Abstract
Since December 2019, the SARS-CoV-2 outbreak that began in Wuhan, China has spread to nearly every continent and become a global health concern. Although much has been discovered about COVID-19 and its pathogenesis, the WHO has identified an immediate need to increase the levels of testing for COVID-19 and identify the stages of the disease accurately for appropriate action to be taken by clinicians and emergency care units. Harnessing technology for accurate diagnosis and staging will improve patient outcomes and minimize serious consequences of false-positive test results. Point-of-care technologies aim to intervene at every stage of the disease to quickly identify infected patients and asymptomatic carriers and stratify them for timely treatment. This requires the tests to be rapid, accurate, sensitive, simple to use and compatible with many body fluids. Mobile platforms are optimal for remote, small-scale deployment, whereas facility-based platforms at hospital centers and laboratory settings offer higher throughput. Here we review evidence-based point-of-care technologies in the context of the entire continuum of COVID-19, from early screening to treatment, and discuss their impact on improving patient outcomes.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is wreaking havoc worldwide and has claimed nearly 400,000 lives as of 15 June 2020 [1]. The rampant nature of the associated disease, COVID-19, has caused a great increase in demand for diagnostic and monitoring modalities because testing is the first line of defense against the pandemic. As learnt from previous viral epidemics – for example, influenza (H1N1) in 2009, Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV) in 2012 and the SARS outbreak during 2003 – rapid and accurate diagnostic testing with point-of-care technologies (POCTs) is beneficial in early identification [2,3]. It is important to understand that the deployment of tests for mass screening implies that the tests are well targeted, suitably reliable (error-free) and high performing in diagnosing the true condition of a patient. Diagnostic tests are actively useful and improve patient outcomes only when they are used in the right settings. A laboratory test may be more accurate in ‘routine’ situations where there is no pandemic, but point-of-care (POC) tests are instituted when the speed of the result seems to be more crucial. The need for rapid screening, triage and isolation of affected populations, the ability to monitor and stratify patients at home, in the clinic and in intensive care units (ICUs), and the associated decisions caregivers must take based on the test results underline the significance of POCTs. The WHO forum responsible for identifying immediate research needs and research gaps for COVID-19 recognized mobilizing research on rapid POC diagnostics for use at the community level and ensuring access to accurate and standardized diagnostics as one of the eight immediate research actions [4]. With primary, secondary and tertiary prevention as goals in this pandemic, the employment of evidence-based POCTs during different phases of the clinical course of COVID-19 is cardinal in monitoring the various stages and stratifying patients appropriately [5].
Current diagnostic tests fall into two main categories: molecular assays and serological assays. The main difference in these lies not only in the sample used for identifying the target viral agents but also in the tests' complexity, speed, scalability and availability for mass screening. La Marca et al. [6] studied the molecular and serological assays in detail in their systematic review and reported several studies using commercial antibody assays on real patients. Molecular assays are primarily nucleic acid amplification tests, diagnostic assays that can be built immediately upon identifying the sequence of the pathogen or viral agent. Serological assays are primarily antibody (IgM/IgG) tests; they are more robust and require a longer time to develop but may take only 15 min to give a result. Rapid POC immunoassays that detect the SARS-CoV-2 antibodies (IgM and IgG) are based on the lateral flow immunoassay technique, in which the patient's sample (typically drawn blood) is placed on a membrane strip with a nanoparticle–antibody conjugate. The proteins in the blood are drawn through the membrane strip by capillary action, and the antigen in the sample binds specifically to the conjugate to give accurate, reliable results. The effectiveness of serological tests with respect to their sensitivity and specificity from the onset of symptoms has been reviewed by Nicol et al. [7]. Udugama et al. [8] described emerging diagnostic platforms, developmental phases of diagnostic tests and the role of smartphone capabilities such as connectivity, databasing and onboard hardware in formulating evidence-based disease response for policy makers and healthcare systems worldwide. The potential for developing biosensors, visualization tools utilizing the principles of nanotechnology, and novel diagnostic tests for SARS-CoV-2 based on blood-based biomarkers has been reviewed by Bhalla et al. [9].
Modern diagnostic tools based on aptamers provide higher detection sensitivity and specificity to the target agents of SARS-CoV-2, such as the nucleocapsid protein [10]. Aptamer-based sensing platforms offer high affinity to the target pathogens; they are cheaper, more flexible and give faster results than antibodies [11,12]. Pinpoint Sciences is currently in the process of developing an aptamer-based rapid diagnostic test for COVID-19 that can give results in less than 30 s [13].
In this article, we consolidate and review various existing POCTs which may be utilized during different clinical phases of COVID-19. We take the approach of monitoring and stratification and segregate the different types of reader and analyzer devices for use in POC settings at home, at work, in clinics and in hospitals. We also gain a cross-sectional insight into the contributions we can make with the body fluid-based POCTs being developed in our lab at UT Dallas.
Clinical course of COVID-19
Understanding the clinical course of the disease caused by SARS-CoV-2 will allow us to categorize the use of existing POCT accordingly [14]. Various descriptions are available for SARS-CoV-2's clinical course; we choose to use the following to assist us in categorizing different available POCTs and their utility, as shown in Figure 1. Table 1 lists the point-of-care technology used at each stage of the clinical course of COVID-19 based on evidence from clinical symptoms.
Uncomplicated mild illness: After exposure to a contact or an asymptomatic carrier of SARS-CoV-2, a patient who develops mild fever, dry cough, sore throat, nasal congestion, malaise, headache and myalgia is known to have uncomplicated mild illness.
Moderate pneumonia: A patient who had mild illness now develops shortness of breath, indicating development of a moderate pneumonia.
Severe pneumonia: Patient with moderate pneumonia who develops tachypnea (increased respiratory rate ≥30 breaths/min) and becomes hypoxic (oxygen saturation ≤90%) on room air is known to have severe pneumonia. At this point the patient is in respiratory distress, signified by hypoxia and tachypnea.
Acute respiratory distress syndrome (ARDS): Worsening of existing respiratory distress, progressing to new-onset respiratory failure, marks the onset of ARDS. Diagnosis of ARDS requires clinical and ventilatory criteria. The reference parameter used is the ratio of partial pressure of oxygen in the alveoli (PaO2) and the fractional inspiration of oxygen (FiO2), PaO2/FiO2 (also known as the P/F ratio). Berlin's criteria are employed to know the severity of ARDS: mild ARDS is when P/F is greater than 200 and less than 300, moderate ARDS is when P/F is between 100 and 200, and severe ARDS is when P/F is less than 100.
Sepsis and septic shock: Sepsis, in the context of COVID-19 refers to the spread of the virus or viral particles into the bloodstream. As blood travels to every part of the body, the virus now reaches all the organs, causing multiorgan involvement. This produces a multitude of signs and symptoms Once blood pressure drops and oxygen delivery to all the organs decreases, the patient is now in septic shock, critically ill.
Figure 1. High-level clinical course of COVID-19 showing progression of various stages of illness from the initial contact phase and illness onset ending with sepsis and septic shock.
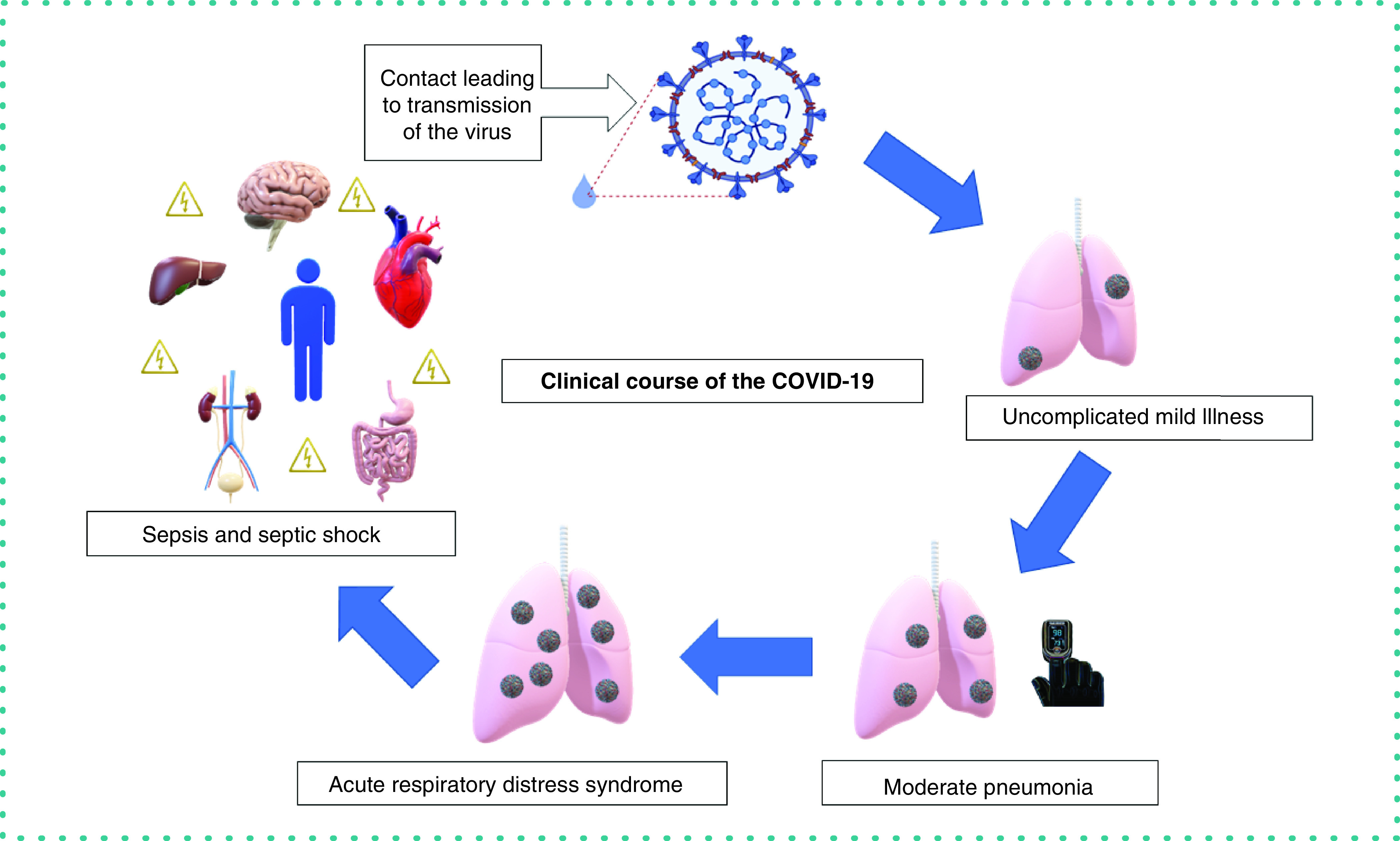
Table 1. Evidence-based employment of point-of-care technologies.
| Evidence (clinical symptoms/signs) | Clinical stage of COVID-19 | Point-of-care technology |
|---|---|---|
| Fever (body temperature) | All stages | Thermometer; esophageal temperature probe (mechanically ventilated patients) |
| Shortness of breath, increased respiratory rate, hypoxia | Moderate and severe pneumonia; ARDS and sepsis/septic shock | Pulse oximetry, forehead oximetry |
| Worsening of already identified respiratory picture or new-onset respiratory failure (PaO2/FiO2*) | ARDS; sepsis and septic shock | Arterial blood gas analyzers, pulse probes, EKG leads |
| Multiorgan involvement: increased heart rate increased respiratory rate decreased urinary output altered mental status |
Sepsis and septic shock | Basic metabolic panel (lactate, bilirubin, creatinine, BUN, electrolytes, cardiac markers; e.g., i-STAT CHEM 8+) capillary blood glucose monitors, noninvasive cardiac output monitoring, bedside bladder ultrasound, hand-held arterial dopplers, bedside echocardiography, bedside lung ultrasound, ultrasounds for line placements, urine dipstick, urine toxicology, EKG leads |
ARDS: Acute respiratory distress syndrome; BUN: Blood urea nitrogen; EKG: Electrocardiogram.
The bridge: translating healthcare needs to the researcher
In the setting of a pandemic respiratory illness, the highest priority of healthcare providers is to triage and isolate the patient to prevent further spread. The COVID-19 pandemic is unique because it has placed a major burden on the hospital infrastructure worldwide.
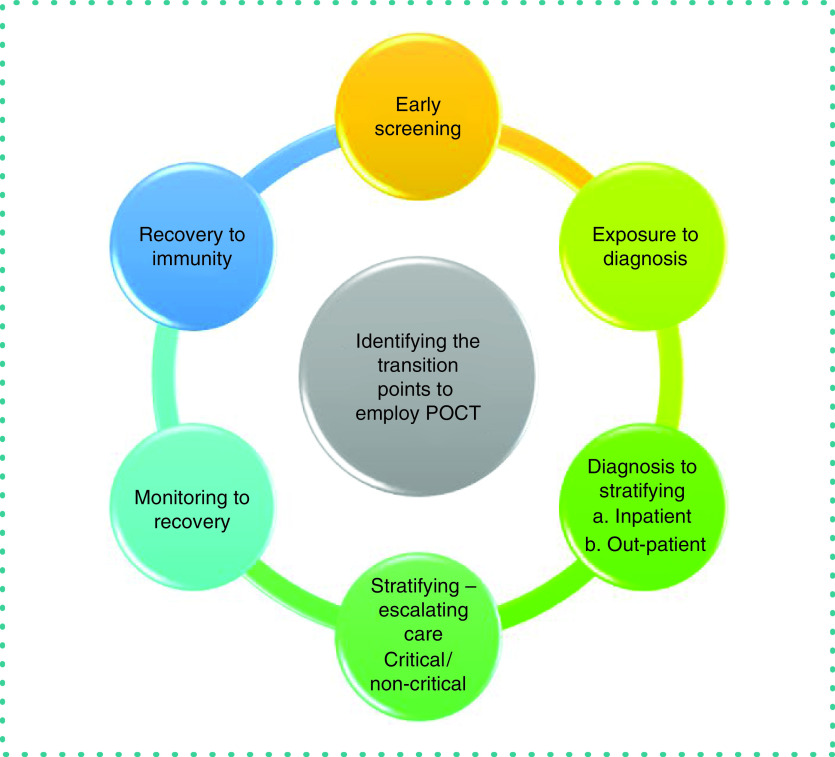
In Figures 2 & 3, we illustrate the COVID-19 continuum to identify a clinician's needs and the transition points in the course of the disease to translate healthcare needs to a researcher. Here we aim to discuss the role of POCTs in identifying the transition points during the course of the disease, which would allow appropriate employment of resources and thereby reduce the load on the healthcare system.
Figure 2. Translating healthcare needs to a researcher's question in times of the COVID-19 pandemic.
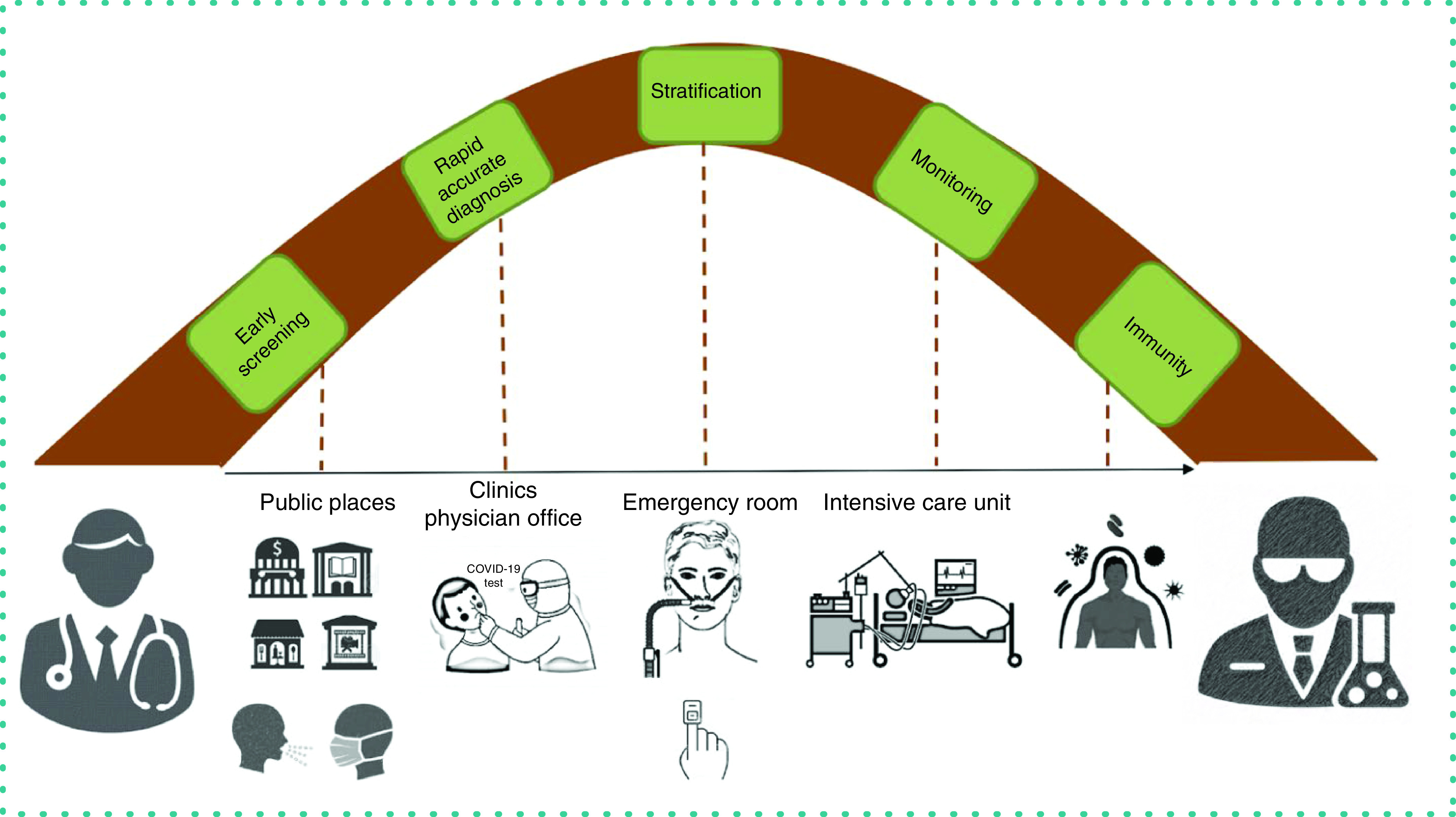
Figure 3. Transition points during the continuum of COVID-19 where point-of-care technologies will have the most impact in delivery of healthcare.
POCT: Point-of-care technology.
Early screening
Early screening refers to the concept of early detection of symptoms to increase the chance of a beneficial public and clinical outcome. With public areas reopening, it is essential to keep the population safe by screening the crowd to identify anyone who is symptomatic.
Contactless thermometers and pulse oximeters or forehead oximeters are suitable for temperature and oxygen saturation detection, respectively. Wearable heart rhythm monitors, either in the form of chest straps and belts or smart watches, will provide heart rate readings. These recordings may allow a person to decide whether to go to the physician's office or clinic for further testing.
Exposure to diagnosis
From the time of exposure to symptom onset and subsequent diagnosis, we need POC devices that allow rapid detection of the SARS-CoV-2 RNA with diagnostic accuracy and decentralized handling. These tests allow healthcare personnel to isolate the patient, reducing the risk of transmission. Nasopharyngeal or oropharyngeal swabs are used to collect upper respiratory tract specimens for use in molecular diagnostic testing of SARS-CoV-2. RT-PCR is the current standard test for qualitative detection. Various test kits have been made available which made the diagnostic process decentralized and easy to handle with rapid results, removing the requirement to run RT-PCR in a centralized laboratory with longer turnaround time.
Diagnosis to stratification
After an individual has been diagnosed with COVID-19, it is crucial to decide the condition of the patient. Essentially this decision is based on a multitude of factors. POCTs such as cutaneous oximetry, pulse oximetry, contactless temperature sensing and measurement of a variety of markers in the blood can be employed to decide the disposition of a patient and whether they require home treatment or inpatient admission.
Stratification to escalating care
The rampant nature of the SARS-CoV-2 pneumonia has been well described in literature and it is crucial that care is escalated at the right time. Increasing oxygen demands and changes in certain parameters in the blood allow physicians to make decisions about whether a patient might need escalation of care; for example, the requirement for noninvasive or invasive ventilation, or moving a patient from a regular room to a critical care setting.
Monitoring to recovery
A wide range of POC devices are employed in healthcare settings on a day-to-day basis. To discuss every POCT used is beyond the scope of this review. In the next section we discuss the various POCTs used to monitor patients admitted to the hospital.
Technology plays a crucial role in at-home monitoring via smart watches/devices that continuously monitor heart rate, respiratory rate, oxygen saturation and body temperature. Wireless transmission of data from these smart devices to phone apps allows healthcare providers to access the information and monitor the situation of these patients remotely.
Follow-up of patients who have recovered from SARS-CoV-2 pneumonia is still being studied for evidence that they have developed immunity against the strain. Identification of antibodies such as IgM and IgG would assist in documenting immunity. Developing POCT in this area would be of great epidemiological significance and may open doors to treatment options.
Evidence-based POCT employment in the times of a pandemic
POCTs often employ biosensors that are used in laboratory analysis to support accurate and rapid diagnosis of infectious diseases. Luppa et al. classified POCTs by several attributes: sensor characteristics, complexity, measuring mode, underlying detection principle and/or sample matrix [15]. Our classification of the use of various POCTs during the clinical course of COVID-19 is presented in Tables 2–4.
Table 2. Point-of-care strip-based devices that provide qualitative output and commercially available devices.
| Application | Sample type | Detection principles | Stage | Setting | Currently available commercial devices |
|---|---|---|---|---|---|
| SARS-CoV-2 detection (viral RNA) | NP swab OP swab Saliva |
Immunochromatography/lateral flow immunoassay PCR |
Any stage of presentation to the healthcare provider | Inpatient and outpatient | SugenTech Respi-Strip |
| Viral antigen detection (N/S antigens, spike receptor binding domain) | Serum | Standard sandwich ELISA | Any stage of presentation to the healthcare provider | Inpatient and outpatient | RayBiotech |
NP: Nasopharyngeal; OP: Oropharyngeal.
Table 3. Point-of-care unit-use analyzers that provide quantitative output and commercially available devices.
| Application | Sample type | Detection principles | Transition point | Setting | Currently available commercial devices |
|---|---|---|---|---|---|
| Glucose detection | Plasma/whole blood | Micromechanical (microcoagulation)/ electrochemical (amperometric) | Monitoring | Home, inpatient and outpatient | OneTouch, Acccheck, FreeStyle Libre 14 day system |
| Basic metabolic panel | Plasma/whole blood | Electrochemical | Stratification and monitoring | Inpatient | i-STAT |
Table 4. Point-of-care benchtop and hand-held analyzers employed at different transition points and commercially available systems.
| Application | Sample type | Detection principles | Transition point | Setting | Currently available commercial systems |
|---|---|---|---|---|---|
| Blood gas analysis | Arterial blood | Potentiometric/amperometric/ optic sensors | Stratification and monitoring | Inpatient | RAPIDPoint 500 Systems |
| Blood gas analysis with CO-oximetry | Arterial and venous blood | Potentiometric/amperometric/ optic sensors + multi-wavelength spectrophotometry | Stratification and monitoring | Inpatient | RAPIDPoint 500 Systems |
| Respiratory rate and heart rate | NA | Cardiac and respiratory motion capture | Monitoring | Inpatient | Hillrom Centrella Smart+ Bed |
| Lung and heart imaging | NA | Ultrasound | Monitoring | Inpatient | GE Healthcare |
Qualitative strip-based devices
These tests often provide qualitative results, are straightforward to interpret and do not require much expertise; one example is the Lateral Flow Immunoassay cassette where the readout device shows either a plus, minus or invalid output. SugenTech developed a rapid diagnostic test to detect SARS-CoV-2 IgG/IgM in blood using lateral flow assay in less than 20 min. The readout is colorimetric, qualitative and equipment-free. Similarly, the COVID-19 Ag Respi-Strip is a rapid SARS-CoV-2 antigen assay for the diagnosis of COVID-19 in 15 min [16]. RayBiotech developed the standard sandwich ELISA based COVID-19/SARS-CoV-2 Nucleocapsid Protein ELISA Kit to detect the N-protein [17].
Unit-use analyzers
Unit-use analyzers are one-time use articles that provide a quantitative readout of parameters after the reaction takes place on test strips. They are patient-friendly and can be used at the patient's bedside in the hospital, at home or in outpatient settings. Examples include the OneTouch and AccuCheck glucometers for measurement of glucose concentration in finger-prick blood samples and the FreeStyle Libre 14-day system for continuous glucose monitoring [18]. The US FDA has approved the use of continuous glucose monitoring devices for use in hospitals. Diabetes is among the top three common comorbidities for COVID-19, along with obesity and hypertension. With the need for continuous monitoring of patient vital signs and key inflammatory markers, and given the steady increase in the number of cases worldwide, unit-use glucometers are advantageous because they allow healthcare workers to obtain glucose readings in a contactless way from the infected patient's bedside. This reduces the number of times the nurses go into the patient's room or change personal protective equipment to obtain the readings without interfering with intensive interventions to treat COVID-19. Continuous glucose readings also help physicians to assess the accurate status of critically ill patients, which is key for timely administration of drugs to treat hyperglycemia. Another example of a unit-use analyzer is the i-STAT system developed by Abbott for the quantification of multiple diagnostic parameters. The range of tests conducted on the i-STAT includes lactate, hematology, chemistries and electrolytes, cardiac markers, endocrinology, blood gases and coagulation [19].
Benchtop POCT analyzers
An example of a POC benchtop blood gas analyzer that meets the challenges of clinicians is the Siemens RAPIDPoint 500 Blood Gas System [20]. It has a rapid response time of around 60 s and tests blood gases, electrolytes, glucose, lactate and full CO-oximetry from samples including whole blood (arterial and venous), pleural fluid and dialysate. The configuration of combined blood gas analysis with CO-oximetry has been implemented in this system. The detection principle for the CO-oximetry unit is based on multi-wavelength spectrophotometry, in which the absorption of multiple wavelengths of light by the blood provides the concentration of hemoglobin and its derivatives [21]. The technological specifications that enhance the clinician's experience are the intuitive touch screen, the connectivity to electronic health records through barcodes, and remote monitoring and control using the RAPIDComm Data management system. The RAPIDComm web application allows the clinician to view the status of the POC instruments and troubleshoot issues.
Bedside POC ultrasound offers clinicians the ability to obtain lung and heart images and observe abdominal fluid accumulation and drainage of pleural fluid; it also provides guidance for central line placement, toward monitoring of mechanically ventilated patients who are the most seriously affected by COVID-19 [22]. Bedside POC ultrasound also reduces radiation exposure compared with repeated radiography.
Continuous measurement with POCT systems
Continuous glucose monitoring systems are one example of essential continuous measurement POCT systems for critically ill ICU patients [23,24]. With a high incidence of COVID-19 being noted in diabetics [25], the use of continuous glucose monitoring and reliable wireless transmission of data is an area to be studied as a means of reducing exposure in an inpatient setting. Respiratory distress is one of the major clinical indications of COVID-19; contact-free continuous heart rate and respiratory rate monitoring is a novel approach that directly assists in the stratification of critically ill patients [26]. Sensors under the mattress capture a patient's cardiac and respiratory motion and translate them to an algorithm which calculates heart and respiratory rates twice every second. Continuous respiratory rate monitoring in this fashion potentially allows earlier identification of patient deterioration and assists with having ICU beds available for critically ill patients. Additional smart bed features include easy-to-interpret automatic alarms and an operator-friendly user interface.
Molecular biology-based POCT devices to detect infectious agents
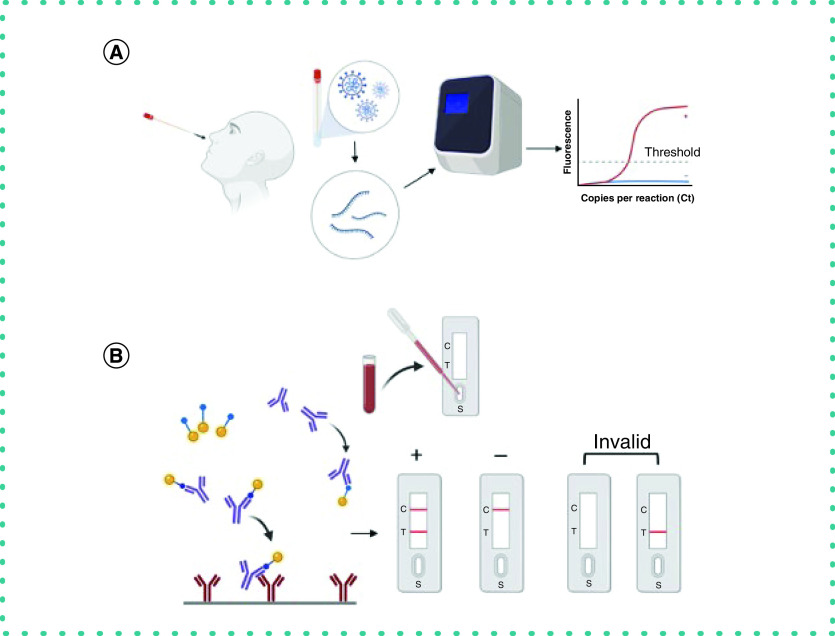
Diagnostic testing for COVID-19 can be categorized in two subsets, as shown in Figure 4: molecular testing (the detection of viral RNA) and serological testing (the detection of IgM and IgG antibodies and viral antigen). In molecular testing, the current reference test for diagnosing an active SARS-CoV-2 infection is an RT-PCR assay. In RT-PCR, viral RNA is extracted and amplified from respiratory samples collected through a nasopharyngeal or oral swab. The common targets for detecting SARS-CoV-2 in the specimen are the envelope gene (E gene), nucleocapsid gene (N gene), spike gene (S gene), ORF1ab gene, and RNA-dependent RNA polymerase (RdRp). Although a POCT, the RT-PCR test is complex and requires a centralized laboratory with expensive equipment and well-trained laboratory technicians (Figure 4A). Most results obtained using PCR provide excellent specificity (up to >95%) when an adequate nasopharyngeal swab specimen is submitted from a symptomatic patient; this helps in confirming or excluding the presence of the disease. Apart from nasopharyngeal specimens, samples from the nares and oropharynx, salivary samples [27], tears [28], anal swabs [29], fecal and urine samples and exhaled breath [30,31] have been studied in patients of different age groups and with different disease severities.
Figure 4. Process of diagnostic testing of COVID.
(A) Molecular testing from nasopharyngeal swab followed by RT-PCR. (B) Serological testing through antibody–antigen interaction from lateral flow immunoassay.
Recent advances have taken place in the development of in vitro assays for COVID-19. In particular, diagnostic kits using RT-PCR, which took a few hours, were replaced with assays which reduced the time to result to 45 min. Abbott's technology decreased the turnaround time to 5 min [32]. Most molecular tests have been approved by the FDA under emergency use authorization (EUA) and are Conformité Européenne (CE) marked.
Serological tests use ELISA to detect the presence of SARS-CoV-2 antibodies in body fluids such as whole blood, plasma or serum. Currently, serological testing is not offered as a part of standard diagnosis of SARS-CoV-2 due to the lack of availability of validated assays. Widespread use of serological tests may not be beneficial for diagnosing COVID-19 in large numbers but is of epidemiological significance for early detection, screening for immunity to disease and also as an adjunct to molecular testing (RT-PCR + antibody detection). One of the limitations for detection of IgG is the cross-reactivity with antibodies produced against SARS-CoV-2. Potential targets for SARS-CoV-2 antigen detection include the glycoprotein spikes on the surface of the viral capsid, N antigen, S antigen and spike receptor binding domain. Currently available kits employ a standard sandwich ELISA format, allowing rapid quantification of the N and S antigens. These assays may be used to quantify antigens on the surface of SARS-CoV-2 and/or pseudovirus, as well as antigens expressed recombinantly. Antigen detection is not currently being offered as standard testing.
Table 5 summarizes some common viral RNA detection tests: the technologies employed, samples used, targets detected and the time from sample to first result (assay time) of 18 commercialized POC diagnostic tests that received EUA in the US as of September 5, 2020. Details were taken from the Foundation of Innovative New Diagnostics database which supplies use cases that enable decision-makers and test developers to come to a common platform [33]. The tests have published clinical trial data and all 18 tests provide a qualitative output with either nasal, nasopharyngeal or oropharyngeal swab samples; they have an average assay time of 2–8 hrs or within the same day. The average sensitivity of the 18 tests is 98% and the average specificity is 99.8%.
Table 5. Commercialized point-of-care diagnostic tests and assays for the detection of SARS-CoV-2 agents with a US regulatory status of Emergency Use Authorization as of 5 September 2020.
| Test name | Technology | Target detected/source | LOD | Assay time | Company |
|---|---|---|---|---|---|
| ID NOW Covid-19 test | Isothermal RT-PCR |
RdRp Control: internal |
125/ml | 5–15 min | Abbott |
| ANDiS SARS-CoV-2 RT-qPCR detection kit | RT-qPCR |
ORF1ab, N, Egenes Control: internal |
5 copies/reaction | 4–6 h | 3D Medicines |
| BioFire COVID-19 test | Rt RT-PCR | Control: external | NA | 45 min | BioFire Defense, LLC |
| BioGX SARS-CoV-2 | RT-PCR |
N genes Control: internal |
NA | NA | BD |
| Go-strips SARS-CoV-2 test | RT-PCR |
ORF1ab, S genes Control: MS2 bacteriophage |
NA | NA | Biomeme |
| LabGun™ COVID 19 Assay PCR Kit | RT-PCR |
RdRp, E genes Control: internal, MS2 bacteriophage |
20 genomic RNA copies/μl | NA | LabGenomics Co., Ltd |
| ARIES SARS-CoV-2 Assay | RT-PCR |
ORF1ab, N genes Control: NA |
NA | 2 h for 12 samples | Luminex Corp. |
| QIAstat-Dx Respiratory Panel 2019-nCoV | RT-PCR |
N gene Control: none |
NA | 20–40 min | QIAGEN GmbH |
| Rheonix COVID-19 MDx Assay | RT-PCR |
N1 gene Control: human RNase P gene |
625 genomic equivalents/ml | Same day | Rheonix, Inc. |
| Xpert Xpress SARS-CoV-2 | PCR-POC |
N2, E genes Control: NA |
250/ml | 45 min | Cepheid |
| Simplexa COVID19 assay | PCR |
ORF1ab, S genes Control: internal |
242/ml | 1 h | DiaSorin Molecular |
| ePlex SARS-CoV-2 test | PCR | NA | 10/ml | 2 h | GenMark Diagnostics |
| Panther Fusion SARS-Cov-2 | PCR |
ORF1abgene Control: NA |
0.01 | 3 h | Hologic |
| OPTI SARS | PCR |
N1, N2 Control: NA |
900/ml | NA | OPTI Medical Systems |
| SARS-CoV-2 Test Kit (Real-time PCR) | PCR |
ORF1ab and N gene Control: NA |
NA | NA | Xiamen Zeesan Biotech Co., Ltd. |
| Cobas SARS-CoV-2 | PCR |
E gene Control: Internal |
150/ml | 3.5 h | Roche |
| DiaPlexQ™ Novel Coronavirus (2019-nCoV) Detection Kit | PCR |
ORF1a, N gene Control: plasmid, positive |
NA | 2 h | Solgent Co. Ltd |
| Allplex 2019-nCoV Assay | PCR |
E, RdRp, N gene Control: RP- V |
1250/ml | 4 h | Seegene |
Data taken from [34].
Table 6 summarizes some commercially available protein tests or serology tests which detect the body's immune response to SARS-CoV-2 infection. The tests are cleared by the FDA and their clinical trial data is published.
Table 6. Commercially available serology tests for detection of antibodies using different measurement principles.
| Test name | Source/target | Measurement principle | Sampling fluid/specimen | Assay time | Company |
|---|---|---|---|---|---|
| Anti-SARS-Cov-2 rapid test | Ig/Spike | LFA | Blood plasma/serum | 15–20 min | Autobio Diagnostics |
| LIAISON SARS-CoV-2 S1/S2 IgG | Ig/Spike | CLIA | Blood plasma/serum | NA | DiaSorin, Inc. |
| Anti-SARS-CoV-2 ELISA (IgG) | IgG, IgA/Spike (S1 domain) | ELISA | Blood plasma/serum | NA | EUROIMMUN US, Inc. |
| RightSign COVID19 IgG/IgM rapid test | Ig/Spike | LFA | Blood plasma/serum | 20 min | Hangzhou Biotech |
| VITROS Immuno diagnostic IgG | Ig/Spike | CLIA | Blood plasma/serum | <1 h | Ortho Clinical |
| Elecsys Anti-SARS-CoV-2 Cobas immuno assay | Ig/Nucleocapsid | ECLIA | Blood plasma/serum | 20 min | Roche |
CLIA: Chemiluminescence immunoassay; ECLIA: Electrochemiluminescence immunoassay; LFA: Lateral flow immunoassay.
Data taken from [34].
Discussion & future perspective
Current immunoassay techniques to detect antibodies in human blood plasma and serum include CLIA, ECLIA, LFA and ELISA. These assays are time consuming, require large sample volumes and sample preprocessing and trained personnel for handling. With every lost minute, the number of asymptomatic carriers is growing and gaps in screening populations in the incubation phase are widening. Screening is essential in several settings: in public places, at home, in offices and in other workplaces. Clinicians must have the capability to diagnose SARS-CoV-2 using rapid tests at various stages of disease progression in clinics, nursing homes, physician offices, emergency rooms and hospitals. Since March 2020, the FDA has granted EUA to more rapid molecular diagnostic tests that can be used in temporary screening locations, doctor's offices, labs and nursing homes. An evidence-based approach for dealing with the pandemic through POCTs will inform medical personnel on the phase of the disease in a specific patient and allow them to stratify accurately. For citizens, the result obtained from a POCT will identify the right measures to take: for example, whether to quarantine or socially distance. Organizations such as the Foundation for Innovative New Diagnostics have enabled the collation of commercially available diagnostic tests from medical manufacturers from different countries. This is a unique platform for collaboration among researchers and new manufacturers to address the rapidly evolving needs of clinicians and hospital systems. POCTs must be simple enough for use in both well-equipped laboratory settings and nontraditional and low-resource settings. This involves standardization, quality assurance and regulatory approval of the POCT for deployment and mass surveillance [35]. As the demands resulting from the pandemic crisis grow, the scale of response and testing must increase proportionately to support efforts of surveillance and epidemiological tracking. Major US commercial diagnostics manufacturers (e.g., Abbott, Thermo Fisher, Roche, Hologic, Cepheid and DiaSorin) are ramping up production efforts of tests by setting weekly targets (up to 6.5 million tests per week) for high-volume testing [36].
Diagnostic tests must require low sample volume and simple methods of sample collection. Minimally invasive sample collection methods and the manufacturability of POC tests will decide their successful integration into device form factors that can be used in various POC settings. Our lab is equipped to address the needs of COVID-19 diagnostics because of our expertise in developing biosensors toward identifying relevant biomarkers from various body fluids such as blood [37], sweat [38,39], saliva, blood and exhaled breath condensate. For example, robust sensing platforms include a rapid electrochemical device using a single drop of sample (<40 μl blood) for point-of-use parathyroid hormone screening, a portable biosensor for cortisol monitoring in a low volume (1–5 μl) of human sweat and a screen-printed graphene oxide textile biosensor for point-of-exposure detection of influenza for at-risk populations [40]. The ability to integrate these sensors into wearable material such as face masks and textiles would enable detection of exposure to SARS-CoV-2 virus before symptoms manifest; this would be particularly useful in the incubation phase for asymptomatic carriers. Mass production of wearable sensors that are conformable to the surface of clothing and other flexible substrate materials can be achieved through established printing technologies such as screen printing, roll-to-roll printing and inkjet printing. Enabling wireless communication of data from these body-worn wearable sensors will enable mass use through Internet of Things reporting platforms. The data can be used in machine learning models to predict geographic areas with abundance of at-risk populations. This would enable policy makers and governments to strategize and make appropriate decisions regarding isolation and distancing at the local level to prevent further spread of infection.
In the current COVID scenario, there is considerable lag in the capacity to detect infections in a timely manner and the spread of disease. Diagnostic testing, which is largely being deployed in centralized laboratory facilities, must rapidly scale up. Laboratory tests are essential in the pandemic response to make critical clinical decisions that affect treatment options, but the availability of POC tests is bound to accelerate clinical decision-making and relieve some workload from these centralized facilities. There is an evident gap in current serological test development of POC or point-of-use sensors which use low sample volumes, require no sample preprocessing, are scalable through manufacturing and are flexible to use with a quick turnaround time to results. Flexible biosensors for detection of a wide range of biomarkers within body fluids, immunity to cross-reactivity, rapid response time and an ability to integrate with noninvasive methods will become attractive research opportunities.
Rapidly deployed laboratory solutions are the only ‘eyes’ for local health authorities to assess the condition of the increasing number of individuals affected by COVID-19. However, any subsequent tests developed must be validated in depth by assessing their accuracy, reproducibility, cross-reactivity, stability, range and limit of detection for the ultimate goal of clinical utility. We also find that serological tests are not considered as standard testing to confirm diagnosis of COVID-19. There is abundant opportunity to make impact through development of rapid and accurate serological tests for diagnosing COVID-19-related biomarkers. Technology would be a major player if integrated effectively with serological tests. We believe that one of the key aspects that would enhance their widespread utility in clinics will be to equip sensing platforms with wireless capability via Bluetooth, Wi-Fi and Zigbee protocols so that the sensor information is transmitted safely and securely for clinicians, enabling an ‘Internet of Diagnostic Things’. Future research in POCT development lies in evidence-based clinical management and design of assays for scalable deployment in times of pandemic response.
Financial & competing interests disclosure
S Prasad and S Muthukumar have a significant interest in Enlisense LLC, a company that may have a commercial interest in the results of this research and technology. The potential individual conflict of interest has been reviewed and managed by The University of Texas at Dallas, and played no role in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the report for publication. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
- 1.Coronavirus disease 2019 (COVID-19) Situation Report 150, 2020. http://www.who.int/docs/default-source/coronaviruse/situation-reports/20200618-covid-19-sitrep-150.pdf?sfvrsn=aa9fe9cf_2
- 2.Louie RF, Kitano T, Brock TK. et al. Point-of-care testing for pandemic influenza and biothreats. Disaster Med. Public Health Prep. 3(2), S193–S202 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Hatchette TF, Bastien N, Berry J. et al. The limitations of point of care testing for pandemic influenza: what clinicians and public health professionals need to know. Can. J. Public Health 100(3), 204–207 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID 19 Public Health Emergency of International Concern (PHEIC) Global research and innovation forum: towards a research roadmap. http://www.who.int/blueprint/priority-diseases/key-action/Global_Research_Forum_FINAL_VERSION_for_web_14_feb_2020.pdf?ua=1
- 5.Picture of America, Prevention. https://www.cdc.gov/pictureofamerica/pdfs/picture_of_america_prevention.pdf
- 6.La Marca A, Capuzzo M, Paglia T. et al. Testing for SARS-CoV-2 (COVID-19): a systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reprod. Biomed. Online 41(3), 483–499 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicol T, Lefeuvre C, Serri O. et al. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech). J. Clin. Virol. 129, 104511 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Udugama B, Kadhiresan P, Kozlowski HN. et al. Diagnosing COVID-19: the disease and tools for detection. ACS Nano 14(4), 3822–3835 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Bhalla N, Pan Y, Yang Z. et al. Opportunities and challenges for biosensors and nanoscale analytical tools for pandemics: COVID-19. ACS Nano 14(7), 7783–7807 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Wu Q, Chen J, Xiaohua N. A DNA aptamer based method for detection of SARS-CoV-2 nucleocapsid protein. Virologica Sinica 35, 351–354 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torabi R, Ranjbar R, Halaji M. et al. Aptamers, the bivalent agents as probes and therapies for coronavirus infections: a systematic review. Mol. Cell. Probes 53, 101636 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Fang X, Liu X. et al. Discovery of sandwich type COVID-19 nucleocapsid protein DNA aptamers. Chem. Commun. 56, 10235–10238 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Pinpoint Science, Inc. https://pinpointscience.com/
- 14.Zhou F, Yu T, Du R. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229), 1054–1062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luppa PB, Müller C, Schlichtiger A. et al. Point-of-care testing (POCT): current techniques and future perspectives. Trends Anal. Chem. 30(6), 887–898 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mertens P, De Vos N, Martiny D. et al. Development and potential usefulness of the COVID-19 Ag Respi-Strip diagnostic assay in a pandemic context. Front. Med. 7, 225 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Empty CI. COVID-19 proteins (2020). https://www.raybiotech.com/covid19-proteins/
- 18.Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019 – United States, February 12–March 28, 2020. Morb. Mortal. Wkly Rep. (69), 382–386 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibasaki M, Ibuki T, Tanaka Y. A portable blood analyzer that uses on-line data management to deliver higher-quality patient information. J. Anesth. 24(4), 643–645 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Nicolas T, Cabrolier N, Bardonnet K. et al. Evaluation of a new blood gas analysis system: rapidPoint 500®. Ann. Biol. Clin. 71(3), 305–311 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Zwart A, Buursma A, Van Kampen EJ. et al. A multi-wavelength spectrophotometric method for the simultaneous determination of five haemoglobin derivatives (1981). [DOI] [PubMed] [Google Scholar]
- 22.Lu W, Zhang S, Chen B. et al. A clinical study of noninvasive assessment of lung lesions in patients with coronavirus disease-19 (COVID-19) by bedside ultrasound. Ultraschall Med. 41(3), 300–307 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Corstjens AM, Ligtenberg JJ, van der Horst IC. et al. Accuracy and feasibility of point-of-care and continuous blood glucose analysis in critically ill ICU patients. Crit. Care 10(5), 1–7 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker L. Characterisation of glucose management in intensive care [Doctoral dissertation]. Massachusetts Institute of Technology, MA, USA: (2019). [Google Scholar]
- 25.Zhu L, She ZG, Cheng X. et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 31(6), 1068–1077 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill-Rom Services, Inc. Smart Beds and Surfaces. http://www.hillrom.com/en-us/products/smart-beds-and-surfaces/centrella-smart-bed/
- 27.Sabino-Silva R, Jardim ACG, Siqueira WL. Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin. Oral Invest. 24(4), 1619–1621 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seah IYJ, Anderson DE, Kang AEZ. et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology 127(7), 977–979 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology 158(6), 1518–1519 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun J, Zhu A, Li H. et al. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerging Microbes Infect. 9(1), 991–993 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung NH, Chu DK, Shiu EY. et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 26(5), 676–680 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Core Laboratory, Abbott. http://www.corelaboratory.abbott/us/en/offerings/segments/infectious-disease/sars-cov-2
- 33.FIND. SARS-COV-2 Diagnostic Pipeline. http://www.finddx.org/covid-19/dx-use-cases/
- 34.Weissleder R, Lee H, Ko J. et al. COVID-19 diagnostics in context. Sci. Transl. Med. 12(546), eabc1931 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Cheng MP, Papenburg J, Desjardins M. et al. Diagnostic testing for severe acute respiratory syndrome-related coronavirus-2: a narrative review. Ann. Int. Med. 172(11), 726–734 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeciBio. http://www.decibio.com/2020/03/24/covid-diagnostics-2020–3/
- 37.Tanak AS, Muthukumar S, Hashim IA. et al. Rapid electrochemical device for single-drop point-of-use screening of parathyroid hormone. Bioelectron. Med. 2(1), 13–27 (2019). [Google Scholar]
- 38.Bhide A, Muthukumar S, Saini A. et al. Simultaneous lancet-free monitoring of alcohol and glucose from low-volumes of perspired human sweat. Sci. Rep. 8(1), 1–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganguly A, Rice P, Lin KC. et al. A combinatorial electrochemical biosensor for sweat biomarker benchmarking. SLAS Technol. 25(1), 25–32 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Kinnamon DS, Krishnan S, Brosler S. et al. Screen printed graphene oxide textile biosensor for applications in inexpensive and wearable point-of-exposure detection of influenza for at-risk populations. J. Electrochem. Soc. 165(8), B3084 (2018). [Google Scholar]