Abstract
Background
Bacterial and fungal infections (BFIs) are frequent in patients with cirrhosis and often trigger acute-on-chronic liver failure (ACLF). This prospective observational study aims to describe the interactions between BFI and ACLF in terms of mortality and related risk factors.
Methods
We performed a 2-center prospective observational study enrolling hospitalized patients with cirrhosis admitted for acute decompensation. Data were recorded at admission and during hospitalization. Survival was recorded up to 1 year.
Results
Among the 516 patients enrolled, 108 (21%) were infected at admission, while an additional 61 patients (12%) developed an infection during hospital stay. In the absence of ACLF, the 1-year mortality rate of patients with BFI did not differ from that of patients without BFI (33% vs 31%; P = .553). In contrast, those with ACLF triggered or complicated by BFI had a significantly higher mortality rate than those who remained free from BFI (75% vs 54%; P = .011). Competing risk analysis showed that the negative impact of ACLF-related BFI on long-term prognosis was independent from Model for End-stage Liver Disease (MELD) incorporating serum sodium concentration score, comorbidity, and basal C-reactive protein level. Finally, multivariable logistic regression showed that higher MELD score (P < .001), QuickSOFA score ≥2 points (P = .007), and secondary bloodstream (P = .022) and multidrug-resistant pathogen isolation (P = .030) were independently associated with ACLF in patients with BFI.
Conclusions
This large prospective study indicated that the adverse impact of BFI on long-term survival in decompensated cirrhosis is not universal but is limited to those patients who also develop ACLF. Both disease severity and microbiological factors predispose infected decompensated patients to ACLF.
Keywords: cirrhosis, bacterial and fungal infections, acute-on-chronic liver failure, mortality
The adverse prognosis of bacterial or fungal infections in patients with decompensated cirrhosis is linked to the development of acute-on-chronic liver failure. Severity, type and microbiological features of infections are main predictors of the development of acute-on-chronic liver failure.
A complex dysfunction endangering both innate and acquired immunity makes patients with liver cirrhosis highly susceptible to bacterial and fungal infections (BFIs) [1]. The occurrence of such a complication is associated with high rates of morbidity, such as acute kidney injury, hepatic encephalopathy, gastrointestinal bleeding, and short-term mortality exceeding 70% in patients developing septic shock [2].
BFIs are also a prevalent precipitating factor of acute-on-chronic liver failure (ACLF), a distinct clinical syndrome characterized by acute decompensation of cirrhosis associated with organ failures and high short-term mortality [3]. Recent retrospective analyses have shown that BFIs precipitating or occurring during the course of ACLF are associated with poor clinical course and high 28- and 90-day mortality [4, 5].
BFIs also appear to adversely affect not only short- and medium-term patient outcomes but also long-term survival. Indeed, a systematic review of studies reporting on the clinical course of cirrhosis after bacterial infections showed that 1-year mortality increased 4-fold, suggesting that the occurrence of bacterial infections could be considered a specific prognostic stage of the disease [6].
However, a large prospective study specifically designed to grasp the ominous interaction between BFI and ACLF in terms of short- and long-term outcome and related risk factors in patients with decompensated cirrhosis is still lacking. Thus, the main aim of the present study was to assess 1-year mortality in infected patients with an acute decompensation of cirrhosis with or without ACLF. Moreover, the risk factors for 1-year mortality, infection-induced ACLF, and development of hospital-acquired infections were investigated.
METHODS
Study Design
The present investigation is a prospective observational study on patients with liver cirrhosis admitted to the hospital who were followed up from the time of admission to 1 year after the first assessment. The investigation was conducted in a tertiary teaching hospital, the S. Orsola-Malpighi University Hospital in Bologna, and a community hospital, the Infermi Hospital in Rimini, from January 2014 to March 2016.
All consecutive patients with liver cirrhosis admitted to the hospital were screened within 36 hours of admission. Inclusion criteria were (i) diagnosis of cirrhosis, based on a composite of clinical signs and findings provided by laboratory test, endoscopy, and radiologic imaging; (ii) presence of acute decompensation of cirrhosis; (iii) age ≥18 years. Exclusion criteria were (i) admission for a scheduled procedure; ii) hepatocellular carcinoma beyond the Milan criteria [7]; (iii) metastatic extrahepatic malignancy; (iv) previous liver transplantation. Patients were monitored for ACLF or BFI development. Survival was recorded up to 1 year. The patients were managed according to international and local guidelines.
Patient Consent Statement
The study protocol was approved by the S. Orsola-Malpighi Hospital and Area Vasta Romagna ethics committees. Written informed consent was obtained from patients or from legal surrogates before enrollment according to the 1975 Declaration of Helsinki.
Definition of Acute Decompensation, Acute-on-Chronic Liver Failure, and Diagnostic Criteria
Acute decompensation of cirrhosis was defined by (i) acute onset of grade 2 or grade 3 ascites, according to the International Club of Ascites classification [8]; (ii) new episode of hepatic encephalopathy in patients with previous normal consciousness and no evidence of an acute neurologic disease; (iii) upper or lower gastrointestinal bleeding; (iv) BFI. Organ failures were defined according to the Chronic Liver Failure Consortium–Sequential Organ Failure Assessment (CLIF-SOFA), and ACLF was diagnosed and classified according to the CLIF consortium criteria [3]. BFI-related ACLF was defined as any case of ACLF diagnosed simultaneous to or occurring within 28 days of BFI diagnosis in patients without any other recognizable precipitating event. Renal dysfunction was diagnosed when the serum creatinine concentration was >1.5 mg/dL.
Every case of BFI was managed and reviewed by an infectious diseases specialist and a hepatologist. Pneumonia was defined as radiologic evidence of a new pulmonary infiltrate or progression of a previous pulmonary infiltrate plus at least 2 of the following criteria: fever >38°C, cough, purulent sputum, dyspnea or >20 breaths per minute, pleuritic chest pain, and a leucocyte count of >10 000/µL or <4000/µL. Urinary tract infection (UTI) was diagnosed in the presence of either of the following criteria: i) flank pain, which must have begun or worsened within 7 days; (ii) costovertebral angle tenderness on examination; (iii) dysuria, urgency, frequency, and/or suprapubic pain plus at least 1 of the following: (i) fever >38°C; (ii) nausea and vomiting. Uncomplicated lower urinary tract infections were excluded. Spontaneous bacterial peritonitis (SBP) was defined by the presence of ≥250 polymorphonuclear cells/µL in ascitic fluid. Intra-abdominal infection (IAI) other than SBP was defined by new onset of fever and/or abdominal pain plus new or worsening radiological images of abscess, bowel perforation, appendicitis, diverticulitis, biliary tract infections, and postsurgical effusion with or without peritonitis. Skin and soft tissue infection (SSTI) was diagnosed in the presence of purulent infections (cutaneous abscesses, furuncles, carbuncles) or in case of nonpurulent infections (cellulitis, erysipelas, or necrotizing infections) [9]. Primary bloodstream infection (BSI) was defined as the growth of a noncommon skin contaminant from ≥1 blood culture (BC) or of a common skin contaminant such as diphtheroids, Bacillus species, Propionibacterium species, coagulase-negative staphylococci (CoNS), or micrococci from ≥2 BCs drawn on separate sites and reporting the same antimicrobial susceptibility test profile in a patient without another identifiable source of infection after a comprehensive diagnostic workup. Episodes in which a potential contaminant (eg, coagulase-negative staphylococci) was only isolated in 1 set of blood cultures without clinical evidence of infection were excluded. Secondary BSIs were defined as any infection of another body site with positive blood cultures, while the term bacteremic infection included both primary and secondary BSIs. Gram-negative bacteria were classified as multidrug-resistant (MDR) according to the European Society of Clinical Microbiology and Infectious Diseases consensus definitions. Patients were classified as having (i) hospital-acquired (HA) infection if infection signs/symptoms started >48 hours after hospital admission or if patients were transferred from another hospital with an already diagnosed HA infection; (ii) health care–associated (HCA) infection according to standard criteria [10]; (iii) community-acquired (CA) infection in all other cases.
Data Collection
Data were collected using an online electronic case report form shared on the study website. The following data were collected at the time of enrollment: demographic characteristics, etiology of cirrhosis, and laboratory and clinical data including the presence of comorbidities according to Charlson score [11]. The Model for End-stage Liver Disease score (MELD) [12], MELD incorporating serum sodium concentration score (MELDNa) [13], Child-Pugh score [14], and CLIF-Acute Decompensation score (CLIF-C AD) [15] were calculated for each patient as appropriate. In patients admitted with or who developed BFI during the hospital stay, the following data were also recorded: infection site, body fluid cultures, and susceptibility data. Infection severity was assessed according to Sepsis-3 criteria and quick-SOFA (qSOFA) score [16].
Statistical Analysis
Continuous data were reported as mean and SD or median and interquartile range (IQR) and analyzed by means of parametric or nonparametric tests as appropriate. Categorical data were reported as absolute number and frequency, while comparisons were performed by the χ 2 test followed by the z-test when >3 groups were analyzed. Mortality was evaluated according to the Kaplan-Meier method followed by the log-rank test. A proportional hazard model considering liver transplant a competing event was also fitted according to the Fine and Gray method to identify predictors of 1-year mortality among parameters recorded at study inclusion or during hospital stay.
Two binary logistic regression models with backward elimination (P > .1) based on likelihood ratios were fitted to identify predictors of HA BFI or BFI-related ACLF.
Further details on the statistical analysis are reported in the Supplementary Data.
RESULTS
Study Population
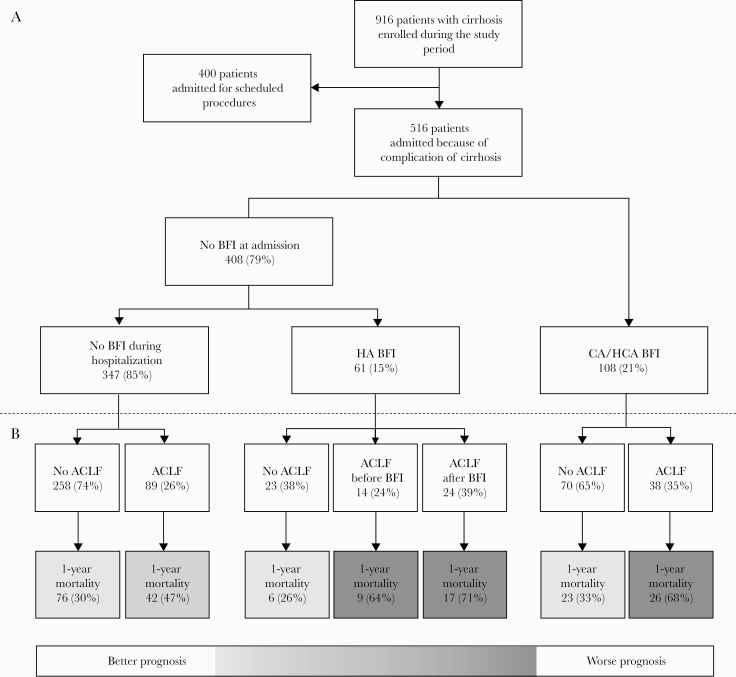
From January 2014 to March 2016, 1140 consecutive hospital admissions to regular wards involving 916 patients with cirrhosis were recorded. Of the 916 patients, 400 were electively admitted for scheduled diagnostic or invasive procedures and were excluded from the current analysis. Thus, the study cohort includes 516 patients admitted because of complications of cirrhosis fulfilling the definition of acute decompensation (Figure 1A).
Figure 1.
Details on patient disposition (A), acute-on-chronic liver failure (ACLF), and 1-year mortality in patients remaining free of bacterial or fungal infection (BFI) during hospitalization, with hospital-acquired (HA) BFI, or with community-acquired (CA)/health care–associated (HCA) BFI (B).
Baseline Comparison of Patients With and Without BFI
Overall, 108 (21%) patients presented CA or HCA BFI at admission. Of the remaining 408 patients (79%), 61 (15%) developed HA BFI. Thus, 169 (33%) patients presented an episode of BFI (Figure 1 A).
Baseline demographic, clinical, and laboratory data of patients free from BFI at admission and during their hospital stay, with BFI at admission, or with HA BFI are reported in Table 1. When compared with those remaining free from BFI, patients with BFI at admission did not differ in terms of age, gender, etiology of cirrhosis, and main clinical features. Conversely, these patients presented with higher WBC count and serum C-reactive protein level (CRP), as well as higher heart rate and lower serum sodium concentration. Finally, MELD-Na and CLIF-C AD scores were the sole prognostic indexes that were more elevated in patients with BFI at admission with respect to those who remained free from BFI.
Table 1.
Demographic and Clinical Characteristics of 516 Patients With Liver Cirrhosis Admitted to the Hospital for Acute Decompensation
| No BFI | BFI at Admission | BFI During Hospitalization | P | |
|---|---|---|---|---|
| No. | 347 | 108 | 61 | |
| Anthropometric data | ||||
| Age, y | 61 (51–72) | 64 (53–74) | 61 (52–72) | .473 |
| Male sex | 209 (60) | 68 (63) | 43 (70) | .306 |
| Etiology of cirrhosis | ||||
| Virala | 142 (41) | 52 (48) | 26 (43) | .415 |
| Alcohol | 66 (19) | 21 (20) | 14 (23) | .775 |
| NASH | 17 (5) | 8 (7) | 4 (6) | .579 |
| Mixed etiologyb | 65 (19) | 11 (10) | 8 (13) | .085 |
| Other | 57 (16) | 16 (15) | 9 (15) | .893 |
| Main clinical features at admission | ||||
| Ascites | 192 (55) | 59 (55) | 39 (64) | .428 |
| HE grades III/IV | 62 (18) | 13 (12) | 8 (13) | .283 |
| Renal dysfunctionc | 61 (18) | 24 (22) | 23 (38)* | .002 |
| GI bleeding | 28 (8) | 2 (2) | 6 (10) | .056 |
| ACLF | 67 (19) | 21 (19) | 18 (30) | .182 |
| Grade 1 | 29 (43) | 12 (57) | 5 (28) | .182 |
| Grade 2 | 35 (52) | 6 (29) | 12 (67) | .050 |
| Grade 3 | 3 (5) | 3 (14) | 1 (6) | .282 |
| Renal failure | 31 (46) | 9 (43) | 13 (72) | .113 |
| Liver failure | 19 (28) | 6 (29) | 7 (39) | .677 |
| Coagulation failure | 12 (18) | 5 (24) | 1 (6) | .301 |
| Brain failure | 25 (37) | 7 (33) | 4 (22) | .485 |
| Respiratory failure | 0 (0) | 0 (0) | 5 (28) | <.001 |
| Circulatory failure | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Biochemical and hemodynamic data | ||||
| WBC, 109/L | 5.2 (3.5–7.4) | 7.8 (5.0–10.9)** | 5.6 (3.6–9.2) | <.001 |
| CRP, mg/dL | 0.93 (0.34–1.76) | 3.94 (1.69–8.15)** | 2.76 (0.62–5.46)** | <.001 |
| Platelets, 109/L | 90 (55–139) | 96 (61–176) | 74 (56–123) | .194 |
| Sodium, mmol/L | 137 (134–140) | 136 (133–138)* | 135 (132–139)* | <.001 |
| Bilirubin, mg/dL | 2.1 (1.1–4.3) | 2.4 (1.1–4.4) | 2.8 (1.5–10.3)* | .018 |
| Creatinine, mg/dL | 0.89 (0.73–1.30) | 1.03 (0.78–1.45) | 1.25 (0.88–1.85)** | <.001 |
| Albumin, mg/dL | 3.2 (2.8–3.6) | 3.0 (2.7–3.4) | 3.1 (2.8–3.5) | .080 |
| INR | 1.40 (1.23–1.56) | 1.39 (1.25–1.67) | 1.46 (1.28–1.73) | .077 |
| MAP, mmHg | 85 (80–93) | 83 (77–97) | 82 (75–90) | .105 |
| HR, bpm | 75 (65–84) | 80 (70–90)* | 80 (70–88)* | <.001 |
| Prognostic scores | ||||
| Child-Pugh score | 8 (7–10) | 8 (7–10) | 9 (8–11)** | .028 |
| Child-Pugh Class | ||||
| Class A | 83 (24) | 25 (23) | 7 (12) | .096 |
| Class B | 160 (46) | 55 (51) | 30 (49) | .655 |
| Class C | 104 (30) | 28 (26) | 24 (39) | .186 |
| MELD | 15 (11–20) | 16 (11–20) | 21 (14–27)** | .001 |
| MELD-Na | 16 (12–22) | 18 (14–24)** | 21 (17–30)** | <.001 |
| CLIF-C-AD | 50 (45–57) | 55 (50–63)* | 54 (49–59)* | <.001 |
| Concomitant medications | ||||
| PPI | 223 (64) | 74 (69) | 43 (71) | .518 |
| Beta-blockers | 150 (43) | 47 (44) | 26 (43) | .994 |
| Rifaximin | 104 (30) | 28 (26) | 20 (34) | .579 |
| Quinolones | 7 (2) | 1 (1) | 1 (2) | .748 |
| Transfer to ICU | 27 (8) | 8 (7) | 8 (13) | .352 |
| Comorbidities | ||||
| CCI | 6.0 (5.0–7.4) | 6.0 (4.8–7.4) | 6.2 (4.4–7.4) | .922 |
| HCC | 76 (22) | 33 (31) | 17 (28) | .156 |
| Diabetes (any stage) | 122 (35) | 39 (36) | 19 (31) | .795 |
Patients were divided according to the presence or lack of a BFI at the time of admission or the development of a hospital-acquired BFI.
*P < .05 vs no BFI; **P < .05 vs all.
Abbreviations: ACLF, acute-on-chronic liver failure; BFI, bacterial or fungal infection; CCI, Charlson comorbidity index; CLIF-C, Chronic Liver Failure Consortium; CRP, C-reactive protein; GI, gastrointestinal; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HE, hepatic encephalopathy; HR, heart rate; ICU, intensive care unit; INR, international normalized ratio; MAP, mean arterial pressure; MELD, Model for End-stage Liver Disease; NASH, non-alcoholic steatohepatitis; PPI, proton pump inhibitor; WBC, white blood cell count.
aViral etiology includes 152 HCV infection, 15 HBV infection, and 53 HBV/HCV coinfection.
bMixed etiology includes viral and alcohol, viral and metabolic, and alcohol and metabolic etiologies.
cRenal dysfunction was defined as serum creatinine level >1.5 mg/dL.
Patients who developed HA BFI did not differ from the other 2 groups as far as age, gender, etiology of cirrhosis, and main clinical features were concerned. However, and notably, they presented higher CRP, heart rate, and serum bilirubin and lower serum sodium concentration than those who remained free from BFI and higher serum creatinine than the other 2 groups.
Finally, frequency of ACLF at admission was similar between patients without BFI, CA, or HCA BFI and those with HA BFI. No differences were also observed regarding the grade of ACLF, while among organ failures, respiratory failure was more frequent in patients developing HA BFI (Table 1).
Clinical and Microbiological Characteristics of Patients With BFI
BFIs were classified as CA in 68 (40%) cases, HCA in 40 (24%), and HA in 61 (36%). Overall, pneumonia was the leading cause of infection (41, 24%), followed by primary BSI (31, 18%), IAI (29, 17%), SBP (26, 15%), UTI (26, 15%), and SSTI (15, 9%) (Supplementary Table 1).
A microbiological diagnosis was obtained in 93 (55%) patients. The etiology of culture-positive infections is reported in Supplementary Table 2. Briefly, gram-negative bacteria were identified in 69 cases (74%), gram-positive cocci in 27 (29%), and fungi in 4 (4%). All fungal infections were episodes of candidemia. Overall, 40 (43%) pathogens were classified as MDR including extended-spectrum beta-lactamase (ESBL)–producing Enterobacteriaceae (19% of all isolates) and carbapenem-resistant Enterobacteriaceae (7% of all isolates). Finally, culture-negative infections were mostly pneumonia, SBP, and SSTI (Supplementary Table 2).
Regarding the severity of BFI, the median SOFA score was 4 [3–7], and qSOFA ≥2 points was found in 25 (15%) patients. Finally, 59 (35%) patients presented with sepsis and 13 (8%) patients with septic shock.
Long-term Outcome and Risk Factors for Mortality
During the 1-year follow-up, 53 (11%) patients underwent liver transplantation and 199 (39%) died after a median time of 89 (33–207) days from inclusion.
The 1-year mortality rate of patients with BFI was higher than in patients without (51% vs 36%; P < .001) (Supplementary Figure 1). However, further analysis revealed that patients who remained free from ACLF, either at admission or during hospitalization, had the lowest mortality, ranging from 26% to 33%, irrespective of BFI, whether CA, HCA, or HA. The highest mortality was seen in patients with ACLF triggered by BFI (68%–71%) or subsequently complicated by HA (64%). Finally, patients with ACLF but free from BFI presented an intermediate mortality rate (47%) (Figure 1 B).
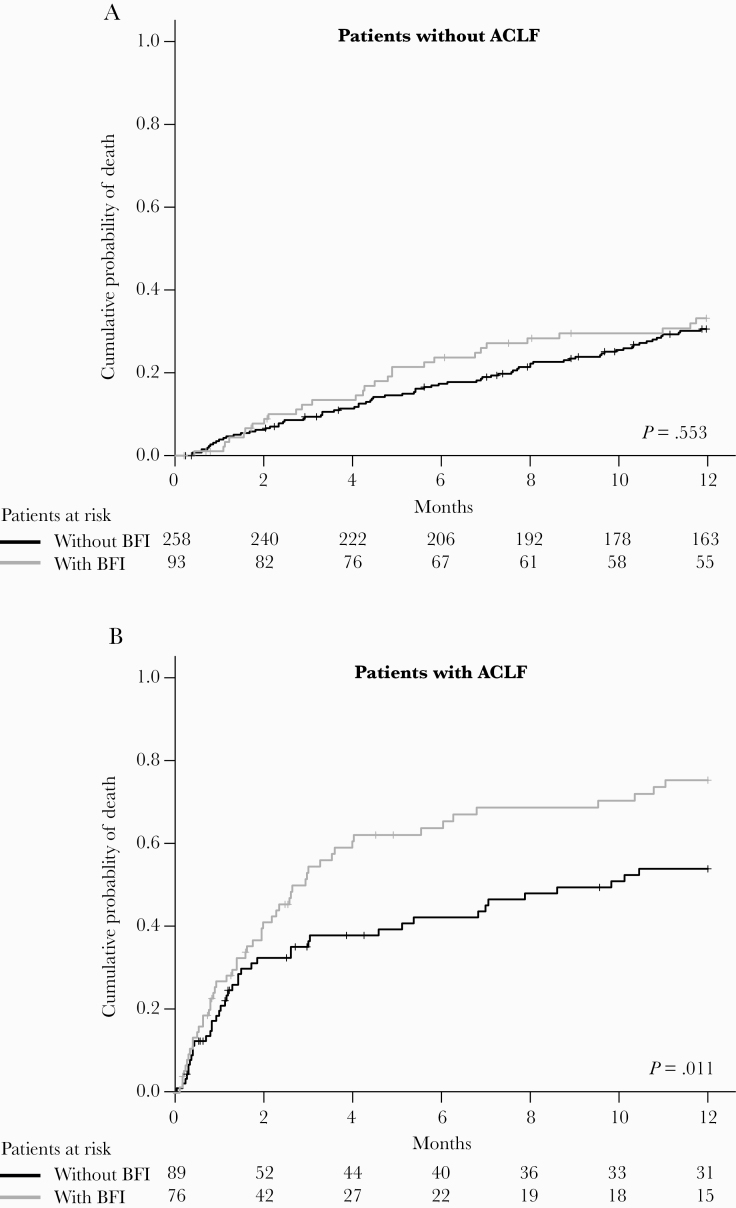
Kaplan-Meyer analysis confirmed that in patients without ACLF the 1-year mortality rate did not differ between those with and without BFI (33% vs 31%; P = .553) (Figure 2A). In contrast, in patients with ACLF, those with ACLF triggered or complicated by BFI had a significantly higher mortality rate than those who remained free from BFI (75% vs 54%; P = .011) (Figure 2 B). Interestingly, while the prognosis of patients with grade 2/3 ACLF was similar in patients with and without BFI (76% vs 81%; P = .738), the presence of BFI deeply affects the prognosis of patients with grade 1 ACLF (71% vs 35%; P < .001) (Supplementary Figure 2).
Figure 2. .
One-year mortality in patients with or without bacterial or fungal infection (BFI). A, Mortality in patients without acute-on-chronic liver failure. B, Mortality in patients with acute-on-chronic liver failure. Comparisons were made by the log-rank test. Abbreviations: ACLF, acute-on-chronic liver failure; BFI, bacterial or fungal infection.
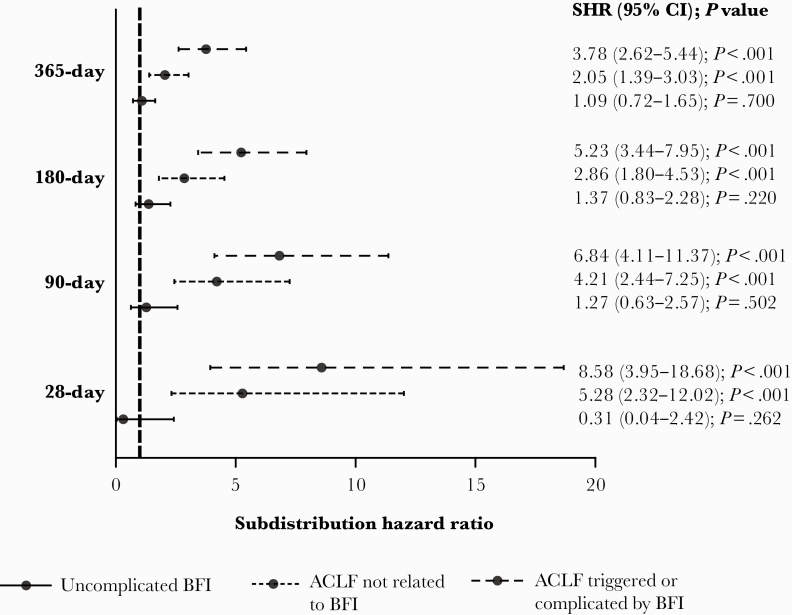
Competing risk regression analysis considering liver transplantation as a competing event for death largely confirmed these findings. In fact, BFI did not influence 28-, 90-, 180-, or 365-day mortality risk in patients without ACLF, while ACLF triggered or complicated by BFI had a higher risk than ACLF free from BFI at all time points (Figure 3).
Figure 3. .
Subdistribution hazard ratio (SHR) for 28-day, 90-day, 180-day, and 1-year mortality related to the presence of uncomplicated bacterial or fungal infection (BFI), acute-on-chronic liver failure (ACLF) triggered or complicated by BFI, or ACLF not associated with BFI according to the competing risk analysis in which liver transplant was considered a competing event.
Factors associated with 1-year mortality were assessed by competing risk regression analysis considering liver transplantation as a competing event. A comparison between survivors and nonsurvivors is reported in Table 2.
Table 2.
Demographic, Biochemical, and Clinical Characteristics of the 516 Patients Included in the Study, Divided According to 1-Year Mortality Status
| Survivors | Nonsurvivors | SHR (95% CI) | P | |
|---|---|---|---|---|
| No. | 317 | 199 | ||
| Anthropometric data | ||||
| Age, y | 59 (50–69) | 67 (58–77) | 1.04 (1.03–1.05) | <.001 |
| Male sex | 203 (64) | 117 (59) | 0.85 (0.64–1.13) | .261 |
| Etiology of cirrhosis | ||||
| Viral | 121 (38) | 99 (50) | 1.43 (1.08–1.89) | .011 |
| Alcohol | 64 (20) | 37 (19) | 0.92 (0.64–1.31) | .639 |
| NASH | 20 (6) | 9 (5) | 0.80 (0.40–1.62) | .538 |
| Mixed etiologya | 59 (19) | 25 (13) | 0.68 (0.45–1.03) | .070 |
| Other | 53 (17) | 29 (15) | 0.88 (0.60–1.31) | .541 |
| BFI and ACLF at admission or during hospitalization | ||||
| BFI at admission | 59 (19) | 49 (25) | 1.51 (1.08–2.11) | .017 |
| BFI during hospitalization | 29 (9) | 32 (16) | 1.87 (1.26–2.79) | .002 |
| All BFI | 88 (28) | 81 (41) | 1.63 (1.23–2.17) | .001 |
| ACLF at admission | 48 (15) | 58 (29) | 2.11 (1.53–2.92) | <.001 |
| Grade 1 | 24 (50) | 22 (38) | 1.34 (0.87–2.06) | .186 |
| Grade 2 | 21 (44) | 32 (55) | 2.56 (1.65–3.97) | <.001 |
| Grade 3 | 3 (6) | 4 (7) | 2.42 (0.65–9.08) | .189 |
| ACLF during hospitalization | 23 (7) | 36 (18) | 2.29 (1.57–3.34) | <.001 |
| Grade 1 | 20 (87) | 20 (56) | 1.49 (0.93–2.37) | .095 |
| Grade 2 | 3 (13) | 9 (25) | 3.08 (1.52–6.22) | .002 |
| Grade 3 | 0 (0) | 7 (19) | 13.18 (7.93–21.91) | <.001 |
| All ACLF | 71 (22) | 94 (47) | 2.68 (2.02–3.56) | <.001 |
| Renal failure | 33 (47) | 50 (53) | 1.30 (0.87–1.94) | .200 |
| Liver failure | 20 (28) | 27 (29) | 1.10 (0.70–1.75) | .670 |
| Coagulation failure | 14 (20) | 20 (21) | 1.16 (0.69–1.93) | .581 |
| Brain failure | 21 (30) | 30 (32) | 1.14 (0.74–1.78) | .547 |
| Respiratory failure | 2 (3) | 12 (13) | 2.41 (1.32–4.42) | .004 |
| Circulatory failure | 0 (0) | 3 (3) | 2.04 (1.27–3.27) | .003 |
| Not complicated BFI | 64 (20) | 29 (15) | 1.09 (0.72–1.65) | .700 |
| BFI and ACLF | 24 (8) | 52 (26) | 3.78 (2.62–5.44) | <.001 |
| ACLF without BFI | 47 (15) | 42 (21) | 2.05 (1.39–3.03) | <.001 |
| Main clinical features at admission | ||||
| Ascites | 154 (49) | 136 (68) | 1.98 (1.47–2.67) | <.001 |
| HE grades III/IV | 39 (12) | 44 (22) | 1.85 (1.30–2.63) | .001 |
| Renal dysfunctionb | 54 (17) | 54 (27) | 1.73 (1.24–2.40) | .001 |
| GI bleeding | 29 (9) | 7 (3) | 0.42 (0.20–0.89) | .024 |
| Biochemical and hemodynamic data at admission | ||||
| WBC, 109/L | 5.2 (3.6–7.9) | 6.2 (4.2–9.3) | 1.06 (1.03–1.10) | .001 |
| CRP, mg/dL | 0.98 (0.34–2.53) | 1.76 (0.84–5.42) | 1.05 (1.02–1.09) | .001 |
| Platelets, 109/L | 90 (58–143) | 89 (55–139) | 1.00 (1.00–1.00) | .574 |
| Sodium, mmol/L | 137 (135–140) | 136 (133–139) | 0.96 (0.93–0.99) | .005 |
| Bilirubin, mg/dL | 1.9 (1.0–3.7) | 3.0 (1.5–6.0) | 1.03 (1.01–1.05) | .007 |
| Creatinine, mg/dL | 0.89 (0.72–1.22) | 1.11 (0.82–1.55) | 1.41 (1.26–1.59) | <.001 |
| Albumin, mg/dL | 3.2 (2.9–3.7) | 3.0 (2.7–3.4) | 0.58 (0.46–0.74) | <.001 |
| INR | 1.37 (1.22–1.54) | 1.42 (1.30–1.68) | 1.27 (1.02–1.59) | .035 |
| MAP, mmHg | 87 (78–93) | 83 (77–91) | 0.99 (0.97–1.00) | .065 |
| HR, bpm | 75 (67–85) | 78 (70–86) | 1.01 (1.00–1.02) | .157 |
| Clinical scores at admission | ||||
| Child-Pugh score | 8 (6–9) | 9 (8–11) | 1.25 (1.17–1.34) | <.001 |
| Child-Pugh Class | ||||
| Class A | 94 (30) | 21 (10) | 0.33 (0.21–0.52) | <.001 |
| Class B | 150 (47) | 95 (48) | 0.97 (0.73–1.28) | .810 |
| Class C | 73 (23) | 83 (42) | 2.11 (1.59–2.81) | <.001 |
| MELD | 14 (10–18) | 18 (14–24) | 1.06 (1.04–1.08) | <.001 |
| MELD-Na | 16 (12–21) | 20 (15–25) | 1.07 (1.05–1.09) | <.001 |
| CLIF-C-AD | 49 (43–55) | 55 (50–62) | 1.06 (1.04–1.08) | <.001 |
| Transfer to ICU | 25 (8) | 18 (9) | 1.24 (0.74–2.10) | .413 |
| Comorbidities | ||||
| CCI | 5.5 (4.4–6.8) | 6.9 (5.8–8.7) | 1.31 (1.24–1.38) | <.001 |
| HCC | 61 (19) | 65 (33) | 1.69 (1.26–2.26) | <.001 |
| Diabetes (any stage) | 105 (33) | 75 (38) | 1.14 (0.86–1.52) | .360 |
For each parameter, the subdistribution hazard ratio with 95% CI for 1-year mortality is reported.
Abbreviations: ACLF, acute-on-chronic liver failure; AD, acute decompensation; CCI, Charlson comorbidity index; CLIF-C, Chronic Liver Failure Consortium; CRP, C-reactive protein; GI, gastrointestinal; HCC, hepatocellular carcinoma; HR, heart rate; ICU, intensive care unit; INR, international normalized ratio; MAP, mean arterial pressure; MELD, Model for End-stage Liver Disease; NASH, non-alcoholic steatohepatitis; WBC, white blood cell count.
aMixed etiology includes viral and alcohol, viral and metabolic, and alcohol and metabolic etiologies.
bRenal dysfunction was defined as serum creatinine level >1.5 mg/dL.
Multivariable analysis showed that only BFIs triggering or complicating ACLF were associated with an increased risk of 1-year mortality (subdistribution hazard ratio [SHR], 2.09; 95% CI, 1.42–3.07; P < .001), independent of MELD-Na (SHR, 1.06; 95% CI, 1.03–1.08; P < .001), Charlson comorbidity index (SHR, 1.31; 95% CI, 1.24–1.39; P < .001), and basal serum CRP level (SHR, 1.06; 95% CI, 1.03–1.09; P < .001) (Supplementary Table 3).
A sensitivity analysis, excluding 126 patients with a diagnosis of HCC at study inclusion, confirmed the results obtained in the whole population. Indeed, BFI triggering or complicating ACLF (SHR, 2.36; 95% CI, 1.50–3.74; P < .001), MELD-Na (SHR, 1.06; 95% CI, 1.03–1.09; P < .001), Charlson comorbidity index (SHR, 1.37; 95% CI, 1.24–1.51; P < .001), and basal serum CRP level (SHR, 1.08; 95% CI, 1.05–1.12; P < .001) were the only independent predictors of 1-year mortality.
Risk Factors for ACLF in Patients With BFI
Overall, ACLF was diagnosed more frequently in patients with BFI than in noninfected patients (76/169 [45%] vs 89/347 [26%]; P < .001). HA infections (38/61; 62%) were more frequently associated with ACLF than CA/HCA infections (38/108, 35%; P = .001). This analysis was performed in patients with ACLF diagnosed simultaneously or following (median delay [IQR], 8 [2–17] days) diagnosis of BFI, excluding the 14 patients who developed BFI after ACLF diagnosis (Figure 2).
Univariate analysis of the demographic, clinical, and microbiological characteristics at BFI diagnosis of patients with uncomplicated infections (n = 93) and those with BFI-triggered ACLF (n = 62) is reported in Supplementary Table 4.
At multivariable analysis, factors independently associated with BFI-triggered ACLF were MELD score (odds ratio [OR], 1.27; 95% CI, 1.16–1.39; P < .001), infection caused by MDR pathogens (OR, 3.02; 95% CI, 1.12–8.16; P = .030), secondary BSI (OR, 5.06; 95% CI, 1.26–20.38; P = .022), and qSOFA score ≥2 points (OR, 8.50; 95% CI, 1.82–39.70; P = .007).
DISCUSSION
The present study confirms that BFIs occur frequently in patients with acute decompensation of cirrhosis requiring hospitalization, are often associated with ACLF either as a precipitating or complicating factor, and carry a poor prognosis. Most available studies dedicated to these topics derive from retrospective evaluations of patient cohorts [4, 17, 18]. Being a prospective, specifically designed investigation, the present study also allowed us further relevant insights into these matters. It also has to be considered that stringent criteria for the diagnosis of BFI were employed as part of a stewardship program involving hepatologists and infectious disease experts. Furthermore, an accurate microbiological characterization of infectious episodes, which was lacking in most previous reports, was ensured. Finally, enrolled patients were followed in regular wards of gastroenterology and internal medicine units, making our results transferrable to most clinical contexts.
The occurrence of BFI is considered an evolutive stage of cirrhosis, as a retrospective systematic review showed that they enhance patient long-term mortality [6]. Our overall results confirmed that the impact of BFI on the mortality of patients with acute decompensation of cirrhosis is maintained over time, as patient 1-year mortality was significantly higher in those with BFI that was either present at admission or developed during hospitalization. However, our results also unveiled that this adverse outcome was confined to infected patients who developed ACLF, either precipitated or complicated by BFI, while no impact on mortality was seen in those with BFI not affected by ACLF. This finding recalls the results of a recent retrospective report where, however, the extent of the follow-up was limited to 90 days [4]. Additionally, fungal infection seems to be particularly related to development of ACLF and therefore associated with poor outcome [19]. Therefore, a first novel finding of the present study is that the occurrence of BFI per se does not influence the long-term survival of patients with cirrhosis, at least in those who are admitted to the hospital because of an acute decompensation of their disease. Indeed, the ominous combination between BFI and ACLF has to occur to enhance their 1-year mortality.
The competing risk analysis of factors associated with mortality considering liver transplantation a competing event confirmed that not BFI per se, but only those triggering or complicating ACLF were independent predictors of mortality, with a >2-fold increase in its hazard ratio, along with and to a greater extent than MELD-Na, Charlson comorbidity index, and basal serum CRP. Notably, in the subset of patients with ACLF the occurrence of BFI, either precipitating or complicating this syndrome, worsens not only short-term (28 days) and medium-term (90 days) mortality, as already reported by retrospective studies [4, 18], but also 1-year mortality. This is a clinically relevant finding, as it allows us to identify ACLF patients surviving to an infection but destined for a poor long-term prognosis, thus requiring close monitoring and specific management. Interestingly, in our series, the negative prognostic impact of BFI was particularly evident in patients with the less severe forms of ACLF, confirming findings from the CANONIC cohort [4].
Of note, among predictors of 1-year mortality, in univariate competing risk regression analysis, GI bleeding was associated with a reduced mortality risk as compared with other decompensation events. This result recalls what was recently reported in another study, suggesting that GI bleeding is not associated with an increased risk of mortality in patients with AD or ACLF [20, 21].
As ACLF takes on a preeminent role in conditioning the outcome of patients with acute decompensation of cirrhosis and BFI, we found it of interest to assess the factors predicting the development of the syndrome. Both clinical and microbiological factors emerged as independent predictors in multivariable analysis, as the severity of the underlying cirrhosis as assessed by MELD score, the severity of infection as assessed by qSOFA score, secondary BSI infections, and infections caused by MDR microorganisms were independently associated with ACLF. Logistic regression showed that, among these factors, secondary BSI and the severity of BFI led to the highest probability of presenting the syndrome.
It has to be acknowledged that our study may be limited by the low number of participating centers. Additionally, enrolling patients only in regular wards, a low number of patients with grade 3 ACLF were recruited. Both factors might reduce the external validity of our findings.
In conclusion, this prospective observational study showed that the negative impact of BFI on the short-, medium-, and long-term mortality of patients with cirrhosis is related to the occurrence of ACLF rather than to BFI per se. Thus, we propose that BFI be considered a prognostic stage of cirrhosis only when associated with the development of ACLF. Finally, both the severity of the underlying cirrhosis and, mainly, the severity, type of infection, and isolation of MDR bacteria are independent predictors of BFI-induced ACLF.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the participating nurses for their time and cooperation (Massimo De Tommaso, Antonella Lattanzi, Roberta Pataro, Marco Rizzuto, Antonella Robbio).
Financial support. This work was supported by a grant from the Italian Ministry of Health (rf-2010-2310623), a grant from the Emilia-Romagna Region (PRUa1GR-2012-002), and by the Fondazione del Monte di Bologna e Ravenna.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. P.C., Ma.Be., M.D., Mi.Ba., Ma.Ba.: study concept interpretation of data, drafting the manuscript; Ma.Ba., Mi.Ba., R.E.L., M.G., A.A., M.R., Ma.Ta., G.Z., L.M., Ma.Tu., S.B., L.N., A.F., R.M.P.: collection of data; Ma.Ba., Mi.Ba.: analysis of data; Mi.Ba., G.V., Ma.Bi., A.F., A.S., F.T., P.V., Ma.Be., P.C.: critical revision for important intellectual content.
References
- 1. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol 2014; 60:1310–24. [DOI] [PubMed] [Google Scholar]
- 2. Gustot T, Durand F, Lebrec D, et al. Severe sepsis in cirrhosis. Hepatology 2009; 50:2022–33. [DOI] [PubMed] [Google Scholar]
- 3. Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013; 144:1426–37, 1437.e1–9. [DOI] [PubMed] [Google Scholar]
- 4. Fernández J, Acevedo J, Wiest R, et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut 2018;. 67:1870–80. [DOI] [PubMed] [Google Scholar]
- 5. Mücke MM, Rumyantseva T, Mücke VT, et al. Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality. Liver Int 2018; 38:645–53. [DOI] [PubMed] [Google Scholar]
- 6. Arvaniti V, D’Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010; 139:1246–56, 1256.e1–5. [DOI] [PubMed] [Google Scholar]
- 7. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–700. [DOI] [PubMed] [Google Scholar]
- 8. Moore KP, Wong F, Gines P, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology 2003; 38:258–66. [DOI] [PubMed] [Google Scholar]
- 9. Stevens DL, Bisno AL, Chambers HF, et al. ; Infectious Diseases Society of America Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–52. [DOI] [PubMed] [Google Scholar]
- 10. Friedman ND, Kaye KS, Stout JE, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137:791–7. [DOI] [PubMed] [Google Scholar]
- 11. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 12. Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001; 33:464–70. [DOI] [PubMed] [Google Scholar]
- 13. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 2008; 359:1018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pugh RNH, Murray-Lyon IM. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60:1971–1974. [DOI] [PubMed] [Google Scholar]
- 15. Jalan R, Pavesi M, Saliba F, et al. ; CANONIC Study Investigators; EASL-CLIF Consortium The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol 2015; 62:831–40. [DOI] [PubMed] [Google Scholar]
- 16. Piano S, Bartoletti M, Tonon M, et al. Assessment of Sepsis-3 criteria and quick SOFA in patients with cirrhosis and bacterial infections. Gut 2018;. 67:1892–9. [DOI] [PubMed] [Google Scholar]
- 17. Sargenti K, Prytz H, Nilsson E, Kalaitzakis E. Predictors of mortality among patients with compensated and decompensated liver cirrhosis: the role of bacterial infections and infection-related acute-on-chronic liver failure. Scand J Gastroenterol 2015; 50:875–83. [DOI] [PubMed] [Google Scholar]
- 18. Shalimar RG, Jadaun SS, Ranjan G, et al. Prevalence, predictors and impact of bacterial infection in acute on chronic liver failure patients. Dig Liver Dis 2018; 50:1225–31. [DOI] [PubMed] [Google Scholar]
- 19. Bajaj JS, Reddy RK, Tandon P, et al. Prediction of fungal infection development and their impact on survival using the NACSELD cohort. Am J Gastroenterol 2018; 113:556–63. [DOI] [PubMed] [Google Scholar]
- 20. Zhao H, Zhao R, Hu J, et al. Upper gastrointestinal hemorrhage in acute-on-chronic liver failure: prevalence, characteristics, and impact on prognosis. Expert Rev Gastroenterol Hepatol 2019; 13:263–9. [DOI] [PubMed] [Google Scholar]
- 21. Mahmud N, Kaplan DE, Taddei TH, Goldberg DS. Incidence and mortality of acute-on-chronic liver failure using two definitions in patients with compensated cirrhosis. Hepatology 2019; 69:2150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.