Abstract
Introduction:
The eighth edition of the American Joint Committee on Cancer (AJCC) melanoma staging system was implemented in the United States on 1 January 2018.
Areas covered:
This article provides an overview of important changes in the eighth edition AJCC staging system from the seventh edition based on analyses of a large international melanoma database. The clinical implications of these changes for melanoma treatment are also discussed.
Expert commentary:
A standardized and contemporary cancer staging system that facilitates accurate risk stratification is essential to guide patient treatment. The eighth edition of the AJCC staging system is currently the most widely accepted approach to melanoma staging and classification at initial diagnosis.
Keywords: Melanoma, TNM, staging, eighth edition, American Joint Committee on Cancer, AJCC, prognosis
1. Introduction
The incidence of cutaneous melanoma in the United States has continued to rise by ~3% per year over the past few decades [1]. The majority of patients with early-stage (I and II) melanoma have an overall favorable prognosis. Patients with stage III melanoma have a prognosis that is rather heterogeneous, and those with stage IV melanoma have historically had a very poor prognosis. Major advances in the treatment options with the introduction of molecularly targeted and immunotherapies have resulted in improved survival for patients with locoregionally advanced and metastatic melanoma [2–5], and more recently for patients in the adjuvant setting [6,7]. A thorough knowledge and understanding of prognostic factors and staging of cutaneous melanoma is crucial for initial patient assessment and treatment sequencing and planning, as well as in the development of surveillance strategies and clinical trial design and analysis.
As our understanding of melanoma biology has improved, the melanoma staging system has been revised a number of times. The seventh edition American Joint Committee on Cancer (AJCC) staging system for cutaneous melanoma was implemented in 2010 following its introduction in 2009 [8,9]. The eighth edition AJCC staging system for cutaneous melanoma was implemented nationwide in the United States on 1 January 2018 [10,11]. Based on analyses of a large international melanoma database, the Melanoma Expert Panel made key changes in the new staging system from the former seventh edition to improve staging and prognostication, risk stratification and selection of patients for clinical trials [10–12]. Details of the composition of the database can be found online in the supporting information section of reference 11.
Here, we review the most important changes in the new AJCC melanoma staging system and their implications for the management of patients with cutaneous melanoma.
2. Highlights of the eighth edition of the AJCC melanoma staging system
The eighth edition AJCC melanoma staging system TNM categories are outlined in Tables 1–3 (juxtaposed with the seventh edition staging system) and the stage groupings are outlined in Tables 4 and 5.
Table 1.
| 8th Edition (implemented 2018) | 7th Edition (implemented 2010) | |||
|---|---|---|---|---|
| T Category | Thickness | Ulceration status | Thickness | Ulceration status |
| TX | N/A | N/A | N/A | N/A |
| T0 | N/A | N/A | N/A | N/A |
| Tis | N/A | N/A | N/A | N/A |
| T1 | ≤1.0 mm | Unknown or unspecified | ≤1.0 mm | Unknown or unspecified |
| T1a | <0.8 mm | Without ulceration | ≤1.0 mm | Without ulceration and <1 mitosis/mm2 |
| T1b | <0.8 mm | With ulceration | ≤1.0 mm | With ulceration or ≥1 mitosis/mm2 |
| 0.8–1.0 mm | With or without ulceration | |||
| T2 | >1.0–2.0 mm | Unknown or unspecified | 1.01–2.00 mm | Unknown or unspecified |
| T2a | >1.0–2.0 mm | Without ulceration | 1.01–2.00 mm | Without ulceration |
| T2b | >1.0–2.0 mm | With ulceration | 1.01–2.00 mm | With ulceration |
| T3 | >2.0–4.0 mm | Unknown or unspecified | 2.01–4.00 mm | Unknown or unspecified |
| T3a | >2.0–4.0 mm | Without ulceration | 2.01–4.00 mm | Without ulceration |
| T3b | >2.0–4.0 mm | With ulceration | 2.01–4.00 mm | With ulceration |
| T4 | >4.0 mm | Unknown or unspecified | >4.00 mm | Unknown or unspecified |
| T4a | >4.0 mm | Without ulceration | >4.00 mm | Without ulceration |
| T4b | >4.0 mm | With ulceration | >4.00 mm | With ulceration |
N/A, not applicable; TX, primary tumor thickness cannot be assessed (e.g. diagnosis by curettage); T0, no evidence of primary tumor (e.g. unknown primary or completely regressed melanoma); Tis, melanoma in situ.
Adapted from and used with permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original and primary source for this information is the AJCC Cancer Staging Manual, 8th Edition (2017), published by Springer International Publishing (Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the Skin. In: Amin AB, Edge SB, Greene, FL, et al. (Eds). AJCC Cancer Staging Manual. 8th Ed. New York: Springer; 2017:563–585).
Adapted from and used with permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original and primary source for this information is the AJCC Cancer Staging Manual, 7th Edition (2009), published by Springer Verlag (Balch CM, Gershenwald JE, Soong SJ, et al. Melanoma of the Skin. In: Edge SB, Byrd D, Compton C, et al. (Eds). AJCC Cancer Staging Manual. 7th Ed. New York: Springer; 2009: 325–344).
Table 3.
| 8th Edition | 7th Edition | |||
|---|---|---|---|---|
| M Category | Anatomic Site | LDH Level | Anatomic Site | LDH Level |
| M0 | No evidence of distant metastasis | Not applicable | No distant metastases | |
| M1 | Evidence of distant metastasis | Evidence of distant metastasis | ||
| M1a | Distant metastasis to skin, soft tissue including muscles, and/or nonregional lymph node | Not recorded or unspecified | Distant skin, subcutaneous, or nodal metastases | Normal |
| M1a(0) | Not elevated | – | – | |
| M1a(1) | Elevated | – | – | |
| M1b | Distant metastasis to lung with or without M1a sites of disease | Not recorded or unspecified | Lung metastases | Normal |
| M1b(0) | Not elevated | – | – | |
| M1b(1) | Elevated | – | – | |
| M1c | Distant metastasis to non-CNS visceral sites with or without M1a or M1b sites of disease | Not recorded or unspecified | All other visceral metastases Any distant metastasis | Normal Elevated |
| M1c(0) | Not elevated | – | – | |
| M1c(1) | Elevated | – | – | |
| M1d | Distant metastasis to CNS with or without M1a, M1b, or M1c sites of disease | Not recorded or unspecified | – | – |
| M1d(0) | Not elevated | – | – | |
| M1d(1) | Elevated | – | – | |
LDH, lactate dehydrogenase; –, No counterpart in AJCC 7th Edition.
Adapted from and used with permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original and primary source for this information is the AJCC Cancer Staging Manual, 8th Edition (2017), published by Springer International Publishing (Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the Skin. In: Amin AB, Edge SB, Greene, FL, et al. (Eds). AJCC Cancer Staging Manual. 8th Ed. New York: Springer; 2017:563–585).
Adapted from and used with permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original and primary source for this information is the AJCC Cancer Staging Manual, 7th Edition (2009), published by Springer Verlag (Balch CM, Gershenwald JE, Soong SJ, et al. Melanoma of the Skin. In: Edge SB, Byrd D, Compton C, et al. (Eds). AJCC Cancer Staging Manual. 7th Ed. New York: Springer; 2009: 325–344).
Table 4.
| 8th Edition | 7th Edition | |||||
|---|---|---|---|---|---|---|
| Clinical Stage Group | T | N | M | T | N | M |
| 0 | Tis | N0 | M0 | Tis | N0 | M0 |
| IA | T1a | N0 | M0 | T1a | N0 | M0 |
| IB | T1b | N0 | M0 | T1b | N0 | M0 |
| T2a | N0 | M0 | T2a | N0 | M0 | |
| IIA | T2b | N0 | M0 | T2b | N0 | M0 |
| T3a | N0 | M0 | T3a | N0 | M0 | |
| IIB | T3b | N0 | M0 | T3b | N0 | M0 |
| T4a | N0 | M0 | T4a | N0 | M0 | |
| IIC | T4b | N0 | M0 | T4b | N0 | M0 |
| III | Any T | ≥N1 | M0 | Any T | N > N0 | M0 |
| IV | Any T | Any N | M1 | Any T | Any N | M1 |
Adapted from and used with permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original and primary source for this information is the AJCC Cancer Staging Manual, 8th Edition (2017), published by Springer International Publishing (Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the Skin. In: Amin AB, Edge SB, Greene, FL, et al. (Eds). AJCC Cancer Staging Manual. 8th Ed. New York: Springer; 2017:563–585).
Adapted from and used with permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original and primary source for this information is the AJCC Cancer Staging Manual, 7th Edition (2009), published by Springer Verlag (Balch CM, Gershenwald JE, Soong SJ, et al. Melanoma of the Skin. In: Edge SB, Byrd D, Compton C, et al. (Eds). AJCC Cancer Staging Manual. 7th Ed. New York: Springer; 2009: 325–344).
Table 5.
| 8th Edition | 7th Edition | |||||
|---|---|---|---|---|---|---|
| Pathological Stage Group | T | N | M | T | N | M |
| IIIA | T1a/b-T2a | N1a or N2a | M0 | T1–4a | N1a or N2a | M0 |
| IIIB | T0 | N1b, N1c | M0 | – | – | – |
| IIIB | T1a/b-T2a | N1b/c or N2b | M0 | T1–4b | N1a or N2a | M0 |
| IIIB | T2b/T3a | N1a-N2b | M0 | T1–4a | N1b, N2b, or N2c | M0 |
| IIIC | T0 | N2b, N2c, N3b, or N3c | M0 | – | – | – |
| IIIC | T1a-T3a | N2c or N3a/b/c | M0 | T1–4b | N1b, N2b, or N2c | M0 |
| IIIC | T3b/T4a | Any N ≥N1 | M0 | Any T | N3 | M0 |
| IIIC | T4b | N1a-N2c | M0 | – | – | – |
| IIID | T4b | N3a/b/c | M0 | – | – | – |
–, No counterpart in AJCC 7th Edition.
Adapted from and used with permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original and primary source for this information is the AJCC Cancer Staging Manual, 8th Edition (2017), published by Springer International Publishing (Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the Skin. In: Amin AB, Edge SB, Greene, FL, et al. (Eds). AJCC Cancer Staging Manual. 8th Ed. New York: Springer; 2017:563–585).
Adapted from and used with permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original and primary source for this information is the AJCC Cancer Staging Manual, 7th Edition (2009), published by Springer Verlag (Balch CM, Gershenwald JE, Soong SJ, et al. Melanoma of the Skin. In: Edge SB, Byrd D, Compton C, et al. (Eds). AJCC Cancer Staging Manual. 7th Ed. New York: Springer; 2009: 325–344.
2.1. Changes to the T category criteria
For the analyses of the international melanoma database which informed the eighth edition AJCC staging system, patients with primary melanoma without evidence of regional or distant metastatic disease were stratified into 8 T subcategories (T1a-T4b) (Table 1, Figure 1). Patients with T1 melanomas were included in these analyses if they had clinical or pathological T1N0 melanomas. Patients with T2-T4 melanomas were included only if they underwent lymphatic mapping and sentinel lymph node (SLN) biopsy and had no tumor-containing SLNs, and no microsatellites, satellites, or in-transit metastases at diagnosis or upon completion of initial treatment (pN0 melanoma).
Figure 1.
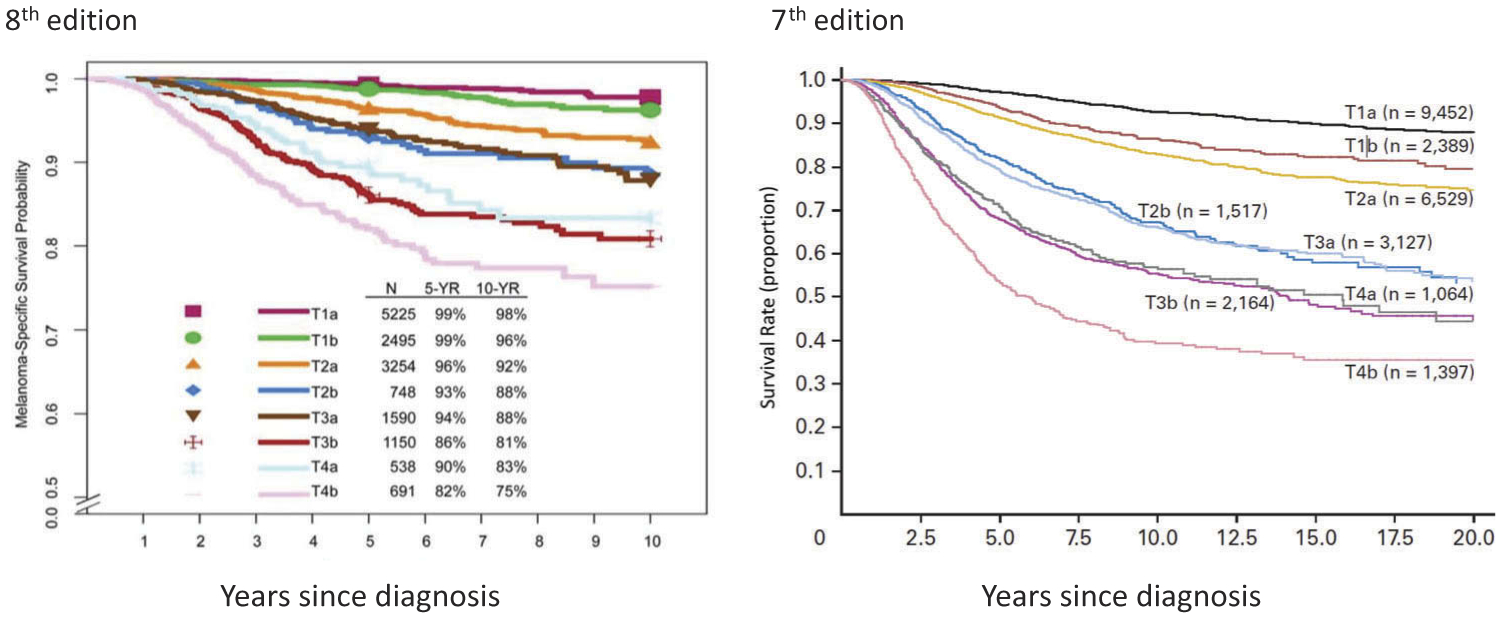
Comparison of AJCC seventh edition and AJCC eighth edition staging systems: melanoma-specific survival of patients with stage I and II melanoma by T subcategory. All patients with T1N0 melanoma were included. Patients with T2 to T4 melanoma were included only if they had negative sentinel lymph nodes. aWith permission from Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; 67:472–491.bWith permission from Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206.
Primary tumor (Breslow) thickness [13] and ulceration [14,15] continue to represent important prognostic factors for survival and define T-category strata in cutaneous melanoma. In the eighth edition, tumor thickness is to be measured to the nearest 0.1mm, not the nearest 0.01 mm (as in prior editions). In the eighth edition, the T category continues to be defined by melanoma thickness thresholds of 1.0, 2.0, and 4.0 mm. Thus, tumors measuring from 0.95 to 1.04 mm would be rounded to 1.0 mm (i.e. T1b) in the eighth edition. Previously, a subset of these melanomas measuring 1.01–1.04 would have been staged as T2 (a: w/o ulceration, b: with ulceration) in the seventh edition [9,10]. The clinical implication, if any, of this small subset of patients who are down-staged under the eighth edition, has not yet been formally explored [16].
Prior studies have suggested a clinically relevant threshold in the region of 0.7–0.8 mm in patients with T1 melanoma [14,16]. In the eighth edition AJCC analyses of the T1 melanoma patient cohort, multivariable analyses of factors predictive of melanoma-specific survival (MSS) [i.e. tumor thickness, ulceration, mitotic rate as a dichotomous variable (<1 mitosis/mm2 vs ≥1 mitosis/mm2)] found that tumor thickness dichotomized as <0.8 mm and 0.8–1.0 mm and ulceration were better predictors of MSS than mitotic rate (as a dichotomous variable) [10,11]. Thus, the definitions of T1a and T1b have been revised such that T1a melanomas include those <0.8 mm without ulceration while T1b melanomas include those 0.8–1 mm with or without ulceration and those <0.8 mm with ulceration.
Although mitotic rate, defined as mitoses per square millimeter, remains a major determinant of prognosis in melanomas of all thickness categories [17–21], it is no longer used as a T-category criterion in the eighth edition of the AJCC melanoma staging system but should be documented for all patients [10].
2.2. Changes to the N category criteria
In the eighth edition staging system, the N category reflects the number and extent of tumor-involved regional nodes, and extent of non-nodal regional metastasis (Table 2, Figure 2). Regional lymph nodes represent the most common first site of metastasis in patients with primary melanoma. Patients without clinical or radiographic evidence of regional lymph node metastasis but who have tumor-involved regional nodal metastasis found at SLN biopsy are defined as having ‘clinically occult’ nodal metastasis (termed ‘microscopic’ nodal metastasis in the seventh edition). Those with ‘clinically detected’ nodal metastasis are patients with tumor-involved regional lymph nodes detected by clinical or radiographic examination (termed ‘macroscopic’ nodal metastasis in the seventh edition).
Table 2.
| 8th Edition | 7th Edition | |||
|---|---|---|---|---|
| N Category | Number of tumor-involved regional lymph nodes and nodal metastatic burden | Presence of in-transit, satellite, and/or microsatellite metastases | Number of tumor-involved regional lymph nodes and nodal metastatic burden | Presence of in-transit, satellite, and/or microsatellite metastases |
| NX | Regional nodes not assessed (e.g. SLNB not performed, regional nodes previously removed for another reason) Exception: pathological N category is not required for T1 melanomas, use cN. |
No | Regional nodes not assessed (e.g. SLNB not performed, regional nodes previously removed for another reason) | No |
| N0 | No regional metastases detected | No | 0 | No |
| N1 | 1 tumor-involved node or in-transit, satellite, and/or microsatellite metastases with no tumor-involved nodes | 1 | No | |
| N1a | 1 clinically occult (i.e. detected by SLNB) | No | 1 with micrometastasis (detected by SLNB) | No |
| N1b | 1 clinically detected | No | 1 with macrometastasis (clinically detectable and pathologically confirmed) | No |
| N1c | No regional lymph node disease | Yes | – | – |
| N2 | 2 or 3 tumor-involved nodes or in-transit, satellite, and/or microsatellite metastases with 1 tumor-involved node | |||
| N2a | 2 or 3 clinically occult (i.e. detected by SLNB) | No | 2–3 with micrometastasis (detected by SLNB) | No |
| N2b | 2 or 3, at least 1 of which was clinically detected | No | 2–3 with macrometastasis (clinically detectable and pathologically confirmed) | No |
| N2c | 1 clinically occult or clinically detected | Yes | 0 | In transit metastases or satellites without metastatic nodes |
| N3 | ≥4 tumor-involved nodes or in-transit, satellite, and/or microsatellite metastases with ≥2 tumor-involved nodes, or any number of matted nodes without or with in-transit, satellite, and/or microsatellite metastases | ≥4 metastatic nodes, or matted nodes, or in transit metastases/satellites with metastatic nodes | ||
| N3a | ≥4 clinically occult (i.e. detected by SLNB) | No | – | – |
| N3b | ≥4, at least 1 of which was clinically detected, or presence of any number of matted nodes | No | – | – |
| N3c | ≥2 clinically occult or clinically detected and/or presence of any number of matted nodes | Yes | – | – |
SLNB, sentinel lymph node biopsy; –, No counterpart in AJCC 7th Edition.
Adapted from and used with permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original and primary source for this information is the AJCC Cancer Staging Manual, 8th Edition (2017), published by Springer International Publishing (Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the Skin. In: Amin AB, Edge SB, Greene, FL, et al. (Eds). AJCC Cancer Staging Manual. 8th Ed. New York: Springer; 2017:563–585).
Adapted from and used with permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original and primary source for this information is the AJCC Cancer Staging Manual, 7th Edition (2009), published by Springer Verlag (Balch CM, Gershenwald JE, Soong SJ, et al. Melanoma of the Skin. In: Edge SB, Byrd D, Compton C, et al. (Eds). AJCC Cancer Staging Manual. 7th Ed. New York: Springer; 2009: 325–344.
Figure 2.
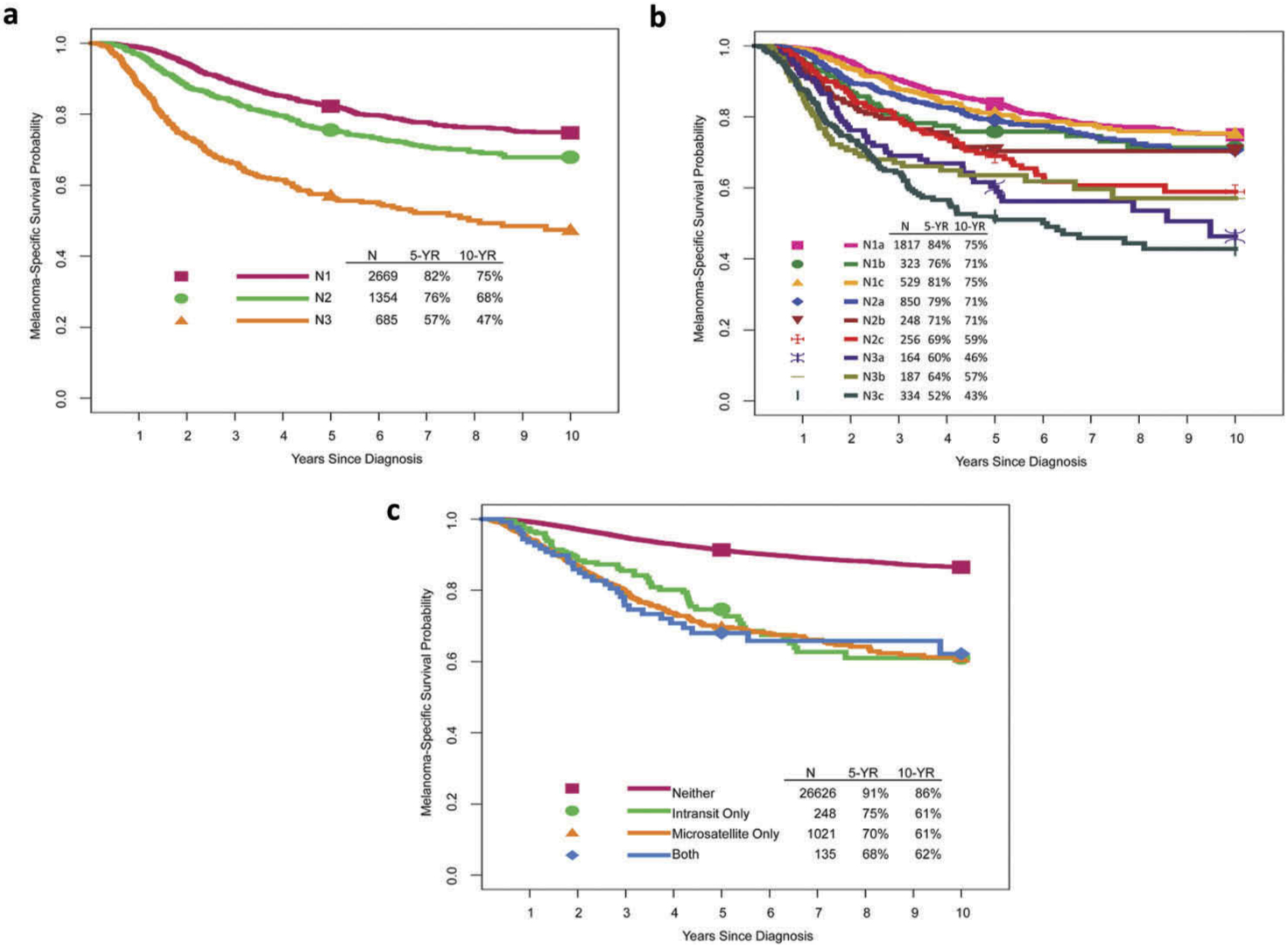
Melanoma-specific survival by (A, B) N categories and (C) presence or absence of microsatellites, satellites, and/or in-transit metastases. With permission from Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; 67:472–491.
Patients with clinically occult nodal metastasis represent the majority of patients with regional metastasis at diagnosis [10] and generally have better survival than those with clinically evident disease (Figure 2b) [22–26]. In these patients, nodal status is a dominant independent predictor of survival [11]. Thus, lymphatic mapping and SLN biopsy constitute important components of melanoma staging to identify occult regional lymph node (stage III) disease among patients who present with clinical stage IB or II cutaneous melanoma.
The number of tumor-involved lymph nodes is also an important predictor of survival (Figure 2b) [11]. Until recently, completion lymph node dissection (CLND) has generally been recommended and performed for patients with a positive SLN biopsy, based in part on results of the Multicenter Selective Lymphadenectomy Trial (MSLT-I) [27], as pathological information from the CLND may contribute important nodal staging and prognostic information to further guide clinical decision-making with respect to adjuvant systemic therapy. However, the recently reported results of the DeCOG-SLT [28] and MSLT-II [29] trials have already been practice-changing [30]. These two multicenter randomized controlled trials were designed to address whether immediate CLND improves survival in patients with clinically occult nodal regional node metastasis compared to nodal observation; neither study demonstrated an overall survival difference. Future staging systems and prognostic models will likely need to be revised as fewer immediate CLNDs are performed for patients with a positive SLN going forward with the resultant loss of CLND-associated staging and prognostic information in order to better guide clinical decision-making regarding adjuvant systemic therapies.
The presence of non-nodal regional (microsatellite, satellite, or in-transit) metastases have been associated with adverse prognosis [31–34] and also represent an N-category criterion in the eighth Edition AJCC staging system (Table 2). Microsatellites are defined as any microscopic focus of metastatic tumor cells in the skin or subcutis adjacent or deep to but discontinuous from the primary tumor [10]. Satellite metastases are classically defined as any foci of clinically evident cutaneous and/or subcutaneous metastases occurring within 2 cm of but discontinuous from the primary melanoma. In-transit metastases are classically defined as clinically evident cutaneous and/or subcutaneous metastases occurring >2 cm from the primary melanoma in the region between the primary melanoma and the regional lymph node basin. In the eighth edition analyses, microsatellites, satellites, and in-transit metastases are associated with similar survival outcomes and were grouped together for staging purposes (Figure 2c).
2.3. Changes to the M category criteria
Patients with stage IV melanoma have historically had poor prognosis with median survival from time of initial stage IV diagnosis of 6–7.5 months and 5-year survival of <10% [35–37]. However, since the introduction of the seventh edition AJCC staging system in 2010, the landscape of treatment options and prognosis for patients with stage IV melanoma has and continues to rapidly evolve with marked gains made. The Melanoma Expert Panel concluded that it was premature to perform a broad-based analytic initiative based on new data from patients treated in recent years for the eighth edition AJCC staging system. In the eighth edition, no M stage subgroups were proposed, although revisions to the M category have been implemented as described below.
The site of distant metastases remains the primary component of the M category (Table 3). The M category definitions are based both on site of distant metastatic disease and serum lactate dehydrogenase (LDH) level. Patients with non-visceral distant metastasis (distant cutaneous, subcutaneous, nodal) are categorized as M1a and have a relatively better prognosis than those with distant metastases to other sites [35,38,39]. Those with lung metastasis are categorized as M1b and have an intermediate prognosis. Patients with non-central nervous system (CNS) visceral metastases have worse prognosis and are categorized as M1c. M1c no longer includes CNS metastasis. A new M1d designation was added that encompasses patients with distant metastasis to the CNS with or without any other distant sites of disease to reflect the poor prognosis of these patients [40,41] and to facilitate clinical trial design and analysis.
Descriptors have been added to each M1 subcategory to designate serum LDH level (‘0’ for ‘not elevated’ and ‘1’ for ‘elevated’). Although LDH remains an adverse predictor of survival [42–48], elevated LDH no longer automatically categorizes a patient as M1c.
2.4. Changes to clinical stage groups
Definitions of clinical stage groups are unchanged between the seventh and eighth edition AJCC melanoma staging systems (Table 4). In the eighth edition, clinical staging includes microstaging after biopsy of the primary melanoma and following clinical and radiographic evaluation (and biopsies as appropriate) for regional and distant metastatic disease.
2.5. Changes to pathological stage I and II subgroups
With respect to pathological stage I and II subgroupings, these remain largely unchanged between the seventh and eighth edition AJCC staging systems (Table 5, Figure 3). The exception is that the definition of stage IA and IB subgroups are refined such that patients with pathological T1bN0M0 melanoma are included in the pathological stage IA subgroup and not the pathological stage IB subgroup as in the seventh edition. This change reflects the overall better prognosis of patients with T1b melanoma with pathologically negative nodes compared to patients with T1b melanoma with clinically negative nodes (some of whom will have pathological positive nodes). Five-year and 10-year MSS rates in the former (pT1bN0M0) group is better (99% and 96%, respectively) compared to the latter (cT1bN0M0) group (97% and 93%, respectively) [11].
Figure 3.
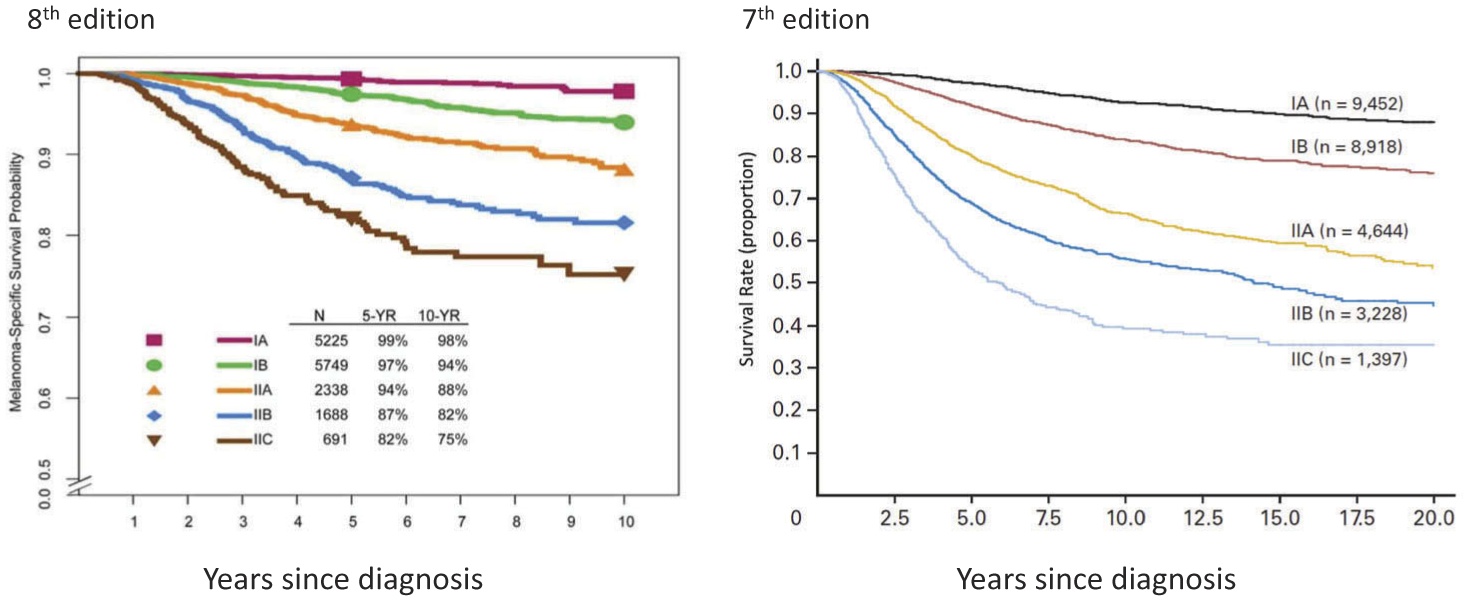
Comparison of AJCC seventh edition and AJCC eighth edition staging systems: melanoma-specific survival of patients by stage I and II subgroups. aWith permission from Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; 67:472–491.bWith permission from Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206.
2.6. Changes to pathological stage III subgroups
In the seventh edition AJCC staging system, stage III subgroups were defined by both primary tumor ulceration and regional lymph node factors (number of nodes involved, microscopic vs macroscopic node involvement). For the eighth edition analysis, the Melanoma Expert Panel tested the hypothesis that more accurate prognostic stage subgroups could be obtained by both T category (i.e. adding tumor thickness along with ulceration) and N-category (number of tumor-involved lymph nodes, whether they were clinically detected or clinically occult, and the presence of microsatellite, satellite, and/or in-transit metastases) factors. Based on these analyses, the Melanoma Expert Panel stratified patients with stage III melanoma into 4 subgroups in the eighth edition (Figures 4 and 5).
Figure 4.
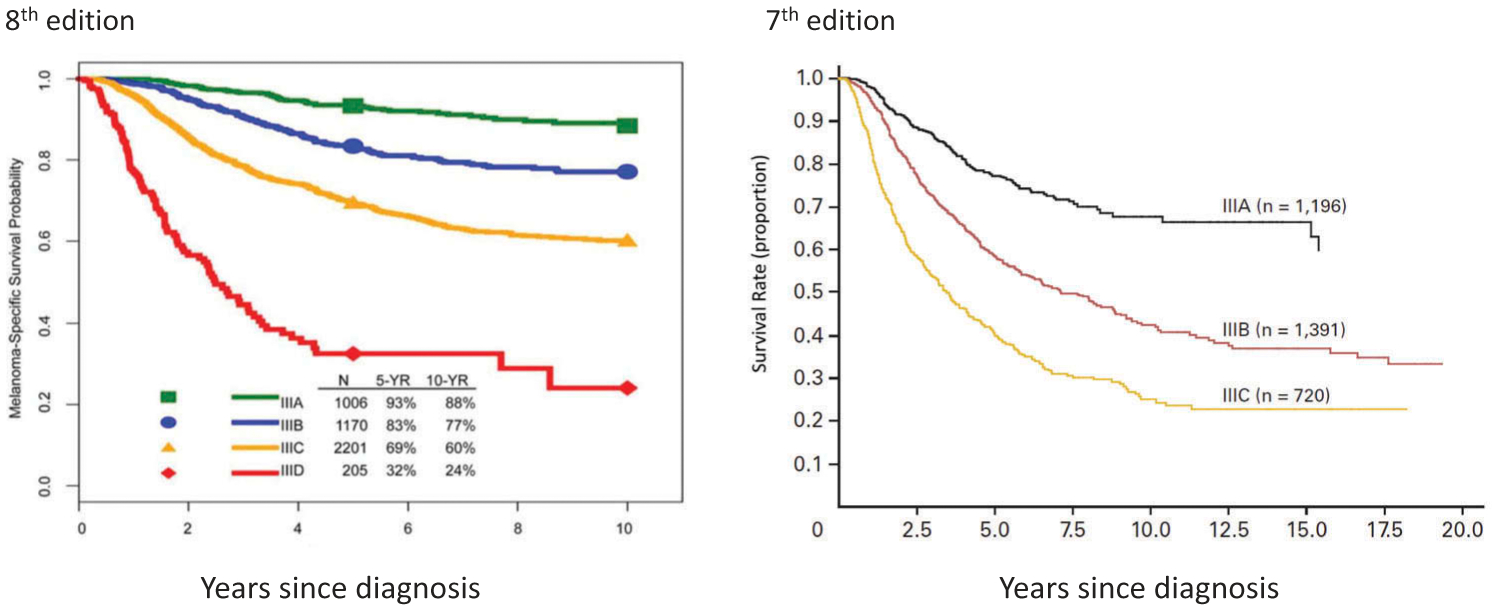
Comparison of AJCC seventh edition and AJCC eighth edition staging systems: melanoma-specific survival of patients by stage III subgroups. aWith permission from Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; 67:472–491.bWith permission from Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206.
Figure 5.
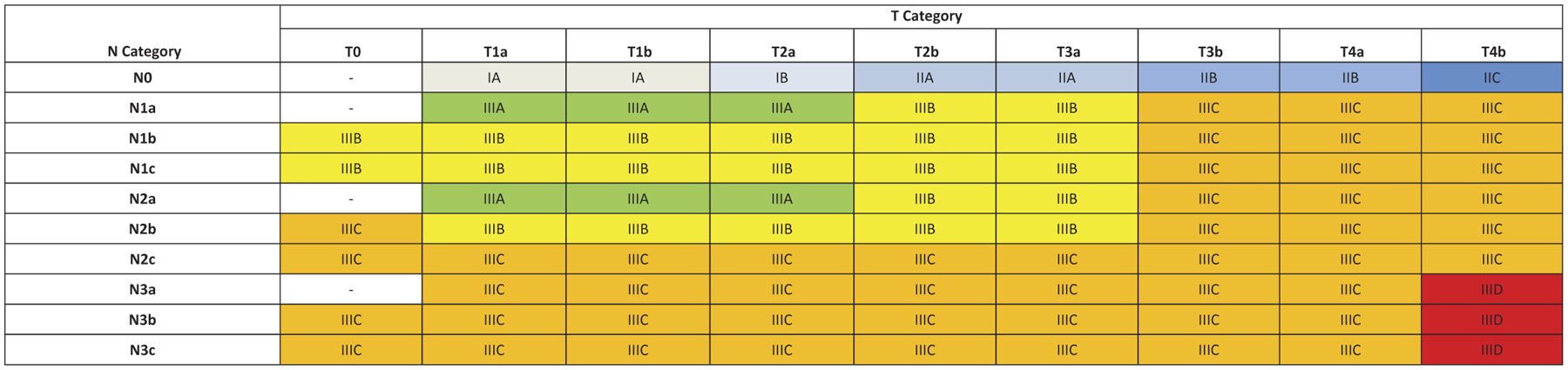
AJCC eighth edition pathological prognostic groups (TNM) for stage I to IV cutaneous melanoma. T0 – no evidence of primary tumor (e.g. unknown primary or completely regressed melanoma); Tis – melanoma in situ; TX – thickness cannot be assessed; NX – Regional nodes not assessed (e.g. SLN biopsy not performed, regional nodes previously removed for another reason). Exception: pathological N category is not required for T1 melanoma, use cN. *Pathological stage is IV for Any T, any N and M1 disease. aAdapted and used with permission from Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; 67:472–491.
2.7. Pathological stage IV group
Although changes were made to the M category criteria in the eighth edition (see above section 2.3), there are no stage subgroups for patients with distant (stage IV) melanoma metastasis.
2.8. Staging patients following neoadjuvant therapy
Neoadjuvant therapy is being increasingly explored in patients with locoregionally advanced and oligo-metastatic melanoma and in subsets of patients in early phase clinical trials enabled surgical resection [49–55]. Results such as these have generated much excitement about developing neoadjuvant strategies for melanoma patients with locally advanced and metastatic disease. To stage patients following neoadjuvant treatment, the eighth edition AJCC staging system includes approaches to classification after definitive systemic or radiation therapy (ycTNM) or after neoadjuvant therapy followed by planned surgery (ypTNM) [56].
2.9. Staging patients following recurrence and/or retreatment
The eighth edition AJCC staging system also includes a classification schema for patients who recur (rTNM) that is divided into ‘r-clinical’ (rcTNM) and ‘r-pathological’ (rpTNM) and which will potentially facilitate improved characterization of an individual’s extent of disease during their melanoma disease course [56].
3. Expert commentary
Overall, the contemporary patient cohort examined in the eighth edition AJCC analyses had higher survival stage for stage compared to those for the sixth and seventh editions (Figures 1, 3, and 4). This is not only due to more accurate nodal staging and risk stratification, but also to changes in the definitions of TNM and pathological stage groupings in the eighth edition AJCC staging system. Here we discuss some of the implications of the eighth edition AJCC staging system for cutaneous melanoma.
3.1. Implications of changes to the T category criteria
Among the differences in the T category criteria between the seventh and eighth editions, the changes in the definitions of T1a and T1b may potentially lead to greater number of patients undergoing SLN biopsy. In the eighth edition AJCC staging system, patients with T1b melanoma include many who in the seventh edition would have previously been classified as T1a. In the seventh edition, patients with melanomas of Breslow thickness 0.75 mm to 1.00 mm without ulceration were classified as T1a. The same patients in the eighth edition are now described as having Breslow thickness 0.8 mm to 1.0 mm without ulceration, are classified as T1b to reflect their worse MSS and increased risk of SLN metastasis (T1b 5–12% vs T1a <5%) [11,57–60] compared to patients with nonulcerated melanomas <0.8 mm (T1a) and should be offered SLN biopsy.
3.2. Implications of changes to the N category criteria and stage III subgroups
As in the seventh edition, there was significant heterogeneity in prognosis for those with stage III regional disease by N category designation in the eighth edition patient cohort (Figures 2 and 4). In the eighth edition, the Melanoma Expert Panel added further granularity throughout the N category by providing clarity of definitions, and increased subcategories from 5 to 9 to reflect factors associated with patient prognosis: (1) extent of regional node tumor involvement [clinically occult (N1a, N2a, N3a) vs clinically detected (N1b, N2b, N3b)], (2) number of tumor-involved regional nodes, and (3) presence of microsatellites, satellites, or in-transit metastases (N1c, N2c, N3c).
In the eighth edition, 4 stage III subgroups were created (compared to 3 in the seventh edition) with additional contributions from primary tumor features and extent of regional node tumor involvement (Table 5). For example, in the seventh edition, patients with up to 3 clinically occult tumor-involved regional lymph nodes and melanoma of any Breslow thickness were either stage IIIA or IIIB depending on presence or absence of primary melanoma ulceration. In the eighth edition, patients with up to 3 clinically occult tumor-involved regional lymph nodes may be IIIA, IIIB, or IIIC depending on primary tumor thickness and presence/absence of ulceration.
In the eighth edition AJCC analyses, patients with stage III had widely variable prognosis, ranging from 93% 5-year MSS for stage IIIA to 32% for stage IIID disease (Figure 4) [11]. In comparison, patients with stage III disease in the seventh edition had overall worse prognosis with 5-year MSS for stage IIIA, IIIB, and IIIC disease of 78%, 59%, and 40%, respectively [8]. These significant differences in prognosis, particularly of patients with stage IIIA and IIIB disease between the seventh and eighth edition staging systems, has important implications for clinical decision, patient counseling, and risk stratifying patients for consideration of possible adjuvant therapy. When interpreting adjuvant therapy clinical trials [6,7,61–64], one must be cognizant that trial participants with stage IIIA/B/C (as defined by the seventh edition staging system) are at higher risk and have worse prognosis than patients with similar stage III subgroup as defined by the eighth edition staging system.
3.3. Implications of changes to the M category criteria
In the eighth edition AJCC staging system, stage IV patients are categorized by site of disease (M1a: non-visceral distant cutaneous, subcutaneous, or nodal sites; M1b: lung; M1c: non-CNS visceral sites; and M1d: CNS sites) (Table 3). Given the poor prognosis associated with the development of CNS metastases in melanoma patients, this group of patients have often been excluded from some clinical trials, while in other studies the presence of CNS disease has been used as a criterion for protocol inclusion and/or stratification [2,6–7,64–71]. With the addition of the new M1d designation to describe patients with distant metastasis to the CNS with or without other distant sites of disease, the eighth edition staging system not only better reflects the poorer prognosis of patients with CNS metastasis but will also facilitate clinical trial design and analysis.
4. Five-year view
A thorough knowledge and understanding of prognostic factors and staging of cutaneous melanoma is crucial for initial patient assessment, treatment planning and sequencing, in the development of surveillance strategies, and for clinical trial design and analysis. The eighth edition of the AJCC melanoma staging system is a standardized and contemporary staging system to facilitate patient risk stratification and guide treatment. Recently reported clinical trials of adjuvant targeted and immune checkpoint therapies in patients with stage III and IV melanoma [6,7] as well as of immediate CLND versus nodal observation in patients with sentinel-node metastasis [29] are practice-changing. Looking ahead, fewer immediate CLNDs will likely be performed, resulting in decrease in staging and prognostic information. Clinical decisions regarding adjuvant systemic therapies will need to be made in the absence of CLND-associated staging and prognostic information. Future staging systems and prognostic models will need to be revised to reflect these changes.
Key issues.
Staging has significant impact on prognostic assessment, treatment decision-making, and clinical trial planning, design, and analysis.
The AJCC melanoma staging system currently represents the most widely accepted approach to staging and classification at initial diagnosis. The eighth edition was implemented nationwide in the United States on January 1, 2018.
Primary tumor thickness and ulceration continue to be important prognostic factors for survival and define T-category strata in the eighth edition AJCC staging system. Mitotic rate is no longer used as a T-category criterion, although it should be documented for all patients
The N category reflects both the number and extent of tumor-involved regional nodes as well as extent of non-nodal regional metastasis.
Stage III groupings are based on both T and N category criteria and increased from three to four subgroups
The site of distant metastases remains the primary component of the M category. A new M1d designation was added to designate metastasis to the CNS and reflects the poor prognosis of these patients.
DeCOG-SLT and MSLT-II are landmark trials that reported no difference in MSS comparing patients with sentinel-node metastasis who underwent immediate CLND versus nodal observation. These results are practice-changing and may lead to fewer immediate CLND and loss of valuable staging and prognostic information. Future staging system and prognostic models will need to reflect such changes in practice.
Funding
This manuscript has been supported in part by The Michael and Patricia Booker Research Endowment; The Robert and Lynne Grossman Family Foundation; and The University of Texas MD Anderson Cancer Center Melanoma Moon Shots Program.
Footnotes
Declaration of interest
JE Gershenwald has served on advisory boards for Merck, Syndax, and Castle Biosciences, unrelated to the content of this article. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Tripp MK, Watson M, Balk SJ, et al. State of the science on prevention and screening to reduce melanoma incidence and mortality: the time is now. CA Cancer J Clin. 2016;66:460–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 5.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–1835. [DOI] [PubMed] [Google Scholar]; • Phase 3, randomized, double-blind trial demonstrating improved recurrence-free survival among patients undergoing resection of stage IIIB, IIIC, or IV melanoma with adjuvant nivolumab compared to adjuvant ipilimumab.
- 7.Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–1823. [DOI] [PubMed] [Google Scholar]; • Phase 3, randomized, double-blind trial demonstrating lower risk of recurrence in patients with stage III melanoma with BRAF V600E or V600k mutations with adjuvant use of combination dabrafenib plus trametinib compared to placebo.
- 8.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This publication from the AJCC Melanoma Staging Committee presents the AJCC seventh edition melanoma staging system and associated analyses.
- 9.Balch CM, Soong SJ, Gershenwald JE, et al. Melanoma of the skin Edge S, Byrd D, Compton C, et al. eds. AJCC Cancer Staging Manual. 7th ed. New York: Springer Verlag; 2009:325–344. [Google Scholar]; • This is the AJCC seventh edition chaper on the staging of cutaneous melanoma.
- 10.Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the Skin Amin M, Edge SB, Greene FL, et al. eds. AJCC Cancer Staging Manual. 8th ed. Switzerland: Springer; 2017:563–585. [Google Scholar]; •• This is the AJCC eighth edition chaper on the staging of cutaneous melanoma. It was implemented nationwide in the United States and in many other regions January 2018.
- 11.Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This publication from members of the AJCC Melanoma Expert Panel and the International Melanoma Database and Discovery Platform presents and discusses the survival analyses of a contemporary international stages I-III database that informed the AJCC eighth edition melanoma staging system and highlights changes from the AJCC seventh edition AJCC staging system.
- 12.Keung E, Balch C, Gershenwald J, et al. Key changes in the AJCC eighth edition melanoma staging system. Melanoma Lett. 2018;36(1):1–9. [Google Scholar]
- 13.Breslow A Tumor thickness, level of invasion and node dissection in stage I cutaneous melanoma. Ann Surg. 1975;182(5):572–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green AC, Baade P, Coory M, et al. 20-year survival among people diagnosed with thin melanomas in Queensland, Australia. J Clin Oncol. 2012;30(13):1462–1467. [DOI] [PubMed] [Google Scholar]
- 15.In ‘t Hout FE, Haydu LE, Murali R, Bonenkamp JJ, et al. Prognostic importance of the extent of ulceration in patients with clinically localized cutaneous melanoma. Ann Surg. 2012;255(6):1165–1170. [DOI] [PubMed] [Google Scholar]
- 16.Gimotty PA, Elder DE, Fraker DL, et al. Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J Clin Oncol. 2007;25(9):1129–1134. [DOI] [PubMed] [Google Scholar]
- 17.Nagore E, Oliver V, Botella-Estrada R, et al. Prognostic factors in localized invasive cutaneous melanoma: high value of mitotic rate, vascular invasion and microscopic satellitosis. Melanoma Res. 2005;15(3):169–177. [DOI] [PubMed] [Google Scholar]
- 18.Busam KJ. The prognostic importance of tumor mitotic rate for patients with primary cutaneous melanoma. Ann Surg Oncol. 2004;11(4):360–361. [DOI] [PubMed] [Google Scholar]
- 19.Nagarajan P, Curry JL, Ning J, et al. Tumor thickness and mitotic rate robustly predict melanoma-specific survival in patients with primary vulvar melanoma: a retrospective review of 100 cases. Clin Cancer Res. 2017;23(8):2093–2104. [DOI] [PubMed] [Google Scholar]
- 20.Mandalà M, Galli F, Cattaneo L, et al. Mitotic rate correlates with sentinel lymph node status and outcome in cutaneous melanoma greater than 1 millimeter in thickness: a multi-institutional study of 1524 cases. J Am Acad Dermatol. 2017;76(2):264–273. [DOI] [PubMed] [Google Scholar]
- 21.Thompson JF, Soong SJ, Balch CM, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. J Clin Oncol. 2011;29(16):2199–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cascinelli N, Belli F, Santinami M, et al. Sentinel lymph node biopsy in cutaneous melanoma: the WHO melanoma program experience. Ann Surg Oncol. 2000;7(6):469–474. [DOI] [PubMed] [Google Scholar]
- 23.Van Akkooi ACJ, Nowecki ZI, Voit C, et al. Sentinel node tumor burden according to the Rotterdam criteria is the most important prognostic factor for survival in melanoma patients: a multicenter study in 388 patients with positive sentinel nodes. Ann Surg. 2008;248(6):949–955. [DOI] [PubMed] [Google Scholar]
- 24.Starz H, Balda BR, Krämer KU, et al. A micromorphometry-based concept for routine classification of sentinel lymph node metastases and its clinical relevance for patients with melanoma. Cancer. 2001;91(11):2110–2121. [PubMed] [Google Scholar]
- 25.Scolyer RA, Li L-XL, McCarthy SW, et al. Micromorphometric features of positive sentinel lymph nodes predict involvement of nonsentinel nodes in patients with melanoma. Am J Clin Pathol. 2004;122(4):532–539. [DOI] [PubMed] [Google Scholar]
- 26.Balch CM, Gershenwald JE, Soong SJ, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28(14):2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17(6):757–767. [DOI] [PubMed] [Google Scholar]
- 29.Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Phase 3, randomized, multicenter trial (MSLT-II) that evaluated the potential survival benefit of completion lymph node dissection in patients with melanoma and sentinel-node metastases versus nodal observation reported an increased rate of regional disease control but not increased melanoma-specific survival among patients who underwent immediate completion lymph node dissection.
- 30.Wong SL, Faries MB, Kennedy EB, et al. Sentinel lymph node biopsy and management of regional lymph nodes in melanoma: American society of clinical oncology and society of surgical oncology clinical practice guideline update. J Clin Oncol. 2018;36(4):399–413. [DOI] [PubMed] [Google Scholar]; • Evidence-based guideline from the American Society of Clinical Oncology and Society of Surgical Oncology on the use of lymphatic mapping and sentinel lymph node biopsy in staging patients with newly diagnosed primary cutaneous melanoma.
- 31.Read RL, Haydu L, Saw RPM, et al. In-transit melanoma metastases: incidence, prognosis, and the role of lymphadenectomy. Ann Surg Oncol. 2015;22(2):475–481. [DOI] [PubMed] [Google Scholar]
- 32.Rao UNM, Ibrahim J, Flaherty LE, et al. Implications of microscopic satellites of the primary and extracapsular lymph node spread in patients with high-risk melanoma: pathologic corollary of Eastern Cooperative Oncology Group Trial E1690. J Clin Oncol. 2002;20(8):2053–2057. [DOI] [PubMed] [Google Scholar]
- 33.Wilmott J, Haydu L, Bagot M, et al. Angiotropism is an independent predictor of microscopic satellites in primary cutaneous melanoma. Histopathology. 2012;61(5):889–898. [DOI] [PubMed] [Google Scholar]
- 34.Van Es SL, Colman M, Thompson JF, et al. Angiotropism is an independent predictor of local recurrence and in-transit metastasis in primary cutaneous melanoma. Am J Surg Pathol. 2008;32(9):1396–1403. [DOI] [PubMed] [Google Scholar]
- 35.Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181(3):193–201. [PubMed] [Google Scholar]
- 36.Manola J, Atkins M, Ibrahim J, et al. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol. 2000;18(22):3782–3793. [DOI] [PubMed] [Google Scholar]
- 37.Unger JM, Flaherty LE, Liu PY, et al. Gender and other survival predictors in patients with metastatic melanoma on Southwest Oncology Group trials. Cancer. 2001;91(6):1148–1155. [DOI] [PubMed] [Google Scholar]
- 38.Brand CU, Ellwanger U, Stroebel W, et al. Prolonged survival of 2 years or longer for patients with disseminated melanoma. An analysis of related prognostic factors. Cancer. 1997;79(12):2345–2353. [PubMed] [Google Scholar]
- 39.Cochran AJ, Bhuta S, Paul E, et al. The shifting patterns of metastatic melanoma. Clin Lab Med. 2000;20(4):759–783. [PubMed] [Google Scholar]
- 40.Staudt M, Lasithiotakis K, Leiter U, et al. Determinants of survival in patients with brain metastases from cutaneous melanoma. Br J Cancer. 2010;102(8):1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117(8):1687–1696. [DOI] [PubMed] [Google Scholar]
- 42.Kelderman S, Heemskerk B, Van Tinteren H, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother. 2014;63(5):449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Efficacy and safety in key patient subgroups of nivolumab (NIVO) alone or combined with ipilimumab (IPI) versus IPI alone in treatment-naive patients with advanced melanoma (MEL) (CheckMate 067). Eur J Cancer. 2015;51(Supp 3):S664–665. [Google Scholar]
- 44.Long GV, Weber JS, Infante JR, et al. Overall survival and durable responses in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib combined with trametinib. J Clin Oncol. 2016;34(8):871–878. [DOI] [PubMed] [Google Scholar]
- 45.Weide B, Martens A, Hassel JC, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. 2016;22(22):5487–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016;17(12):1743–1754. [DOI] [PubMed] [Google Scholar]
- 47.Nosrati A, Tsai KK, Goldinger SM, et al. Evaluation of clinicopatho-logical factors in PD-1 response: derivation and validation of a prediction scale for response to PD-1 monotherapy. Br J Cancer. 2017;116(9):1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menzies AM, Wilmott JS, Drummond M, et al. Clinicopathologic features associated with efficacy and long-term survival in metastatic melanoma patients treated with BRAF or combined BRAF and MEK inhibitors. Cancer. 2015;121(21):3826–3835. [DOI] [PubMed] [Google Scholar]
- 49.van Ziejl MCT, Van Den Eertwegh AJ, Haanen JB, et al. (Neo)adjuvant systemic therapy for melanoma. Eur J Surg Oncol. 2017;43(3):534–543. [DOI] [PubMed] [Google Scholar]
- 50.Keung EZ, Ukponmwan EU, Cogdill AP, et al. The rationale and emerging use of neoadjuvant immune checkpoint blockade for solid malignancies. Ann Surg Oncol. 2018;25(7):1814–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amaria RN, Prieto PA, Tetzlaff MT, et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: a single-centre, open-label, randomised, phase 2 trial. Lancet Oncol. 2018;19(2):181–193. [DOI] [PubMed] [Google Scholar]
- 52.Ascierto PA, Eggermont AMM. Neoadjuvant therapy in melanoma: the next step? Lancet Oncol. 2018;19(2):151–153. [DOI] [PubMed] [Google Scholar]
- 53.Jakub JW, Racz JM, Hieken TJ, et al. Neoadjuvant systemic therapy for regionally advanced melanoma. J Surg Oncol. 2018;117(6):1164–1169. [DOI] [PubMed] [Google Scholar]
- 54.Amaral T, Tampouri I, Garbe C. How to use neoadjuvant medical treatment to maximize surgery in melanoma. Expert Rev Anticancer Ther. 2018;18(2):121–130. [DOI] [PubMed] [Google Scholar]
- 55.Menzies AM, Rozeman E, Amaria RN, et al. Preliminary results from the international neoadjuvant melanoma consortium (INMC). J Clin Oncol. 2017;35(15):Suppl 9581. [Google Scholar]
- 56.Gress DM, Edge SB, Greene FL, et al. Principles of Cancer Staging In: Amin M, Edge SB, Greene FL, et al., eds. AJCC Cancer Staging Manual. 8th Switzerland: Springer; 2017. p. 3–30. [Google Scholar]
- 57.Andtbacka RHI, Gershenwald JE. Role of sentinel lymph node biopsy in patients with thin melanoma. J Natl Compr Canc Netw. 2009;7(3):308–317. [DOI] [PubMed] [Google Scholar]
- 58.Cordeiro E, Gervais M-K, Shah PS, et al. Sentinel lymph node biopsy in thin cutaneous melanoma: a systematic review and meta-analysis. Ann Surg Oncol. 2016;23(13):4178–4188. [DOI] [PubMed] [Google Scholar]
- 59.Han D, Zager JS, Shyr Y, et al. Clinicopathologic predictors of sentinel lymph node metastasis in thin melanoma. J Clin Oncol. 2013;31(35):4387–4393. [DOI] [PubMed] [Google Scholar]
- 60.Murali R, Haydu LE, Quinn MJ, et al. Sentinel lymph node biopsy in patients with thin primary cutaneous melanoma. Ann Surg. 2012;255(1):128–133. [DOI] [PubMed] [Google Scholar]
- 61.Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. [DOI] [PubMed] [Google Scholar]
- 62.Eggermont AMM, Chiarion-Sileni V, Grob -J-J, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eggermont AMM, The DR. complete overhaul of adjuvant therapies for high-risk melanoma and its consequences for staging and management of melanoma patients. Eur J Cancer. 2017;2017(86):101–105. [DOI] [PubMed] [Google Scholar]
- 64.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–465. [DOI] [PubMed] [Google Scholar]
- 65.Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–1876. [DOI] [PubMed] [Google Scholar]
- 69.Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095. [DOI] [PubMed] [Google Scholar]
- 70.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. [DOI] [PubMed] [Google Scholar]
- 71.Robert C, Thomas L, Bondarenko I. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. [DOI] [PubMed] [Google Scholar]


