Abstract
Background
Mortality related to bloodstream infections (BSIs) is high. The epidemiology of BSIs is changing due to the increase in multidrug resistance, and it is unclear whether the presence of multidrug-resistant (MDR) organisms, per se, is an independent risk factor for mortality. Our objectives were, first, to describe the epidemiology and outcome of BSIs and, second, to determine the risk factors associated with mortality among patients with BSI.
Methods
This research used a single-center retrospective observational study design. Patients were identified through microbiological reports. Data on medical history, clinical condition, bacteria, antimicrobial therapy, and mortality were collected. The primary outcome was crude mortality at 30 days. The relationships between mortality and demographic, clinical, and microbiological variables were analyzed by multivariate analysis.
Results
A total of 1049 inpatients were included. MDR bacteria were isolated in 27.83% of patients, where 2.14% corresponded to an extremely drug-resistant (XDR) isolate. The crude mortality rates at days 7, 30, and 90 were 12.11%, 25.17%, and 36.13%, respectively. Pitt score >2, lung and abdomen as site of infection, and XDR Pseudomonas aeruginosa were independent risk factors for 7-, 30-, and 90-day mortality. Charlson score >4, carbapenem-resistant Klebsiella pneumoniae, and XDR Acinetobacter baumannii were independent risk factors for 30- and 90-day mortality. Infection by XDR gram-negative bacteria, Charlson score >4, and immunosuppression were independent risk factors for mortality in patients who were stable at the time of BSI.
Conclusions
BSI is an event with an extreme impact on mortality. Patients with severe clinical condition are at higher risk of death. The presence of XDR gram-negative bacteria in blood is strongly and independently associated with patient death.
Keywords: bloodstream infection, gram-negative bacteria, hospital, MDR, P. aeruginosa, XDR
Bloodstream infections (BSIs) are a major cause of morbidity and mortality worldwide, constituting a growing public health concern [1–3].
Mortality associated with BSI ranges from 14% for community-onset BSI to 30% for patients with severe comorbidities, such as cirrhosis, onco-hematologic diseases, or solid-organ transplants [4–7].
BSIs in patients admitted to the intensive care unit (ICU) have particularly high mortality rates, ranging between 40% and 60% [8].
According to the latest data collected by the European Centre for Disease Control (ECDC), Italy is among the European countries with a high frequency of multidrug-resistant (MDR) invasive organisms [9]. This high and stable level of antimicrobial resistance is of particular concern due to limited antibiotic treatment options. Although there is a wide consensus that critically ill patients are at higher risk of dying from BSI [8, 10], it remains unclear whether the resistance pattern, per se, may contribute to increased mortality, and results in the literature are contradictory. Some studies have reported that mortality related to MDR organisms is higher than that related to nonresistant organisms [5, 11]. Conversely, other studies suggest that antibiotic resistance in BSI does not adversely affect the outcome for patients, representing only a small fraction of deaths [12, 13].
The purpose of this study was to describe the current epidemiology and outcomes of BSIs in a large cohort of patients with positive blood cultures in a tertiary care university teaching hospital. Our secondary objective was to determine which independent variables were associated with mortality among patients with BSI and whether multidrug-resistant bacteria constituted a risk for mortality.
METHODS
An observational monocentric cohort study was conducted at the 700-bed University Hospital Modena, Northern Italy. All consecutive patients aged 18 years and older with at least 1 episode of BSI during hospitalization between November 1, 2015, and June 30, 2017, were retrospectively included, such that the target of minimally 1000 patients was reached. The occurrence of BSIs was determined through microbiological laboratory reports.
BSI episodes occurring in the same patients were only considered when caused by different species [14]. Data were collected by reviewing all patient files for each BSI. This included patient history, comorbidities (Charlson comorbidity index) [15], and the use of antibiotics. For each BSI episode, the following data were collected: clinical conditions at the time of BSI, grade of clinical severity (Pitt bacteremia score) [16], device presence (central venous catheter or urinary catheter), state of immunosuppression (transplant, AIDS, chronic treatment with steroids), and source of BSI. Information about empirical antibiotic therapy and subsequent therapy changes was collected.
Definitions
BSI was defined as a positive blood culture (or cultures), with an isolate of the same species grown in at least 1 blood culture bottle, obtained from a patient with a compatible clinical syndrome (ie, a patient who had evidence of 1 or more of the following signs and symptoms, with no other recognized cause: fever [>38°C], hypothermia [<36°C core], chills, hypotension, oliguria, and/or elevated lactate levels).
A positive blood culture for an organism that was previously isolated within 30 days was considered a unique BSI episode [17].
Coagulase-negative staphylococci (CoNS), Corynebacterium spp., and Propionibacterium spp. were regarded as contaminants unless isolated from 2 or more separate blood culture sets. Definitions and criteria were formulated according to the ECDC hospital-acquired infections guidelines [18].
Hospital-acquired BSI was defined as a positive blood culture obtained on day ≥3 after hospital admission [18]. All other BSIs were defined as being community-onset BSIs.
Polymicrobial BSI was defined as isolation of >1 bacterial species from the same blood culture.
Source of BSI was determined by 2 ID physicians (A.S. and E.F.) independently reviewing the patient files. The criteria used for determining the source of BSI were identification of the same isolate from another infection site or strong clinical evidence that the bloodstream infection was secondary to another infection site.
Appropriateness of Therapy
Empirical antibiotic therapy was considered appropriate if the identified microorganisms were susceptible, in vitro, to at least 1 of the antibiotics used and if the doses were in agreement with the recommendations by national evidence-based guidelines on antibiotic therapy. Appropriateness was categorized as “appropriate at 48 hours” if targeted therapy against the isolated bacteria was started within the first 2 days after BSI and “inappropriate” if the patients did not receive any correct therapy within the whole BSI episode.
Patients who died within 2 days were included, by definition, in the group of patients who did not receive appropriate therapy. Targeted therapy could only be started after the culture results became known. Hence, in these patients, it was only possible to assess empirical therapy.
Outcome was assessed at the patient level. Mortality was assessed at 30, 60, or 90 days using information from hospital records that were linked with a municipal records database. Our hospital information system allows us access to patient personal data (living or deceased status, date of death). For this reason, we have no patients lost to follow-up in mortality analyses.
Microbiology
Blood cultures were processed using the BACTEC system (Becton Dickinson). Microorganism identification was performed using Matrix Assisted Laser Desorption Ionization Time-of-Flight mass spectrometry (bioMérieux’s VITEK MS), and susceptibility was determined by VITEK 2 (VITEK MS, bioMérieux, Marcy l’Etoile, France). Susceptibility data for all bacterial isolates were available. Extended-spectrum beta-lactamase (ESBL) detection for Klebsiella spp., Proteus spp., and E. coli was performed using BD BBL Sensi-Disc ESBL Confirmatory Test discs. For Enterobacter spp., resistance to third-generation cephalosporins was detected with Kirby-Bauer disk diffusion or broth dilution.
Minimum inhibistory concentrations were interpreted according to current EUCAST clinical breakpoints [19]. Presence of carbapenem resistance was identified by the use of a rapid molecular diagnostic test (Xpert Carba-R), with a qualitative PCR test for the detection and differentiation of KPC, NDM, VIM, OXA-48, and IMP in <24 hours. The on-call infectious disease specialist was alerted of the positivity of this rapid test by the laboratory (Monday to Saturday, 8 am–8 pm).
A pathogen was classified as multidrug-resistant (MDR), extensively drug-resistant (XDR), or pandrug-resistant (PDR), according to the definitions of the ECDC and the US Centers for Disease Control and Prevention (CDC) [20]. ESKAPE categories (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) were used for the comparison between community-onset and hospital-acquired BSI and mortality analyses [21]. In 1 of the subanalyses, we additionally identified a difficult-to-treat (DTR resistance for gram-negative BSI) category [17]. Information regarding combined nonsusceptibility to carbapenems, extended-spectrum cephalosporins, and fluoroquinolones was required to determine DTR status.
Statistical Analysis
Continuous variables were described using mean (SD) or median (interquartile range), according to normal or non-normal distribution, respectively. Categorical variables were summarized as absolute counts and relative frequencies. First, a comparison between community-onset and hospital-acquired infection was made using the Student t test, Mann-Whitney U test, chi-square test, or Fisher exact test, as appropriate. Statistical significance was set at an alpha error <.05. Bonferroni correction was applied to all comparisons in the descriptive analysis (Table 1).
Table 1.
Patient Demographic, Clinical, and Microbiological Characteristics of BSI (n = 1526), According to Onset of BSI
| All Patients | Community-Onset | Hospital-Acquired | P | |
|---|---|---|---|---|
| Variables | 460 | 630 | 419 | |
| Male sex | 589 (56.14) | 332 (52.73) | 257 (61.35) | .006 |
| Age, mean (SD), y | 69.2 (16) | 70.2 (16) | 67.5 (16) | .002 |
| Long-term care facilities | 38 (3.62) | 27 (4.24) | 11 (2.68) | .159 |
| Immunosuppression | 229 (21.83) | 128 (20.35) | 101 (24.14) | .328 |
| Central venous catheter | 292 (27.84) | 90 (14.23) | 202 (48.26) | <.001 |
| Urinary catheter | 247 (23.55) | 82 (13.07) | 165 (39.35) | <.001 |
| Charlson comorbidity index, mean (SD) | 6.09 (3.16) | 6.0 (3.23) | 6.2 (3.01) | .39 |
| Pitt bacteremia score, mean (SD) | 1.64 (1.81) | 1.3 (1.62) | 3.5 (1.94) | <.001 |
| Previous BSI episodes, mean (SD) | 0.3 (0.75) | 0.3 (0.83) | 0.3 (0.74) | .17 |
| Ward | With Bonferroni-adj. significance level considered for P value <.007 | <.001 | ||
| Internal med | 348 (33.12) | 240 (38.14) | 108 (25.71) | <.001 |
| Hematology | 43 (4.13) | 11 (1.75) | 32 (7.64) | <.001 |
| Dialysis ward | 25 (2.38) | 25 (3.94) | 0 (0) | <.001 |
| Oncology | 152 (14.49) | 107 (16.94) | 45 (10.72) | <.001 |
| Other med | 285 (27.12) | 163 (25.83) | 122 (29.12) | .34 |
| Surgery | 115 (10.92) | 43 (6.81) | 72 (17.14) | <.001 |
| ICU | 81 (7.73) | 41 (6.56) | 40 (9.52) | .07 |
| Source of infection | With Bonferroni-adj. significance level considered for P value <.004 | <.001 | ||
| Urinary catheter | 81 (7.72) | 45 (7.14) | 36 (8.59) | .41 |
| Urinary tract | 190 (18.11) | 164 (26.01) | 26 (6.21) | <.001 |
| Abdomen | 118 (11.25) | 65 (10.33) | 53 (12.65) | .27 |
| Biliary tract | 59 (5.62) | 39 (6.18) | 20 (4.74) | .34 |
| Pulmonary | 179 (17.06) | 123 (19.52) | 56 (13.36) | .009 |
| Skin and soft tissue | 36 (3.43) | 17 (2.75) | 19 (4.53) | .12 |
| Central venous catheter | 163 (15.54) | 46 (7.35) | 117 (27.92) | <.001 |
| Other (including unknown source) | 142 (13.53) | 82 (13.01) | 60 (14.32) | .93 |
| Microbiology | With Bonferroni-adj. significance level considered for P value <.006 | <.001 | ||
| CoNS | 135 (12.82) | 51 (8.13) | 84 (20.05) | <.001 |
| Enterococci | 96 (9.15) | 41 (6.54) | 55 (13.18) | <.001 |
| Staphylococcus aureus | 127 (12.11) | 73 (11.59) | 54 (12.89) | .59 |
| Streptococci | 96 (9.15) | 78 (12.31) | 18 (4.34) | <.001 |
| Other gram-pos | 15 (1.43) | 10 (1.59) | 5 (1.19) | .45 |
| Enterobacterales | 469 (44.78) | 313 (49.66) | 156 (37.22) | <.001 |
| Pseudomonas spp. | 53 (5.05) | 32 (5.08) | 21 (5.01) | .23 |
| Other nonfermenting gram-neg | 26 (2.47) | 15 (2.38) | 11 (2.62) | .76 |
| Other gram-neg | 32 (3.05) | 21 (3.36) | 11 (2.63) | .51 |
| Polymicrobial | 101 (9.63) | 49 (7.72) | 52 (12.44) | .03 |
| Resistance | With Bonferroni-adj. significance level considered for P value <.012 | <.001 | ||
| NO R | 733 (69.88) | 485 (76.98) | 248 (59.19) | .04§ |
| MDR | 292 (27.84) | 139 (22.06) | 153 (35.52) | <.001§ |
| XDR | 22 (2.09) | 4 (1.86) | 18 (8.87) | <.001§ |
| PDR | 2 (0.19) | 2 (0.53) | 0 (0) | .52 |
| III-gen ceph-res Enterobacterales (other than Enterobacter) | 117 (11.15) | 64 (10.15) | 53 (12.64) | .67 |
| ESKAPE categories | With Bonferroni-adj. significance level considered for P value <.007 | <.001 | ||
| VRE | 4 (0.38) | 1 (0.16) | 3 (0.72) | .15 |
| MRSA | 33 (3.15) | 23 (3.59) | 10 (2.38) | .25 |
| CRKP | 12 (1.14) | 5 (0.71) | 7 (1.62) | .19 |
| Acinetobacter XDR | 11 (1.05) | 1 (0.47) | 10 (4.67) | <.001 |
| Pseudomonas XDR | 4 (0.38) | 1 (0.16) | 3 (0.72) | .31 |
| III-gen ceph-res Enterobacter spp. | 11 (1.05) | 6 (0.95) | 5 (1.19) | .35 |
Data presented as No (%), unless otherwise indicated.
Abbreviations: CRKP, carbapenem-resistant Klebsiella pneumoniae; CoNS, coagulase-negative Staphylococcus; ICU, intensive care unit; III-gen ceph-res, third-generation cephalosporin-resistant; MDR, multidrug-resistant; MDR, multidrug-resistant; Med, medicine; MRSA, methicillin-resistant Staphylococcus aureus; Other, includes unknown origin; Neg, negative; NO R, resistant to <3 antibiotic categories; PDR, pandrug-resistant; Pos, positive; VRE, vancomycin-resistant Enterococcus faecium; XDR, extensively drug-resistant.
Second, survival analysis was performed using crude mortality rates at 7, 30, and 90 days.
Patient survival curves were prepared by the Kaplan-Meier method, and univariate survival distributions were compared using the log-rank test. In patients with a polymicrobial BSI, we selected the isolate with resistance against the largest number of antibiotics according to class.
To assess the relationship between mortality and a set of independent variables, univariate and multivariate survival analyses were used (Cox proportional hazards model); further, adjusted hazard ratios (HRs) and 95% CIs were calculated. Variables were selected based on a literature review. For the multivariate analysis, proxy variables were removed. We performed a proportional hazards assumption test based on Schoenfeld residuals, both for univariate and multivariate analyses. The global test resulted in nonsignificant P values.
All statistical analyses were performed using STATA 13.1 software for Mac (StataCorp Ltd, College Station, TX, USA). Each patient with BSI was considered only once, using data from the last BSI episode before each outcome.
RESULTS
During the study period of 21 months, 1526 BSI episodes were identified in 1049 patients. Of these, 419 (39.94%) BSIs were hospital-acquired. A total of 589 (56.14%) patients were male. The mean (SD) age was 69 (±16) years. Table 1 describes the patient characteristics, clinical features, and microbiology of BSIs by epidemiological type (community-onset or hospital-acquired). Patients with hospital-acquired BSIs had an indwelling catheter significantly more often and higher clinical severity at onset.
Microbiology
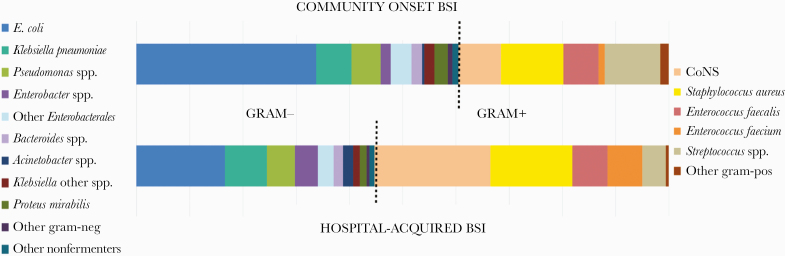
A total of 1598 bacteria were isolated from blood, of which 850 (53.19%) were gram-negative and 748 (46.80%) were gram-positive. Figure 1 shows the bacterial epidemiology of BSIs according to onset (ie, community or hospital).
Figure 1.
Bacterial epidemiology of bloodstream infections (n = 1598 isolates). Abbreviations: BSI, bloodstream infection, CoNS, coagulase-negative staphylococci.
The most common gram-negative isolates in community-onset BSI were Escherichia coli (33.78%), K. pneumoniae (6.62%), and P. aeruginosa (5.14%), while S. aureus was the most common gram-positive isolate in community-onset BSI (11.59%), followed by Streptococcus spp. (10.36%) and CoNS (8.04%). In hospital-acquired BSI, the most common gram-negative isolates were E. coli (16.62%), K. pneumoniae (7.79%), and P. aeruginosa (5.13%). The gram-positive isolates in hospital-acquired BSI were mostly commonly CoNS (21.77%), followed by S. aureus (15.43%) and Streptococcus spp. (4.46%).
A total of 733 out of 1049 (69.88%) patients had a BSI caused by bacteria, with resistance in <3 antimicrobial categories (NO R); 292 (27.84%) patients had 1 bacterial isolate classified as MDR, 22 (2.09%) as XDR, and 2 patients (0.19%) as PDR. Among the gram-positive isolates, there were no bacteria classified as XDR or PDR. Overall, MDR and XDR bacterial isolates were more frequent in hospital-acquired than community-onset BSIs. XDR A. baumannii were also more prevalent in hospital-acquired than in community-onset BSIs.
S. aureus was resistant to methicillin (MRSA) in 21.45%, and E. faecium was resistant to vancomycin (VRE) in 11.63% of isolates. K. pneumoniae resistance to fluoroquinolones was 37.28%, and K. pneumoniae resistance to third-generation cephalosporins was 40.81%. K. pneumoniae resistance to carbapenems and colistin was 14.82% and 5.38%, respectively. E. coli resistance to third-generation cephalosporins was 22.94%. P. aeruginosa showed resistance to piperacillin-tazobactam in 30.01%, to quinolones in 39.75%, and to carbapenems in 24.14%. A. baumannii had combined resistance to fluoroquinolones, carbapenems, and aminoglycosides in 72.03% of isolates. Twenty-two point zero nine percent of Enterobacter spp. were resistant to third-generation cephalosporins.
A total of 79.94% of patients received appropriate antibiotic therapy within 48 hours; for 6.48% of patients, therapy was appropriate after 48 hours, while 13.52% of patients did not receive appropriate therapy during the entire BSI episode. The mean duration of appropriate therapy in patients with gram-negative bacteria (SD) was 10.8 (9.5) days, while for patients with gram-positive bacteria, the mean duration (SD) was 12.9 (12.3) days.
Mortality Analyses
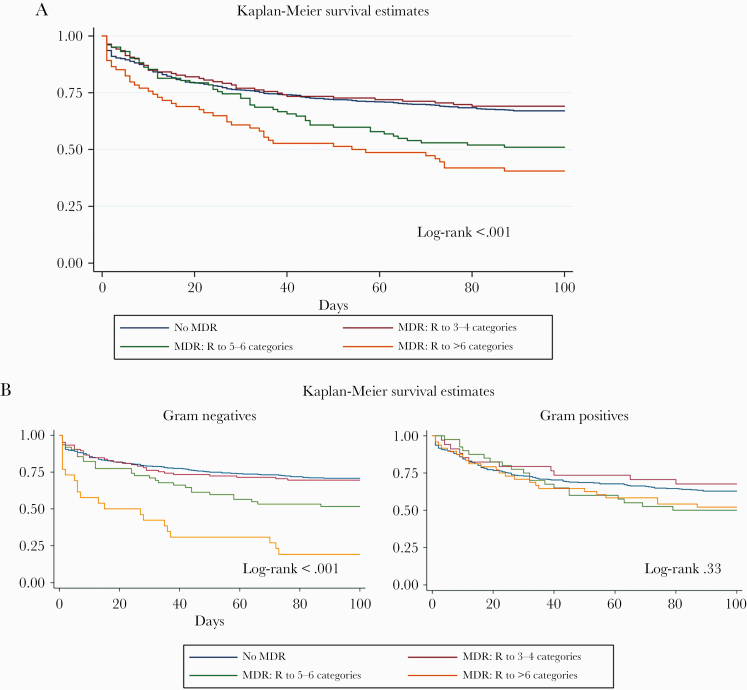
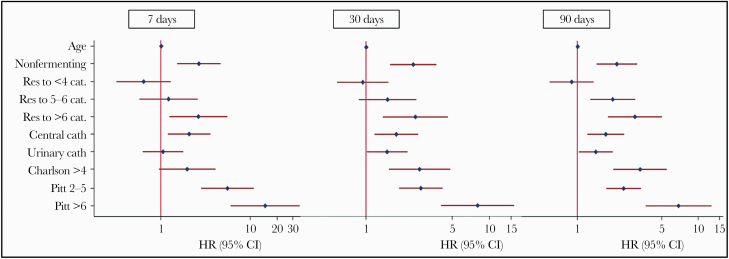
Crude mortality rates at days 7, 30, and 90 after first BSI episode were 12.11%, 25.17%, and 36.13%, respectively. Mortality was higher in patients with hospital-acquired BSI, with a rate of 39% at 90 days compared with 30% for community-onset BSIs (P < .01). Figure 2A shows the survival curves for all patients, classified according to resistance of blood isolates against a number of antibiotic categories. Mortality increased significantly for patients with isolates resistant to >4 categories of antibiotics (log-rank P < .001). Survival curves of patients categorized according to gram-negative or -positive isolates show that differences were significant only for patients with gram-negative isolates (Figure 2B). The univariate analysis is shown in the Supplementary Data. For the multivariate analysis, we considered number of resistances a proxy of ESKAPE categories, being in the ICU a proxy for Pitt score, and microbiological species a proxy for specific ESKAPE categories. Table 2 shows the risk factors for mortality at 7, 30, and 90 days in the multivariate analysis. The strongest independent risk factor for mortality at 7 days was a Pitt score >6, with a hazard ratio (HR) of 11.30. Respiratory tract and abdomen as infection source were independent risk factors for mortality, with HRs of 4.95 and 5.07, respectively. Infection with Pseudomonas XDR had an HR of 6.72. Patients who did not receive any appropriate therapy for BSI had an HR of 3.24.
Figure 2.
A, Patient survival curves according to antibiotic resistance category of isolates (all patients n = 1049). B, Survival curves of patients with gram-negative (n = 580) or gram-positive (n = 469) BSI isolates, according to antibiotic resistance category. Abbreviations: BSI, bloodstream infection; Central cath, central venous catheter; MDR, multidrug-resistant; No MDR, R <3 categories; PDR, pandrug-resistant; Res >6 categories, XDR and PDR categories for gram-negative bacteria; XDR, extensively drug-resistant.
Table 2.
Multivariate Analysis for Mortality (n = 1049)
| 7 d | 30 d | 90 d | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | P | 95% CI | HR | P | 95% CI | HR | P | 95% CI |
| Age | 1.01 | .19 | 0.99–1.02 | 1.00 | .29 | 0.99–1.01 | 1.00 | .15 | 0.99–1.01 |
| Hospital-acquired BSI | 0.94 | .75 | 0.61–1.43 | 1.02 | .84 | 0.77–1.36 | 1.11 | .36 | 0.88–1.41 |
| Previous BSI | 0.95 | .73 | 0.75–1.22 | 1.04 | .53 | 0.90–1.19 | 1.02 | .64 | 0.91–1.16 |
| Central venous catheter | 1.50 | .10 | 0.92–2.46 | 1.49 | .02 | 1.06–2.10 | 1.39 | .02 | 1.03–1.87 |
| Urinary catheter | 1.14 | .58 | 0.59–1.69 | 1.23 | .23 | 0.87–1.74 | 1.38 | .02 | 1.03–1.85 |
| Charlson comorbidity index | |||||||||
| Charlson CI <4 | 1 (ref.) | ||||||||
| Charlson CI 4 | 1.22 | .70 | 0.32–4.98 | 2.23 | .15 | 0.74–6.60 | 2.36 | .06 | 0.95–5.86 |
| Charlson CI 5–7 | 1.88 | .28 | 0.58–6.0 | 3.96 | .006 | 1.48–10.58 | 4.46 | <.001 | 1.95–10.27 |
| Charlson CI >8 | 2.23 | .18 | 0.68–7.29 | 5.69 | .001 | 2.10–15.39 | 6.52 | <.001 | 2.81–15.9 |
| Pitt score | |||||||||
| Pitt score <2 | 1 (ref.) | ||||||||
| Pitt score 2–5 | 4.80 | <.001 | 2.90–8.00 | 2.95 | <.001 | 2.20–3.95 | 2.44 | <.001 | 1.94–3.06 |
| Pitt score >6 | 11.30 | <.001 | 5.35–23.8 | 6.49 | <.001 | 3.76–11.2 | 4.53 | <.001 | 2.75–7.47 |
| Source of infection | |||||||||
| Urinary catheter | 1 (ref.) | ||||||||
| Urinary tract | 1.4 | .59 | 0.40–4.89 | 0.75 | .41 | 0.38–1.49 | 1.04 | .87 | 0.60–1.80 |
| Abdomen | 5.07 | .004 | 1.65–15.5 | 2.52 | .002 | 1.42–4.47 | 2.74 | <.001 | 1.70–1.43 |
| Biliary tract | 0.63 | .68 | 0.06–5.7 | 0.47 | .19 | 0.15–1.47 | 0.59 | .24 | 0.25–1.41 |
| Pulmonary | 4.95 | .005 | 1.62–15.08 | 2.23 | .006 | 1.26–3.97 | 2.66 | <.001 | 1.64–4.29 |
| Skin and soft tissue | 2.82 | .13 | 0.72–11.0 | 1.65 | .22 | 0.73–3.73 | 2.13 | .034 | 1.05–4.30 |
| Central venous catheter | 1.87 | .33 | 0.53–6.30 | 0.91 | .80 | 0.47–1.78 | 1.30 | .33 | 0.75–2.25 |
| ESKAPE categories | |||||||||
| No ESKAPE bacteria | 1 (ref.) | ||||||||
| VRE (n = 4) | 1.14 | .87 | 0.21–6.04 | 1.27 | .74 | 0.28–5.63 | 1.20 | .79 | 0.28–5.04 |
| MRSA (n = 33) | 0.62 | .43 | 0.19–2.02 | 0.61 | .20 | 0.29–1.29 | 0.83 | .52 | 0.48–1.45 |
| CRKP (n = 12) | 1.66 | .28 | 0.65–4.24 | 2.08 | .04 | 1.01–4.30 | 2.51 | .005 | 1.31–4.78 |
| Acinetobacter XDR (n = 11) | 1.62 | .28 | 0.66–3.99 | 2.08 | .05 | 0.97–4.45 | 2.33 | .025 | 1.11–4.88 |
| Pseudomonas XDR (n = 4) | 6.72 | .016 | 1.43–31.6 | 3.53 | .04 | 1.06–11.7 | 3.09 | .06 | 0.94–10.0 |
| III-gen ceph-res Enterobacter spp. (n = 11) | 1.03 | .9 | 0.50–2.10 | 0.79 | .33 | 0.50–1.26 | 1.06 | .72 | 0.74–1.51 |
| Appropriate treatment within 2 d | 1 (ref.) | ||||||||
| Appropriate after 2 d | 0.18 | .025 | 0.04–0.80 | 1.01 | .94 | 0.60–1.70 | 1.06 | .76 | 0.70–1.60 |
| Inappropriate treatment | 3.24 | <.001 | 1.98–5.16 | 2.39 | <.001 | 1.62–3.53 | 2.11 | <.001 | 1.50–2.90 |
Abbreviations: BSI, bloodstream infection; cat, categories; CI, comorbidity index; CRKP, carbapenem-resistant Klebsiella pneumoniae; Enter, Enterobacter; III-gen ceph-res, third-generation cephalosporin-resistant; MDR, multidrug-resistant; MRSA, methicillin-resistant Staphylococcus aureus; R, resistance; VRE, vancomycin-resistant Enterococcus faecium; XDR, extensively drug-resistant.
Regarding 30- and 90-day mortality, all significant risk factors at day 7 were confirmed to be independently associated with death. Moreover, at day 30 and day 90, patients with a Charlson score >4 had significantly higher HRs. Furthermore, carbapenem-resistant K. pneumoniae and Acinetobacter XDR were also independent risk factors. Third-generation cephalosporin-resistant Enterobacterales spp. were not significantly associated with mortality.
To identify the risk factors for mortality, according to gram staining of causative organisms, we conducted a subanalysis. The populations of 580 patients with gram-negative BSI and 469 patients with gram-positive BSI were compared. Regarding gram-negative infection, the risk factors for mortality were similar to those for the total population (Figure 3). Neither the number of MDR categories nor bacterial species were significantly associated with mortality (Supplementary Figure 1). In the subanalysis, we included a DTR (difficult-to-treat resistance [17]) category for gram-negative BSIs in order to further identify the gram-negative BSIs at highest risk for mortality. DTR was confirmed to be an independent risk factor for 7-, 30-, and 90-day mortality (Supplementary Table 2).
Figure 3.
Multivariate analysis for mortality in patients with gram-negative BSI (n = 580). Abbreviations: BSI, bloodstream infection; HR, hazard ratio; PDR, pandrug-resistant; Res >6 categories, XDR and PDR categories for gram-negative bacteria; XDR, extensively drug-resistant.
Supplementary Figure 2 shows the 7-, 30-, and 90-day mortality rates according to ESKAPE categories [21] and the ECDC definition for MDR and XDR bacteria [20], and it shows gram-negative BSIs according to DTR category [17].
A further subanalysis was conducted on patients not in critical condition. For this, only patients with a Pitt score <2 were selected and categorized according to gram-negative or -positive isolates. In this analysis, the outcome was 30- and 90-day mortality due to the low numbers of patients who had died by day 7. Table 3A describes 295 patients with Pitt score <2 and gram-negative BSI. The analysis identified infection with XDR bacteria, Charlson score >4, and immunosuppression to be independent risk factors for mortality in patients who were stable at the time of BSI. Table 3B describes 236 patients with gram-positive BSI and Pitt score <2. The only significant risk factor for mortality was Charlson score >4. Excluding polymicrobial BSIs in the mortality analyses showed similar results (data not shown).
Table 3.
Multivariate Analyses for Mortality in Patients With Pitt Score <2 According to Gram-Negative and Gram-Positive BSI
| A, Patients With Gram-Negative Isolates (n = 295) | 30 d | 90 d | ||||
|---|---|---|---|---|---|---|
| Variables | HR | P | 95% CI | HR | P | 95% CI |
| Age | 0.98 | .14 | 0.95–1.00 | 0.99 | .58 | 0.97–1.01 |
| Immunosuppression | 1.69 | .14 | 0.82–3.47 | 1.98 | .016 | 1.13–3.45 |
| Charlson CI >4 vs <4 | 7.73 | .001 | 2.32–25.7 | 7.30 | <.001 | 2.79–19.0 |
| MDR vs no MDR | 1.19 | .63 | 0.56–2.51 | 1.54 | .11 | 0.89–1.86 |
| XDR vs no MDR | 7.25 | .002 | 2.07–25.3 | 7.25 | .001 | 2.13–24.6 |
| B, Patients with gram-positive isolates (n = 236) | 30 d | 90 d | ||||
| Variables | HR | P | 95% CI | HR | P | 95% CI |
| Age | 1.01 | .23 | 0.98–1.05 | 1.01 | .15 | 0.99–1.03 |
| Immunosuppression | 0.57 | .27 | 0.21–1.56 | 0.96 | .90 | 1.52–1.77 |
| Charlson CI >4 vs <4 | 4.19 | .03 | 1.12–15.6 | 4.30 | .001 | 1.79–10.3 |
| MDR vs no MDR | 1.38 | .41 | 0.63–3.03 | 1.17 | .58 | 0.66–2.05 |
Abbreviations: BSI, bloodstream infection; CI, comorbidity index; MDR, multidrug-resistant; XDR, extensively drug-resistant.
DISCUSSION
The main finding of this cohort study in a major teaching hospital was that BSIs caused by MDR bacteria did not significantly increase mortality. On the other hand, BSI caused by XDR gram-negative bacteria was a strong predictor of death. Many patients died in the first week of hospital stay, and more than one-fifth in the first months after BSI. Crude mortality was superior in patients with hospital-acquired BSI compared with community-onset BSI.
In the subanalysis considering all of the noncritical patients, we demonstrated that BSIs caused by an XDR gram-negative microorganism were associated with an increased risk of death compared with BSIs due to bacteria with susceptibility to at least 3 antibiotic categories. Poor general condition of the patient (Charlson comorbidity index >4), however, remained an important factor for determining mortality due to infection caused by both gram-negatives and -positives.
We confirmed that BSIs are events with a severe impact on mortality [14, 22]. An elevated Pitt bacteremia score for severity represented the strongest significant risk factor for mortality. There is a wide concordance in the literature on the association of Pitt score with clinical severity [23, 24]. Although the mortality rates vary in different studies, they all demonstrate that BSIs represent health-threatening events. Interestingly, only XDR status was shown to increase (by 4.8 times) the risk for 7-day mortality, and this trend was similar for outcomes at 30 and 90 days.
The observation that MDR did not increase the risk of mortality for all examined periods could provide a possible explanation for the conflicting results of previous studies: Barrasa-Villar et al. compared 324 patients with infection by MDR with 676 controls with infection by susceptible organisms and concluded that infections caused by MDR increased mortality compared with those caused by susceptible strains [25]. On the other hand, Blot et al. compared critically ill patients with nosocomial bacteremia caused by antibiotic-susceptible and -resistant gram-negative bacteria and found that resistance was not associated with higher mortality [13]. In a Finnish cohort, Kontula et al. stated that MDR bacteria could not be interpreted as a risk for severe outcome in patients [26]. However, the proportion of BSIs caused by MDR microbes in these 2 North European countries could be considered too low to give a full picture of the impact of MDR. A larger study in patients admitted to European ICUs evaluated clinical outcomes of nosocomial infections and concluded that common patterns of antibiotic resistance only made a small contribution to the overall effect of these infections [27].
Another possible explanation for the discordance between these studies and ours could be that the other studies did not differentiate MDR bacteria from XDR bacteria. In particular, we demonstrated that XDR Pseudomonas remained independently associated with mortality for all study end points, with a risk of death 6 times higher for short-term mortality (7 days) and 3 times higher at 30 days. Our data are in line with 2 previous studies that concluded that mortality was associated with P. aeruginosa bacteremia [25, 28].
In our cohort, patients with XDR A. baumannii and carbapenem-resistant K. pneumoniae BSI had only a 2-fold risk of death compared with nonresistant bacteria of the same species (not included in the ESKAPE resistance definition). However, this impact was seen only at 30 days after BSI and not earlier. This could be explained by the lower virulence of Acinetobacter and Klebsiella spp. compared with Pseudomonas spp.[29]. In our subanalysis involving DTR gram-negative BSIs, this particular category of resistant bacteria presented as a risk factor for mortality. This result was in line with the American multicentric retrospective cohort analysis, which evaluated outcomes of patients with DTR in gram-negative bacteremia. In this major article, involving 173 US hospitals and 45 011 episodes, DTR was associated with decreased survival [17]. This study strengthens our hypothesis that resistance against multiple categories of antibiotics could have an impact on mortality per se.
E. coli was the single most common BSI isolate, with major prevalence of E. coli in community-onset BSI. Not surprisingly, gram-positive bacteria were more prevalent in nosocomial bacteremia, with CoNS as the major isolate, followed by S. aureus, as shown in previous studies [22, 30]. Interestingly, in our population, the prevalence of P. aeruginosa was similar in hospital-acquired and community-onset BSIs; this finding differs from those of studies conducted in the United States and Europe [22, 31].
Evaluating patients with BSIs caused by ESKAPE bacteria, we found that almost all XDR A. baumannii BSIs, three-quarters of XDR P. aeruginosa, and more than half of CR K. pneumoniae were acquired in the hospital. Comparing our BSI epidemiology data with the national data of invasive isolates in the last ECDC report [32], the rates of E. coli resistance to third-generation cephalosporins and K. pneumoniae resistance to fluoroquinolones, third-generation cephalosporins, and, above all, to carbapenems were lower than the national averages. Additionally, the decrease in resistance rates in our hospital can be explained by intensive antimicrobial stewardship and the infection control programs that have been in place since 2013 [33]. Finally, S. aureus and E. faecium also showed lower levels of resistance to methicillin and vancomycin when compared with the rest of Italy.
This is the first contemporary study examining mortality data on consecutive patients admitted to the hospital with community-onset and hospital-acquired BSIs. One strength of this study is its size. The cohort of >1000 patients with 21 months of observation is the largest conducted in Italy [34–37], providing not only a very relevant picture of Italian epidemiology, but also accurately showing the greater mortality burden of BSI linked to antimicrobial resistance.
The lack of uniform definitions has been a main limitation of previous published studies focusing on BSI outcomes in both hospital and community settings [25, 38–40]. In the present cohort, antimicrobial resistance was defined using the ECDC classification [20], dividing causative organisms into MDR and XDR, as well as ESKAPE pathogen resistance [21]. These definitions allowed us to perform an outcome evaluation after a precise stratification of different bacteria and patterns of resistance. Another important strength of our study is that we adjusted mortality rates for treatment factors, differentiating appropriate and inappropriate therapy and evaluating the number of days before starting appropriate therapy. In our patients with ESKAPE isolates, no significant differences were found. This could be explained by the use of the rapid molecular diagnostic test for carbapenem resistance and an efficient alert system, which reduced the time to starting appropriate therapy.
Finally, in this study, all baseline conditions of the patients were collected, allowing us to identify the severity of illness (Pitt bacteremia score) at BSI onset as the major independent factor related to mortality for all end points. We also demonstrated that underlying comorbidity conditions (as represented by Charlson score) of patients with BSI was a factor related to death. Patients with an increased number of comorbidities had a higher risk of poor outcome (especially death) after the first month following BSI. In addition, mortality data after discharge were accessible in the hospital system for all patients. The subanalysis on noncritical patients allowed us to identify risk factors for mortality that were not connected to severe conditions at BSI onset.
This study has some limitations: First, the quality of recorded data might have been affected by the retrospective design, although we examined mortality data for a significant number of consecutive patients. Second, we differentiated BSI as community- or hospital-acquired without identifying a category of “health care–associated” BSI (eg, patients living in long-term care facilities, patients with recent access to the hospital, patients in dialysis or followed in oncology day hospitals); however, these patients comprised only a small proportion of the total population. Third, we collected data from a single hospital, providing internal, but not external, validity. In addition, in the study period, there could have been suboptimal therapies for XDR-GNB, not including the novel agents against gram-negative bacteria that have recently become available. In our study, we found an association between mortality and XDR-GNB, independent of therapy, but further studies are needed to understand the potential role of novel agents against gram-negative bacteria. Finally, as the MDR BSIs had broad heterogeneity regarding causative agents and the combination of antimicrobial classes defining MDR, it remains uncertain as to whether the registered lack of association between MDR and mortality truly applies to all of the different BSI subgroups, due to different agents and/or different MDR profiles, in terms of the classes defining MDR. For this reason, the clinical implications of these findings may remain uncertain and be difficult to extrapolate to clinical practice.
CONCLUSIONS
Our study described 1526 BSIs in >1000 consecutive patients accessing a tertiary Italian hospital. The data confirmed that BSI is an event with an extreme impact on mortality. Patients with a severe clinical condition at BSI onset were those at higher risk of death. BSIs caused by MDR bacteria were not independently associated with increased mortality, but those caused by XDR bacteria had a strong association with death, independent of clinical severity. More specifically, XDR P. aeruginosa, XDR A. baumannii, and CR K. pneumoniae resistant to carbapenems were independently associated with crude mortality.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. No external funding was received.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Each author made substantial contributions to this paper. Antonella Santoro, Erica Franceschini, Marianna Meschiari, Giovanni Guaraldi, Mical Paul, Inge C. Gyssens, Cristina Mussini: conception and design of the study. Marianna Menozzi, Stefano Zona: analysis and interpretation of data. Claudia Venturelli, Margherita Digaetano, Carlotta Rogati: acquisition of data. Antonella Santoro, Inge C. Gyssens: writing the manuscript. Giovanni Guaraldi, Mical Paul, Inge C. Gyssens, Cristina Mussini: revision and final approval.
Ethical approval. The design of the study was approved by local ethical committees, and it conforms to standards currently applied in Italy (protocol number: AOU0008848/20; authorizing body: “Comitato Etico dell’Area Vasta Emilia Nord”).
Patient consent. In accordance with this approval, in large monocentric, retrospective, and noninterventional studies, patient consent is not required for data that already exist.
References
- 1. Søgaard M, Nørgaard M, Dethlefsen C, Schønheyder HC. Temporal changes in the incidence and 30-day mortality associated with bacteremia in hospitalized patients from 1992 through 2006: a population-based cohort study. Clin Infect Dis 2011; 52:61–9. [DOI] [PubMed] [Google Scholar]
- 2. Wilson J, Elgohari S, Livermore DM, et al. Trends among pathogens reported as causing bacteraemia in England, 2004-2008. Clin Microbiol Infect 2011; 17:451–8. [DOI] [PubMed] [Google Scholar]
- 3. Deen J, von Seidlein L, Andersen F, et al. Community-acquired bacterial bloodstream infections in developing countries in South and Southeast Asia: a systematic review. Lancet Infect Dis 2012; 12:480–7. [DOI] [PubMed] [Google Scholar]
- 4. Bartoletti M, Giannella M, Caraceni P, et al. Epidemiology and outcomes of bloodstream infection in patients with cirrhosis. J Hepatol 2014; 61:51–8. [DOI] [PubMed] [Google Scholar]
- 5. Trecarichi EM, Pagano L, Candoni A, et al. ; HeMABIS Registry—SEIFEM Group, Italy Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: an Italian multicentre prospective survey. Clin Microbiol Infect 2015; 21:337–43. [DOI] [PubMed] [Google Scholar]
- 6. Islas-Muñoz B, Volkow-Fernández P, Ibanes-Gutiérrez C, et al. Bloodstream infections in cancer patients. Risk factors associated with mortality. Int J Infect Dis 2018; 71:59–64. [DOI] [PubMed] [Google Scholar]
- 7. Silva M Jr, Marra AR, Pereira CA, et al. Bloodstream infection after kidney transplantation: epidemiology, microbiology, associated risk factors, and outcome. Transplantation 2010; 90:581–7. [DOI] [PubMed] [Google Scholar]
- 8. Bassetti M, Righi E, Carnelutti A. Bloodstream infections in the intensive care unit. Virulence 2016; 7:267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe 2017. 2018. Available at: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2017. Accessed 24 January 2020.
- 10. Russotto V, Cortegiani A, Graziano G, et al. Bloodstream infections in intensive care unit patients: distribution and antibiotic resistance of bacteria. Infect Drug Resist 2015; 8:287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alexopoulou A, Vasilieva L, Agiasotelli D, et al. Extensively drug-resistant bacteria are an independent predictive factor of mortality in 130 patients with spontaneous bacterial peritonitis or spontaneous bacteremia. World J Gastroenterol 2016; 22:4049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lye DC, Earnest A, Ling ML, et al. The impact of multidrug resistance in healthcare-associated and nosocomial gram-negative bacteraemia on mortality and length of stay: cohort study. Clin Microbiol Infect 2012; 18:502–8. [DOI] [PubMed] [Google Scholar]
- 13. Blot S, Vandewoude K, De Bacquer D, Colardyn F. Nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in critically ill patients: clinical outcome and length of hospitalization. Clin Infect Dis 2002; 34:1600–6. [DOI] [PubMed] [Google Scholar]
- 14. Rodríguez-Baño J, López-Prieto MD, Portillo MM, et al. ; SAEI/SAMPAC Bacteraemia Group Epidemiology and clinical features of community-acquired, healthcare-associated and nosocomial bloodstream infections in tertiary-care and community hospitals. Clin Microbiol Infect 2010; 16:1408–13. [DOI] [PubMed] [Google Scholar]
- 15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 16. Rhee JY, Kwon KT, Ki HK, et al. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the Pitt bacteremia score and the Acute Physiology and Chronic Health Evaluation II scoring systems. Shock 2009; 31:146–50. [DOI] [PubMed] [Google Scholar]
- 17. Kadri SS, Adjemian J, Lai YL, et al. ; National Institutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH–ARORI) Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis 2018; 67:1803–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. European Centre for Disease Prevention and Control. Questions and answers—point prevalence survey on healthcare-associated infections in acute care hospitals. Available at: https://www.ecdc.europa.eu/en/healthcare-associated-infections-acute-care-hospitals/facts/qa. Accessed 5 May 2020.
- 19. Leclercq R, Cantón R, Brown DF, et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin Microbiol Infect 2013; 19:141–60. [DOI] [PubMed] [Google Scholar]
- 20. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–81. [DOI] [PubMed] [Google Scholar]
- 21. Goto M, McDanel JS, Jones MM, et al. Antimicrobial nonsusceptibility of gram-negative bloodstream isolates, Veterans Health Administration system, United States, 2003–2013. Emerg Infect Dis 2017; 23:1815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kollef MH, Zilberberg MD, Shorr AF, et al. Epidemiology, microbiology and outcomes of healthcare-associated and community-acquired bacteremia: a multicenter cohort study. J Infect 2011; 62:130–5. [DOI] [PubMed] [Google Scholar]
- 23. Rodríguez-Baño J, Picón E, Gijón P, et al. Risk factors and prognosis of nosocomial bloodstream infections caused by extended-spectrum-beta-lactamase-producing Escherichia coli. J Clin Microbiol 2010; 48:1726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Michalopoulos A, Falagas ME, Karatza DC, et al. Epidemiologic, clinical characteristics, and risk factors for adverse outcome in multiresistant gram-negative primary bacteremia of critically ill patients. Am J Infect Control 2011; 39:396–400. [DOI] [PubMed] [Google Scholar]
- 25. Barrasa-Villar JI, Aibar-Remón C, Prieto-Andrés P, et al. Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin Infect Dis 2017; 65:644–52. [DOI] [PubMed] [Google Scholar]
- 26. Kontula KSK, Skogberg K, Ollgren J, et al. The outcome and timing of death of 17 767 nosocomial bloodstream infections in acute care hospitals in Finland during 1999-2014. Eur J Clin Microbiol Infect Dis 2018; 37:945–52. [DOI] [PubMed] [Google Scholar]
- 27. Lambert ML, Suetens C, Savey A, et al. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis 2011; 11:30–8. [DOI] [PubMed] [Google Scholar]
- 28. Dantas RC, Ferreira ML, Gontijo-Filho PP, Ribas RM. Pseudomonas aeruginosa bacteraemia: independent risk factors for mortality and impact of resistance on outcome. J Med Microbiol 2014; 63:1679–87. [DOI] [PubMed] [Google Scholar]
- 29. Sligl WI, Dragan T, Smith SW. Nosocomial gram-negative bacteremia in intensive care: epidemiology, antimicrobial susceptibilities, and outcomes. Int J Infect Dis 2015; 37:129–34. [DOI] [PubMed] [Google Scholar]
- 30. Hoenigl M, Wagner J, Raggam RB, et al. Characteristics of hospital-acquired and community-onset blood stream infections, South-East Austria. PLoS One 2014; 9:e104702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diekema DJ, Beekmann SE, Chapin KC, et al. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J Clin Microbiol 2003; 41:3655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2016. 2017. Available at: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2016. Accessed 29 January 2020.
- 33. Bedini A, Venturelli C, Meschiari M, et al. Impact of an antimicrobial stewardship programme on the trend of carbapenem prescriptions, carbapenem resistance rates and candidaemia at an Italian tertiary care hospital. Paper presented at: ECCMID 2019; 13–16 August 2019;. Amsterdam, the Netherlands. [Google Scholar]
- 34. Delle Rose D, Pezzotti P, Fontana C, et al. An in-depth analysis of nosocomial bloodstream infections due to gram-negative bacilli: clinical features, microbiological characteristics and predictors of mortality in a 1 year, prospective study in a large tertiary care Italian hospital. Infect Dis (Lond) 2019; 51:12–22. [DOI] [PubMed] [Google Scholar]
- 35. Cattaneo C, Di Blasi R, Skert C, et al. ; SEIFEM Group Bloodstream infections in haematological cancer patients colonized by multidrug-resistant bacteria. Ann Hematol 2018; 97:1717–26. [DOI] [PubMed] [Google Scholar]
- 36. Caselli D, Cesaro S, Fagioli F, et al. ; Infectious Diseases Study Group of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Incidence of colonization and bloodstream infection with carbapenem-resistant Enterobacteriaceae in children receiving antineoplastic chemotherapy in Italy. Infect Dis (Lond) 2016; 48:152–5. [DOI] [PubMed] [Google Scholar]
- 37. Busani S, Serafini G, Mantovani E, et al. Mortality in patients with septic shock by multidrug resistant bacteria: risk factors and impact of sepsis treatments. J Intensive Care Med 2019; 34:48–54. [DOI] [PubMed] [Google Scholar]
- 38. Laupland KB, Church DL. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev 2014; 27:647–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laupland KB, Gregson DB, Flemons WW, et al. Burden of community-onset bloodstream infection: a population-based assessment. Epidemiol Infect 2007; 135:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kanoksil M, Jatapai A, Peacock SJ, Limmathurotsakul D. Epidemiology, microbiology and mortality associated with community-acquired bacteremia in northeast Thailand: a multicenter surveillance study. PLoS One 2013; 8:e54714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.