Abstract
Background
The Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) revised their Clostridioides difficile infection (CDI) severity classification criteria in 2017 to include an absolute serum creatinine (SCr) value above a threshold (≥1.5 mg/dL) rather than a relative increase from baseline (≥1.5 times the premorbid level). To date, how to best define kidney injury as a CDI disease severity marker has not been validated to assess severe outcomes associated with CDI.
Methods
This multicenter cohort study included adult hospitalized patients with CDI. Patients were assessed for the presence of acute kidney injury (AKI), chronic kidney disease (CKD), and CDI severity using the 2010 and 2017 IDSA/SHEA CDI guidelines. Primary outcome was all-cause inpatient mortality.
Results
The final study cohort consisted of 770 CDI episodes from 705 unique patients aged 65 ± 17 years (female, 54%; CKD, 36.5%; AKI, 29.6%). Eighty-two episodes (10.6%) showed discordant severity classification results due to the inclusion of more patients with preexisting CKD in the severe disease category using an absolute SCr threshold criterion. The absolute SCr criterion better correlated with all-cause mortality (odds ratio [OR], 4.04; 95% confidence interval [CI], 1.76–9.28; P = .001) than the relative increase in SCr (OR, 1.34; 95% CI, 0.62–2.89; P = .46). This corresponded to an increased likelihood of the 2017 CDI severity classification criteria to predict mortality (OR, 5.33; 95% CI, 1.81–15.72; P = .002) compared with the 2010 criteria (OR, 2.71; 95% CI, 1.16–6.32; P = .02).
Conclusions
Our findings support the 2017 IDSA/SHEA CDI severity classification criteria of a single pretreatment SCr in future CDI guideline updates.
Keywords: acute kidney injury, Clostridium difficile, chronic kidney disease, diarrhea, serum creatinine
The changing criteria for kidney injury from a relative change to an absolute serum creatinine threshold adopted by the 2017 IDSA/SHEA Clostridioides difficile infection (CDI) guideline changed the disease severity classification for 82 of 770 patients (10.7%).
Clostridioides difficile infection (CDI) is the most common healthcare-associated infection in the United States, and it causes an estimated 12 800 deaths annually [1, 2]. Severity definitions for CDI are different, but they most commonly include elevated serum creatinine (SCr) values as a biomarker for kidney injury [3–8]. Elevated SCr values measure acute kidney injury (AKI) caused by CDI-induced diarrhea as well as preexisting chronic kidney disease (CKD), both of which have been associated with poor CDI outcomes including mortality [9–26]. The updated 2017 Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) CDI severity classification criteria include an absolute SCr value above a threshold (≥1.5 mg/dL) as opposed to a relative increase from baseline (≥1.5 times the premorbid level) previously recommended in the 2010 guideline [4, 6]. This change allowed clinicians to more easily classify patients at the point of care, because premorbid (baseline) SCr levels were unable to be assessed in approximately 13% of patients diagnosed with CDI, particularly in those with community-associated CDI [27]. The guideline panel noted that these revised criteria require validation and that they would not be able to detect AKI in patients with preexisting renal insufficiency [6]. However, the revised SCr criterion may have benefit by including patients with underlying CKD, which is a population at risk for poor outcomes.
For several years, our research group has conducted a clinical trial investigating CDI in hospitalized patients [28, 29]. Leftover stool samples were collected for strain typing, and corresponding clinical data, including SCr values, have been collected to allow determination of the 2010 and 2017 IDSA/SHEA CDI severity classification. This provided us the unique opportunity to validate the change in the SCr criterion between the 2010 and 2017 guidelines. The goals of this study were threefold: (1) describe the number of patients diagnosed with CDI that have concomitant AKI, CKD, and/or require chronic renal replacement therapy; (2) assess the impact of the revised SCr criterion on the number of CDI cases classified as severe; and (3) assess the ability of both the 2010 and 2017 IDSA/SHEA SCr criteria to predict inpatient mortality.
METHODS
Study Population
This multicenter cohort study was conducted using data collected from 2016 to 2018 in 2 large Houston, Texas area health systems (12 hospitals in total) as part of an ongoing clinical study of patients with CDI. Patients with positive C difficile toxin tests were identified from the clinical microbiology laboratory of the health system. The study included all patients ≥18 years of age with CDI who had documented values for baseline SCr, SCr within 24 hours of CDI diagnosis, and white blood cell (WBC) count within 24 hours of CDI diagnosis. Patients with diarrhea due to laxative use were excluded.
Patient Consent Statement
The study was approved by the University of Houston Committee for the Protection of Human Subjects with a waiver of informed consent (Instititutional Review Board study 00000128).
Definitions
Patients’ electronic medical records (EMRs) (Epic Systems Co., Verona, WI) were reviewed retrospectively for demographic information, underlying comorbidities, laboratory data, and clinical outcomes. Laboratory data, including albumin level, eosinophil count, SCr, temperature, and WBC count, were recorded as the most extreme value within 24 hours of CDI diagnosis. Hypoalbuminemia was defined as a serum albumin level <2.5 mg/dL [7], and eosinopenia was defined as an eosinophil count of 0.0 cells/μL [30]. The Charlson comorbidity index (CCI) score was calculated using comorbidities documented on or before the date of hospital admission [31]. Residence before admission was reported as home versus nonhome.
Patients were tested for CDI at the discretion of the treating physician and medical team. The standard-of-care C difficile diagnostic in all 12 hospitals during the majority of the study time frame was a nucleic acid amplification test (NAAT) in patients with unexplained and new-onset diarrhea (≥3 unformed stools in 24 hours) with the exception of 1 hospital system (4 hospitals) that changed to an enzyme-linked immunosorbent assay 2 months before the end of the study period. Recurrent CDI (rCDI) and healthcare facility-onset CDI (HO-CDI) cases were defined per the US Centers for Disease Control and Prevention Multidrug-Resistant Organism and Clostridioides difficile Infection (MDRO/CDI) module [32]. Two episodes of CDI in the same patient were considered to be distinct events if they occurred >8 weeks apart, and the same patient was eligible for inclusion multiple times. Clinical definitions of CDI severity were defined per the 2010 and 2017 IDSA/SHEA CDI guidelines [4, 6]. Because the criteria for severe, complicated CDI (fulminant CDI) did not change from 2010 to 2017, these patients were included in the severe group regardless of their SCr or WBC values.
Acute kidney injury and CKD were defined per the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [33, 34]. Glomerular filtration rate (GFR) was estimated using the 2009 CKD-EPI creatinine equation [34]. The need for chronic renal replacement therapy, defined as hemodialysis or peritoneal dialysis, was determined by searching the EMRs for documentation before hospital admission. Although KDIGO specifies that a decrease in GFR must be sustained for >3 months before the diagnosis of CKD can be confirmed, this study used a single SCr value to calculate GFR and classify CKD, and all patients requiring chronic renal replacement therapy before admission were classified as having KDIGO CKD category G5 regardless of their GFR. Baseline SCr levels were determined by choosing the most current SCr value within one year before the current hospital admission. Because data regarding the initiation of renal replacement therapy during hospitalization were not collected and urinary output was not consistently available for all patients, AKI determinations were based solely on the change in SCr from baseline. In addition, although KDIGO specifies that the increase in SCr from baseline must be known or presumed to have occurred within the prior 7 days, baseline SCr values were used regardless of time before CDI diagnosis.
Outcomes
The primary study outcome used to assess the correlation between the 2010 versus 2017 IDSA/SHEA CDI guideline severity classification criteria was all-cause inpatient mortality.
Sample Collection and Ribotyping
Leftover stool samples from patients diagnosed with CDI were collected and brought to a centralized research laboratory at the University of Houston. Stool samples were then plated onto C difficile-selective cefoxitin-cycloserine-fructose agar plates and anaerobically incubated for 48–72 hours. Colonies were identified as C difficile by polymerase chain reaction (PCR). Fluorescent PCR ribotyping was performed as previously described [35]. The library contains >100 known ribotypes (https://thewalklab.com/tools/) but does not distinguish between ribotypes 014 and 020, ribotypes 053 and 163, and ribotypes 078 and 126; therefore, these are reported as combined ribotypes (eg, 014-020, 053-163, and 078-126).
Statistical Analysis
For the primary outcome analyses, logistic regression models were developed modeling all-cause inpatient mortality as a function of CDI severity and other relevant covariates. To prevent overfitting the model, variables from the univariate analysis with a P < .20 were included in the multivariable model. A stepwise backwards elimination procedure was performed by which variables with a P > .05 were removed one at a time, and the partial likelihood ratio test was used to compare the new, smaller model to the old model. All variables with a P < .05 were included in the final model and defined as statistically significant. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. For all subgroup and post hoc analyses, the same covariates identified in the primary outcome analyses were included in multivariable models. Our ongoing ribotyping study also provided us an opportunity to assess the 2010 versus 2017 CDI severity classification criteria for their ability to predict infection with ribotype 027, a known hypervirulent strain associated with poor clinical outcomes [35]. Classification and regression tree (CART) analysis was performed using the “rpart” package in R, version 3.6.1 (The R Foundation for Statistical Computing, Vienna, Austria). All other statistical analyses were performed using STATA, version 15.1 (StataCorp LLC, College Station, TX), and results of the multivariable, subgroup, and post hoc analyses were visualized using the “forestplot” and “ggplot2” packages in R.
RESULTS
Patient Characteristics and Proportion of Patients With Concomitant Kidney Disease
A total of 887 CDI episodes were identified: 91 episodes (10.3%) were missing patient baseline SCr values, 4 were missing SCr values within 24 hours of CDI diagnosis, 6 were missing WBC counts within 24 hours of CDI diagnosis, and 16 tests were duplicates or represented a test for cure. The final study cohort consisted of 770 CDI episodes from 705 unique patients in 12 institutions. Of these, 418 (54.3%) episodes had matching ribotype data (Supplementary Figure S1). The mean age of the cohort was 65 ± 17 years, 54% were female, and the median CCI score was 2 (Table 1). Kidney disease was common and included those with preexisting CKD (total, 36.5%; category G3, 15.3%; category G4, 3.1%; category G5, 18.1%), AKI at the time of CDI diagnosis (total, 29.6%; stage 1, 15.7%; stage 2, 9.0%; stage 3, 4.9%), and chronic renal replacement therapy (17.8% all classified as end-stage renal disease [KDIGO CKD category G5]). Furthermore, 113 of 228 (49.6%) patients with AKI at the time of CDI diagnosis had preexisting CKD.
Table 1.
Patient Demographics, Comorbidities, and Laboratory Parameters
| Cohort | |
|---|---|
| Variable | (n = 770) |
| Age, mean (±SD), years | 65.3 (16.7) |
| Female, no. (%) | 418 (54.3) |
| Race/ethnicity, no. (%) | |
| White, non-Hispanic | 453 (58.8) |
| Black, non-Hispanic | 158 (20.5) |
| Hispanic | 116 (15.1) |
| Asian | 18 (2.3) |
| Othera | 25 (3.3) |
| Admitted from home, no. (%) | 593 (77.0) |
| CCI, median (IQR) | 2 (1–4) |
| SOT, no. (%) | 66 (8.6) |
| HSCT, no. (%) | 2 (0.3) |
| History of CDI ever, no. (%) | 217 (28.2) |
| CDI diagnostic testing method, no. (%) | |
| NAAT | 747 (97.0) |
| EIA | 23 (3.0) |
| HO-CDI, no. (%) | 331 (43.0) |
| rCDI, no. (%) | 94 (12.2) |
| Temperature, mean (±SD), °Fd | 98.8 (1.4) |
| SCr (baseline), median (IQR), mg/dL | 0.90 (0.66–1.32) |
| Collected within 30 days, no. (%) | 417 (54.2) |
| Collected within 31–90 days, no. (%) | 110 (14.3) |
| Collected within 91–365 days, no. (%) | 243 (31.5) |
| SCr (within 24 hours of diagnosis), median (IQR), mg/dL | 1.10 (0.74–2.20) |
| WBC, median (IQR), cells/μL | 10 900 (7200–16 400) |
| Eosinophils, median (IQR), cells/μLc | 80 (10–190) |
| Albumin, mean (±SD), g/dLb | 3.0 (0.7) |
Abbreviations: CCI, Charlson comorbidity index; CDI, Clostridioides difficile infection; EIA, enzyme-linked immunosorbent assay; HO-CDI, healthcare facility-onset CDI; HSCT, hematopoietic stem cell transplantation; IQR, interquartile range; NAAT, nucleic acid amplification test; no., number; rCDI, recurrent CDI; SCr, serum creatinine; SD, standard deviation; SOT, solid organ transplantation; WBC, white blood cells.
aAmerican Indian, Alaska Native, Native Hawaiian, Pacific Islander, other race/ethnicity, or ≥2 races/ethnicities.
bn = 159 missing values.
cn = 6 missing values.
dn = 2 missing values.
Impact of Changing Serum Creatinine Criterion on Clostridioides difficile Infection Severity
Eighty-two episodes (10.6%) showed discordant severity classification results after the change from a relative increase in SCr to an absolute SCr threshold in the IDSA/SHEA SCr criterion. This was due to the inclusion of patients with preexisting CKD when using the latter definition. There was a concomitant decrease in severity from severe (2010) to nonsevere (2017) for an additional 38 (4.9%) patients with baseline SCr values below 0.99 mg/dL because even a 50% increase in SCr from baseline did not place them at or above the 1.5 mg/dL threshold. Overall, this resulted in an increase from 375 (48.7%) CDI episodes classified as severe per the 2010 guideline to 419 (54.4%) episodes classified as severe per the revised 2017 guideline (Table 2). Finally, 172 of 228 patients (75.4%) with a SCr ≥1.5 times their baseline value also had a SCr ≥1.5 mg/dL, indicating that the absolute SCr threshold suggested in the 2017 guideline identified the majority of patients with AKI at the time of CDI diagnosis.
Table 2.
Severity Classification Based on 2010 vs 2017 Severity Classification Criteria
| 2010 Severity Criteria | 2017 Severity Criteria | |||
|---|---|---|---|---|
| SCr ≥1.5× Baseline | SCr <1.5× Baseline | SCr ≥1.5 mg/dL | SCr <1.5 mg/dL | |
| WBC ≥15 000 cells/μL | Severe | Severe | Severe | Severe |
| n = 94 | n = 147 | n = 113 | n = 128 | |
| WBC <15 000 cells/μL | Severe | Mild-to-Moderate | Severe | Nonsevere |
| n = 134 | n = 395 | n = 178 | n = 351 | |
Abbreviations: SCr, serum creatinine; WBC, white blood cells.
Primary Outcome Analyses
Overall, inpatient mortality occurred in 5.2% (40 of 770) of the cohort. Using the 2010 guideline, the mortality rate was 3.0% (12 of 395) in patients with mild or moderate CDI and 7.5% (28 of 375) in patients with severe CDI. Using the 2017 guideline, the mortality rate was 2.0% (7 of 351) in patients with nonsevere CDI and 7.9% (33 of 419) in patients with severe CDI. Furthermore, the mortality rate was 2.2% (7 of 313) in episodes classified as nonsevere by both guidelines, 4.2% (5 of 120) in those classified as severe by only 1 guideline, and 8.3% (28 of 337) in those classified severe by both guidelines.
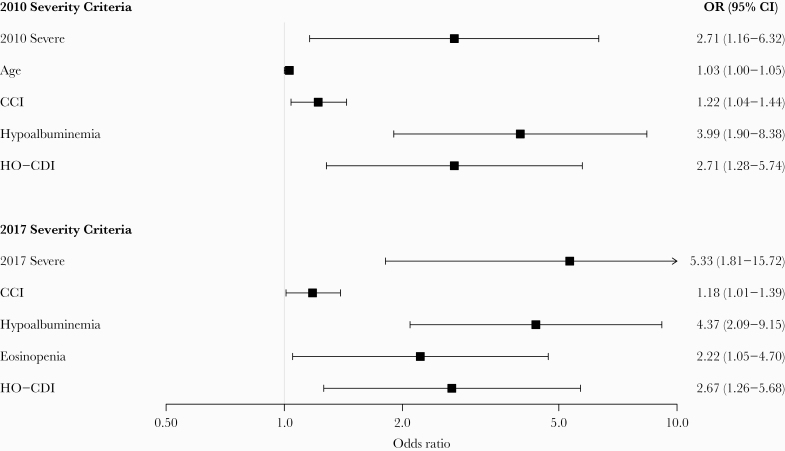
In univariate analysis, the odds of inpatient mortality were higher among patients with severe CDI as classified by either the 2010 or 2017 severity classification criteria (Table 3). In the best fit multivariable logistic regression model, severe CDI classified per the 2010 severity classification criteria was an independent predictor of inpatient mortality (OR, 2.71; 95% CI, 1.16–6.32; P = .02) after adjusting for age, CCI score, hypoalbuminemia, and HO-CDI. Likewise, severe CDI as classified per the revised 2017 severity classification criteria was an independent predictor of inpatient mortality (OR, 5.33; 95% CI, 1.81–15.72; P = .002) after adjusting for CCI score, hypoalbuminemia, eosinopenia, and HO-CDI (Figure 1).
Table 3.
Univariate Analysis for Predictors of Inpatient Mortality
| Univariate Analysis | ||
|---|---|---|
| Covariate | OR (95% CI) | P Value |
| Age (per 1-year increase) | 1.03 (1.01–1.05) | .01 |
| CCI (per 1-unit increase) | 1.20 (1.05–1.36) | .007 |
| SOT | 0.55 (0.13–2.32) | .41 |
| 2010 “Severe” | 2.58 (1.29–5.14) | .007 |
| SCr ≥ 1.5× baseline | 1.63 (0.85–3.13) | .14 |
| 2017 “Severe” | 4.20 (1.83–9.62) | .001 |
| SCr ≥1.5 mg/dL | 3.67 (1.86–7.23) | <.001 |
| WBC ≥15 000 cells/μL | 3.97 (2.05–7.67) | <.001 |
| Albumin <2.5 mg/dL | 6.02 (2.99–12.12) | <.001 |
| Eosinopenia | 1.96 (1.01–3.83) | .05 |
| Temperature (per 1-degree increase) | 1.00 (0.79–1.26) | .97 |
| EIA positive | 1.78 (0.40–7.86) | .45 |
| HO-CDI | 2.59 (1.30–5.04) | .005 |
| rCDI | 0.36 (0.09–1.54) | .17 |
Abbreviations: CCI, Charlson comorbidity index; CI, confidence interval; EIA, enzyme-linked immunosorbent assay; HO-CDI, healthcare facility-onset Clostridioides difficile infection; OR, odds ratio; rCDI, recurrent C difficile infection; SCr, serum creatinine; SOT, solid organ transplantation; WBC, white blood cells.
Figure 1.
Multivariable analyses for predictors of inpatient mortality. CCI, Charlson comorbidity index; CI, confidence interval; HO-CDI, healthcare facility-onset Clostridioides difficile infection; OR, odds ratio.
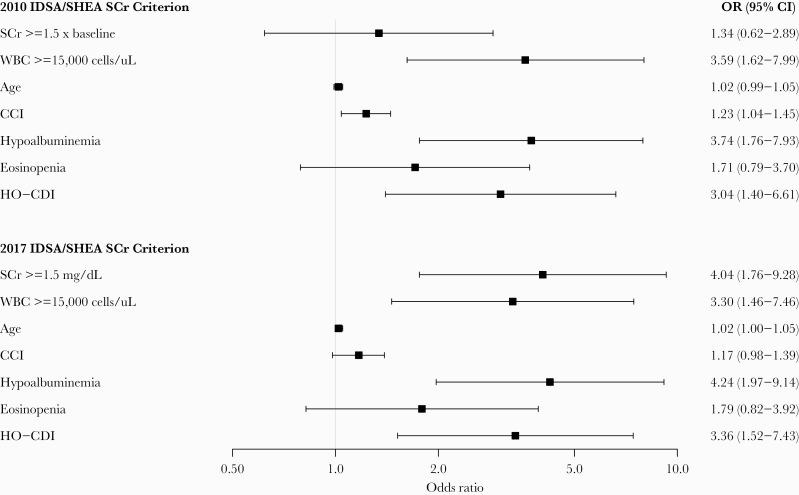
To observe the effects of each SCr criterion on inpatient mortality individually, 2 multivariable logistic regression models were developed with inpatient mortality as the outcome variable and a relative change in SCr per the 2010 guideline or an absolute SCr threshold per the 2017 guideline as predictor variables (Figure 2). Each model also controlled for age, CCI score, leukocytosis, hypoalbuminemia, eosinopenia, and HO-CDI. The absolute SCr threshold suggested by the 2017 severity classification criteria was better correlated with inpatient mortality (OR, 4.04; 95% CI, 1.76–9.28; P = .001) than the relative increase in SCr using the 2010 severity classification criteria (OR, 1.34; 95% CI, 0.62–2.89; P = .46).
Figure 2.
Multivariable analysis of individual criterion for association with inpatient mortality. CCI, Charlson comorbidity index; CI, confidence interval; HO-CDI, healthcare facility-onset Clostridioides difficile infection; OR, odds ratio; SCr, serum creatinine; WBC, white blood cells.
Subgroup and Post Hoc Analyses
As previously stated, 75.4% of patients with a SCr ≥1.5 times their baseline value also had a SCr ≥1.5 mg/dL. A post hoc analysis was performed to determine whether a different SCr threshold could better capture those with AKI at the time of CDI diagnosis. The CART analysis identified a SCr threshold of ≥1.36 mg/dL, which identified 81.6% (186 of 228) of patients with AKI. Four different SCr thresholds (1.0, 1.36, 1.5, and 2.0 mg/dL) were tested for their ability to identify AKI. The incidence of AKI as well as the corresponding sensitivity, specificity, negative predictive value, and positive predictive value of each SCr threshold can be seen in Supplementary Figure S2 and Supplementary Table S1, respectively. Overall, the CART-derived SCr threshold of ≥1.36 mg/dL performed the best with a sensitivity of 81.6% and a specificity of 75.5%. To ensure the CART-derived threshold was able to predict mortality, it was placed into a multivariable logistic regression model identical to those performed in the primary analyses. Similar to the 2017 IDSA/SHEA SCr threshold, the CART-derived SCr threshold was an independent predictor of mortality (OR, 3.20; 95% CI, 1.40–7.31; P = .006) (Supplementary Figure S3).
Four hundred eighteen CDI episodes also had ribotyping data. For these subgroup analyses, ribotypes were classified as ribotype 027 (RT027; 55 of 418 [13.2%]) or non-RT027 (363 of 418 [86.8%]). An evaluation was done to assess whether the 2010 or 2017 severity classification criteria could predict infection with this hypervirulent strain. Two logistic regression models were built; both included age and CCI score along with severe CDI as classified by either the 2010 or 2017 criteria. The odds of severe CDI predicting infection by RT027 were statistically significant in both models although the 2017 severity classification criteria (OR, 3.63; 95% CI, 1.78–7.41; P < .001) were more predictive than the 2010 criteria (OR, 2.45; 95% CI, 1.31–4.60; P = .005).
Discussion
The previous CDI severity classification criteria from the 2010 IDSA/SHEA CDI guideline included a ≥1.5× relative increase in SCr value from baseline as a severity criterion [4]. The decision to choose a relative increase in SCr as a CDI severity criterion was based on the logic that “an elevated SCr level may indicate severe diarrhea with subsequent dehydration or inadequate renal perfusion” [4]. However, Shah et al [27] demonstrated that 15% of patients lacked adequate data to determine CDI severity at the point of care, which was largely due to a lack of baseline SCr data. Partly based on this reference, the CDI severity classification criteria was modified for the 2017 IDSA/SHEA CDI guideline to include an absolute SCr value above a threshold (≥1.5 mg/dL) rather than a relative increase from baseline [4, 6]. This change had the benefit of being readily available clinically and also able to identify patients with preexisting CKD as well as AKI at the time of CDI diagnosis if the kidney injury resulted in a SCr above the threshold value. However, the guideline panel noted that these revised criteria require validation and that they would not be able to detect AKI in patients with preexisting renal insufficiency [6]. Our ongoing clinical trial of hospitalized patients with CDI gave us the opportunity to validate the decision to change the SCr criterion in the 2017 guideline compared with the 2010 guideline. Strengths of our study include a multicenter study design, standardized data definitions and collection procedures, and ongoing strain typing allowing for translational research subgroup analyses.
In this study, a large proportion of patients had preexisting CKD (281 of 770; 36.5%) including 137 of these 281 patients (48.8%) that required chronic renal replacement therapy before CDI diagnosis. Although we hypothesized this subgroup of patients to be at highest risk of being misclassified as having severe CDI due to the SCr criterion revision, the guideline revision lead to an increase in severity for only 82 (10.6%) patients. In addition, our study suggests that this simplified SCr criterion is an acceptable surrogate for AKI because it was able to identify 75.4% of patients with AKI at the time of CDI diagnosis. The CART-derived SCr threshold of ≥1.36 mg/dL was very close to the currently recommended SCr threshold value of ≥1.5 mg/dL with marginal differences noted between the 2 values.
Severity classification criteria should most importantly identify variables at disease presentation that predict poor outcomes. For example, the Acute Physiology and Chronic Health Evaluation (APACHE) II and the Sequential Organ Failure Assessment (SOFA) scores were developed and subsequently validated to predict mortality among various infectious diseases populations [36, 37]. Although investigators have used various definitions, AKI has been associated with poor CDI outcomes including mortality [9–11]. For example, Tay et al [38] demonstrated an association between the 2010 severity classification criteria and all-cause 30-day mortality (OR, 3.18; 95% CI, 1.52–6.66; P = .002). Likewise, CKD has also been associated with poor CDI outcomes in a multitude of studies [12–26]. Using data from the fidaxomicin phase III clinical trials, Bauer et al [39] observed an association between WBC ≥15 000 cells/μL and SCr ≥1.5 mg/dL assessed separately and treatment failure and CDI recurrence (SCr only). Mortality was not included as an outcome in this study. Taken together, these data suggest that a SCr criterion that identifies both AKI and CKD patients would likely improve the performance of severity classification criteria to identify patients at high risk for mortality. Our study confirmed these previous findings and indicate that the association between mortality and severe CDI per the 2010 IDSA/SHEA severity classification criteria was driven primarily by the WBC criterion (Figure 2). In contrast, the revised SCr criterion from the 2017 guideline was independently associated with increased odds of mortality when analyzed as a single criterion and improved the performance of the WBC variable included in the 2017 IDSA/SHEA severity classification criteria. When plotting our sample estimates next to previous retrospective findings (Supplementary Table S2), there is considerable overlap (Supplementary Figure S4), which suggests that our findings are externally valid. Thus, these findings support the continued use of the 2017 severity classification criteria in future guideline updates, including in patients with preexisting renal insufficiency.
This study has certain limitations. We purposely chose 1 objective primary outcome (all-cause inpatient mortality) to limit bias associated with the outcome evaluation. Further studies assessing CDI attributable mortality, initial clinical cure, and CDI recurrence will be required. In addition, we chose only to evaluate 2 measures of kidney injury, namely, those proposed by the 2010 and 2017 IDSA/SHEA CDI guidelines. Other methods to evaluate kidney injury, including evaluation at different time points or resolution of kidney injury, may improve upon our analyses. However, the ease of collection and availability of a SCr value at the time of CDI diagnosis makes this an especially attractive variable to use in severity classification criteria. Finally, almost all CDI episodes were diagnosed using a NAAT (747 of 770; 97.0%). The use of an NAAT has been shown to identify patients with less severe disease and may lead to false-positive results in patients colonized with C difficile [40]. The effect of this would likely be a decrease in mortality and bias our results towards the null.
Conclusions
In conclusion, the 2017 IDSA/SHEA CDI severity classification criteria better correlated with inpatient mortality than the 2010 severity classification criteria, which supports the continued use of these revised criteria in future guideline updates.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support.This work was funded by the Society of Infectious Diseases Pharmacists and the National Institutes of Health National Institute of Allergy and Infectious Diseases (U01AI124290-01)
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Magill SS, O’Leary E, Janelle SJ, et al. ; Emerging Infections Program Hospital Prevalence Survey Team. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med 2018; 379:1732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States (U.S.), 2019. Atlanta: U.S. Department of Health and Human Services, CDC; 2019. [Google Scholar]
- 3. Bauer MP, Kuijper EJ, van Dissel JT; European Society of Clinical Microbiology and Infectious Diseases European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficile infection (CDI). Clin Microbiol Infect 2009; 15:1067–79. [DOI] [PubMed] [Google Scholar]
- 4. Cohen SH, Gerding DN, Johnson S, et al. ; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–55. [DOI] [PubMed] [Google Scholar]
- 5. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108:478–98; quiz 499. [DOI] [PubMed] [Google Scholar]
- 6. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:987–94. [DOI] [PubMed] [Google Scholar]
- 7. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–7. [DOI] [PubMed] [Google Scholar]
- 8. Johnson S, Louie TJ, Gerding DN, et al. ; Polymer Alternative for CDI Treatment (PACT) investigators. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 2014; 59:345–54. [DOI] [PubMed] [Google Scholar]
- 9. Gujja D, Friedenberg FK. Predictors of serious complications due to Clostridium difficile infection. Aliment Pharmacol Ther 2009; 29:635–42. [DOI] [PubMed] [Google Scholar]
- 10. Lungulescu OA, Cao W, Gatskevich E, et al. CSI: a severity index for Clostridium difficile infection at the time of admission. J Hosp Infect 2011; 79:151–4. [DOI] [PubMed] [Google Scholar]
- 11. Kassam Z, Cribb Fabersunne C, Smith MB, et al. Clostridium difficile associated risk of death score (CARDS): a novel severity score to predict mortality among hospitalised patients with C. difficile infection. Aliment Pharmacol Ther 2016; 43:725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yousuf K, Saklayen MG, Markert RJ, et al. Clostridium difficile-associated diarrhea and chronic renal insufficiency. South Med J 2002; 95:681–3. [PubMed] [Google Scholar]
- 13. Pépin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. Can Med Assoc J 2004; 171:466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henrich TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis 2009; 15:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dudukgian H, Sie E, Gonzalez-Ruiz C, Etzioni DA, Kaiser AM. C. difficile colitis: predictors of fatal outcome. J Gastrointest Surg 2010; 14:315–22. [DOI] [PubMed] [Google Scholar]
- 16. Wilson V, Cheek L, Satta G, et al. Predictors of death after Clostridium difficile infection: a report on 128 strain-typed cases from a teaching hospital in the United Kingdom. Clin Infect Dis 2010; 50:e77–81. [DOI] [PubMed] [Google Scholar]
- 17. Fujitani S, George WL, Murthy AR. Comparison of clinical severity score indices for Clostridium difficile infection. Infect Control Hosp Epidemiol 2011; 32:220–8. [DOI] [PubMed] [Google Scholar]
- 18. Manek K, Williams V, Callery S, Daneman N. Reducing the risk of severe complications among patients with Clostridium difficile infection. Can J Gastroenterol 2011; 25:368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Welfare MR, Lalayiannis LC, Martin KE, et al. Co-morbidities as predictors of mortality in Clostridium difficile infection and derivation of the ARC predictive score. J Hosp Infect 2011; 79:359–63. [DOI] [PubMed] [Google Scholar]
- 20. Wenisch JM, Schmid D, Kuo HW, et al. Hospital-acquired Clostridium difficile infection: determinants for severe disease. Eur J Clin Microbiol Infect Dis 2012; 31:1923–30. [DOI] [PubMed] [Google Scholar]
- 21. Mullane KM, Cornely OA, Crook DW, et al. Renal impairment and clinical outcomes of Clostridium difficile infection in two randomized trials. Am J Nephrol 2013; 38:1–11. [DOI] [PubMed] [Google Scholar]
- 22. Hensgens MP, Dekkers OM, Goorhuis A, et al. Predicting a complicated course of Clostridium difficile infection at the bedside. Clin Microbiol Infect 2014; 20:O301–8. [DOI] [PubMed] [Google Scholar]
- 23. Rodríguez-Pardo D, Almirante B, Bartolomé RM, et al. ; Barcelona Clostridium difficile Study Group. Epidemiology of Clostridium difficile infection and risk factors for unfavorable clinical outcomes: results of a hospital-based study in Barcelona, Spain. J Clin Microbiol 2013; 51:1465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim SC, Seo MY, Lee JY, et al. Advanced chronic kidney disease: a strong risk factor for Clostridium difficile infection. Korean J Intern Med 2016; 31:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Figh ML, Zoog ESL, Moore RA, et al. External validation of Velazquez-Gomez Severity Score Index and ATLAS Scores and the identification of risk factors associated with mortality in Clostridium difficile infections. Am Surg 2017; 83:1347–51. [PubMed] [Google Scholar]
- 26. Shin J, Wi YM, Lee YJ. Metronidazole therapy as initial treatment of infection in patients with chronic kidney disease in Korea. Epidemiol Infect 2019; 147:e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shah DN, Bhatt NS, Welch JK, et al. Defining acute renal dysfunction as a criterion for the severity of Clostridium difficile infection in patients with community-onset vs hospital-onset infection. J Hosp Infect 2013; 83:294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Endres BT, Begum K, Sun H, et al. Epidemic Clostridioides difficile ribotype 027 lineages: comparisons of Texas versus worldwide strains. Open Forum Infect Dis 2019; 6:ofz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gonzales-Luna AJ, Carlson TJ, Dotson KM, et al. PCR ribotypes of Clostridioides difficile across Texas from 2011 to 2018 including emergence of ribotype 255. Emerg Microbes Infect 2020; 9:341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carlson TJ, Endres BT, Le Pham J, et al. Eosinopenia and binary toxin increase mortality in hospitalized patients with Clostridioides difficile infection. Open Forum Infect Dis 2020; 7:ofz552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention (CDC). Multidrug-Resistant Organism and Clostridioides difficile Infection (MDRO/CDI) Module. Atlanta: U.S. Department of Health and Human Services, CDC; 2019. [Google Scholar]
- 33. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2:1–138. [Google Scholar]
- 34. Kidney Disease: Improving Global Outcomes (KDIGO) Chronic Kidney Disease Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150. [DOI] [PubMed] [Google Scholar]
- 35. Reveles KR, Dotson KM, Gonzales-Luna AJ, et al. Clostridioides (formerly Clostridium) difficile infection during hospitalization increases the likelihood of non-home patient discharge. Clin Infect Dis 2019; 68:1887–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–29. [PubMed] [Google Scholar]
- 37. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 1996; 22:707–10. [DOI] [PubMed] [Google Scholar]
- 38. Tay HL, Chow A, Ng TM, Lye DC. Risk factors and treatment outcomes of severe Clostridioides difficile infection in Singapore. Sci Rep 2019; 9:13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bauer MP, Hensgens MP, Miller MA, et al. Renal failure and leukocytosis are predictors of a complicated course of Clostridium difficile infection if measured on day of diagnosis. Clin Infect Dis 2012; 55 Suppl 2:S149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koo HL, Van JN, Zhao M, et al. Real-time polymerase chain reaction detection of asymptomatic Clostridium difficile colonization and rising C. difficile-associated disease rates. Infect Control Hosp Epidemiol 2014; 35:667–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.