Abstract
Background:
Diabetic neuropathy is the most common long-term complications of diabetes, frequently presenting as painful diabetic peripheral neuropathy (PDPN), which can significantly impair patients’ quality of life (QOL). This study set to estimate the prevalence of PNPD and health-related quality of life (HRQoL) in the setting of primary health care in Saudi Arabia.
Methods:
This study was conducted in primary health-care centers affiliated with the National Guard Health Affairs in Western Saudi Arabia. Arabic version of the Douleur Neuropathique 4 questionnaire was administered on diabetic patients to screen for neuropathic pain and short-form 12 questionnaire to assess HRQoL.
Results:
The study screened (n = 349) Type 2 diabetic patients. The prevalence of PDPN was 33.2%. PDRN was more likely to affect females (adjusted odds ratio [“AOR”] =1.96, P = 0.024), and those living with diabetes for over 15 years (AOR = 2.26, P = 0.039), and those on insulin treatment (AOR: 2.33, P = 0.010) alone or in combination (AOR = 1.78, P = 0.034). Both physical and mental components (MCs) of QOL scores were significantly higher in diabetic patients without PDPN compared to those with it; 49.57 ± 9.31 versus 40.77 ± 8.14 for physical component QOL and 51.72 ± 9.36 versus 44.35 ± 8.12 for MC QOL, P < 0.001.
Discussion and Conclusion:
Painful peripheral neuropathy is relatively common among type 2 diabetic patients in Western Saudi Arabia and impacts both physical and MCs of the QOL of affected patients.
Keywords: Diabetic, health-related quality of life, painful diabetic neuropathy, Saudi Arabia, Western region
Introduction
Diabetes mellitus (DM) constitutes a growing global problem. It is a chronic disease with huge personal sufferings and negative financial implications. DM affects 425 million people worldwide, over 8% of all adults of 20–79 years age. This very proportion is expected to rise to 10% and nearly 629 million of people will have DM by 2045.[1]
The current Saudi Arabian DM prevalence is 18.5%,[2] substantially surpassing the global (8.8%) and the regional (10.7%) figures. Hence, Saudi Arabia is among the countries with the highest magnitude of the disease.[3]
Diabetic neuropathy is the most common long-term complications of DM, and it is the leading initiating factor for foot ulceration, Charcot neuroarthropathy, and lower extremity amputation.[4] One of the common types of diabetic neuropathy is painful diabetic peripheral neuropathy (PDPN), described as a superficial burning pain associated with other sensory symptoms that affect the lower extremities.[5,6,7] PDPN is extremely common in Saudi Arabia and is estimated to affect 65.3% of diabetic patients.[8] PDPN significantly alters the patients’ quality of life (QOL).[9,10,11,12] Notably, QOL is defined by the World Health Organization as “an individual's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns.”[13] The prevalence of PDPN among Saudi patients in the primary care setting was estimated in a recent investigation at 35%,[14] although their use Michigan Neuropathy Screening Instrument, instead of the more accurate Douleur Neuropathique 4 (DN4) tool, remains questionable as it may have underestimated prevalence measures.[15] The regional estimated for PDPN varied considerably according to setting, population, country, and tool used. They ranged between 13.7% in Iran,[16] 32.2% in Kuwait,[17] 23% in Turkey,[18] and 53.7% among Egyptian, Lebanese and Jordanian patients,[19] and 42.2% in Libya.[20] Similar picture in terms of PDNP point prevalence emerged among international surveys. Proportion of DM patients who developed PDPN was 44.5% in Nigeria[21] and 30.6% in Taiwan[22] for instance. Studies across the world established an inverse association between PDNP and health-related quality of life (HRQoL). Substantial reduction in HRQoL, both physical and mental components (MCs), was noted in the samples of DM patients from Spain,[23] France,[24] and Belgium.[9]
No published data, to the best of our knowledge, are available on PDPN prevalence, or impact on QOL, among diabetic patients who live in the Western Region, Kingdom of Saudi Arabia (KSA). This study aimed to estimate the prevalence of PDPN, its impact on patients’ QOL and to determine its predictors.
Methodology
This was a descriptive, cross-sectional investigation conducted between May and December 2019 on diabetic patients selected from diabetic patients Type 2 who attended the local primary health-care centers affiliated with the National Guard Health Affairs in Western Saudi Arabia (namely, King Khalid Residential City Clinic [Taif Housing] in Taif city, Sharia center in Makkah city, Specialized polyclinic, Al-Iskan center and Bahra center in Jeddah city). The study was approved at King Abdul-Aziz Medical City, Ministry of National Guard-Health Affairs Jeddah.
Patients were included if they were over 25 and under 65 and had confirmed the diagnosis of Type 2 DM. They were excluded if they had comorbid neurological disorders, malignancy, history of nerve root compression, other pain conditions unrelated to diabetic peripheral neuropathy, or alcoholism.
Based on 65.3% PDPN prevalence,[8] and 80% power, 5% significance, a sample size of (n = 348) was required for the study.
We adopted the random cluster sampling technique. Patients were sampled randomly and equally (n = 87) from each of the four centers.
The data were collected by using an interview-administered questionnaire consisting of 3 parts. The first part constituted the personal demographic and clinical data about diabetes. The second part includes the Arabic version of the DN4 questionnaire which is valid and reliable screening tool for neuropathic pain whose Cronbach's value was 0.67. DN4 includes 10 items; 7 items are based on an interview with the patient that related to pain quality (i.e., sensory and pain descriptors) and 3 items based on the clinical examination. A score of 4 was found to be the best cutoff for the diagnosis of neuropathic pain.[25] The third part includes the application of Arabic HRQoL short-form (SF12) questionnaire, whose Cronbach's was 0.84 considered very good reliability level.[26] It has two components: physical component (PC) and a MC. The scoring system used for scoring of the SF12 questionnaire was based on work by Ware et al.[27]
With the consent of target institutions and treating physicians, all patients fitting the inclusion criteria were recruited while waiting for their turn in the clinics waiting areas on random days after making sure the diabetes clinics were available. Questionnaires were filled out through a face-to-face interview for each patient. Interviews took an average of 10–15 min to be completed. Interviews were conducted on different days of the week and at varying times to ensure a representative cross-sectional sample of patients. The nurses in charge at the clinics introduced the study to all eligible patients present in the waiting areas before visiting the physicians during the follow-up visits. Some of trained medical interns were involved to help in data collection. They underwent comprehensive training in questionnaire administration and interviewing techniques. In addition, weekly meetings of the research team were held to ensure the inter-rater reliability and the standardization of data collection protocol. Each participant was interviewed only once.
Health-care providers responsible for the treatment of interviewed patients were not present during the interviews. The participants were assured that any information they reveal will remain confidential and will be strictly used for research purposes only. Patients were not paid to take part in the study and were informed that they are free to decline answering any questions they will not be comfortable with. Signed consent forms were obtained from all participating patients. Research data, both soft and hard copies, were maintained in a secure location and/or unit within NGHA premises and were only accessible by the Research Team.
The data were checked for completeness, and responses were coded and entered into the Statistical Package for the Social Sciences (SPSS) software version 25 for Windows. Then results were tabulated, graphically and statistically analyzed. Categorical variables were described in the form of frequency and percentage, whereas continuous variables were summarized by mean and standard deviation. Comparisons between the variables were made by using the Chi-squared test (bivariate analysis). Multivariate logistic regression model was utilized to evaluate the impact of covariates on PDPN. P = 0.05 was used to determine the statistical significance.
The study was granted ethical approval on April 23, 2019, by Biomedical Ethics Section of King Abdullah International Medical Research Centre's International Review Board Office, affiliated to the Health Affairs Department of the Ministry of National Guard; Memo Ref No. IRBC/0560/19.
Results
A total of n = 349 patients with DM participated in this investigation. Over 43.9% of them exceeded 55 years in age. Females were 56.4% and 46.2% were illiterates, whereas 7.8% were university or above graduated [Table 1]. Of all participants, 54.1% were obese and 35.2% were overweight, as illustrated in Figure 1.
Table 1.
Demographic characteristics of the patients
| n (%) | |
|---|---|
| Age (years) | |
| 25-35 | 5 (1.4) |
| 36-45 | 42 (12.0) |
| 46-55 | 114 (32.7) |
| >55 | 188 (43.9) |
| Gender | |
| Male | 152 (43.6) |
| Female | 197 (56.4) |
| Educational level (n=348) | |
| Illiterate | 161 (46.2) |
| Elementary school | 72 (20.7) |
| Intermediate school | 42 (12.1) |
| High school | 46 (13.2) |
| University/above | 27 (7.8) |
Figure 1.
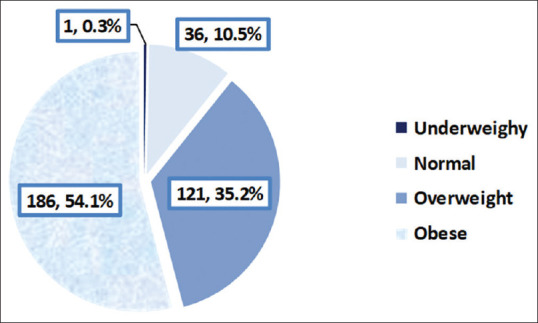
Body mass index of the participants
The duration of diabetes ranged between 5 and 10 years among 30.6% of the patients, while it exceeded 15 years in 27.5% of them. Glycated hemoglobin, unfortunately, exceeded 7% among most of the participants (72.8%). More than half of the patients (56.3%) were treated by oral hypoglycemic drugs only, whereas 9.8% were treated by insulin only and 32.2% were treated by a combination of both Table 2 summarizes the diabetes-related characteristics of the participants.
Table 2.
Diabetes-related characteristics of the participants
| n (%) | |
|---|---|
| Duration of diabetes (years) | |
| <5 | 91 (26.1) |
| 5-10 | 107 (30.6) |
| 11-15 | 55 (15.8) |
| >15 | 96 (27.5) |
| Glycated hemoglobin (%) (n=342) | |
| <7 | 93 (27.2) |
| 7-8 | 96 (28.1) |
| 8.1-9.5 | 77 (22.5) |
| >9.5 | 76 (22.2) |
| Medication for diabetes (n=348) | |
| None | 6 (1.7) |
| Oral hypoglycemic | 196 (56.3) |
| Insulin | 34 (9.8) |
| Oral hypoglycemic and insulin | 112 (32.2) |
Participants’ responses to the DN4 questionnaire are on display in Table 3. Burning sensation was reported by 43% of DM patients and numbness in the same area of pain by 46.4%. Physical examination revealed hypoesthesia to touch in the same pain location in 12.9% of patients. The pain was accentuated by brushing in 4.6% of cases. The prevalence of PDPN in our DM sample was 33.2%, as shown in Figure 2.
Table 3.
Response of the participants to the Douleur Neuropathique 4 questionnaire
| Yes, n (%) | No, n (%) | |
|---|---|---|
| Does the pain have one or more of the following characteristics? | ||
| Burning | 150 (43.0) | 199 (57.0) |
| Painful cold | 94 (26.9) | 255 (73.1) |
| Electric shocks | 79 (22.6) | 270 (77.4) |
| Is the pain associated with one or more of the following symptoms in the same area? | ||
| Tingling | 124 (35.5) | 225 (64.5) |
| Pins and needles | 111 (31.8) | 238 (68.2) |
| Numbness | 162 (46.4) | 187 (53.6) |
| Itching | 66 (18.9) | 283 (81.1) |
| Is the pain located in an area where the physical examination may reveal one or more of the following characteristics? | ||
| Hypoesthesia to touch | 45 (12.9) | 304 (87.1) |
| Hypoesthesia to pinprick | 38 (10.9) | 311 (89.1) |
| In the painful area, can the pain be caused or increased by: Brushing? | 16 (4.6) | 333 (95.4) |
Figure 2.
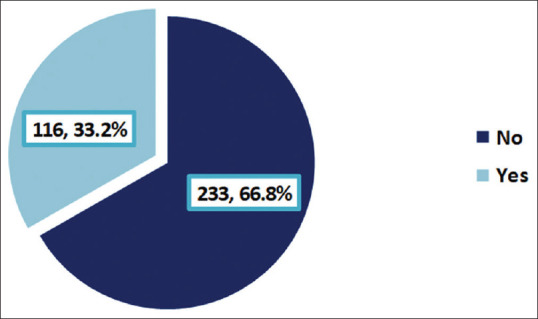
Prevalence of painful diabetic peripheral neuropathy among Type 2 diabetic patients
Women were more likely to have PDPN compared to men (40.6% vs. 23.7%, P = 0.001). Illiterate patients had the highest rate of PDPN (41.6%), whereas those with intermediate school education had the lowest rate (19%), P = 0.007. Concerning body mass index (BMI), normal participants had the highest rate of PDPN (44.4%), whereas overweight participants had the lowest rate (19.8%), P = 0.001. More than half (50.5%) of patients whose duration of diabetes exceeded 15 years compared to 18.7% of those whose duration of diabetes ranged between 5 and 10 years had PDPN (P < 0.001). More than half of patients treated with insulin (55.9%) compared to those without treatment and 23.6% of those treated with only oral hypoglycemic drugs had PDPN, (P < 0.001) [Table 4].
Table 4.
Factors associated with painful peripheral neuropathy: Bivariate analysis
| Painful diabetic peripheral neuropathy | P* | ||
|---|---|---|---|
| No (n=233), n (%) | Yes (n=116), n (%) | ||
| Age (years) | |||
| 25-35 (n=5) | 4 (80.0) | 1 (20.0) | 0.181 |
| 36-45 (n=42) | 31 (73.8) | 11 (26.2) | |
| 46-55 (n=114) | 82 (71.9) | 32 (28.1) | |
| >55 (n=188) | 116 (61.7) | 72 (38.3) | |
| Gender | |||
| Male (n=152) | 116 (76.3) | 36 (23.7) | 0.001 |
| Female (n=197) | 117 (59.4) | 80 (40.6) | |
| Educational level (n=348) | |||
| Illiterate (n=116) | 94 (58.4) | 67 (41.6) | 0.007 |
| Elementary school (n=72) | 55 (76.4) | 17 (23.6) | |
| Intermediate school (n=42) | 34 (81.0) | 8 (19.0) | |
| High-school (n=46) | 28 (60.9) | 18 (39.1) | |
| University/above (n=27) | 21 (77.8) | 6 (22.2) | |
| Body mass index (n=343) | |||
| Normal (n=36) | 20 (55.6) | 16 (44.4) | 0.001 |
| Overweight (n=121) | 97 (80.2) | 24 (19.8) | |
| Obese (n=186) | 113 (60.8) | 73 (39.2) | |
| Duration of diabetes (years) | |||
| <5 (n=86) | 65 (75.6) | 21 (24.4) | <0.001 |
| 5-10 (n=107) | 87 (81.3) | 20 (18.7) | |
| 11-15 (n=55) | 31 (56.4) | 24 (43.6) | |
| >15 (n=95) | 47 (49.5) | 48 (50.5) | |
| Glycated hemoglobin (%) (n=342) | |||
| <7 (n=91) | 69 (75.8) | 22 (24.2) | 0.212 |
| 7-8 (n=95) | 64 (67.4) | 31 (32.6) | |
| 8.1-9.5 (n=75) | 46 (61.3) | 29 (38.7) | |
| >9.5 (n=76) | 49 (64.5) | 27 (35.5) | |
| Medication for diabetes (n=348) | |||
| None (n=6) | 6 (100) | 0 (0.0) | <0.001 |
| Oral hypoglycemic (n=191) | 146 (76.4) | 45 (23.6) | |
| Insulin (n=34) | 15 (44.1) | 19 (55.9) | |
| Oral hypoglycemic and insulin (n=111) | 63 (56.8) | 48 (43.2) | |
*Pearson Chi-square
Multivariate logistic regression analysis revealed that after controlling for confounding effect, females were at higher risk to develop PDPN compared to males (adjusted odds ratio [“AOR”]: 1.96; 95% confidence interval [CI]: 1.09–3.50, P = 0.024). Compared to patients whose duration of diabetes was <5 years, those with duration exceeded 15 years were over double risk to have PDPN (AOR: 2.26; 95% CI: 1.04–4.93, P = 0.039). Considering patients who did not take treatment as a reference category, those treated either insulin alone or a combination of insulin and oral hypoglycemic were more likely to develop PDPN (AOR: 2.33; 95% CI: 1.59–3.42, P = 0.010 and AOR: 1.78; 95% CI: 1.51–2.09, P = 0.034, respectively). Patients’ educational level and BMI were not significantly associated with the development of PDPN. The results are on display in [Table 5].
Table 5.
Predictors of painful diabetic peripheral neuropathy: Results of multivariate logistic regression analysis
| Adjusted OR | 95% CI | P | |
|---|---|---|---|
| Gender | |||
| Male (n=152)a | 1.0 | - | |
| Female (n=197) | 1.96 | 1.09-3.50 | 0.024 |
| Duration of diabetes (years) | |||
| <5 (n=86)a | 1.0 | - | |
| 5-10 (n=107) | 0.61 | 0.45-2.03 | 0.202 |
| 11-15 (n=55) | 1.99 | 0.87-4.56 | 0.104 |
| >15 (n=95) | 2.26 | 1.04-4.93 | 0.039 |
| Medication for diabetes (n=348) | |||
| None (n=6) | 1.0 | - | |
| Oral hypoglycemic (n=191) | 1.13 | 0.91-2.42 | 0.171 |
| Insulin (n=34) | 2.33 | 1.59-3.42 | 0.010 |
| Oral hypoglycemic and insulin (n=111) | 1.78 | 1.51-2.09 | 0.034 |
aReference category. OR: Odds ratio, CI: Confidence interval
As illustrated in Table 6, both physical and MCs of QOL scores were significantly higher in diabetic patients without PDPN compared to those with it; 49.57 ± 9.31 versus 40.77 ± 8.14 for PC QOL and 51.72 ± 9.36 versus 44.35 ± 8.12 for MC QOL, P < 0.001.
Table 6.
Quality of life among diabetic patients, according to the presence of painful diabetic neuropathy
| Mean±SD | P | ||
|---|---|---|---|
| Patients without PDPN | Patients with PDPN | ||
| PC QOL | 49.57±9.31 | 40.77±8.14 | <0.001 |
| MC QOL | 51.72±9.36 | 44.35±8.12 | <0.001 |
SD: Standard deviation; QOL: Quality of life, PDPN: Painful diabetic peripheral neuropathy, PC: Physical component, MC: Mental component
Discussion
The present study is considered the first in the western region of KSA to explore PDPN frequency and associated factors, uncovered prevalence of PDPN among Type 2 diabetic patients was 33.2%; consistent with the 35% and 37.1% figures reported in recent studies from Riyadh,[14] and collective Gulf states;[19] but well below the 65.3% rate reported in earlier Saudi studies.[8] This is clearly an artifact of using different diagnostic tools in detection of PDPN. International surveys suffered same difficulty as USA PDPN prevalence rates ranged between 11% and 25%,[28] whereas UK rates were in the region of 33%.[29] Substantial heterogeneity was noted in the consequence of utilizing different tools to diagnose PDPN as well as variation in the characteristics of samples, methodology of studies, and inclusion criteria.[30]
One important key fining in or study is that female gender, DM duration, and treatment with either insulin alone or in combination with oral hypoglycemic were, collectively, the factors that exerted significant impact on PDNP. Both educational level and BMI, although significant in the unadjusted analysis, were not associated with PDNP at the multivariate level analysis. Our results tally well with regional and international studies as the longer duration of diabetes increase PDNP susceptibility.[8,20,31,32,33,34,35]
Our results, in agreement with previous research findings,[14] insulin therapy was an independent risk factor for PDPN. This could well reflect the severity of the disease and poor glycemic control. The finding that females were at higher risk for PDPN was also observed by others[9,21] and could be attributed to the more sedentary life and higher BMI compared to males.
In our current study, both physical and MCs of QOL scores were lower in diabetic patients with PDPN. The same has been observed in studies carried out in Spain,[22] Belgium,[9] USA,[36] Greece,[37] and France,[24] utilizing the same tool used in the present study (HRQoL-SF12). This deterioration in both physical and MCs of QOL is mostly attributed to adverse effects of the disease on patients such as limited daily activities, erectile dysfunction, pain sensation, and poor quality of sleep. Therefore, there is a need to regularly evaluate the HRQoL of diabetic patients with peripheral neuropathy.[36,38]
This current study has several strengths that addressed past researchers’ shortcomings. First, we used DN4; a notoriously reliable, valid, and easily to use diagnostic tool for PDPN. Second, we utilized a reliable and valid tool to assess physical and MCs of QoL in diabetic patients (namely, SF-12). Third, our patients were carefully recruited from primary health-care centers, to represent the overall population of diabetic patients usually seen by family physicians. The results of our study are relevant to family physicians who manage the majority of patients with diabetes in the community. Fourth, we applied robust statistical method to compare with PDNP patients with diabetic patients without peripheral neuropathy to identify and qualitatively evaluate potential risk factors. However, the study has a range of limitations that need to be taken onboard before generalizing its results. We did not include some of the important risk factors that could affect the development of PDPN such as hypertension and lipid profile, particularly triglyceride and cholesterol. Moreover, full neurological examination was not performed to confirm the diagnosis. Furthermore, our investigation evaluated the point prevalence, given its cross-sectional nature. Future research should employ a longitudinal design, with huge sample sizes, and preferably, big data and machine learning methods.
Conclusion
We summarize the key points as follows:
PDNP affects almost a third of DM patients in Western Saudi Arabia
Female patients, those with longer duration of the disease and treated with either insulin alone or a combination of insulin and oral hypoglycemic were more likely to have PNDP
PNDP negatively impacts both physical and MCs of the QOL of affected DM patients
Training should be provided to health-care workers and family physician in terms of identification (by use of DN4), treatment, and prevention of PDPN
Regular assessment of QOL of DM patient should be included in any routine clinical encounter
Further studies should be more comprehensive in terms of risk factors and larger in scale.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors wish to acknowledge the leadership teams of the primary health centers that authorized data collection work of this study to be carried at their premises. The authors are also deeply indebted to every patient that took the time to complete the questionnaire associated with this study.
References
- 1.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.IDF. [Last accessed on 31 Mar 2020]. Available from: https://idforg/our-network/regions-members/middle-east-andnorth-africa/members/46-saudi-arabiahtml .
- 3.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–24. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 5.Chong MS, Hester J. Diabetic painful neuropathy: Current and future treatment options. Drugs. 2007;67:569–85. doi: 10.2165/00003495-200767040-00006. [DOI] [PubMed] [Google Scholar]
- 6.Sommer C. Painful neuropathies. Curr Opin Neurol. 2003;16:623–8. doi: 10.1097/01.wco.0000093106.34793.06. [DOI] [PubMed] [Google Scholar]
- 7.Kästenbauer T, Irsigler P, Sauseng S, Grimm A, Prager R. The prevalence of symptoms of sensorimotor and autonomic neuropathy in Type 1 and Type 2 diabetic subjects. J Diabetes Complications. 2004;18:27–31. doi: 10.1016/S1056-8727(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 8.Halawa MR, Karawagh A, Zeidan A, Mahmoud AE, Sakr M, Hegazy A. Prevalence of painful diabetic peripheral neuropathy among patients suffering from diabetes mellitus in Saudi Arabia. Curr Med Res Opin. 2010;26:337–43. doi: 10.1185/03007990903471940. [DOI] [PubMed] [Google Scholar]
- 9.Ligda G, Ploubidis D, Foteli S, Kontou PI, Nikolaou C, Tentolouris N. Quality of life in subjects with type 2 diabetes mellitus with diabetic retinopathy: A case-control study. Diabetes Metab Syndr. 2019;13:947–52. doi: 10.1016/j.dsx.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Tonetto IF, Baptista MH, Gomides DD, Pace AE. Quality of life of people with diabetes mellitus. Rev Esc Enferm USP. 2019;53:e03424. doi: 10.1590/S1980-220X2018002803424. [DOI] [PubMed] [Google Scholar]
- 11.Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: Clinical and quality-of-life issues. Mayo Clin Proc. 2006;81:S3–11. doi: 10.1016/s0025-6196(11)61474-2. [DOI] [PubMed] [Google Scholar]
- 12.Mokhtari Z, Gheshlagh RG, Kurdi A. Health-related quality of life in Iranian patients with type 2 diabetes: An updated meta-analysis. Diabetes Metab Syndr. 2019;13:402–7. doi: 10.1016/j.dsx.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 13.The World Health Organization Quality of Life assessment (WHOQOL): Position paper from the World Health Organization. Soc Sci Med. 1995;41:1403–9. doi: 10.1016/0277-9536(95)00112-k. [DOI] [PubMed] [Google Scholar]
- 14.Algeffari MA. Painful Diabetic Peripheral Neuropathy among Saudi Diabetic Patients is Common but Under-recognized: Multicenter Cross-sectional study at primary health care setting. J Family Community Med. 2018;25:43–7. doi: 10.4103/jfcm.JFCM_145_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sendi RA, Mahrus AM, Saeed RM, Mohammed MA, Al-Dubai SAR. Diabetic peripheral neuropathy among Saudi diabetic patients: A multicenter cross-sectional study at primary health care setting. J Family Med Prim Care. 2020;9:197–201. doi: 10.4103/jfmpc.jfmpc_927_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salman Roghani R, Delbari A, Asadi-Lari M, Rashedi V, Lökk J. Neuropathic pain prevalence of older adults in an Urban Area of Iran: A population-based study. Pain Res Treat. 2019;2019:9015695. doi: 10.1155/2019/9015695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zghoul N, Ross EL, Edwards RR, Ahmed A, Jamison RN. Prevalence of chronic pain with neuropathic characteristics: A randomized telephone survey among medical center patients in Kuwait. J Pain Res. 2017;10:679–87. doi: 10.2147/JPR.S123966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Celik S, Yenidunya G, Temel E, Purisa S, Uzum AK, Gul N, et al. Utility of DN4 questionnaire in assessment of neuropathic pain and its clinical correlations in Turkish patients with diabetes mellitus. Prim Care Diabetes. 2016;10:259–64. doi: 10.1016/j.pcd.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Jambart S, Ammache Z, Haddad F, Younes A, Hassoun A, Abdalla K, et al. Prevalence of painful diabetic peripheral neuropathy among patients with diabetes mellitus in the Middle East region. J Int Med Res. 2011;39:366–77. doi: 10.1177/147323001103900204. [DOI] [PubMed] [Google Scholar]
- 20.Garoushi S, Johnson MI, Tashani OA. A cross-sectional study to estimate the point prevalence of painful diabetic neuropathy in Eastern Libya. BMC Public Health. 2019;19:78. doi: 10.1186/s12889-018-6374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young EE, Nwatu CB, Ekenze OS, Onodugo OD, Onyenekwe BM, Ugwu ET, et al. Prevalence of painful diabetic peripheral neuropathy among patients with diabetes attending a tertiary outpatient diabetes clinic in Nigeria. J Adv Med Med Res. 2019;28:1–10. [Google Scholar]
- 22.Jane SW, Lin MS, Chiu WN, Beaton RD, Chen MY. Prevalence, discomfort and self-relief behaviours of painful diabetic neuropathy in Taiwan: A cross-sectional study. BMJ Open. 2016;6:e011897. doi: 10.1136/bmjopen-2016-011897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madariaga Muñoz MC, Villegas Estévez F, Jiménez López AJ, Cabezón Álvarez A, Soler López B. Evaluation of quality of life and satisfaction of patients with neuropathic pain and breakthrough pain: Economic impact based on quality of life. Pain Res Treat. 2018;2018:5394021. doi: 10.1155/2018/5394021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouhassira D, Letanoux M, Hartemann A. Chronic pain with neuropathic characteristics in diabetic patients: A French cross-sectional study. PLoS One. 2013;8:e74195. doi: 10.1371/journal.pone.0074195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terkawi AS, Abolkhair A, Didier B, Alzhahrani T, Alsohaibani M, Terkawi YS, et al. Development and validation of Arabic version of the douleur neuropathique 4 questionnaire. Saudi J Anaesth. 2017;11:S31–9. doi: 10.4103/sja.SJA_97_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Shehri AH, Taha AZ, Bahnassy AA, Salah M. Health-related quality of life in type 2 diabetic patients. Ann Saudi Med. 2008;28:352–60. doi: 10.5144/0256-4947.2008.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ware JE, Kosinski MA, Keller SD. SF-12 how to Score the SF-12 Physical and Mental Health Summary Scales. Lincoln, RI: QualityMetric Inc; 1998. [Google Scholar]
- 28.Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2012;28(Suppl 1):8–14. doi: 10.1002/dmrr.2239. [DOI] [PubMed] [Google Scholar]
- 29.Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34:2220–4. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: Epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008;9:660–74. doi: 10.1111/j.1526-4637.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 31.Aouiche S, Ouerdane K, Frioui M, Ait Boudaoud A, Ragguem A, Boudiba A. Painful diabetic neuropathy : Frequency, risk factors, and severity in a cohort of 400 diabetic patients in Algeria. Mé Maladies Métaboliques. 2014;8:211–5. [Google Scholar]
- 32.Petropoulos IN, Azmi S, Khan A, Ponirakis G, Malik RA. Diabetic neuropathy and painful diabetic neuropathy in the Middle East and North Africa (MENA) region: Much work needs to be done. J Taibah Univer Med Sci. 2016;11:284–94. [Google Scholar]
- 33.Hebert HL, Abirami V, Nicola T, Blair HS. Risk factors for neuropathic pain in diabetes mellitus. Pain. 2017;158:560–8. doi: 10.1097/j.pain.0000000000000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aizarani C, Amir AA, Benchouk Z, Abu Al-Samen MA, Farghaly M, Adnan Kandil A, et al. The dos and don'ts of painful diabetic peripheral neuropathy: Primary care guidelines for the Middle East and North Africa. Middle East J Fam Med. 2017;7:4–18. [Google Scholar]
- 35.Erbas T, Ertas M, Yucel A, Keskinaslan A, Senocak M TURNEP Study Group. Prevalence of peripheral neuropathy and painful peripheral neuropathy in Turkish diabetic patients. J Clin Neurophysiol. 2011;28:51–5. doi: 10.1097/WNP.0b013e3182051334. [DOI] [PubMed] [Google Scholar]
- 36.Smith SC, Lamping DL, Maclaine GD. Measuring health-related quality of life in diabetic peripheral neuropathy: A systematic review. Diabetes Res Clin Pract. 2012;96:261–70. doi: 10.1016/j.diabres.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Lyrakos NG, Hatziagelaki E, Damigos D, Papazafiropoulou KA, Bousboulas S, Batistaki C. Predictors of health-related quality of life in diabetic neuropathy type ii diabetic patients in Greece. Health Sci J. 2013;7:327–41. [Google Scholar]
- 38.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: A systematic review and meta-analysis. Diabet Med. 2006;23:1165–73. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]


