Abstract
Background
Tuberculous meningitis (TBM) is a medical emergency, yet there are no standardized treatment guidelines for the medical or neurosurgical management of these patients and little data on neurocritical care. We conducted an international survey to understand current medical and neurosurgical TBM management and resource availability to provide baseline data needed for future multicenter trials addressing unanswered clinical research questions and the establishment of standardized guidelines.
Methods
An online survey of 77 questions covering medical and neurosurgical TBM management aimed at clinicians/nurses treating TBM was distributed as an anonymous link through email invitation, international organizations’ membership distribution, and direct links on organizational webpages or social media. The survey remained open for 5 months. Data were summarized with frequencies and percentages.
Results
The survey had 222 responses from 43 countries representing 6 continents. Most respondents were from tertiary care facilities, with broad access to medical and neurosurgical resources. There was significant heterogeneity in general supportive care, and TBM-specific management demonstrated considerable divergence from current standard-of-care practices. The lack of standardized guidelines was identified as a major challenge in TBM management. General and neurocritical care were largely absent. Resources for bedside supportive care and noninvasive monitoring were broadly accessible.
Conclusions
These findings suggest that current TBM management could be improved by the establishment of internationally accepted treatment guidelines based on available evidence, and that numerous centers have resources available to participate in future multicenter trials, even for basic interventions, that may further improve patient outcomes globally.
Keywords: international guidelines, survey, tuberculous meningitis
Tuberculous meningitis is a medical emergency, yet no standardized treatment guidelines exist. We surveyed treatment and resource availability from 43 countries, which demonstrated heterogeneity and divergence from standard of care. Resources for bedside supportive care and monitoring were broadly accessible.
Tuberculous meningitis (TBM) is the most fatal form of tuberculosis (TB) in adult and pediatric patients [1]. Treatment largely focuses on pharmacological intervention, including antibiotics and host-directed therapies. However, patient outcomes remain poor and treatment regimens are heterogeneous in terms of duration, dosing, and drugs used [2–7]. The treatment of TBM-associated hydrocephalus is also variable with some centers tailoring treatment based on whether the hydrocephalus is communicating, whereas others use surgical intervention uniformly [8, 9]. The evidence base to establish standardized medical and neurosurgical treatment guidelines is limited, and more work is required to establish comprehensive international guidelines.
Little to no investigation into supportive medical management and neurocritical care strategies has been conducted for TBM, despite evidence of ongoing brain injury in patients receiving antibiotic and steroid therapy [10]. Research from other forms of brain injury demonstrates the benefit of neurocritical care to patient outcome [11]. Critical care involves monitoring and responding to systemic variables (ie, temperature, nutrition, blood pressure) and cerebral variables (ie, intracranial pressure [ICP] and brain oxygenation), which can affect outcomes [12, 13]. Application of similar critical care measures in TBM could potentially improve outcome, and warrant investigation [14].
We conducted a survey of the current medical and neurosurgical measures available and used internationally. The aim was to establish what practices are currently being used, the degree of heterogeneity thereof, and the availability of resources. To date, this information has not been gathered on a global scale and would be valuable for the establishment of evidence-based guidelines for the acute medical and neurosurgical management of TBM through future multicenter research.
Participant Consent Statement
The survey began with written informed consent, and participants had to electronically indicate their willingness to participate. This survey was approved by the Johns Hopkins Medicine Institutional Review Board (IRB00152756) and the University of Cape Town Scientific and Human Research Ethics Committees (HREC 683/2017).
METHODS
The survey themes and questions were developed based on current management strategies in TBM and piloted by a group within the Tuberculous Meningitis International Research Consortium. The survey was compiled in English using the Qualtrics program (https://login.qualtrics.com/login) and was translated into Chinese by collaborators. The survey included 76 questions in 12 sections; the first 2 sections covered demographics and resources, which enabled skip logic to show the participant only questions relevant to their resources. The remaining sections focused on acute medical and neurosurgical management in the first 2 weeks of presentation, including general questions, neuroimaging, laboratory acquisition, and management of hydrocephalus, hyponatremia, oxygenation/ventilation, temperature, nutrition, hemoglobin, blood pressure, and pharmaceuticals. Most questions were closed-ended but allowed participants to “check all that apply.” There were 4 open-ended questions to allow respondents to enter their location and to comment on topics not addressed by previous questions. Open-ended questions were analyzed using qualitative directed content analysis to generate themes, and grouping was reached by consensus by 2 principal investigators [15]. The complete survey can be found in the Supplementary Material (Supplementary Text S1).
Distribution of the survey began on May 1, 2018 and involved a combination of individual email invitations, international organizations’ membership distribution, and direct links on organizational webpages or social media (ie, Twitter) via an anonymous link. The international organizations included the World Federation of Pediatric Intensive and Critical Care Societies (WFPICCS), World Federation Neurosurgery Society (WFNS), Pediatric Acute Lung Injury and Sepsis Investigators (PALISI)’s Global Health subgroup, International Society for Pediatric Neurosurgery (ISPN), Pediatric Neurocritical Care Research Group (PNCRG), Society of Neurosurgeons of South Africa (SNSA), German Society of Tropical Paediatrics and International Child Health (GTP), American Academy of Pediatrics’ Section on International Child Health (AAP SOICH), Pakistan Neurology Society (PNS), World Federation of Societies of Intensive and Critical Care Medicine (WFICC), and International Child Neurology Association (ICNA). Some authors were also survey respondents. The survey was closed on October 2, 2018.
Data were analyzed using IBM SPSS Statistics (IBM Corporation, Armonk, NY), and figures were made with CorelDRAW (Corel Corporation, Ottawa, CA). Respondent answers were summarized with frequencies and percentages for categorical variables. Partial responses were analyzed if ≥75% of questions were completed, but only surveys that were 100% completed had an identified location. Percentages were calculated based on the number of questions answered and applicable to the respondents’ resource availability.
RESULTS
Survey Demographics
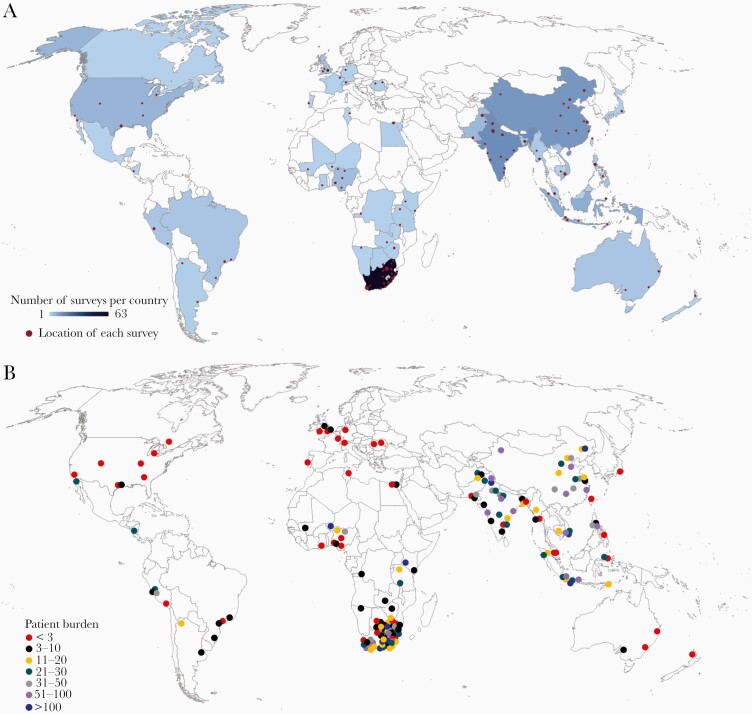
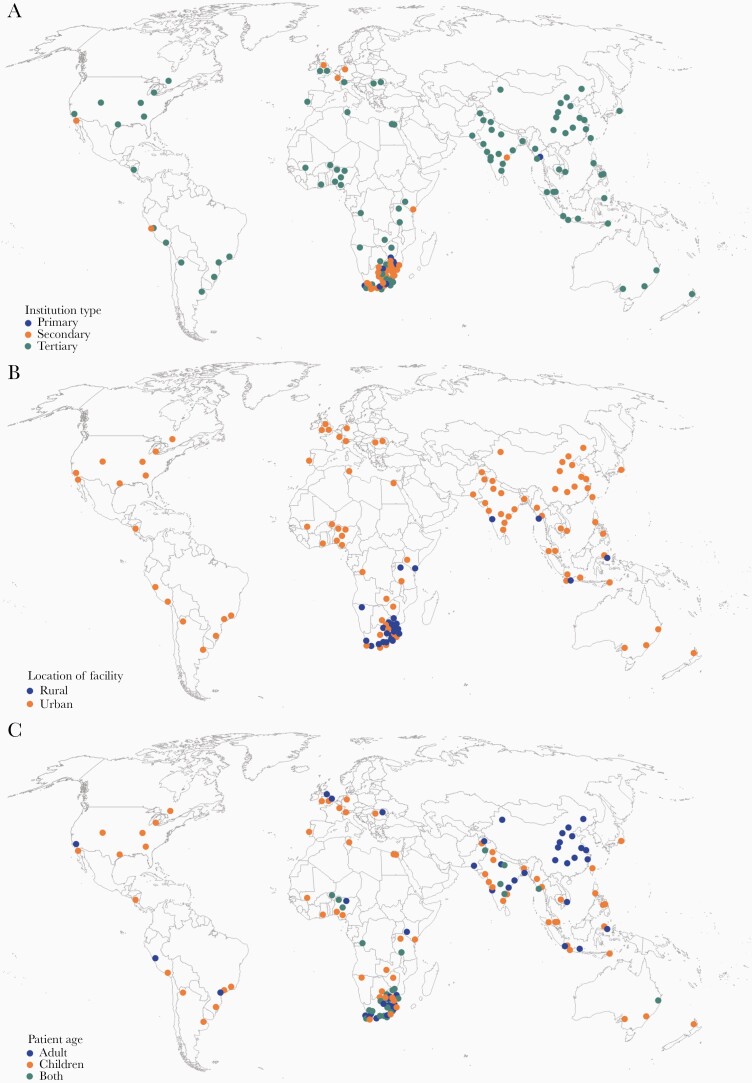
The survey was completed by 199 respondents from 43 countries representing 6 continents, with an additional 23 partial responses without a location included. South Africa, India, China, Indonesia, and Pakistan were the countries with the highest survey response (Figure 1 and Supplementary Table S1). The majority of participants were from tertiary care facilities in urban environments and almost half cared solely for children (Figure 2). Pediatric neurology (21.6%), neurosurgery (12.2%), adult infectious diseases (11.7%), and adult neurology (10.8%) were the top specialties, with >90% reporting active involvement in patient care (Supplementary Table S2). Half of respondents saw >11 patients a year, with 35% seeing >20 annually (Figure 1 and Supplementary Table S2). Most patients were hospitalized for >10 days. Supplementary Table S2 lists all survey respondent demographics.
Figure 1.
Survey respondent location and their reported patient burden. (A) Map of survey respondents, with red dot indicating location and size corresponding with number of respondents from each city. Darker blue indicates more responses per country. (B) Number of tuberculous meningitis patients per year cared for by respondents.
Figure 2.
International distribution of respondents. Global maps demonstrating survey respondent distribution by (A) type of facility (primary, secondary, tertiary), (B) location of facility (urban, rural), and (C) patient age (pediatric, adult, both).
Resources Availability and Utilization
Table 1 summarizes the availability and utilization of resources, with detailed information in Supplementary Material (Supplementary Tables S3 to S11). Laboratory studies, including blood, urine, and cerebrospinal fluid (CSF), were available to almost all participant sites and used largely at admission or when clinically indicated (Table 1 and Supplementary Table S3). Empiric diagnosis (based on clinical presentation, imaging, and/or routine CSF characteristics) was most common in conjunction with culture, Gram stain/acid-fast bacilli smear, or polymerase chain reaction (PCR), with the majority of PCR reported as GeneXpert (98%; Cepheid, Sunnyvale, CA). Neuroimaging was available to 90% of respondents, but admission imaging was performed by only 63%. Access to an intensive care unit (ICU) and to supportive noninvasive monitoring, such as blood pressure, oxygenation (ie, pulse oximetry), and temperature, was common. When ICU care was available, respondents reported high patient to nurse ratios, low ICU admission rates, and barriers to ICU care, most commonly bed shortage (Supplementary Table S11). Although currently there are no international guidelines available for TBM management, 40.5% of respondents reported access to a protocol for TBM, most commonly an institutional/hospital protocol (Supplementary Table S12).
Table 1.
Resource Availability and Utilization
| Availability | Utilization | |||
|---|---|---|---|---|
| Resource | Yes (%) | Yes (%) | Frequency | |
| Intensive Care Unit | ||||
| Admission | 183/222 (82.4) | 129/183 (70.5) | ||
| Noninvasive Monitoring | Hourly | Every 4 Hours | ||
| Blood pressure | 217/222 (97.7) | 33/217 (15.2) | 67/217 (30.9) | |
| Pulse oximetry | 214/221 (96.8) | |||
| End-tidal carbon dioxide | 143/219 (65.3) | 95/143 (66.4) | ||
| Temperature | 216/221 (97.7) | 27/216 (12.5) | 143/216 (66.2) | |
| Respiratory Supporta | ||||
| None (room air) | 77/217 (35.5) | |||
| Nasal cannula | 211/221 (95.4) | 100/211 (47.4) | ||
| High-flow nasal cannula | 140/218 (64.2) | 10/140 (7.1) | ||
| Continuous positive airway pressure | 178/219 (81.3) | 2/178 (1.1) | ||
| Bilevel positive airway pressure | 153/218 (70.2) | 2/153 (1.3) | ||
| Ventilator | 192/220 (87.2) | 22/192 (11.5) | ||
| Medical Intervention | ||||
| Transfusion | 213/222 (95.9) | 167/213 (78.4) | ||
| Intravenous antibiotic | 218/222 (98.2) | |||
| Intravenous antiretroviral | 211/222 (95) | |||
| Noninvasive Neuroimaginga | Admission Only | ≥2 Occasions | ||
| All imaging | 127/202 (62.9) | 124/202 (61.4) | ||
| Computed tomography | 203/222 (91.4) | 166/203 (81.8) | ||
| Magnetic resonance imaging | 172/222 (77.5) | 117/172 (68) | ||
| Cranial ultrasound | 148/220 (67.3) | 22/148 (14.9) | ||
| Angiography | 142/222 (63.9) | 14/142 (9.9) | ||
| Ocular ultrasound | 74/212 (34.9) | |||
| Noninvasive Neuromonitoringa | ||||
| Near-infrared spectroscopy | 38/215 (17.7) | |||
| Transcranial doppler | 90/219 (41.1) | |||
| Electroencephalogram | 132/220 (60) | |||
| Neurosurgery | ||||
| Consult | 199/221 (90) | |||
| Neurosurgical Interventiona | Measure ICP | Treat ICP | ||
| Ventriculoperitoneal shunt | 167/222 (75.2) | 122/167 (73.1) | ||
| Lumbar drain | 136/220 (61.8) | 41/136 (30.1) | ||
| Lumbar puncture | 156/209 (74.6) | 79/222 (35.6) | ||
| External ventricular drain | 135/220 (61.4) | 71/135 (52.9) | 107/135 (79.3) | |
| Endoscopic third ventriculostomy | 109/217 (50.2) | 47/179 (26.3) | ||
| Invasive Neuromonitoring | ||||
| Intraparenchymal intracranial pressure catheter | 80/216 (37) | |||
| Partial brain tissue oxygen tension | 29/215 (13.5) | |||
| System Resources | ||||
| Treatment protocol | 90/222 (40.5) | |||
| Any medical record | 160/222 (72) | |||
| Electronic medical record | 62/222 (27.9) | |||
| Research database | 76/222 (34.2) | |||
| Blood Laboratory Testsa | Admission | If Clinically Indicated | ||
| All laboratory tests | 215/221 (97.2) | |||
| Basic metabolic profileb | 107/214 (50) | 78/214 (36.4) | ||
| Liver function testc | 103/214 (48.1) | 86/214 (40.1) | ||
| Kidney function testd | 109/214 (50.9) | 79/214 (36.9) | ||
| Complete blood counte | 117/220 (53.2) | 79/220 (35.9) | ||
| Blood gas | 63/212 (29.7) | 128/212 (60.4) | ||
| Coagulationf | 70/214 (32.7) | 130/214 (60.7) | ||
| Serum osmolality | 29/211 (13.7) | 127/211 (60.2) | ||
| HIV testing | 206/222 (92.8) | |||
| Urine Laboratory Testsa | Admission | Clinically Indicated | ||
| All laboratory tests | 214/220 (97.2) | |||
| Urine sodium | 12/211 (5.6) | 131/211 (60.2) | ||
| Urine osmolality | 14/212 (6.6) | 131/212 (61.8) | ||
| CSF Laboratory Testsa | Admission | Every LP | Clinically Indicated | |
| All laboratory tests | 215/222 (96.8) | |||
| Protein | 141/213 (66.2) | 79/213 (37) | 66/213 (30.9) | |
| Cell count | 140/213 (65.7) | 78/213 (36.6) | 65/213 (30.5) | |
| Glucose | 140/212 (66) | 79/212 (37.2) | 65/212 (30.6) | |
| Gram stain/culture | 143/212 (67.5) | 78/212 (36.7) | 66/212 (31.1) | |
| TBM Diagnosticsa,g | Admission | |||
| Empiric | 142/221 (64.2) | |||
| Culture | 133/221 (60.1) | |||
| Gram stainh | 113/221 (51.1) | |||
| Polymerase chain reactioni | 135/221 (61) | |||
| Interferon gamma release assay | 9/221 (4.1) | |||
| Adenosine deaminase | 2/221 (0.9) | |||
| Biopsy | 1/221 (0.5) | |||
Abbreviations: CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; ICP, intracranial pressure; LP, lumbar puncture; TBM, tuberculous meningitis.
NOTE: The response “yes” to availability of resource combines respondents that answered “always” and “sometimes” to access. All blank cells reflect data that was not available or applicable, such as frequency of utilization for continuous monitoring (eg, pulse oximetry).
aMore than 1 response was allowed per question.
bSodium, potassium, and glucose.
cAlanine transaminase and aspartate transaminase.
dCreatinine and blood urea nitrogen.
eFull blood count, hemoglobin, and platelet.
fProthrombin time and partial thromboplastin time.
gOnly utilization, not availability, of TBM diagnostic was surveyed here.
hGram stain includes acid-fast bacilli smear.
iAll but 4 of the polymerase chain reactions were GeneXpert (Cepheid, Sunnyvale, CA).
Due to the high burden of disease and high survey response rate, a subanalysis of responses from tertiary care centers in China, India, and South Africa was performed for TBM-specific resource availability and utilization (Table 2). Although access to neuroimaging, ICU care, and neurosurgical management were similar across all 3 countries, South African centers used these resources less. In addition, centers in China (100%) and India (70.6%) more commonly reported managing patients according to a protocol, whereas this was reported in less than half of South African centers (40.7%).
Table 2.
TBM-Specific Resource Availability and Utilization in Tertiary Centers in China, India, and South Africa
| Resource | China | India | South Africa |
|---|---|---|---|
| Yes (%) | Yes (%) | Yes (%) | |
| Resource Availability | |||
| General | |||
| Intensive care unit | 15/16 (93.8) | 17/17 (100) | 26/27 (96.3) |
| Treatment protocol | 16/16 (100) | 12/17 (70.6) | 11/27 (40.7) |
| Noninvasive Neuroimaginga | |||
| Computed tomography | 16/16 (100) | 16/17 (94.1) | 26/27 (96.3) |
| Magnetic resonance imaging | 16/16 (100) | 16/17 (94.1) | 24/27 (88.9) |
| Neurosurgerya | |||
| Neurosurgery consult | 15/16 (93.8) | 17/17 (100) | 26/27 (96.3) |
| Ventriculoperitoneal shunt | 13/16 (81.3) | 16/17 (94.1) | 23/27 (85.2) |
| Lumbar drain | 13/15 (86.7)* | 14/17 (82.4) | 18/27 (66.7) |
| External ventricular drain | 15/16 (93.8) | 15/17 (88.2) | 20/27 (74.1) |
| Endoscopic third ventriculostomy | 10/16 (62.5) | 16/17 (94.1) | 16/26 (61.5)* |
| Laboratory Tests | |||
| All blood laboratory tests | 16/16 (100) | 16/17 (94.1) | 26/27 (96.3) |
| All urine laboratory tests | 16/16 (100) | 16/17 (94.1) | 26/27 (96.3) |
| All CSF laboratory tests | 16/16 (100) | 16/17 (94.1) | 26/27 (96.3) |
| Resource Utilization | |||
| Noninvasive Neuroimaginga | |||
| Computed tomography | 11/16 (68.8) | 12/16 (75) | 25/26 (96.2) |
| Magnetic resonance imaging | 16/16 (100) | 15/16 (93.8) | 5/26 (19.2) |
| TBM Diagnosisa,b | |||
| Empiric | 4/16 (25) | 9/17 (52.9) | 17/27 (63) |
| Culture | 15/16 (93.8) | 7/17 (41.2) | 14/27 (51.9) |
| Gram stainc | 16/16 (100) | 5/17 (29.4) | 9/27 (33.3) |
| Polymerase chain reactiond | 10/16 (62.5) | 10/17 (58.8) | 14/27 (51.9) |
| ICP Monitoringa | |||
| External ventricular drain transduced | 0/15 (0)* | 4/14 (28.6)* | 4/26 (15.4)* |
| Lumbar puncture | 15/15 (100)* | 12/14 (85.7)* | 26/26 (100)* |
| Intraparenchymal intracranial pressure catheter | 0/15 (0)* | 5/14 (35.7)* | 1/26 (3.8)* |
| ICP Managementa | |||
| Ventriculoperitoneal shunt | 10/14 (71.4)* | 15/17 (88.2) | 17/25 (68)* |
| External ventricular drain | 11/14 (78.6)* | 15/17 (88.2) | 13/25 (52)* |
| Endoscopic third ventriculostomy | 2/14 (14.3)* | 7/17 (41.2) | 5/25 (20)* |
| Lumbar drain | 8/14 (57.1)* | 3/17 (17.6) | 2/25 (8)* |
| Lumbar puncture | 8/16 (50) | 4/17 (23.5) | 16/27 (59.3) |
| Diuretics | 15/16 (93.8) | 10/17 (58.8) | 12/27 (44.4) |
| Osmotics | 16/16 (100) | 17/17 (100.0) | 16/27 (59.3) |
Abbreviations: CSF, cerebrospinal fluid; ICP, intracranial pressure; TBM, tuberculous meningitis.
NOTE: The availability of resource combines respondents that answered “always” and “sometimes” to access.
aMore than 1 response was allowed per question.
bOnly utilization, not availability, of TBM diagnostics.
cGram stain includes acid-fast bacilli smear.
dAll but 1 of the polymerase chain reactions were GeneXpert (Cepheid, Sunnyvale, CA).
*Indicates questions for which some responses were missing.
General Supportive Management
The most common target values in medical management reported by respondents demonstrated significant heterogeneity (see Supplementary Tables S5 for summary and S6 to S10 for complete lists).
Hemodynamics
Blood pressure could be monitored by almost all respondents and was monitored several times per day (Tables 1 and Supplementary Tables S6). Hypotension was treated with fluid boluses or vasoactive medications, of which dopamine was the most common (Supplementary Table S6).
Oxygen/Ventilation
Pulse oximetry was more frequently available than end-tidal carbon dioxide (ETCO2) and used to monitor adequate ventilation (Tables 1 and Supplementary Table S7). Nasal cannula/prongs were the most common respiratory support devices (Table 1).
Temperature
Temperature was frequently measured, at least every 3–4 hours, by transcutaneous, ear, or oral thermometers (Tables 1 and Supplementary Table S8). Paracetamol and ibuprofen were commonly used to treat hyperpyrexia (Supplementary Table S8).
Nutrition
Nutrition was largely given orally or administered via nasogastric tube. Oral feeding safety was assessed by clinical exam or less commonly by a speech consultant or swallow test (Supplementary Table S9). Glucose levels were monitored daily.
Anaemia and Transfusion
Most respondents reported the ability to transfuse TBM patients, although the goal hemoglobin demonstrated heterogeneity across centers (Supplementary Tables S5 and S10).
Tuberculous Meningitis-Specific Management
Tuberculous meningitis-specific patient management was heterogeneous, and some respondents reported divergence from current standard of care as summarized in Table 3.
Table 3.
Clinical Management of TBMa
| Clinical Management | Standard of Care | Reference | Survey Results |
|---|---|---|---|
| TBM Diagnosis | |||
| Empiric TBM diagnosis | • Empiric diagnosis is based on CSF parameters, imaging, clinical exam, and history | • As referenced in Panel 1 of Marais et al [29] | • Only ~66% report obtaining CSF studies at admission, but 97% have access to CSF laboratory tests |
| • As referenced in Table 2 of Wilkinson et al [1] | • Only 60% report performing a Gram stain, culture or PCR on CSF | ||
| • Only 63% report performing neuroimaging at admission, but 91% have access to CT and 76% to MRI | |||
| Antimicrobial Management | |||
| First-line antibiotics | • First-line antibiotics for drug-susceptible TBM are isoniazid, rifampin, pyrazinamide, and ethambutol | • WHO’s Guidelines for Treatment of Drug-Susceptible Tuberculosis and Patient Care [30] | • Only 78% use all 4 first-line drugs |
| Antiretroviral (ART) in ART-naive HIV coinfection | • In newly diagnosed HIV, TB treatment should be initiated first, followed by ART (strong recommendation, high certainty in the evidence as per WHO) | • WHO’s Guidelines for Treatment of Drug-Susceptible Tuberculosis and Patient Care [30] | • 11% initiate ART simultaneously with TB treatment instead of waiting until after TB treatment initiation |
| • ART should be initiated regardless of CD4 cell count (strong recommendation, high certainty in the evidence as per WHO) | • Török et al [19] | • 8% only initiate ART depending on CD4 cell count | |
| Host-Directed Therapy Management | |||
| Corticosteroid initiation | • Adjuvant corticosteroids are recommended at treatment initiation (strong recommendation, moderate certainty in the evidence) | • WHO’s Guidelines for Treatment of Drug-Susceptible Tuberculosis and Patient Care [30] | • 3% never use corticosteroids |
| • Schoeman et al [31] | |||
| • Corticosteroids are recommended regardless of the severity of illness and have decreased mortality in adults and children | • Thwaites et al [2] | ||
| Corticosteroid taper | • Corticosteroid should be tapered over 6–8 weeks (strong recommendation, moderate certainty in the evidence as per WHO) | • WHO’s Guidelines for Treatment of Drug-Susceptible Tuberculosis and Patient Care [30] | • Only 24% taper corticosteroids over 6–8 weeks |
| Hydrocephalus Diagnosis | |||
| Hydrocephalus | • Hydrocephalus increases the risk of poor outcome, especially if undiagnosed | • As reviewed by Wen et al [32] | • Only 63% perform neuroimaging at admission, 77% during hospitalization, and 61% for ≥2 occasions |
Abbreviations: CSF, cerebrospinal fluid; CT, computed tomography; HIV, human immunodeficiency virus; MRI, magnetic resonance imaging; PCR, polymerase chain reaction; TB, tuberculosis; TBM, tuberculous meningitis; WHO, World Health Organization.
aClinical management (ie, empiric TBM diagnosis, antimicrobial usage, host-directed therapy management, and hydrocephalus diagnosis) reported by survey respondents compared with current standard of care in literature.
Pharmaceutical Management
Pharmaceutical management included antibiotics, antiretrovirals (ARTs), corticosteroids, and other host-directed therapies (Supplementary Table S13). Approximately 70% of respondents used all first-line antibiotics (per the World Health Organization), and over 40% used at least 1 second-line antibiotic, mostly fluoroquinolones [16]. Antibiotic duration was commonly 12 months, and steroid duration was 6 weeks usually with a 1- to 2-week taper. Most respondents waited 2 to 4 weeks after antibiotic initiation before starting ARTs in human immunodeficiency virus (HIV) coinfected patients; however, 11% did not. Corticosteroids were used by 97% of respondents, although choice of corticosteroid was heterogeneous.
Intracranial Pressure and Hydrocephalus Management
More than 80% of respondents reported measuring ICP, mostly with a lumbar puncture ([LP] Table 1 and Supplementary Table S14). Lumbar punctures were most often repeated when clinically indicated, although only 58.3% recorded opening pressure with each LP. Most respondents tested for communicating hydrocephalus, most commonly using imaging. Air encephalogram and a column test were less commonly used (Supplementary Table S14). Ventriculoperitoneal shunts and external ventricular drains were the most common neurosurgical interventions for elevated ICP (Supplementary Table S14). Medical treatments of raised ICP included raising the head of the bed, seizure control, and hypertonic saline or mannitol, and less commonly temperature control, diuretics, hyperventilation, and frequent LPs (Supplementary Table S14).
Hyponatremia Management
Clinical examination and urine output were used to determine fluid balance (Supplementary Figure S1A). Treatment of hyponatremia was most often initiated for serum sodium <130 and hypertonic saline was most commonly used, irrespective of the underlying etiology (Supplementary Figure S1B and C). However, fluid restriction was more common in syndrome of inappropriate antidiuretic hormone ([SIADH] 61.8%) than cerebral salt wasting ([CSW] 31.8%). Rarely, hyponatremia was treated with transfusions, fludrocortisone, or vasopressin/vasopressin receptor analogs (Supplementary Table S15 and Supplementary Figure S1C).
Open-Ended Questions
Three open-ended questions allowed respondents to highlight themes not addressed by the survey, with representative quotes listed in Supplementary Table S16.
“What other imaging, laboratory or clinical parameters do you monitor in TBM patients that were not previously asked about?”—Three themes emerged: (1) neurological clinical exam and neuromonitoring, (2) investigation of extrapulmonary sites, and (3) TB screening of contacts.
“What other treatments (that were not previously asked about) do you use in patients with TBM?”—The common themes were as follows: (1) additional antimicrobials, (2) other pharmaceutical management, (3) ancillary support (ie, physical/occupational therapy and social work), and (4) supportive technology and/or invasive procedures (ie, biopsy/resection, dialysis, and gastrostomy tube).
“ What are the biggest challenges facing your facility in managing patients with TBM?”—Common concerns were as follows: (1) diagnostic challenges, (2) inadequate access to specialized neurosurgical/neurocritical care, (3) general lack of experience and standardized guidelines, (4) management of HIV and comorbidities, (5) antimicrobial management (ie, access, drug delivery/brain penetration, duration of therapy, drug resistance, compliance, and drug toxicities), (6) limited resources (human, physical, and financial), and (7) socioeconomic constraints.
DISCUSSION
The survey had broad global reach with representation from high-burden countries. Two main findings were as follows: (1) notable heterogeneity across all domains of TBM management, including access and utilization of resources, with some respondents diverging from current standards of care; and (2) demonstration of geographic resource availability that could aid in future clinical trials to establish treatment guidelines. Persistent knowledge gaps in medical and neurosurgical care are summarized in Table 4, including clinical trials (both past and ongoing) and required resources to address these questions (see Supplementary Table S17 for expanded version of Table 4).
Table 4.
Outstanding Clinical Research Questions in the Medical and Surgical Management of TBM
| Clinical Management | Research Question | Additional Resourcesa | Randomized Controlled Trialsb |
|---|---|---|---|
| Antimicrobial Management | |||
| Antibiotic choice and dosage | • What is the optimal antibiotic regimen for TBM? | • Laboratory | • Past Publications (Clinical Trial Name) |
| ◦Serum and CSF collection | ◦Te Brake et al [20]; Ruslami et al [3]; Dian et al [21]; Heemskerk et al [4] | ||
| • What is the optimal dose in adults and children? | ◦Long-term sample storage | • Ongoing Study by Clinical Trial Name | |
| ◦Drug concentration analysis | ◦Optimizing Antituberculosis Therapy in Adults with Tuberculous Meningitis; TBM-KIDS; INTENSE-TBM; LASER-TBM; ALTER; SIMPLE; Harvest Trial—Improving Outcomes from TB Meningitis with High Dose Oral Rifampicin; Is Levofloxacin Better than Rifampicin in the Treatment of Tuberculous Meningitis, a Randomized Control Study; ESCALATE; High Dose Oral and Intravenous Rifampicin for Improved Survival of Adult Tuberculous Meningitis: a Phase II Open-Label Randomised Controlled Trial | ||
| • Pharmaceuticals | |||
| ◦Antibiotics | |||
| Antibiotic duration | • What is the optimal treatment duration in adults and children? | • Laboratory | • Past Publications (Clinical Trial Name) |
| ◦As above | ◦Reviewed in Jullien et al [33]c | ||
| • Pharmaceuticals | • Ongoing Study by Clinical Trial Name | ||
| ◦Antibiotics | ◦SURE; Determination of Efficacy of 9 Months, 12 Months and 18 Months of Anti-tubercular Treatment (ATT) for Tuberculous Meningitis (TBM): a Randomized Controlled, Noninferiority Trial | ||
| Antibiotic route of administration | • What is the optimal route of administration, oral or intravenous? | • Laboratory | • Past Publications (Clinical Trial Name) |
| ◦As above | ◦Ruslami et al [3] | ||
| • Pharmaceuticals | • Ongoing Study by Clinical Trial Name | ||
| ◦Antibiotics | ◦LASER-TBM; High Dose Oral and Intravenous Rifampicin for Improved Survival of Adult Tuberculous Meningitis: a Phase II Open-Label Randomised Controlled Trial | ||
| Antiretroviral (ART) initiation | • What is the optimal timing of ART initiation? | • Laboratory | • Past Publications (Clinical Trial Name) |
| ◦As above | ◦Török et al [19]; Laureillard et al [34]d | ||
| • Pharmaceuticals | • Ongoing Study by Clinical Trial Name | ||
| ◦ARTs | ◦None | ||
| Host-Directed Therapy | |||
| Corticosteroid duration | • What is the optimal duration of corticosteroid use? | • Laboratory | • Past Publications (Clinical Trial Name) |
| ◦As above | ◦Török et al [35] | ||
| • Pharmaceuticals | • Ongoing Study by Clinical Trial Name | ||
| ◦Corticosteroids | ◦Leukotriene A4 Hydrolase Stratified Trial of Adjunctive Corticosteroids for HIV-uninfected Adults with Tuberculous Meningitis; The ACT HIV Trial | ||
| Corticosteroids route of administration | • What is the optimal route of administration, oral or intravenous? | • Laboratory | • Past Publications (Clinical Trial Name) |
| ◦As above | ◦None | ||
| • Pharmaceuticals | • Ongoing Study by Clinical Trial Name | ||
| ◦Corticosteroids | ◦Comparison of Regimens of Dexamethasone in Tubercular Meningitis—a Randomized Control Trial | ||
| Corticosteroid taper | • What is the optimal duration of corticosteroid taper? | • Laboratory | • Past Publications (Clinical Trial Name) |
| ◦As above | ◦None | ||
| • Pharmaceuticals | • Ongoing Study by Clinical Trial Name | ||
| ◦Corticosteroids | ◦None | ||
| Aspirin | • What is the role of aspirin as a host-directed therapy? | • Laboratory | • Past Publications (Clinical Trial Name) |
| ◦As above | ◦Mai et al [22]; Schoeman et al [6]e | ||
| • What is the optimal dose and duration? | • Pharmaceuticals | • Ongoing Study by Clinical Trial Name | |
| ◦Aspirin | ◦INTENSE-TBM; LASER-TBM; SURE | ||
| Thalidomide | • What is the role of thalidomide as a host-directed therapy? | • Laboratory | • Past Publications (Clinical Trial Name) |
| ◦As above | ◦Schoeman et al [7]e | ||
| • What is the optimal dose and duration? | • Pharmaceuticals | • Ongoing Study by Clinical Trial Name | |
| ◦Thalidomide | ◦None | ||
| Other host-directed therapies | • What is the role of other host-directed therapies in TBM? | • Laboratory | • Past Publications (Clinical Trial Name) |
| ◦As above | ◦None | ||
| • Pharmaceuticals | • Ongoing Study by Clinical Trial Name | ||
| ◦Other (ie, Indomethacin) | ◦An Open Label Randomized Study to Evaluate Effectiveness of Indomethacin in Tuberculous Meningitis | ||
| Intracranial Pressure (ICP) Management | |||
| ICP management | • How should ICP be measured? | • Neurosurgical Expertise | • Past Publications (Clinical Trial Name) |
| • How frequently should ICP be measured? | ◦ICP monitoring | ◦None | |
| • How should ICP be managed? | • Neuromonitoring | • Ongoing Study by Clinical Trial Name | |
| • What is the optimal ICP target in TBM? | ◦ICP (brain oxygenation) | • None | |
| • Neuroimaging | |||
| • Should the target be age specific? | • Equipment | ||
| ◦Manometer | |||
| • Cardiovascular Monitoring | |||
| ◦Blood pressure, heart rate | |||
| • Pharmaceuticals | |||
| ◦Diuretic, sedation/paralytic, antiepileptic, osmotic agent | |||
| Hydrocephalus diagnosis | • How should communicating vs noncommunicating hydrocephalus be diagnosed? | • Neurosurgical Expertise | • Past Publications (Clinical Trial Name) |
| • Neuroimaging | ◦None | ||
| ◦CT, MRI, X-ray (skull) | • Ongoing Study by Clinical Trial Name | ||
| • Equipment | ◦None | ||
| ◦Manometer | |||
| Hydrocephalus medical and surgical management | • What should be the standard of care in medical and surgical management of hydrocephalus? | • Neurosurgical Expertise | • Past Publications (Clinical Trial Name) |
| ◦Surgical intervention | ◦None | ||
| • Neuromonitoring | • Ongoing Study by Clinical Trial Name | ||
| ◦ICP monitor | ◦None | ||
| • What are the indications for medical vs surgical management? | • Neuroimaging | ||
| • Equipment | |||
| ◦Manometer | |||
| • Pharmaceuticals | |||
| ◦Diuretic | |||
| Hyponatremia Management | |||
| Hyponatremia diagnosis | • How should the etiology of hyponatremia be investigated? | • Laboratory | • Past Publications (Clinical Trial Name) |
| ◦Electrolytes (serum/urine) | ◦Misra et al [28]; Misra et al [36]c | ||
| • Fluid Balance Monitoring | • Ongoing Study by Clinical Trial Name | ||
| ◦Invasive or noninvasive | ◦None | ||
| Hyponatremia management | • What should be the standard of care in hyponatremia management when etiology is cerebral salt wasting or syndrome of inappropriate antidiuretic hormone? | • Laboratory | • Past Publications (Clinical Trial Name) |
| ◦Electrolytes (serum/urine) | ◦Misra et al [37] | ||
| • Fluid Balance Monitoring | • Ongoing Study by Clinical Trial Name | ||
| ◦Invasive or noninvasive | ◦None | ||
| • Pharmaceuticals | |||
| ◦Sodium supplementation | |||
| Neurocritical Care Management | |||
| Temperature management | • How should temperature be monitored and frequency? | • Temperature Monitoring | • Past Publications (Clinical Trial Name) |
| ◦Continuous or noncontinuous | ◦None | ||
| • What is the target temperature and how should temperature be managed? | • Pharmaceuticals | • Ongoing Study by Clinical Trial Name | |
| ◦Antimicrobials, corticosteroids, antipyretics | ◦None | ||
| Blood pressure (BP) management | • How should BP be monitored? | • Blood Pressure Monitoring | • Past Publications (Clinical Trial Name) |
| • What is the target BP? | ◦Invasive or noninvasive | ◦None | |
| • How should BP be managed? | • Neuromonitoring | • Ongoing Study by Clinical Trial Name | |
| ◦ICP to establish goal CPP | ◦None | ||
| • Pharmaceuticals | |||
| ◦Vasopressor | |||
| Respiratory management | • How should ventilation/oxygenation be monitored and managed? | • General Monitoring | • Past Publications (Clinical Trial Name) |
| ◦BP, pulse oximetry | ◦None | ||
| • Neuromonitoring | • Ongoing Study by Clinical Trial Name | ||
| • What is the target end-tidal carbon dioxide level (ETCO2)? | ◦Consider brain oxygenation | ◦None | |
| • Equipment | |||
| ◦Ventilator, nasal cannula, ETCO2, arterial blood gas | |||
| • What is the target oxygen? | • Pharmaceuticals | ||
| ◦Sedation/paralytics | |||
| Seizure management | • How should seizures be monitored? | • Noninvasive Monitoring | • Past Publications (Clinical Trial Name) |
| ◦Temperature, EEG | ◦None | ||
| • How should seizures be managed? | • Neuromonitoring | • Ongoing Study by Clinical Trial Name | |
| • Laboratory | ◦None | ||
| ◦Electrolytes (serum) | |||
| • Pharmaceuticals | |||
| ◦Antiepileptic |
Abbreviations: ATT, antitubercular treatment; CPP, cerebral perfusion pressure; CSF, cerebrospinal fluid; CT, computed tomography; EEG, electroencephalogram; HIV, human immunodeficiency virus; MRI, magnetic resonance imaging; RCT, randomized controlled trial; TBM, tuberculous meningitis.
NOTE: Summary of outstanding questions regarding the medical management of TBM, the necessary resources, and relevant past and ongoing RCTS. Previous clinical trials and their associated publications highlight RCTs and publications since 2010 unless no RCTs exist or only earlier studies are available.
aAdditional resources are those beyond resources for diagnosis, patient management (general clinical care, monitoring, and staff), trial support.
bIn Supplementary Table S17, RCT acronyms are spelled out and listed with clinical trial identifier and reflect those registered by the National Institutes of Health’s US National Library of Medicine (clinicaltrials.gov) or World Health Organization’s International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/Default.aspx).
cNo RCTs available.
dThis RCT included all patients with TB, including pulmonary and TBM.
eNo clinical trial identifier available but included due to paucity of data in pediatrics.
Access to Resources
This survey demonstrated that many laboratory tests, neuroimaging, and noninvasive monitoring were broadly accessible. This provides a promising framework for future trials to address unanswered management questions, although trial funding would be needed to compensate for resource limitations. Access to resources alone does not account for all variability in utilization, which was demonstrated by the subanalysis of China, India, and South Africa where South African tertiary centers used ICU care, neuroimaging, and neurosurgery less, possibly due to a higher burden on available resources. It is regrettable that few respondents reported using a research database. Databases that can be shared across centers would be invaluable in the development of evidence-based guidelines, and approaches to standardize data and sample collection for comparison across centers have been published by the Tuberculous Meningitis International Research Consortium [17, 18].
Current Patient Management
Lack of standardized international TBM treatment protocols represents one of the “biggest challenges” in management. National or organizational protocols mentioned did not deal with supportive management, and few suggested neurosurgical intervention. Furthermore, as demonstrated in Table 3, numerous respondents diverged from current standards of care in terms of diagnosis and clinical management. Despite almost universal access to laboratory investigations, one third of respondents reported not testing CSF on admission. Cerebrospinal fluid is vital to the empiric and laboratory diagnosis of TBM and raises the question of whether there is over- and underdiagnosis of TBM. Likewise, neuroimaging is key to empiric diagnosis and the assessment of hydrocephalus, yet it was used by only 63% of respondents, although it was accessible to 91%. In addition, only 78% of respondents used all 4 first-line antibiotics for drug-susceptible TBM and 3% did not use corticosteroids, although this is the internationally accepted standard of care. Furthermore, 11% of respondents initiated ART simultaneously with antibiotics, even though this is known to increase the risk of an immune reconstitution inflammatory syndrome response in ART-naive patients [19]. These departures from the standard of care could be a symptom of the absence of internationally accepted guidelines. It is interesting to note that in the subanalysis of tertiary centers across China, India, and South Africa, China and India reported broader use of treatment protocols and more uniform use of resources. Establishment of clear guidelines for optimal TBM management could improve care now and in the future.
Addressing Unanswered Clinical Research Questions
Pharmaceutical Care
Survey data show heterogeneity in the choice of antibiotics, route of administration, dosage, and duration of treatment. Similarly host-directed therapies were used variably. Numerous studies to address these questions are already underway (Table 4), and findings suggest that higher doses of rifampicin and intravenous administration may be beneficial [3, 20, 21]. Work on corticosteroids aims to understand how they improve mortality and determine whether host-associated characteristics play a role. In HIV-coinfected patients, research on how to optimally incorporate steroids in the treatment regimen is ongoing. New additions to host-directed therapy such as aspirin are being tested, and novel targets are likely to be elucidated by further research [22].
Neurocritical and Supportive Care
Supportive general and neurocritical care are the standard of care for numerous forms of brain injury [11–13]. However, it appears rare in TBM despite the high rate of neurological injury, and respondents reported this lack of specialized neurocritical care as one of the biggest challenges to TBM management. Neurocritical care is not limited to the ICU and can include basic, but effective, treatment at ward level. Resources for bedside supportive management and noninvasive monitoring were available in most respondents’ centers. Therefore, basic interventions could be feasible for TBM-endemic settings (Table 4). For example, blood pressure, pulse oximetry, and temperature monitoring are widely available, suggesting that goals to avoid hypotension, hypoxia, and hyperpyrexia could be investigated. Although TBM is often characterized by weight loss and malnutrition affects antibiotic pharmacokinetics, TBM studies investigating early feeding or avoidance of hyperglycemia have not been done despite their ability to improve outcomes in TBI [23, 24]. Data to establish the benefit of simple interventions like these in TBM may require clinical trials in better-resourced environments, but their application would be broadly appropriate. Until a sufficient evidence base for the neurocritical and supportive care of TBM can be established, a checklist may be a beneficial first step [25].
Although 84% of respondents reported measuring ICP, fewer reported how this was done and <50% regularly recorded the opening pressure, suggesting that ICP is not systematically measured (Supplementary Table S14). Given the prevalence of hydrocephalus with associated raised ICP, these data suggest a need for protocols to monitor ICP, including location, frequency of measurement, and treatment thresholds (Table 4) [26]. Medical interventions for raised ICP, such as raising the head of the bed, controlling seizures, and utilizing osmotic agents, were reported by 70%–80% of respondents and could therefore be studied more systematically.
Differentiating between SIADH and CSW as the etiology of hyponatremia is a challenging but important step to implement appropriate intervention due to their divergent treatment (Table 4 and Supplementary Figure 1) [9]. Hypertonic saline may be a suitable treatment for both conditions, and our data demonstrate its frequent use [9]. However, 30% of respondents used fluid restriction even when they did not suspect SIADH. Furthermore, although respondents considered SIADH to be the predominant etiology, recent studies have demonstrated that CSW occurs more frequently in TBM and is associated with severe infarction [27, 28]. Multicenter studies to establish a recommended diagnostic approach for SIADH and CSW, and trial treatment options are needed.
Neurosurgical Care
Currently there is limited evidence for the nature and timing of neurosurgery for TBM hydrocephalus [8]. Consequently, practices are heterogeneous and accurate data on neurosurgical complications and success rates are lacking. The use of shunts or endoscopic third ventriculostomy and the selection of patients most likely to benefit requires multicenter study. Many respondents used imaging to test for hydrocephalus communication, yet there are no data that support the accuracy of imaging in determining this. The relevance, diagnosis, and advantages of medical versus surgical management of hydrocephalus communication warrant investigation (Table 4) [9].
Limitations
Responses were largely from tertiary centers and may not reflect the rural and primary/secondary setting. However, the data show that most referrals are to tertiary centers. The use of organizational contacts within the TBM field could have contributed to sampling bias, and this likely skewed the data by the high number of respondents from neurosurgery, pediatrics, and South Africa, although wide distribution of the survey was available on social media. This is unlikely to have obscured the heterogeneity of management protocols or resource access, but neurosurgical treatment for hydrocephalus may be overrepresented. In addition, other complications, such as drug-induced liver toxicity or paradoxical reactions, may have revealed similarly heterogenous management but were beyond the scope of this survey. We were unable to determine the survey hit rate due to the use of an anonymous link and utilization of list-serve distribution. Survey completion was subjective and consistency in intraindividual respondent answers cannot be guaranteed. Due to resource constraints, we were not able to translate this survey into multiple languages. However, English is commonly used by medical professionals worldwide. We were unable to back-translate the Chinese version, but answers were entered directly into the Qualtrics program to maintain response format.
CONCLUSIONS
The management of TBM is highly variable, even with respect to current standard of care, and the use of neurocritical care is limited. Numerous sites have access to important resources to participate in single-center and multicenter clinical trials to establish evidence-based treatment guidelines and investigate the value of supportive medical and neurosurgical measures on patient outcomes. Guidelines based on current evidence could help improve management now, and serve as the framework on which to build.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank international organizations that helped with distribution of the survey to their members, including World Federation of Pediatric Intensive and Critical Care Societies, World Federation Neurosurgery Society, Pediatric Acute Lung Injury and Sepsis Investigators, International Society for Pediatric Neurosurgery, Pediatric Neurocritical Care Research Group, Society of Neurosurgeons of South Africa, German Society of Tropical Paediatrics and International Child Health, American Academy of Pediatrics’ Section on International Child Health, Pakistan Neurology Society, World Federation of Societies of Intensive and Critical Care Medicine, and International Child Neurology Association. We also thank the members of the focus group from the Tuberculous Meningitis International Research Consortium for valuable input on content.
Author contributions. E. W. T. and U. R. conceptualized, drafted, and distributed the survey, analyzed the data, and wrote the manuscript. S. M., J. A. S., R. v. C., A. R. G., R. R., R. S. S., F. V. C., and J. W. reviewed and edited survey draft. W. Z., F. S., and X. Z. translated, distributed, and input responses into Qualtrics from China. All coauthors edited the manuscript.
Financial support. This work was supported by the National Institutes of Health (NIH), and the National Institute of Allergy and Infectious Diseases (NIAID) (grant K08-AI139371) (E. W. T.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Elizabeth W Tucker, Center for Infection and Inflammation Imaging Research, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Center for Tuberculosis Research, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Division of Pediatric Critical Care, Johns Hopkins All Children’s Hospital, St. Petersburg, Florida, USA.
Suzaan Marais, Division of Neurology, Department of Medicine and Neuroscience Institute, University of Cape Town, Cape Town, South Africa.
James A Seddon, Department of Infectious Diseases, Imperial College London, London, United Kingdom; Desmond Tutu TB Centre, Department of Paediatrics and Child Health, Stellenbosch University, Cape Town, South Africa.
Reinout van Crevel, Department of Internal Medicine and Radboud Center for Infectious Diseases, Radboud University Medical Center, Nijmegen, the Netherlands; Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom.
Ahmad Rizal Ganiem, Department of Neurology, Hasan Sadikin Hospital, Faculty of Medicine Universitas Padjadjaran, Bandung, Indonesia.
Rovina Ruslami, Department of Neurology, Hasan Sadikin Hospital, Faculty of Medicine Universitas Padjadjaran, Bandung, Indonesia.
Wenhong Zhang, Departments of Infectious Diseases, Huashan Hospital, Fudan University, Shanghai, China; National Clinical Research Center for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai, China.
Feng Sun, Departments of Infectious Diseases, Huashan Hospital, Fudan University, Shanghai, China; National Clinical Research Center for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai, China.
Xian Zhou, Departments of Infectious Diseases, Huashan Hospital, Fudan University, Shanghai, China; National Clinical Research Center for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai, China.
Regan S Solomons, Department of Paediatrics and Child Health, Stellenbosch University, Cape Town, South Africa.
Fiona V Cresswell, Clinical Research Department, London School of Hygiene and Tropical Medicine, London, United Kingdom; Infectious Disease Institute, Mulago College of Health Sciences, Kampala, Uganda; MRC-UVRI LSHTM Uganda Research Unit, Entebbe, Uganda.
Jo Wilmshurst, Neuroscience Institute and Department of Paediatric Neurology, University of Cape Town, Red Cross War Memorial Children’s Hospital, Cape Town, South Africa.
Ursula Rohlwink, Division of Neurosurgery, Department of Surgery and Neuroscience Institute, University of Cape Town, Faculty of Health Sciences, Cape Town, South Africa; The Francis Crick Institute, London, United Kingdom.
References
- 1. Wilkinson RJ, Rohlwink U, Misra UK, et al. ; Tuberculous Meningitis International Research Consortium . Tuberculous meningitis. Nat Rev Neurol 2017; 13:581–98. [DOI] [PubMed] [Google Scholar]
- 2. Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 2004; 351:1741–51. [DOI] [PubMed] [Google Scholar]
- 3. Ruslami R, Ganiem AR, Dian S, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis 2013; 13:27–35. [DOI] [PubMed] [Google Scholar]
- 4. Heemskerk AD, Bang ND, Mai NT, et al. Intensified antituberculosis therapy in adults with tuberculous meningitis. N Engl J Med 2016; 374:124–34. [DOI] [PubMed] [Google Scholar]
- 5. van Toorn R, Schaaf HS, Laubscher JA, et al. Short intensified treatment in children with drug-susceptible tuberculous meningitis. Pediatr Infect Dis J 2014; 33:248–52. [DOI] [PubMed] [Google Scholar]
- 6. Schoeman JF, Janse van Rensburg A, Laubscher JA, Springer P. The role of aspirin in childhood tuberculous meningitis. J Child Neurol 2011; 26:956–62. [DOI] [PubMed] [Google Scholar]
- 7. Schoeman JF, Springer P, van Rensburg AJ, et al. Adjunctive thalidomide therapy for childhood tuberculous meningitis: results of a randomized study. J Child Neurol 2004; 19:250–7. [DOI] [PubMed] [Google Scholar]
- 8. Goyal P, Srivastava C, Ojha BK, et al. A randomized study of ventriculoperitoneal shunt versus endoscopic third ventriculostomy for the management of tubercular meningitis with hydrocephalus. Childs Nerv Syst 2014; 30:851–7. [DOI] [PubMed] [Google Scholar]
- 9. Figaji AA, Fieggen AG. The neurosurgical and acute care management of tuberculous meningitis: evidence and current practice. Tuberculosis (Edinb) 2010; 90:393–400. [DOI] [PubMed] [Google Scholar]
- 10. Rohlwink UK, Mauff K, Wilkinson KA, et al. Biomarkers of cerebral injury and inflammation in pediatric tuberculous meningitis. Clin Infect Dis 2017; 65:1298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elf K, Nilsson P, Enblad P. Outcome after traumatic brain injury improved by an organized secondary insult program and standardized neurointensive care. Crit Care Med 2002; 30:2129–34. [DOI] [PubMed] [Google Scholar]
- 12. Kochanek PM, Tasker RC, Carney N, et al. Guidelines for the management of pediatric severe traumatic brain injury, third edition: update of the brain trauma foundation guidelines, executive summary. Neurosurgery 2019; 84:1169–78. [DOI] [PubMed] [Google Scholar]
- 13. Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 2017; 80:6–15. [DOI] [PubMed] [Google Scholar]
- 14. Donovan J, Figaji A, Imran D, et al. The neurocritical care of tuberculous meningitis. Lancet Neurol 2019; 18:771–83. [DOI] [PubMed] [Google Scholar]
- 15. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005; 15:1277–88. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization. Global Tuberculosis Report 2018. Geneva: World Health Organization; 2018. [Google Scholar]
- 17. Marais BJ, Heemskerk AD, Marais SS, et al. Standardized methods for enhanced quality and comparability of tuberculous meningitis studies. Clin Infect Dis 2016; 64:501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rohlwink UK, Chow FC, Wasserman S, et al. ; Tuberculous Meningitis International Research Consortium . Standardized approaches for clinical sampling and endpoint ascertainment in tuberculous meningitis studies. Wellcome Open Res 2019; 4:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Török ME, Yen NTB, Chau TTH, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)-associated tuberculous meningitis. Clin Infect Dis 2011; 52:1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Te Brake L, Dian S, Ganiem AR, et al. Pharmacokinetic/pharmacodynamic analysis of an intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis. Int J Antimicrob Agents 2015; 45:496–503. [DOI] [PubMed] [Google Scholar]
- 21. Dian S, Yunivita V, Ganiem AR, et al. Double-blind, randomized, placebo-controlled Phase II dose-finding study to evaluate high-dose rifampin for tuberculous meningitis. Antimicrob Agents Chemother 2018; 62:e01014–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mai NT, Dobbs N, Phu NH, et al. A randomised double blind placebo controlled phase 2 trial of adjunctive aspirin for tuberculous meningitis in HIV-uninfected adults. Elife 2018; 7:e33478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramachandran G, Hemanth Kumar AK, Bhavani PK, et al. Age, nutritional status and INH acetylator status affect pharmacokinetics of anti-tuberculosis drugs in children. Int J Tuberc Lung Dis 2013; 17:800–6. [DOI] [PubMed] [Google Scholar]
- 24. Vavilala MS, Kernic MA, Wang J, et al. ; Pediatric Guideline Adherence and Outcomes Study . Acute care clinical indicators associated with discharge outcomes in children with severe traumatic brain injury. Crit Care Med 2014; 42:2258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Donovan J, Rohlwink UK, Tucker EW, et al. ; Tuberculous Meningitis International Research Consortium . Checklists to guide the supportive and critical care of tuberculous meningitis. Wellcome Open Res 2019; 4:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rohlwink UK, Donald K, Gavine B, et al. Clinical characteristics and neurodevelopmental outcomes of children with tuberculous meningitis and hydrocephalus. Dev Med Child Neurol 2016; 58:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Misra UK, Kalita J, Kumar M, Neyaz Z. Hypovolemia due to cerebral salt wasting may contribute to stroke in tuberculous meningitis. QJM 2018; 111:455–60. [DOI] [PubMed] [Google Scholar]
- 28. Misra UK, Kalita J, Bhoi SK, Singh RK. A study of hyponatremia in tuberculous meningitis. J Neurol Sci 2016; 367:152–7. [DOI] [PubMed] [Google Scholar]
- 29. Marais S, Thwaites G, Schoeman JF, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis 2010; 10:803–12. [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization. Guidelines for Treatment of Drug-Susceptible Tuberculosis and Patient Care. Geneva: World Health Organization; 2017. [Google Scholar]
- 31. Schoeman JF, Van Zyl LE, Laubscher JA, Donald PR. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics 1997; 99:226–31. [DOI] [PubMed] [Google Scholar]
- 32. Wen L, Li M, Xu T, et al. Clinical features, outcomes and prognostic factors of tuberculous meningitis in adults worldwide: systematic review and meta-analysis. J Neurol 2019; 266:3009–21. [DOI] [PubMed] [Google Scholar]
- 33. Jullien S, Ryan H, Modi M, Bhatia R. Six months therapy for tuberculous meningitis. Cochrane Database Syst Rev 2016; 9:CD012091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laureillard D, Marcy O, Madec Y, et al. ; CAMELIA (ANRS 1295—CIPRA KH001) Study Team . Paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome after early initiation of antiretroviral therapy in a randomized clinical trial. AIDS 2013; 27:2577–86. [DOI] [PubMed] [Google Scholar]
- 35. Török ME, Bang ND, Chau TTH, et al. Dexamethasone and long-term outcome of tuberculous meningitis in Vietnamese adults and adolescents. PLoS One 2011; 6:e27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Misra UK, Kalita J, Kumar M, Tripathi A. A study of atrial and brain natriuretic peptides in tuberculous meningitis and acute encephalitis. Int J Tuberc Lung Dis 2018; 22:452–7. [DOI] [PubMed] [Google Scholar]
- 37. Misra UK, Kalita J, Kumar M. Safety and efficacy of fludrocortisone in the treatment of cerebral salt wasting in patients with tuberculous meningitis: a randomized clinical trial. JAMA Neurol 2018; 75: 1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.