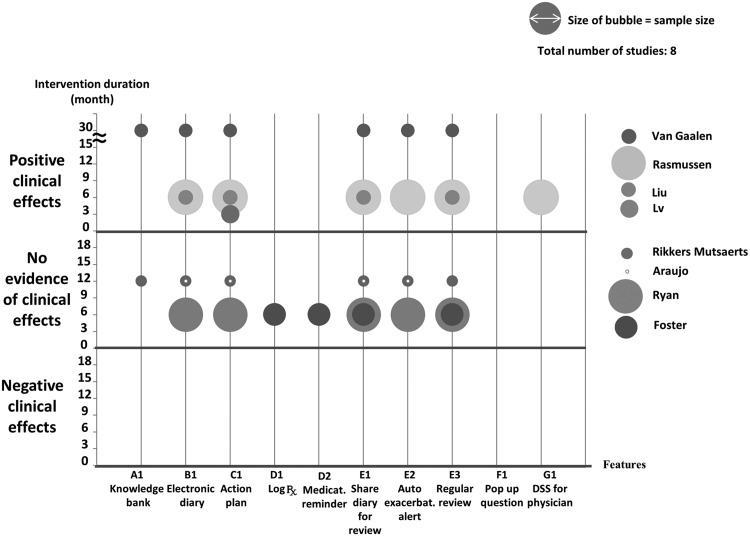
We performed narrative synthesis of heterogeneous studies. We plotted the app features and their associations with outcomes, sample size, and intervention duration on a bubble plot. This plot enables identification of a combination of features for effective clinical outcomes and/or adoption and sustainability.
Interpretation
The results of the data synthesis were discussed within the multidisciplinary team, which included expertise in e-health, ICT, and asthma self-management.
RESULTS
Included studies
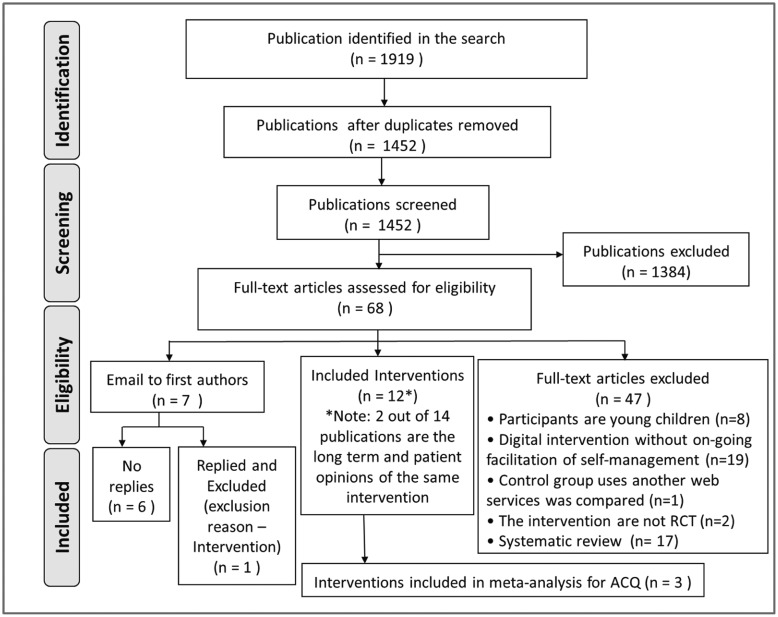
The identified papers, the screening process, and the final number of studies included are detailed in the PRISMA flowchart (Figure 1 ). In summary, out of 1919 papers, 14 were finally included,14–27 reporting 12 different studies. Van Gaalen et al.15 is a long-term follow-up of Meer et al.,21 and Cruz-Correia et al.23 presents the adherence and feasibility data of Araujo et al.18
Figure 1.
PRISMA flow diagram.
Characteristics of included studies
The detailed table of characteristics is presented in Supplementary Appendix B and summarized in Table 2. The 12 interventions12–27 were conducted from 2005 to 2014 across the world: 2 in the Netherlands15,16 and 1 each in Australia,14 Croatia,25 China,17 Denmark,24 Portugal,18 Singapore,20 Taiwan,19 Turkey,27 the United Kingdom,26 and the United States.22 The studies are all RCTs, including a cluster RCT14 and a crossover RCT.18
Table 2.
Clinical outcomes of the included interventions.
| Author [bias] | Trial | Participant characteristics | Inclusion criteria | Clinical effectiveness outcomes | Self-efficacy, adoption, and adherence outcome |
|---|---|---|---|---|---|
| Cingi (2015) Turkey RCT [Unclear risk] | Mobile APP vs Usual care FU 3 months | Secondary care patients: n = (I: 68; C: 68) Age: I: 32 yrs (SD: 3.7); C: 34.5 yrs (SD: 8.2) | Mild to severe persistent asthma, owned a smartphone at least 6 months prior to enrollment. | *Asthma control: Compared to control group, more patients achieved a well-controlled asthma score (ACT > 19) than in the control group (I: 49% vs C: 27%, P < .05). | Adherence: App group inputted 90 (70–154) sets of data; 86% of communications were between 08.00 and 18.00. Attrition was greater in the control group (I, 8 vs C, 39). |
| Foster (2014) Australia Cluster RCT [Unclear risk] | Personalized adherence discussion (PAD) vs SmartTrack reminder (IRF) vsIRF + PAD vs Usual care FU 6 months | Primary care patients: PAD, n = 24; IRF, n = 35; PAD + IRD, n = 41; C, n = 43 Age: PAD: 42.3 yrs (SD: 15.6); IRF: 40.0 yrs (SD: 13.7); PAD + IRD: 39.7 yrs (SD: 17.7); C: 40.0 yrs (SD: 14.1) % Female: PAD: 63%; IRF: 54%; PAD + IRD: 49%; C: 78% | Suboptimal asthma control and prescribed twice-daily ICS/LABA for 1 month or more | *Asthma control: No between-group differences in ACT (P = .14) or between reminder and nonreminder groups. *Medication adherence: Adherence declined in all groups over 6 months (PAD from 62% to 35% vs IRF from 80% to 60%; IRF + PAD from 85% to 68%; UC from 62% to 29%). Exacerbations: No between-group differences in patients with > 1 severe exacerbations (P = .06). Quality of life: No between-group differences in mini AQLQ (P = .26). | N/A |
| Van Gaalen (2013) Netherlands RCT [low risk] | Web monitoring + education vs Usual care 30-month FU of Meer trial | Primary and secondary care patients: n = I: 47; C: 60 Age: I: 36 yrs (SD: 8.7); C: 37 yrs (SD: 8.0) % Female I: 74%; C: 68% | Patients from Meer agreeing to 30-month FU | Asthma control: Significant but attenuated between group improvement in ACQ score at 30 months (adj mean df−0.33 [−0.61, −0.05]). *Quality of life: Significant but attenuated between-group improvement in AQLQ score at 30 months (adj mean diff 0.29 [0.01–0.57]). | N/A |
| Meer (2009) Netherlands RCT [low risk] | Web monitoring + education vs Usual care 12 months RCT | Primary and secondary care patients: n = I: 101; C: 99 Age: I: 36 yrs (range 19–50); C: 37 yrs (range 18–50)] % Female: I: 68%; C: 71% | Physician-diagnosed asthma on ICS for ≥ 3 months, access to Internet, Dutch speaking. | Asthma control: Compared to controls, Web group had improved ACQ at 12 months (I: −0.54 [−0.65 to −0.42] vs C: −0.06 [−0.18 to 0.05]). *Quality of life: Compared to controls, Web group had improved AQLQ at 12 months (I: 0.56 [0.43 –0.68] vs C: 0.18 [0.05–0.31]). Medication adherence: No between-group difference in self-reported medication adherence | Adherence: Average of 34.8 website log files received from each patient in the Web group at 12 months. No reports on data in the control group. |
| Araújo (2012) Portugal Crossover RCT [unclear risk] | Paper-Web vs Web-paper FU 48 weeks | Secondary care patients: n = I: 12; C: 9 Age: I: 26 yrs (SD 6.2); C: 32 yrs (SD 12.2) % Female: I: 67%; C: 78% | Moderate/severe asthma for ≥ 6 months using ICS/LABA in a single inhaler and FEV1 > 50% predicted. | *Asthma control: No between-group difference in ACQ-5 (mean diff −0.2 [−0.63 to 0.27], P = .42). Quality of life: No between-group difference in mini-AQLQ (mean diff −0.1 [−0.33 to 0.49] P = .68). | N/A |
| Cruz-Correia (2007) Portugal Crossover RCT [unclear risk] | Same intervention as Araújo | Refer to Araújo | Refer to Araújo | This publication showed patient’s opinions and adherence to monitoring tool only. Clinical effectiveness reported in Araujo. | Adherence: Paper diary completion was better than Web records (I: 48% vs C: 95%, P < .001), but use of electronic PEF meter was similar in both groups (I: 50% vs C: 50%). 63% of patients were “very interested” in continuing to use the app. |
| Lv (2012) China, Guangzhou RCT [unclear risk] | SMS messages vs Verbal education vs Usual care FU 12 weeks | Secondary care patients: n = SMS: 30; Verbal: 14; C: 27 Age: SMS: 36 yrs (SD: 11); verbal: 41 yrs (SD: 12); C: 37 yrs (SD: 12)] % Female: SMS: 33.3%; verbal: 50.0%; C: 48.1% | Asthma for ≥ 3 months (positive bronchodilator reversibility or bronchodilator provocation test). | Quality of life: compared the traditional (16.52 [SD: 21.10]) and control group (4.21 [SD: 30.98]), SMS group had the highest mean change in AQLQ(S) (31.40 [SD: 30.42]) P = .008. Medication adherence: No between-group difference in medication adherence (SMS: 80% vs verbal: 74.1% vs control: 50%, P = .113). | *Perceived control of asthma: significant different in PACQ-6 score between SMS group and control group (P = .018). |
| Rikkers-Mutsaerts (2012) Netherlands RCT [high risk in general] | Web-based self-management vs Usual care FU 12 months | Primary and secondary patients: n = I: 46; C: 44 Age: I: 13.4 yrs (12–17); C: 13.8 yrs (12–17) % Female: I: 57%; C: 43% | Mild-severe persistent asthma, ICS in previous year, access to Internet, and Dutch speaking. | Asthma control: No between-group difference in change in ACQ at 12 months (−0.05 [−0.35, 0.25]) *Quality of life: No between-group difference in change in PAQLQ at 12 months (−0.05 [−0.50, 0.41]). Medication adherence: No between-group difference in self-reported medication adherence at 12 months (P = .12). | Adherence: Average of 19.9 website log files received from each patient in the Web group at 12 months. No information on data recording in the control group. Attrition was greater in the Web group (I: 11/46 vs C: 4/44). |
| Ryan (2012) UK RCT [low risk] | Mobile self-management app vs Usual care FU 6 months | Primary care patients: n = I: 145; C: 143 Age: I: 46.6 yrs (SD: 18); C: 51.1 yrs (SD: 17.7) % Female: I: 66%; C: 59% | Poorly controlled asthma, had or willing to borrow a compatible mobile phone handset. | *Asthma control: No between-group difference in change in ACQ (mean diff−0.02 [−0.23 to 0.19]). Quality of life: No between-group difference in change in mini AQLQ (mean diff 0.10 [−0.16, 0.34]). Exacerbation: No between-group difference in A&E attendance (P = .08), admissions (P = .32), unscheduled GP consultation (P = .07), steroid courses (P = .79), acute exacerbations (P = .84). | *Self-efficacy: No between-group difference in change in KASE-AQ self-efficacy mean diff 2.0 (−0.3, 4.2); attitude mean diff −0.2 (−1.6, 1.6) Adherence: Of 27 lost to follow-up, 5 patients because of telemonitoring problems. |
| Liu (2011) Taiwan RCT [unclear risk] | Mobile app vs Usual care FU 6 months | Secondary care patients: n = I: 43; C: 46 Age: I: 50.4 yrs (SD: 1.9); C: 54.0 yrs (SD: 2.4) % Female: I: 48.8%; C: 52.2% | Moderate to severe persistent asthma. | Asthma control: Compared to control group. mean FEV1 increased at 6 months; I: 65.2 l/min (SEM: 3.2%) vs C: 56.5 (SEM: 2.8) P < .05). Quality of life: SF-12 (physical) improved in mobile app group from baseline 41.6 (SEM: 1.5) to 45.5 (SEM: 1.4) at 6 months. No significant changes in SF-12 (mental). | Adherence: Percentage of participants recording data decreased over time in both groups (I: 71.7% vs C: 76.7%) at 6 months. Of the 11 patients who withdrew, 4 could not use the app and 2 had problems with the app. |
| Prabhakaran (2010) RCT Singapore [high risk] | SMS symptom monitoring vs Usual care FU 3 months | Secondary care patients: n = I: 60; C:60 Age: I: 37 yrs (SD: 12); C: 40 yrs (SD: 13) % Female: I: 65%; C: 53% | Previous hospital admission, owned a mobile phone, knew how to use SMS, and understood English. | *Asthma control: No between-group difference in proportion with ACT ≥ 20 at 3 months (I: 36% vs C: 28%, P = .113). Exacerbation: No between-group difference in proportion of patients with reduction in A&E visits (I: 85% vs C: 95%, P = .063), admissions (I: 92% vs C: 93%, P = .50), or nebulizations (I: 86% vs C: 96%, P = .053). | Adherence: Of the 2 patients who withdrew, 1 was dissatisfied with the SMS service. |
| Jacobson (2009) US RCT [unclear risk] | Electronic asthma monitoring system (AMS) vs Usual care FU 6 months | Primary care patients: n = I: 29; C: 30 Age: I: 8–15 yrs; C: 8–15 yrs % Female: I: 51.7%; C: 50.0% | Moderate/severe asthma, ≥ 2 ED visits or 1 hospitalization. | *Exacerbation: No between-group difference in percentage of patients with emergency department visits (P = .8) and hospitalizations (P = .6). | Adherence: Compared to control group, data were received on more days in the AMS group (I: 211 days vs C: 136.6 days). |
| Rasmussen (2005) Denmark RCT [unclear risk] | Web management tool (Web) vs Specialist care (S) vs Usual care (GP) FU 6 months | Community-based patients: n = I: 29; S: 88; GP: 80 Age: Web: 28 yrs (18–44); S: 30 yrs (19–45); GP: 30 yrs (20–45) % Female: Web: 68%; S: 66%; GP: 73% | Asthma diagnosed, and living in the catchment area of University Hospital of Copenhagen | Asthma control: OR of improved symptoms: (Web vs S 2.64 [1.43–4.88], Web vs GP 3.26 [1.71–6.19], S vs GP 1.23 [0.66–2.30]). Quality of life: OR of improved AQLQ: (Web vs S 2.21 [1.09–4.47], Web vs GP 2.10 [102–4.31], S vs GP 0.95 [0.43–2.07]). | Adoption: Web group showed largest improvement in use of action plan (Web: from 2% to 88%; S: from 3% to 55%; GP: from 0% to 6%) compared to specialist and GP groups. |
| Ostojic (2005) Croatia RCT [unclear risk] | SMS transmission of monitoring data vs Usual care FU 6 months | Secondary care patients: n = I: 8; C: 8 Age: I: 24.8 yrs (SD: 6.3); C: 24.5 yrs (SD: 7.1) % Female: I: 37%; C: 50% | Persistent asthma for at least 6 months, and were being treated with ICS and LABA, experienced in SMS. | Asthma control: Compared to control group, SMS group had lower control cough symptom score: I: 1.42 (SD: 0.28) vs C: 1.85 (SD: 0.43), (P < .05), and night symptom score: I: 0.85 (SD: 0.32) vs C: 1.22 (SD: 0.23) (P < .05). Exacerbation: No between-group difference in number of office visits (I: 21 vs C: 15) or hospital admissions (I: 2 vs C: 7). | Adherence: 1769 sets of data were received by SMS. No reports on recording of data in the control group. |
Studies are listed by year of publication in order to reflect the rapidly evolving technological environment. 3G was available in the market in 2001 (technically approved in 20001); the first Apple app and Android app were available in the market in 20082 and 2009,3 respectively.
Abbreviations: Validated measures of asthma control: ACQ: Asthma Control Questionnaire; ACT: Asthma Control Test. Validated measures of asthma-related quality of life: AQLQ: Asthma Quality-of-Life Questionnaire; PAQLQ: Pediatric Asthma Quality-of-Life questionnaire; PCAQ-6: Perceived Control of Asthma Questionnaire; KASE-AQ: Knowledge, Attitude, and Self-Efficacy Asthma Questionnaire.
ICS: inhaled corticosteroid; LABA: long-acting beta-agonist; PEF: peak expiratory flow; GP: general practitioner;
I: intervention group; C: control group.
*Primary outcome; FU: follow-up; OR: odds ratio, SD: standard deviation, SEM: standard error of the mean.
References: (1) International Telecommunication Union. About mobile technology and IMT-2000. http://www.itu.int/osg/spu/imt-2000/technology.html Accessed June 2016); (2) Apple UK and Ireland Public Relations. Apple press info July 14, 2008. http://www.apple.com/uk/pr/library/2008/07/14iPhone-App-Store-Downloads-Top-10-Million-in-First-Weekend.html Accessed June 2016; (3) Eric Chu. Android Develop blogs: Android market update: support for priced applications. February 13, 2009. http://android-developers.blogspot.co.uk/2009/02/android-market-update-support-for.html Accessed June 2016.
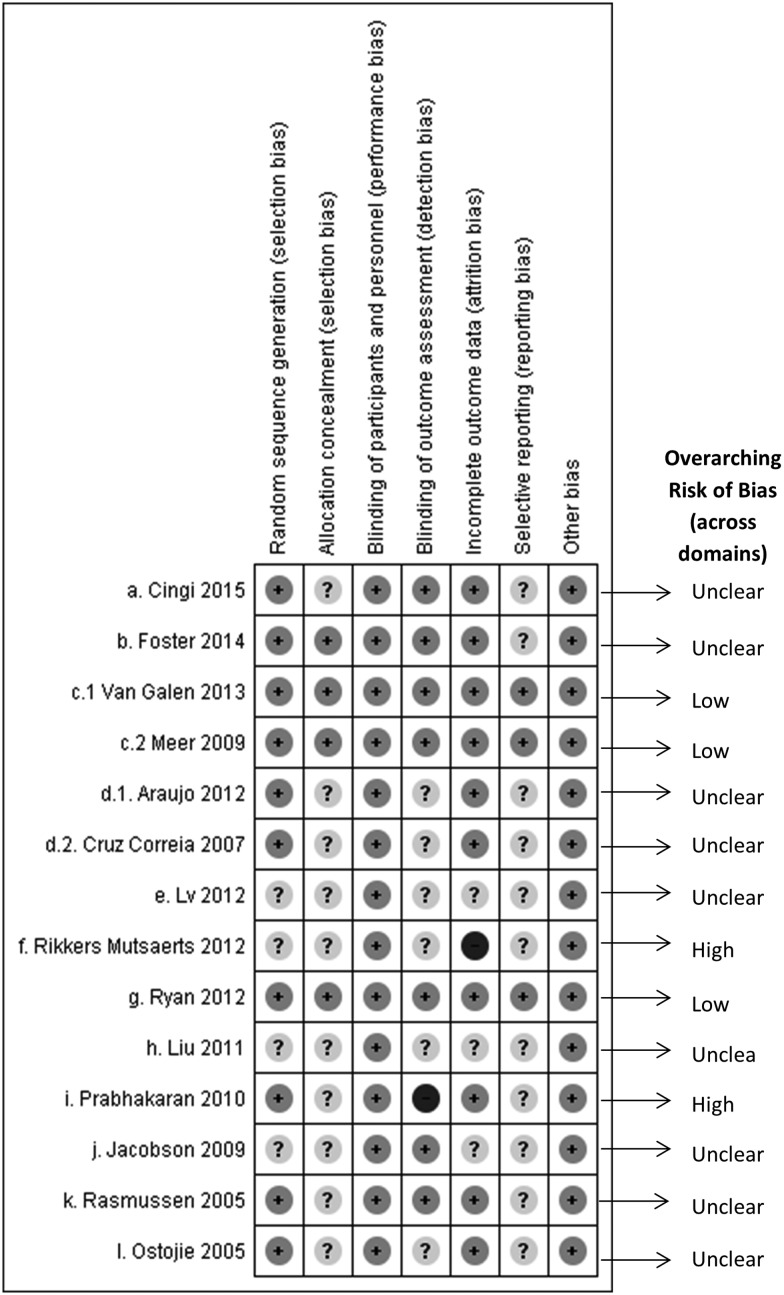
The risk of bias across interventions is summarized in Figure 2 .
Figure 2.
Risk of bias across interventions.
Participants
The number of participants for each intervention ranged from 16 to 300, and participants were recruited from primary and/or secondary care, with mild/moderate, severe persistent, or poorly controlled asthma, or patients admitted to the hospital. Most studies included teenagers and adults, although 1 intervention22 also included children from 8 years of age. Six interventions15,16,20,25–27 additionally required patients to have access to the Internet or own a mobile phone with mobile network capability and/or know how to use short messaging service (SMS).
Interventions
Of the 12 ICT interventions, there were 3 mobile phone apps,19,26,27 4 Web applications15,16,18,24 (one of which used peak flow monitoring), 3 SMSs,17,20,25 1 electronic inhaler reminder system connected with a Web application,14 and 1 customized asthma monitoring system with 4 keys for data entry and transmission by telephone line.22
Comparisons
In most studies, the comparator was patients without access to any ICT system to support their asthma self-management, but 1 had 2 comparator groups (usual care and verbal self-management advice)24 and 1 had 2 components (reminders and professional consultation skills training) compared or combined in 4 groups.14
Clinical outcomes
Clinical outcomes are summarized in Table 2, with further details in Supplementary Appendix B.
Meta-analysis for asthma control
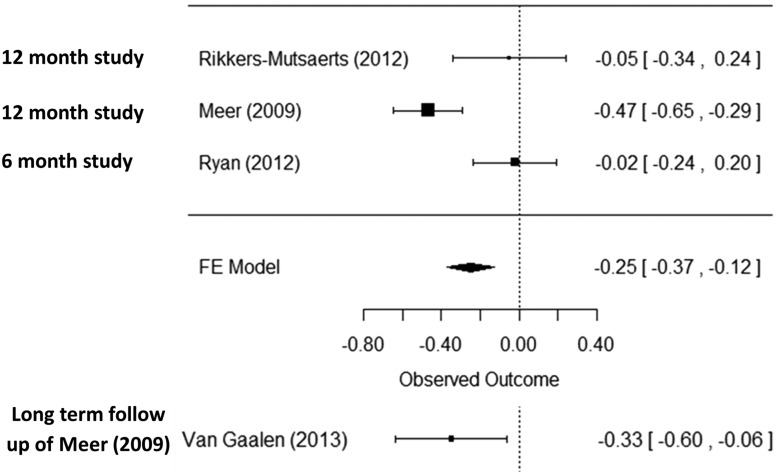
Four publications15,16,21,26 reported asthma control using the Asthma Control Questionnaire (ACQ), 2 of which are included in the meta-analysis. One study, Araujo et al.,18 was excluded, because it used a shorter version of the ACQ (ACQ-5), which meant that it was not appropriate to combine this study with the other RCTs that used the full version of the ACQ. There was statistically significantly improved asthma control in the intervention group (mean difference −0.25, [95% CI, −0.37 to −0.12]), but the confidence interval did not include the minimum clinically important difference of 0.528 (see forest plot, Figure 3 ). In addition, van Gaalen et al.,15 the follow-up study of Meer et al.,21 reported ACQ. The between-group difference was maintained, albeit attenuated (−0.33, 95% CI, −0.61 to 0.05) for the 107 patients (60.8% of the participants in the original trial) who contributed data at 30 months.15
Figure 3.
Forest plot for meta-analysis of asthma control and asthma control outcome of long-term follow-up study of Meer.
Narrative synthesis: asthma control
In 6 of 11 studies15,17,19,24,25 researchers reported improved asthma control over time scales of 3–30 months in the intervention groups. The interventions consisted of 2 mobile apps, 2 web applications, and 2 SMSs. A common feature was an electronic diary that could be shared with health care professionals for regular review. Of the 6 interventions, 125 was at low risk of bias, while 515,17,19,24 showed unclear risk of bias.
Quality of life
Although 8 studies14–19,24,26 reported asthma-related quality of life, heterogeneity of study design and outcome measure precluded meaningful meta-analysis. Four interventions15,17,19,24 (50%) found that quality of life improved over 6–30 months. The interventions were Web applications with common features of an electronic diary, an action plan, and regular supportive reviews by health care professionals. Of the 4 effective interventions, 1 study was at low risk of bias15 and 3 were at unclear risk of bias.17,19,24
Exacerbations
Five interventions14,20,22,25,26 reported 6 outcomes relevant to exacerbations (hospital admissions, emergency department visits, unscheduled visits to practices, steroid courses, numbers of patients with 1 or more severe exacerbations, and practice visits triggered by an exacerbation alert generated by the ICT system). The interventions were mobile app, smart inhaler, handheld asthma monitoring device, and SMSs.
None of the interventions were associated with a significant reduction in exacerbation-related outcomes. Three of the studies22,25,26 presented data on proportion of patients with a hospital admission over 3–6 months, but the rates were very close to zero (0.02%, 0.17%, and 0.25%), so that meta-analysis was unhelpful. Of 5 interventions, 3 studies were at unclear risk of bias,14,22,25 1 was at low risk of bias,26 and 1 was at high risk of bias.20
Application features in the included interventions
Characteristics of the application features
There were 10 application features in the 12 interventions, details of which are summarized in Table 3. These were categorized into 7 themes: education, asthma diary, action plan, medication adherence, facilitating professional support, raising patients’ awareness of asthma control, and decision support for the health care professional. Eleven of the 12 interventions included more than 1 feature. Four interventions included 5 or more features. Eight included an asthma diary, 9 an action plan, and 11 professional support. Only 1 intervention24 contained a decision support system for the health care professional.
Table 3.
Application features of the included interventions.
| Themes (n = 7) [% of interventions that contained features related to the theme] | Application features (n = 10) | Cingi (2015) | Foster (2014) | Meer (2009), Van Gaalen (2013) | Cruz-Correia (2007), Araujo (2012) | Lv (2012) | Rikkers-Mutsaerts (2012) | Ryan (2012) | Liu (2011) | Prabhakaran (2010) | Jacobson (2009) | Rasmussen (2005) | Ostojic (2005) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. Education [3, 25%] | A1. Provides links of online learning resources (eg, asthma information, news, FAQs) with face-to-face education by specialized nurse | ✓ | × | ✓ | × | × | ✓ | × | × | × | × | × | × |
| B. Asthma diary [8, 67%] | B1. Provides electronic diary to log symptoms, PEF or FEV1, ACQ for decision making during intervention | Health status (7-point scale) with emoticon | × | FEV1 and ACQ | symptom, PEF, FEV1 | × | FEV1 and ACQ | symptom, PEF, and drug use | symptom, PEFR, PEFR variability, use of relievers | × | × | symptom, PEF, rescue medication | PEF |
| C. Action Plan [9, 75%] | C1. Provides advice (mapped on 3 color zone/status and treatment adjustment advise) | × | × | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | × | ✓ | ✓ | ✓ |
| D. Medication adherence [2, 17%] | D1. Log daily prescribed medication | ✓ | ✓ | × | × | × | × | × | × | × | × | × | × |
| D2. Reminder for medication | × | ✓ | × | × | × | × | × | × | × | × | × | × | |
| E. Facilitating professional support [11, 92%] | E1. Shares electronic diary/report to professional for review via shared database | ✓ | ✓ | ✓ | ✓ | × | ✓ | ✓ | ✓ | × | ✓ | ✓ | ✓ |
| E2. Identify exacerbation /urgent messages | Patient self-report to physician, triggered an voice notification in physician’s app | × | System suggested patient to contact physician, patient chose to contact physician | System detected asthma not under controlled, auto alert generated to physician | × | System suggested patient to contact physician, patient chose to contact physician | System detected asthma not under controlled, auto alert generated to physician | × | System detected asthma not under controlled, auto alert generated to physician | Physician/case manager reviewed patient’s logged data and contact patient | Patient not uncontrolled were keep tracked by the decision support system, physician contacted patients for treatment adjustment | Physician reviewed patient’s logged data and contact patient | |
| E3. Regular consultation by professional | ✓ | ✓ | × | × | × | ✓ | ✓ | × | × | × | × | × | |
| F. Raising patient’s awareness of asthma control [2, 17%] | F1. Pop up questions and feedbacks | × | × | × | × | × | × | × | × | ✓ | ✓ | × | × |
| G. Decision Supports for physician [1, 8%] | G1. DSS for the physician | × | × | × | × | × | × | × | × | × | × | ✓ | × |
Application features associated with health-related outcomes of the included interventions
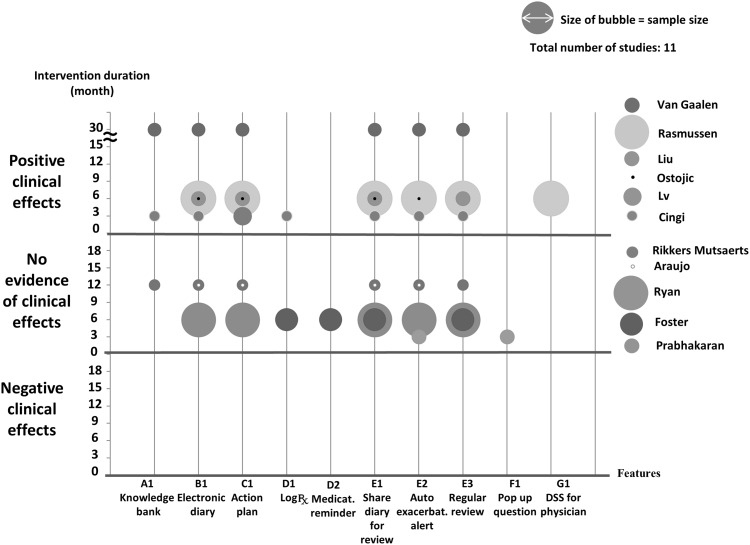
To synthesize the impacts of the application features on health-related outcomes while considering the sample size and duration of each study, we prepared bubble plots (see Figures 4 and 5 ). The effect on asthma control and quality of life was inconsistent, although there were no examples of harm. There was no significant clinical impact (either positive or negative) on exacerbations.14,20,22,25,26 Most of the interventions included multiple features such as self-monitoring and action plans, but outcomes were variable. One study that focused on medication adherence with reminders and treatment logs improved adherence but none of the clinical outcomes.14 One study that incorporated feedback and decision support for physicians24 improved asthma control and quality of life.
Figure 4.
Clinical outcome: asthma control.
Figure 5.
Clinical outcome: quality of life.
Adoption and adherence to usage
Action plan ownership
Within the 12 studies, only 124 reported action plan ownership in the 3 study groups. A significant increase in use of an action plan from baseline to end of study was reported in both intervention groups (Web-based monitoring, from 2% to 88%; Web-based specialist support, from 3% to 55%) compared to a smaller increase in the usual care group (from 0% to 6%).
Self-efficacy
Only 1 study reported self-efficacy.26 The intervention was a mobile app that provided patients with an asthma diary, an action plan, and structured support from health care professionals for 6 months. No significant difference was reported in self-efficacy between the intervention and control group, which had similar professional support (KASE-AQ, self-efficacy score; mean difference 2.0 [95% CI, −0.3 to 4.2]).
Adoption of and adherence to intervention
There were no interventions that explicitly reported adoption of the ICT system, and it is impossible to gauge directly in a trial because, by definition, everyone in the intervention group received the ICT system. However, usage data may give an indication of the general level of interest in the ICT system, and adherence to the system may be inferred by looking at differential attrition rates in the intervention/control groups and reasons for withdrawal. Eight studies reported the data transmitted during the studies and/or reasons for attrition because of problems with the ICT system. Details are summarized in Table 2.
Of the 8 interventions, only 2 (Araujo et al.18 and Jacobson et al.22) reported the data transmitted in the control and intervention groups. Araujo et al.18 reported that there was no significant difference in adherence to electronic peak flow monitoring between the Web application group and paper-based monitoring. At the end of the trial, 12 of the 18 participants in the crossover trial were “very interested” in continuing to monitor their asthma using the Web application. Another study, Jacobson et al.,22 reported 2.85 times more data received from the intervention group than the paper-based group. Araujo et al.18 used a Web application, while Jacobson et al.22 used a customized embedded system. They both had the application features of an action plan and facilitated support from health care professionals.
Three interventions explicitly reported the number of patients who were lost to follow-up or withdrew because of problems with the ICT system; these were Ryan et al.26 (n = 5, “telemonitoring problem”), Liu et al.19 (n = 4, “couldn’t use the app”; n = 2, had a “problem with the app”), and Prabhakaran et al.20 (n = 1, “dissatisfied with the service”). Ryan et al.26 and Liu et al.19 were mobile app interventions while Prabhakaran et al.20 was an SMS application. They both had an asthma diary, an action plan, and support from health care professionals.
DISCUSSION
Summary of findings
Our meta-analysis of 3 trials showed a positive effect on asthma control, and a 30-month follow-up study showed that this effect was sustained, albeit attenuated. Within the 12 studies,12–27 we identified 10 common features grouped into 7 themes. Most of the interventions included multiple features of self-monitoring and action plans. The effect of the features on health-related outcomes (asthma control, quality of life, exacerbations) and medication adherence varied, though importantly there were no examples of harm. There was no significant clinical impact (either positive or negative) on exacerbations.14,20,22,25,26 The impact of the different features on adoption of and adherence to the system was not possible to gauge directly, but reasons for attrition highlighted the importance of reliable user-friendly systems.
Strengths and limitations
Our systematic review provides an evidence-based review explicitly of the ICT features included in recent interventions (since 2000) and their associations with asthma health-related outcomes. We performed an updated search in early April 2016.
Nevertheless, in the fast-moving field of ICT, this may still have missed some contemporary features.
There are some methodological limitations. First, due to resource and time constraints, a single review was performed at the initial screening stage, although we implemented robust training and quality control processes during review in order to minimize potential inaccuracies. Second, we did not translate papers that were not written in English, though only 1 study (Kokubu et al.29 in Japanese) was identified. Third, the included trials focused primarily on health outcomes and the interventions included multiple features, so they could not provide evidence on the individual application features associated, though our grouping of the features may be useful for further research.
Interpretations in relation to published literature
Our findings are in line with other reviews,30,31 which show that ICT interventions to support asthma self-management have an inconsistent impact on asthma control and quality of life. The core elements of effective self-management recommended by the British asthma guidelines3 are education, a PAAP, and regular professional review. Two15,27 of the 3 interventions15,16,27 incorporating these showed improvement in asthma control. A recent review32 suggested that providing instruction on better health care management and sharing data with a designated professional were the most valuable features of health care apps for users. Interventions with these features (see the bubble plot, Figures 4 and 5) found that impact on asthma control and quality of life varied, and there was no significant impact on exacerbations.
The inconsistent clinical outcomes from the 11 studies, despite incorporating similar features, highlight the importance of context in determining whether an intervention is effective. This resonates with the findings of a systematic review of studies implementing supported asthma self-management, which concluded that a whole-systems approach (ie, explicitly addressing patient, professional, and organizational factors) showed the most consistent improvement in clinical outcomes.33 Of the 12 studies in this review, the 11 studies with application features focused solely on patients showed inconsistent impacts on clinical outcomes; the 1 study with features targeted at both patients and health care professionals improved both asthma control and quality of life.
Implications for clinical care and future research
Our findings suggest that mobile apps have the potential to be effective in supporting self-management and are an option that may be preferred by some people and their clinicians. However, these studies of multifaceted interventions did not provide clear evidence on which of the range of ICT features were essential for effectiveness. Furthermore, the lack of technical specifications of the ICT systems evaluated in the clinically focused publications with health outcomes did not allow understanding of the design factors of the systems, which may have affected how they operated or were used by patients and professionals. Finally, no matter how well designed the ICT is, it will not be effective if patients do not adopt it and continue to use it. The challenge for researchers and technology developers now is to explore the dynamic needs and preferences of people with asthma and evaluate the features associated with improved adoption of and adherence to mobile apps.
CONCLUSION
Mobile apps, incorporating an action plan and other self-monitoring features, are an effective option for supporting self-management, which resonates with the widespread adoption of technology in this digital era. However, there is insufficient evidence to identify the important application features that attract and encourage patients to continue using the app. Further development in this field will require robust studies that not only establish the long-term effectiveness but also evaluate the specific features associated with improved adoption of and adherence to the mobile app.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Marshall Dozier for her advice on the search strategy.
Funding
CyH is funded by a PhD studentship from the Chief Scientist Office (Scotland) (AUKCAR/14/01). This work was carried out with the support of the Asthma UK Centre for Applied Research (AUK-AC-2012‐01). The views expressed in this publication are those of the authors and not necessarily those of the Chief Scientist Office (Scotland).
Competing Interests
None.
Contributors
CyH, RW, BM, and HP designed the systematic review. CyH screened titles and abstracts of references identified in the databases, and HP reviewed the selection. TJ acted as second reviewer. CyH undertook the data extraction and synthesized the data with HP. RP was the statistical advisor. HP reviewed the data. CyH and HP wrote the initial draft of the manuscript. All authors reviewed the content.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
REFERENCES
- 1. World Health Organization. Facts About Asthma. Updated November 2013. http://www.who.int/mediacentre/factsheets/fs307/en/. Accessed September 13, 2016. [Google Scholar]
- 2. British Thoracic Society, Scottish Intercollegiate Guideline Network. A National Clinical Guideline: The British Guideline on the Management of Asthma. Revised 2014. http://www.sign.ac.uk/guidelines/fulltext/141/index.html. Accessed September 13, 2016. [Google Scholar]
- 3. Levy ML, Andrews R, Buckingham R et al. Royal College of Physicians. Why asthma still kills: the National Review of Asthma Deaths (NRAD) Confidential Enquiry Report London: RCP. 2014. https://www.rcplondon.ac.uk/sites/default/files/why-asthma-still-kills-full-report.pdf. Accessed September 13, 2016. [Google Scholar]
- 4. Taylor SJC, Pinnock H, Epiphaniou E et al. A rapid synthesis of the evidence on interventions supporting self-management for people with long-term conditions. Health Serv Deliv Res. 2014;2:54. [PubMed] [Google Scholar]
- 5. Gibson PG, Powell H, Coughlan J et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev. 2003;(1):CD001117. [DOI] [PubMed] [Google Scholar]
- 6. Wiener-Ogilvie S, Pinnock H, Huby G et al. Do practices comply with key recommendation of the British Asthma Guideline, and if not, why not? Prim Care Respir J. 2007;16(6):369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ring N, Jepson R, Hoskins G et al. Understanding what helps or hinders asthma action plan use: a systematic review and synthesis of the qualitative literature. Pat Ed Counsel. 2011;85:e131–43. [DOI] [PubMed] [Google Scholar]
- 8. Kielmann T, Huby G, Powell A, Sheikh A et al. From support to boundary: a qualitative study of the border between self-care and professional care. Pat Ed Counsel. 2010;79:55–61. [DOI] [PubMed] [Google Scholar]
- 9. Nuffield Trust. Delivering the Benefits of Digital Heath Care http://www.nuffieldtrust.org.uk/publications/delivering-benefits-digital-health-care. Accessed September 13, 2016. [Google Scholar]
- 10. Paul K, Dustin TD. Health app use among US mobile phone owners: a National Survey. JMIR mHealth. 2015;3(4):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 http://handbook.cochrane.org/. Accessed September 13, 2016. [Google Scholar]
- 12. International Telecommunication Union. About Mobile Technology and IMT-2000 http://www.itu.int/osg/spu/imt-2000/technology.html. Accessed September 13, 2016. [Google Scholar]
- 13. R Core Team. R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing; 2015. http://www.R-project.org/. Accessed September 13, 2016. [Google Scholar]
- 14. Foster JM, Usherwood T, Smith L et al. Inhaler reminders improve adherence with controller treatment in primary care patients with asthma. J Allergy Clin Immunol. 2014;134(6):1260–68. [DOI] [PubMed] [Google Scholar]
- 15. Van Gaalen JL, Beerthuizen T, van der Meer V et al. Long-term outcomes of internet-based self-management support in adults with asthma: randomized controlled trial. J Med Internet Res. 2013;15(9):40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rikkers-Mutsaerts ERVM, Winters AE, Bakker MJ et al. Internet-based self-management compared with usual care in adolescents with asthma: a randomized controlled trial. Pediatric Pulmonol. 2012;47(12):1170–9. [DOI] [PubMed] [Google Scholar]
- 17. Lv YH, Zhao HJ, Liang ZY et al. A mobile phone short message service improves perceived control of asthma: a randomized controlled trial. Telemed E-Health. 2012;18(6):420–26. [DOI] [PubMed] [Google Scholar]
- 18. Araújo L, Jacinto T, Moreira A et al. Clinical efficacy of web-based versus standard asthma self-management. J Invest Allerg Clin. 2012;22(1):28–34. [PubMed] [Google Scholar]
- 19. Liu WT, Huang CD, Wang CH et al. A mobile telephone–based interactive self-care system improves asthma control. Eur Respir J. 2011;37(2):310–17. [DOI] [PubMed] [Google Scholar]
- 20. Prabhakaran L, Chee WY, Chua KC et al. The use of text messaging to improve asthma control: a pilot study using the mobile phone short messaging service (SMS). J Telemed Telecare. 2010;16(5):286–90. [DOI] [PubMed] [Google Scholar]
- 21. Meer V, Bakker MJ, Hout WB et al. Internet-based self-management plus education compared with usual care in asthma: a randomized trial. Ann Internal Med. 2009;151(2):110–20. [DOI] [PubMed] [Google Scholar]
- 22. Jacobson JS, Lieblein A, Fierman AH et al. Randomized trial of an electronic asthma monitoring system among New York City children. Am J Managed Care. 2009;15(11):809–14. [PubMed] [Google Scholar]
- 23. Cruz-Correia R, Fonseca J, Lima L et al. Web-based or paper-based self-management tools for asthma: patients’ opinions and quality of data in a randomized crossover study. Stud Health Technol Inform. 2007;127:178–89. [PubMed] [Google Scholar]
- 24. Rasmussen LM, Phanareth K, Nolte H et al. Internet-based monitoring of asthma: a long-term, randomised clinical study of 300 asthmatic subjects. J Allerg Clin Immun. 2005;115:1137–42. [DOI] [PubMed] [Google Scholar]
- 25. Ostojic V, Cvoriscec B, Ostojic SB et al. Improving asthma control through telemedicine: a study of short-message service. Telemed J E-health. 2005;11(1):28–35. [DOI] [PubMed] [Google Scholar]
- 26. Ryan D, Price D, Musgrave SD et al. Clinical and cost effectiveness of mobile phone supported self monitoring of asthma: multicentre randomised controlled trial. BMJ. 2012;344:e1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cingi C, Yorgancioglu A, Cingi CC et al. The “physician on call patient engagement trial” (POPET): measuring the impact of a mobile patient engagement application on health outcomes and quality of life in allergic rhinitis and asthma patients. Int Forum Allergy Rhinol. 2015;5(6):487–97. [DOI] [PubMed] [Google Scholar]
- 28. Helen K, Reddel D, Robin T et al. An Official American Thoracic Society/European Respiratory Society Statement: Asthma Control and Exacerbations. Standardizing Endpoints for Clinical Asthma Trials and Clinical Practice. Am J Respir Crit Care Med. 2009;180:59–99. [DOI] [PubMed] [Google Scholar]
- 29. Kokubu F, Nakajima S, Ito K et al. Hospitalization reduction by an asthma tele-medicine system. [Japanese]. Arerugi [Allergy] 2000;49(1):19–31. [PubMed] [Google Scholar]
- 30. Morrison D, Wyke S, Agur K et al. Digital asthma self-management interventions: a systematic review. Eysenbach G, ed. J Med Internet Res. 2014;16(2):e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McLean S, Chandler D, Nurmatov D et al. Telehealthcare for asthma. Cochrane Database Syst Rev. 201010):CD007717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mendiola MF, Kalnicki M, Lindenauer S. Valuable features in mobile health apps for patients and consumers: content analysis of apps and user ratings, JMIR Mhealth Uhealth. 2015;3(2):e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pinnock H, Epiphaniou E, Pearce G et al. Implementing supported self-management for asthma: a systematic review of implementation studies. BMC Med. 2015;13:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.